Abstract
Despite its 0.5–1% lifetime prevalence in men and its general societal relevance, neuroimaging investigations in pedophilia are scarce. Preliminary findings indicate abnormal brain structure and function. However, no study has yet linked structural alterations in pedophiles to both connectional and functional properties of the aberrant hotspots. The relationship between morphological alterations and brain function in pedophilia as well as their contribution to its psychopathology thus remain unclear. First, we assessed bimodal connectivity of structurally altered candidate regions using meta‐analytic connectivity modeling (MACM) and resting‐state correlations employing openly accessible data. We compared the ensuing connectivity maps to the activation likelihood estimation (ALE) maps of a recent quantitative meta‐analysis of brain activity during processing of sexual stimuli. Second, we functionally characterized the structurally altered regions employing meta‐data of a large‐scale neuroimaging database. Candidate regions were functionally connected to key areas for processing of sexual stimuli. Moreover, we found that the functional role of structurally altered brain regions in pedophilia relates to nonsexual emotional as well as neurocognitive and executive functions, previously reported to be impaired in pedophiles. Our results suggest that structural brain alterations affect neural networks for sexual processing by way of disrupted functional connectivity, which may entail abnormal sexual arousal patterns. The findings moreover indicate that structural alterations account for common affective and neurocognitive impairments in pedophilia. The present multimodal integration of brain structure and function analyses links sexual and nonsexual psychopathology in pedophilia. Hum Brain Mapp 36:2374–2386, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: pedophilia, meta‐analytic connectivity modeling (MACM), functional magnetic resonance imaging (fMRI), resting state, functional connectivity, neuroimaging
INTRODUCTION
There is growing evidence that pedophilia is linked to both structural and functional brain abnormalities [Mohnke et al., 2014]. In spite of a considerable lifetime prevalence of approximately 0.5–1% in the male population [Mokros et al., 2012], similar to schizophrenia, and its general societal relevance, neuroimaging investigations of this paraphilia are surprisingly scarce.
Only a small number of functional magnetic resonance imaging (fMRI) studies investigated dysfunctional brain activations during visual sexual stimulation in pedophilia. These have been located predominantly to cortical and subcortical regions that are closely related to the generation of sexual arousal [Poeppl et al., 2014; Stoléru et al., 2012]. More specifically, pedophiles featured abnormal neural activity in the left dorsolateral prefrontal cortex (DLPFC) and the hypothalamus [Walter et al., 2007], the right amygdala [Sartorius et al., 2008], thalamus, pallidum, and striatum [Schiffer et al., 2008a], cingulate and bilateral insular cortex [Poeppl et al., 2011], as well as in the orbitofrontal cortex (OFC) and right DLPFC [Schiffer et al., 2008b]. Notably, there is a considerable variability in findings between studies. Yet, a recent study suggests that functional imaging of brain response patterns during visual sexual stimulation might enable diagnostic classification of pedophilia with high sensitivity and specificity [Ponseti et al., 2012].
On the level of individual patients, neurological case reports demonstrated that pedophilic behavior can follow both focal (i.e., various types of tumors and lesions) and systemic brain diseases (e.g., dementia, Parkinson's syndrome). This indicates at least a contributing role of structural brain alterations to the etiology of sexual interest in children. In the respective case reports, morphological alterations comprised the frontal lobes, anterior temporal lobes, and amygdalae, non‐motor basal ganglia, hypothalamus, and septal nuclei. However, the anatomical location of impairment varied considerably between case studies. Hence, neurological disorders may merely unmask a predisposition to sexual interest in children through different mechanisms, for example, disinhibition, sexual preoccupation, or hypersexuality [Mendez and Shapira, 2011].
These qualitative neurological case reports have been complemented by quantitative voxel‐based morphometric studies that demonstrated volume reductions of the right amygdala, hypothalamus, and septal regions [Poeppl et al., 2013; Schiltz et al., 2007], structural deficits of temporal cortices and fiber bundles [Cantor et al., 2008; Schiffer et al., 2007], and morphologic abnormalities of OFC and basal ganglia [Schiffer et al., 2007]. Further alterations appeared in areas in the parietal lobe [Cantor et al., 2008; Schiffer et al., 2007] as well as the cingulate cortex, insula, and cerebellum [Schiffer et al., 2007], when comparing pedophilic with nonpedophilic men. Similar to previous reports of functional abnormalities, these morphometric results show a considerable variability between studies. Only the finding of decreased right amygdala volume has been replicated [Poeppl et al., 2013; Schiltz et al., 2007]. Such topographical heterogeneity may at first glance appear contradictory. Diverging findings might however be explained by inclusion of different patient samples with diverse features of pedophilia, given evidence that specific neuroanatomical deficits may correlate with certain clinical “phenotypic” characteristics of pedophiles [Poeppl et al., 2013]. In this way, the seemingly conflicting results could be read as complementary rather than contradictory. However, it should also be noted that there are considerable differences in sample sizes between studies. While three previous studies have each investigated less than 20 individuals [Poeppl et al., 2013; Schiffer et al., 2007; Schiltz et al., 2007], only one study included a sample as large as 65 pedophilic subjects [Cantor et al., 2008]. This sample size enabled a whole‐brain analysis [Cantor et al., 2008], which contrasts the current dominance of region‐of‐interest (ROI) approaches [Poeppl et al., 2013; Schiffer et al., 2007; Schiltz et al., 2007]. Inconsistencies between results may also arise from differences in statistical power.
The conjunction of insight from neuroimaging research on pedophilia indicates both abnormal brain structure and abnormal brain function during processing of sexual stimuli. The finding of volume reductions in white matter (WM) