Abstract
Background
Rapid growth in the provision of cardiac imaging tests has led to concerns about overuse. Little is known about the degree to which health care delivery system characteristics influence use and variation in echocardiography.
Methods
We analyzed administrative claims of veterans with heart failure older than 65 years from 2007 to 2010 across 34 metropolitan service areas (MSAs). We compared overall rates and geographic variation in use of transthoracic echocardiography (TTE) between veterans who used the Veterans Health Administration (VA) and propensity-matched veterans who used Medicare. “Dual users” were excluded.
Results
There were no significant differences in clinical characteristics or mortality between the propensity-matched cohorts (overall n = 30,404 veterans, mean age 76 years, mortality rate 52%). The Medicare cohort had a significantly higher overall rate of TTE use compared with the VA cohort (1.25 vs 0.38 TTEs per person-year, incidence rate ratio 2.89 [95% CI 2.80-3.00], both P< .001), but a similar coefficient of variation across MSAs (0.36 [95% CI 0.27-045] vs 0.48 [95% CI 0.37-0.59]). There was a moderate to strong correlation in variation at the MSA level between cohorts (Spearman r = 0.58, P < .001).
Conclusion
Overall rates of TTE use were significantly higher in a Medicare cohort compared with a propensity score-matched VA cohort of veterans with heart failure living in urban areas, with similar relative degrees of geographic variation and moderate to strong regional correlation. Rates of TTE use may be strongly influenced by health care system characteristics, but local practice styles influence echocardiography rates irrespective of health system.
Growth in costs of health care for cardiovascular patients has been driven largely by a substantial increase in the rate of diagnostic imaging. Among Medicare beneficiaries, annual rates of echocardiography doubled and annual rates of nuclear stress tests tripled between 1999 and 2006, accounting for nearly half of the total growth in the costs of cardiology services.1 Furthermore, there is substantial geographic variation in the use of cardiac imaging that may not be explained entirely by differences in clinical appropriateness.2,3 These findings raise concern that rising rates of cardiac imaging could be attributable in part to overuse.4,5 As a result, professional societies have attempted to better elucidate the complex mechanisms that drive imaging rates.6–8 However, the degree to which differing characteristics of health care delivery systems account for overall cardiac imaging volume and geographic variation in cardiac imaging use remains unclear.
To address this question, we compared overall rates, degree of geographic variation, and correlation in local rates of use of resting transthoracic echocardiography (TTE) among veterans with heart failure (HF) who use services provided by the Veterans Health Administration (VA) vs similar veterans who use nonfederal health systems reimbursed by FFS Medicare. The VA health system is an integrated health care delivery system operating under a fixed global budget. Several studies have shown that the quality of care patients receive within the VA system is high, and outcomes are equal to or better than non-VA patients.9–12
Using administrative claims from VA and Medicare of propensity-matched veterans with HF older than 65 years from 2007 to 2010, we examined use rates and assessed whether the degree of geographic variation in use of TTE was higher among veterans receiving care primarily from Medicare vs the VA.
Methods
Study design and data source
We conducted a cohort study comparing rates of echocardiography among veterans who received care from the VA vs from hospitals participating in fee-for-service (FFS) Medicare from 2007 to 2010. All VA-enrolled or VA-eligible veterans who had at least 1 inpatient hospitalization or at least 2 outpatient encounters for HF between 2002 and 2011 were included. We obtained all VA administrative records, as well as comprehensive Medicare FFS administrative claims from the Outpatient, Carrier, and MedPAR files for each veteran in the cohort during this period. There were over 2 million veterans with HF included in this database, which was assembled as part of a VA Health Services Research and Development-funded study. Demographic data for all patients included dates of birth and death, sex, and zip code of residence. We used 2010 US Census per-capita income data mapped to counties (based on zip code of residence) to estimate the median household income for each veteran. Comorbid conditions were assessed from administrative claims using algorithms from the Centers for Medicare and Medicaid Service's Chronic Conditions Data Warehouse.13
Geographic selection
The analysis was restricted to veterans living in the largest major metropolitan service areas (MSAs; n = 36) in the United States, defined as cities and their surrounding suburbs with populations more than 1,000,000 people based on the 2010 US Census, and that had at least 1 VA medical center. Metropolitan service areas with populations more than 1,000,000 but no major VA medical center (n = 2) were excluded.
Prior studies of geographic variation have used hospital referral regions (HRRs) defined by the Dartmouth Atlas project as the geographic unit.14 However, we chose to use MSAs to concentrate on practice patterns in metropolitan areas because HRRs were developed to reflect referral patterns among Medicare populations that occasionally include a much broader surrounding area.
VA and Medicare cohort selection
We included only veterans 65 years and older because Medicare coverage is nearly universal after that age. Inclusion criteria for the VA and Medicare cohorts were designed to include veterans who chose to receive their health care exclusively within either the VA or alternatively at nonfederal hospitals participating in FFS Medicare. Therefore, we excluded any patient who had claims filed with both VA and Medicare. Furthermore, we limited our VA cohort to include only patients who were potential recipients of cardiac imaging within VA by excluding those veterans who used the VA for only supplementary medical services such as prescription renewal. The VA cohort therefore included only patients who had 3 or more outpatient visits at the VA within a 12-month period, received at least 1 medical or surgical procedure within the VA during the study period, or had at least 1 hospitalization within the VA during the study period. Veterans who entered (ie, received an initial diagnosis of HF during the 2007-2010 study period) and exited the cohort (ie, at date of death) during the study period were accounted for by appropriate measures of follow-up time and censoring.
Propensity score calculation and matching
To minimize differences between cohorts, we used propensity scores to match veterans in the Medicare cohort to veterans in the VA cohort.15 Propensity scores were calculated as probabilistic measures reflecting the likelihood of a veteran to use the VA vs Medicare, based on demographic and clinical characteristics. The propensity score was calculated using multivariate logistic regression that included the following independent variables: age, sex, and median household income; presence/absence of ischemic heart disease, chronic kidney disease, chronic pulmonary disease, diabetes mellitus, hypertension, stroke/transient ischemic attack, and lung, colorectal, prostate, or breast cancer; receipt of cardiac procedures including percutaneous coronary intervention, surgical valve repair/replacement, coronary artery bypass graft, heart transplant, or implantation of a circulatory support device; and a dummy variable for year of diagnosis of HF. The success of the propensity score is determined by its ability to balance independent covariates between the patients who used the VA vs Medicare. This covariate balance was assessed using standard mean differences.
To create our matched cohorts, veterans who used the VA were matched to patients who had a similar propensity to use the VA but instead used Medicare. Matching was performed using a 1-to-1 “nearest-neighbor” matching algorithm without replacement,16 and with maximal caliper distance of 25% of the standard deviation of all propensity scores. In addition,wespecifiedanexact match at the MSA level to ensure equal numbers of veterans in each cohort within each MSA. We then tested whether rates of death, use of implantable cardioverter-defibrillators (ICDs), and hospitalizations for HF (outcomes not included in the calculation of propensity scores because they could potentially be affected by use of echocardiography) were different between the matched cohorts.
Procedure identification
Receipt of a TTE was identified by Current Procedural Technology (CPT) codes 93303-93325, or by International Classification of Diseases, Ninth Revision (ICD-9) code 88.72. Treadmill or pharmacologic stress TTEs were not included in the analysis. For each patient in the cohort, we summed the total number of TTEs performed over all study years and removed any potential duplicate references to the same study (ie, same patient, CPT code, and date of study). We included both inpatient and outpatient studies.
Coronary artery bypass graft surgery was identified by ICD-9 codes 36.1× or 36.2×, valve repair/replacement surgery by codes 35.1× or 35.2×, and heart transplant or circulatory assist device implantation by codes 37.5× or 37.6×. Percutaneous coronary intervention was identified by ICD-9 codes 00.66, 36.01-36.07, and 36.09 or CPT codes 92980-92984, 92995, 92996, G0290, and G0291. Receipt of an ICD was identified by ICD-9 codes 00.51, 00.54, 37.94, and 37.98 or CPT code 33249. Hospitalizations with a primary discharge diagnosis of HF were identified by ICD-9 codes 398.91, 402.01, 402.11, 402.91, 404.01, 404.11, 404.91, 404.03, 404.13, 404.93, 425.4, 428.0, 428.1, 428.20, 428.21, 428.22, 428.23, 428.30, 428.31, 428.32, 428.33, 428.40, 428.41, 428.42, 428.43, or 428.9.
Statistical analysis
Standard paired summary statistics were used to compare the baseline patient-level characteristics between the propensity-matched cohorts. Rates of mortality, hospitalizations for HF, and ICD use were compared between the matched groups using McNemar tests. Differences in survival were assessed using the Kaplan-Meier method and log-rank tests. Rates of TTEs per person-year for each MSA by cohort were calculated by dividing the total number of TTEs among the population within a given region during the study period by the eligible veteran population within that region. We used a Spearman rank-order correlation coefficient to test whether there was a correlation between the MSA-level rates of TTE use for the VA and Medicare cohorts.
To compare the overall TTE use rates across MSAs between the VA cohort and the propensity-matched Medicare cohort, we used 2 methods. First, we compared the mean overall rates of TTE use between the VA and Medicare cohorts using a paired t test. Next, to account for variability between MSA-level and patient-level characteristics, we used a multilevel mixed-effects negative binomial regression model. Specifically,we used the number of TTEs per veteran as the dependent variable; age, sex, income, presence/absence of ischemic heart disease, chronic kidney disease, chronic obstructive pulmonary disease, diabetes mellitus, hypertension, stroke/transient ischemic attack, cancer, receipt of cardiac surgery, and an indicator for cohort as the independent variables; an exposure term for number of years present in the cohort; and random effects specified at the MSA level.
We explored the degree of regional variation between the VA and Medicare cohorts in 2 ways. First, we calculated the difference between the maximum and minimum MSA-level procedure rates for each cohort. Second, we compared the coefficients of variation of the mean rates of TTE use across MSAs between the VA and Medicare cohorts.
Statistical analyses were conducted using Stata version 13.1 (College Station, TX). P < .05 (2 sided) was considered statistically significant. The institutional review board at the Philadelphia Veterans Affairs Medical Center approved the study.
Dr Kini received support from the National Institutes of Health (5T32HL007843-18). Dr Groeneveld received support from the VA's Health Services Research and Development Service (Grant Nos. 1I0HX000523 and 1I01HX001630). The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, and its final contents.
Results
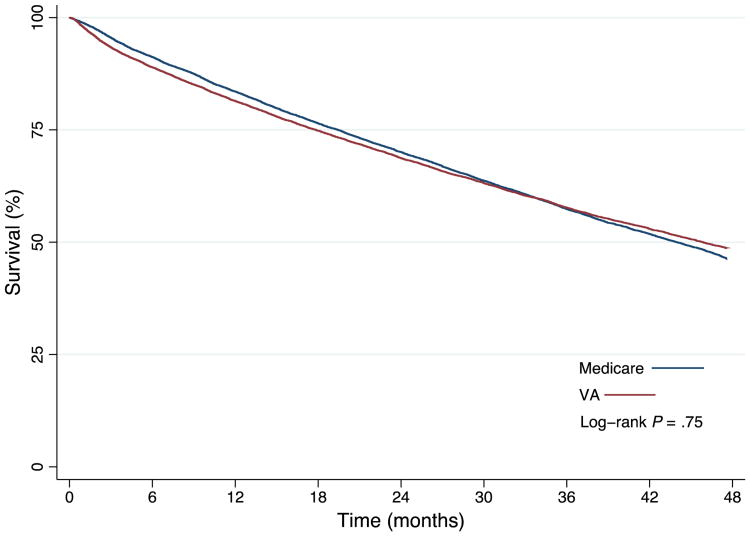
The primary cohort was selected from a pool of 15,330 VA veterans and 224,525 Medicare veterans living in urban areas who were older than 65 years with HF. Propensity score matching yielded a primary VA cohort that included 15,202 veterans and 40,158 person-years, and a primary Medicare cohort that included 15,202 veterans and 40,094 person-years. All patient-level characteristics were statistically balanced after matching (Table I). Geographically, all veterans were matched within the 34 MSAs, which were broadly distributed across 30 states. There was no significant difference in mortality between the VA and Medicare cohorts (52.0% vs 52.4%, log-rank P = .75) (Figure 1) or ICD use between the VA and Medicare cohorts (6.9% vs 7.1%, P = .41). However, the proportion of veterans who experienced at least 1 hospitalization for HF was greater in the VA cohort compared with the Medicare cohort (17% vs 12%, P < .001) (Table I).
Table I. Characteristics of the VA and propensity-matched medicare cohorts*.
| VA cohort (n = 15,202) | Medicare cohort (n = 15,202) | P | |
|---|---|---|---|
| Age (y) | .27 | ||
| 65-69 | 22 | 21 | |
| 70-74 | 21 | 21 | |
| 75-79 | 23 | 23 | |
| 80-84 | 20 | 21 | |
| 85-100 | 14 | 14 | |
| Women | 2 | 2 | .63 |
| Median household income ($) | .19 | ||
| <35,000 | 7 | 8 | |
| 35,000-44,999 | 21 | 21 | |
| 45,000-54,999 | 46 | 44 | |
| 55,000-64,999 | 14 | 14 | |
| 65,000-74,999 | 7 | 7 | |
| >75,000 | 5 | 6 | |
| Comorbidities | |||
| Ischemic heart disease | 53 | 53 | .73 |
| Chronic kidney disease | 18 | 18 | .45 |
| Pulmonary disease | 29 | 29 | .26 |
| Diabetes mellitus | 36 | 35 | .09 |
| Hypertension | 80 | 81 | .12 |
| Stroke/transient ischemic attack | 8 | 8 | .83 |
| Cancer (colorectal, lung, prostate, or breast) | 16 | 16 | .40 |
| Cardiac procedures | |||
| Percutaneous coronary intervention | 18 | 18 | .59 |
| ICD | 7 | 7 | .41 |
| Coronary artery bypass | 2 | 2 | .86 |
| Valve repair/replacement | 1 | 1 | .58 |
| Transplant/ventricular assist device | <1 | <1 | .33 |
| Year of diagnosis of HF | .17 | ||
| Before 2007 | 39 | 40 | |
| 2007 | 15 | 16 | |
| 2008 | 15 | 15 | |
| 2009 | 16 | 15 | |
| 2010 | 16 | 15 | |
| Hospitalized for HF during study period | 17 | 12 | <.01 |
| Died during study period | 52 | 52 | .23 |
Data are percentages.
Figure 1.
Kaplan-Meier survival analysis by health system. There was no significant difference in mortality between veterans in the VA cohort compared with veterans in the Medicare cohort (52.0% vs 52.4%, log-rank P = .75).
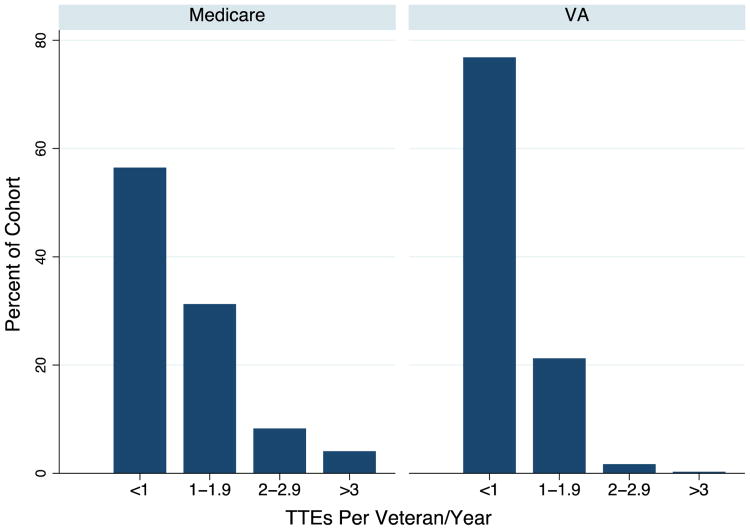
Overall, the propensity-matched Medicare cohort had a significantly higher rate of TTE use compared with the VA cohort (1.25 [95% CI 1.24-1.27] vs 0.38 [95% CI 0.37-0.39] TTEs per person-year, P < .001) (Table II). After accounting for variability of patient-level and MSA-level characteristics in a multilevel mixed-effects model, the Medicare cohort continued to have a significantly higher rate of TTE use compared with the VA cohort (incidence rate ratio 2.89 [95% CI 2.80-3.00], P < .001). Frequency of testing was also significantly higher in the Medicare cohort; among patients who received TTEs, 3,553 (44%) of 8,161 had one or more TTEs annually during the study period, compared with 1,059 (23%) of 4,572 in the VA cohort (P < .001) (Figure 2).
Table II. Echocardiography use in Medicare and VA cohorts.
| Adjusted mean use* (echocardiograms per veteran/y) | Geographic variation in use* | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Medicare | VA | P | Medicare CV | VA CV | MSA-level correlation |
| 1.25 (1.24-1.27) | 0.38 (0.37-0.39) | <.001 | 0.36 (0.27-0.45) | 0.48 (0.37-0.59) | r = 0.58 (P < .001) |
Abbreviation: CV, Coefficient of variation.
Data are point estimates with 95% CIs.
Figure 2.
Annual use of echocardiography per veteran by health system. This figure displays the number of TTEs received each year by veterans in the VA and Medicare cohorts, among veterans who received at least 1 TTE during the study period.
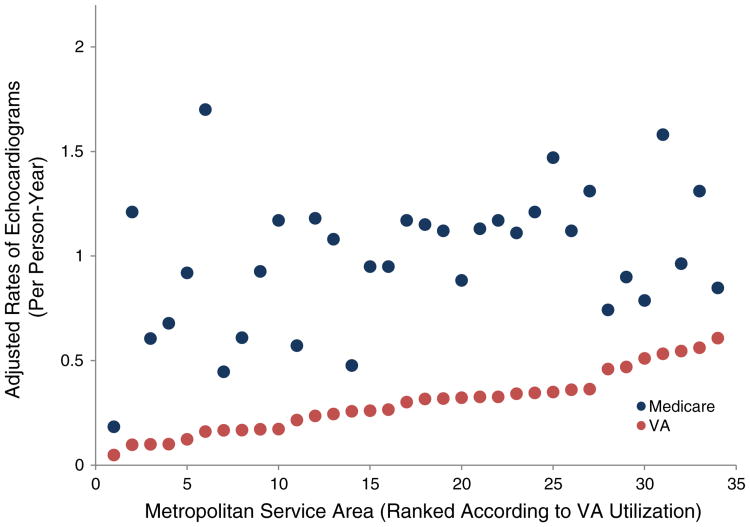
We found that although the range of the MSA-level rates of TTE use was greater in the Medicare cohort, the coefficients of variation were similar. Across the 34 MSAs, the rate of TTE use per person-year among the VA cohort ranged from 0.05 to 0.61 (range 0.56), whereas the rate among the Medicare cohort ranged from 0.23 to 1.95 (range 1.72). However, the coefficient of variation of the MSA-level rates was not significantly different in the Medicare cohort compared with the VA cohort (0.36 [95% CI 0.27-0.45] vs 0.48 [95% CI 0.37-0.59]) (Table II), indicating that the relative degree of variation was similar between cohorts. Finally, we found that there was a moderate to strong Spearman rank correlation in TTE use between the VA and Medicare cohorts (r = 0.58, P < .001) (Figure 3).
Figure 3.
Geographic variation and correlation in use of echocardiography. For each cohort, adjusted mean rate of echocardiography (y-axis) is displayed according MSA (x-axis). Spearman correlation coefficient = 0.58 (P < .001).
Discussion
We compared overall rates and geographic variation in the use of echocardiography between propensity-matched veterans with HF who used the VA vs Medicare across 34 MSAs. We found that mean echo rates were significantly higher among veterans who used Medicare, despite similar clinical characteristics and mortality rates. We also found that the MSA-level TTE rates exhibited similar relative degrees of variation and were moderately to strongly correlated between the cohorts. Our findings suggest that overall TTE use rates are strongly influenced by health care system, but local practice style influences care for both VA and Medicare patients.
This study has several strengths. First, we directly compared rates of imaging testing between similar groups of patients who use 2 separate health care financing and delivery systems. Most prior studies exploring use and variation in cardiovascular procedure rates have done so by examining Medicare or commercially insured populations.17–20 Second, we used major MSAs with large VA medical centers as our geographic unit of analysis rather than HRRs defined by the Dartmouth Atlas project,14 which concentrated our study on practice patterns in large metropolitan areas where veterans who live near VA and nonfederal hospitals may choose to use either system. Third, we matched veterans by propensity score to minimize differences in demographic, clinical, and geographic characteristics between the VA and Medicare cohorts. This provided a unique and potentially more robust analysis of the drivers of use and variation.
Optimizing the value of diagnostic cardiac imaging tests has proved challenging. Imaging tests may be particularly prone to overuse because these tests are generally low risk, easily available as a result of technology proliferation, and potentially lucrative to providers and health systems, and the appropriate use criteria encompass a wide variety of clinical scenarios.21,22 Furthermore, although imaging testing is an integral part of cardiovascular care, there is little evidence that frequent use of imaging testing directly leads to improved patient outcomes.8,23 Our study is consistent with another study showing that imaging use is higher in Medicare compared with the VA24 despite similarities in patient-level characteristics and mortality, raising the possibility that the difference could be explained in part due to overuse driven by structures of care delivery that include FFS incentives. Another possible explanation is the high degree of fragmentation of care in Medicare, leading to frequent duplication of testing.25 However, the difference in use could also be due to underuse of testing at the VA because some features of the VA system such as constrained budgets and restrictions on expansion may disincentivize use of health care resources and lead to delays in scheduling of testing. In addition, patients in the VA cohort had a higher rate of hospitalization for HF, which could indicate that more frequent testing might be associated with less need for acute care. Other unique features of the VA health care system including structural integration (including a nationally available electronic medical record), salaried physicians, and low emphasis on clinical revenue generation could also contribute to lower cardiovascular imaging use rates in VA. Our study suggests that Medicare Accountable Care Organizations that exhibit similar characteristics may be successful in reducing imaging-related health care costs without compromising quality.26–29
Our finding of moderate to strong correlation of regional rates of TTE use between VA and Medicare suggests that local practice styles contributed substantially to local rates of echocardiography. This finding is consistent with some prior research,30,31 but our study extends current knowledge in 2 ways. First, prior studies have largely compared patterns of procedure use between FFS and capitated reimbursement systems,19,32 rather than entirely distinct payment and care delivery systems such as VA and Medicare. Second, our study found a stronger correlation in regional rates than prior studies that have examined correlation in cancer-related imaging,24 invasive cardiovascular procedures,19 or number of hospitalizations32 between different health systems. Our finding of stronger correlation may be because TTE use is highly discretionary, and discretionary practices may be more correlated within local groups than practices that are more standardized. Stronger correlation may also be due to the fact that medical training often occurs in VA facilities that are associated with local University medical centers with similar practice patterns. Other possible explanations based on prior research include regional patterns in referral or testing biases based on risk aversion,33,34 differences in supply,35 or differences in physician training.36
Although the range of TTE use rates was higher in the Medicare cohort compared with the VA cohort, there was no difference in the coefficient of variation, suggesting that the relative degree of geographic variation was similar for both cohorts. Although some studies have suggested that physician-level factors including payment mechanisms may influence regional variations,37–39 our study findings are consistent with others that found that wide variation exists despite radically different financial incentives.19,24 Indeed, a recent Institute of Medicine report found that wide variation in spending and procedure use exists and is unexplained by age, sex, health status, insurance plan factors, or market-level characteristics.40 Because the report was based on data largely from FFS Medicare and commercial insurance providers, our study extends this prior research by showing that integrated, fixed budget systems such as the VA might exhibit a similar relative degree of variation compared with other payment systems. Since its transformation in the 1990s, the VA health care system has emphasized features such as accountability, integrated care delivery, quality measurement, performance incentives, and global budgets.41 If significant variation exists despite this emphasis and variation is locally correlated between FFS and fixed-budget health systems, policies targeting high-use areas may not effectively foster more efficient care even if they reduce geographic variation.
Our study has several limitations. First, unmeasured differences between VA and Medicare patients could have contributed to the difference in use of TTE. Although our propensity score match is likely to have controlled for some of these differences (particularly differences that would have significantly affected mortality), certain clinical characteristics such as type of HF (ie, preserved vs reduced ejection fraction), stage of HF or severity of symptoms, whether the cause of death was HF, and patient preferences could not be measured. Second, we were unable to assess the degree to which use and variation were affected by clinical appropriateness of echocardiograms. Third, veterans who choose to use the VA as their primary source of medical care after age 65 years may have inherently different socioeconomic and clinical characteristics than veterans that choose FFS Medicare as their primary insurer, and the “correct” rate of use of echocardiography may be different in these populations.
Conclusion
Echocardiography use rates were significantly higher in a Medicare cohort compared with a propensity-matched VA cohort of veterans with HF, suggesting that TTE use rates may be strongly influenced by the differing characteristics of health care systems. The relative degree of geographic variation was similar and regionally correlated between the VA and Medicare cohorts, suggesting that local practice styles influence imaging rates irrespective of payment models. Novel reimbursement policies that emphasize current characteristics of the VA health care system including accountability, integrated care delivery, quality measurement, and global budgets may be an effective means of controlling growth of imaging testing rates while preserving quality of imaging services.
Footnotes
Conflicts of interest: None.
References
- 1.Andrus BW, Welch HG. Medicare services provided by cardiologists in the United States: 1999-2008. Circulation Cardiovascular Quality and Outcomes. 2012;5(1):31–6. doi: 10.1161/CIRCOUTCOMES.111.961813. [DOI] [PubMed] [Google Scholar]
- 2.Pearlman AS, Ryan T, Picard MH, et al. Evolving trends in the use of echocardiography: a study of Medicare beneficiaries. Journal of the American College of Cardiology. 2007;49(23):2283–91. doi: 10.1016/j.jacc.2007.02.048. [DOI] [PubMed] [Google Scholar]
- 3.Lucas FL, Wennberg DE, Malenka DJ. Variations in the use of echocardiography. Effective Clinical Practice. 1999;2:71–5. [PubMed] [Google Scholar]
- 4.Iglehart JK. Health insurers and medical-imaging policy—a work in progress. New England Journal of Medicine. 2009;360(10):1030–7. doi: 10.1056/NEJMhpr0808703. [DOI] [PubMed] [Google Scholar]
- 5.Medicare Payment Advisory Commission (MedPAC) Report to the Congress: aligning incentives in medicare. Washington, DC: MedPAC; 2010. [Google Scholar]
- 6.Douglas PS, Garcia MJ, Haines DE, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography. Journal of the American Society of Echocardiography. 2011;24(3):229–67. [Google Scholar]
- 7.Wolk MJ, Bailey SR, Doherty JU, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease. Journal of the American College of Cardiology. 2014;63:380–406. doi: 10.1016/j.jacc.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Mark DB, Anderson JL, Brinker JA, et al. ACC/AHA/A-SE/ASNC/HRS/IAC/Mended Hearts/NASCI/RSNA/SAIP/SCAI/SCCT/SCMR/SNMMI 2014 health policy statement on use of noninvasive cardiovascular imaging: a report of the American College of Cardiology Clinical Quality Committee. Journal of the American College of Cardiology. 2014;63:698–721. doi: 10.1016/j.jacc.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Petersen LA, Normand SL, Daley J, et al. Outcome of myocardial infarction in Veterans Health Administration patients as compared with medicare patients. The New England Journal of Medicine. 2000;343(26):1934–41. doi: 10.1056/NEJM200012283432606. [DOI] [PubMed] [Google Scholar]
- 10.Asch SM, McGlynn EA, Hogan MM, et al. Comparison of quality of care for patients in the Veterans Health Administration and patients in a national sample. Annals of Internal Medicine. 2004;141(12):938–45. doi: 10.7326/0003-4819-141-12-200412210-00010. [DOI] [PubMed] [Google Scholar]
- 11.Selim AJ, Berlowitz D, Kazis LE, et al. Comparison of health outcomes for male seniors in the Veterans Health Administration and Medicare Advantage plans. Health Services Research. 2010;45(2):376–96. doi: 10.1111/j.1475-6773.2009.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keating NL, Landrum MB, Lamont EB, et al. Quality of care for older patients with cancer in the Veterans Health Administration versus the private sector: a cohort study. Annals of Internal Medicine. 2011;154(11):727–36. doi: 10.7326/0003-4819-154-11-201106070-00004. [DOI] [PubMed] [Google Scholar]
- 13.Chronic Conditions Warehouse Categories. Chronic Conditions Data Warehouse. [Accessed 27 Feb, 2015]; https://www.ccwdata.org/web/guest/condition-categories.
- 14.Dartmouth Institute for Health Policy and Clinical Practice, The Dartmouth Atlas of Health Care. Dartmouth Atlas website. [Accessed February 26, 2015]; www.dartmouthatlas.org.
- 15.Guo S, Fraser M. Propensity score analysis: statistical methods and applications. Thousand Oaks, CA: Sage Publications; 2010. [Google Scholar]
- 16.Leuven E, Sianesi B. PSMATCH2: Stata module which implements full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. [Accessed March 4, 2015]; https://ideas.repec.org/c/boc/bocode/s432001.html.
- 17.Ko DT, Wang Y, Alter DA, et al. Regional variation in cardiac catheterization appropriateness and baseline risk after acute myocardial infarction. Journal of the American College of Cardiology. 2008;51(7):716–23. doi: 10.1016/j.jacc.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 18.Federspiel JJ, Mudrick DW, Shah BR, et al. Patterns and predictors of stress testing modality after percutaneous coronary stenting: data from the NCDR. Journal of the American College of Cardiology: Cardiovascular Imaging. 2012;5(10):969–80. doi: 10.1016/j.jcmg.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matlock DD, Groeneveld PW, Sidney S, et al. Geographic variation in cardiovascular procedure use among Medicare fee-for-service vs Medicare Advantage beneficiaries. JAMA. 2013;310(2):155–62. doi: 10.1001/jama.2013.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farmer SA, Lenzo J, Magid DJ, et al. Hospital-level variation in use of cardiovascular testing for adults with incident heart failure: findings from the cardiovascular research network heart failure study. Journal of the American College of Cardiology: Cardiovascular Imaging. 2014;7(7):690–700. doi: 10.1016/j.jcmg.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhave NM, Mansour IN, Veronesi F, et al. Use of a Web-based application of the American College of Cardiology Foundation/American Society of Echocardiography Appropriateness Use Criteria for Transthoracic Echocardiography: a pilot study. Journal of the American Society of Echocardiography. 2011;24:271–6. doi: 10.1016/j.echo.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 22.Fonseca R, Negishi K, Otahal P, et al. Temporal changes in appropriateness of cardiac imaging. Journal of the American College of Cardiology. 2015;65:763–73. doi: 10.1016/j.jacc.2014.11.057. [DOI] [PubMed] [Google Scholar]
- 23.Ferrari VA, Whitman B, Blankenship JC, et al. Cardiovascular imaging payment and reimbursement systems: understanding the past and present in order to guide the future. Journal of the American College of Cardiology: Cardiovascular Imaging. 2014;7(3):324–32. doi: 10.1016/j.jcmg.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 24.McWilliams JM, Dalton JB, Landrum MB, et al. Geographic variation in cancer-related imaging: Veterans Affairs health care system versus Medicare. Annals of Internal Medicine. 2014;161(11):794–802. doi: 10.7326/M14-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welch HG, Hayes KJ, Frost C. Repeat testing among medicare beneficiaries. Archives of Internal Medicine. 2012;172(22):1745–51. doi: 10.1001/2013.jamainternmed.727. [DOI] [PubMed] [Google Scholar]
- 26.Center for Medicare & Medicaid Innovation. Washington, DC: Department of Health & Human Services, Centers for Medicare & Medicaid Services; 2011. [Accessed February 26, 2015]. Pioneer Accountable Care Organization (ACO) model request for application. http://innovations.cms.gov/Files/x/Pioneer-ACO-Model-Request-For-Applications-document.pdf. [Google Scholar]
- 27.Centers for Medicare & Medicaid Services (CMS), HHS. Medicare program; Medicare Shared Savings Program: Accountable Care Organizations. Final rule Federal Register. 2011;76:67802–990. [PubMed] [Google Scholar]
- 28.Center for Medicare & Medicaid Innovation. Innovation models. [Accessed February 26, 2015]; http://innovation.cms.gov/initiatives/index.html#views?models.
- 29.Centers for Medicare & Medicaid Services. Hospital Value-Based Purchasing Program. [Accessed February 26, 2015]; www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/hospital-value-based-purchasing/index.html.
- 30.Barnato AE, Bost JE, Farrell MH, et al. Relationship between staff perceptions of hospital norms and hospital-level end-of-life treatment intensity. Journal of Palliative Medicine. 2007;10(5):1093–100. doi: 10.1089/jpm.2006.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin CY, Farrell MH, Lave JR, et al. Organizational determinants of hospital end-of-life treatment intensity. Medical Care. 2009;47(5):524–30. doi: 10.1097/MLR.0b013e31819261bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baker LC, Fisher ES, Wennberg JE. Variations in hospital resource use for Medicare and privately insured populations in California. Health Affairs (Project Hope) 2008;27(2):w123–34. doi: 10.1377/hlthaff.27.2.w123. [DOI] [PubMed] [Google Scholar]
- 33.Pearson SD, Goldman L, Orav EJ, et al. Triage decisions for emergency department patients with chest pain: do physicians' risk attitudes make the difference? Journal of General Internal Medicine. 1995;10(10):557–64. doi: 10.1007/BF02640365. [DOI] [PubMed] [Google Scholar]
- 34.Franks P, Williams GC, Zwanziger J, et al. Why do physicians vary so widely in their referral rates? Journal of General Internal Medicine. 2000;15(3):163–8. doi: 10.1046/j.1525-1497.2000.04079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wennberg D, Dickens J, Jr, Soule D, et al. The relationship between the supply of cardiac catheterization laboratories, cardiologists and the use of invasive cardiac procedures in northern New England. Journal of Health Services Research & Policy. 1997;2(2):75–80. doi: 10.1177/135581969700200204. [DOI] [PubMed] [Google Scholar]
- 36.Newhouse JP, Garber AM. Geographic variation in health care spending in the United States: insights from an Institute of Medicine report. JAMA. 2013;310(12):1227–8. doi: 10.1001/jama.2013.278139. [DOI] [PubMed] [Google Scholar]
- 37.Fisher ES, Wennberg DE, Stukel TA, et al. The implications of regional variations in Medicare spending, part 1: the content, quality, and accessibility of care. Annals of Internal Medicine. 2003;138(4):273–87. doi: 10.7326/0003-4819-138-4-200302180-00006. [DOI] [PubMed] [Google Scholar]
- 38.Fisher ES, Wennberg DE, Stukel TA, et al. The implications of regional variations in Medicare spending, part 2: health outcomes and satisfaction with care. Annals of Internal Medicine. 2003;138(4):288–98. doi: 10.7326/0003-4819-138-4-200302180-00007. [DOI] [PubMed] [Google Scholar]
- 39.Sutherland JM, Fisher ES, Skinner JS. Getting past denial—the high cost of health care in the United States. New England Journal of Medicine. 2009;361(13):1227–30. doi: 10.1056/NEJMp0907172. [DOI] [PubMed] [Google Scholar]
- 40.Institute of Medicine Committee on Geographic Variation in Health Care Spending and Promotion of High-Value Care. Washington, DC: National Academies Press; 2013. [Accessed February 27, 2015]. Variation in health care spending: target decision making, not geography. http://books.nap.edu/openbook.php?record_id=18393. [PubMed] [Google Scholar]
- 41.Kizer KW, Demakis JG, Feussner JR. Reinventing VA health care: systematizing quality improvement and quality innovation. Medical Care. 2000;38:I7–I16. [PubMed] [Google Scholar]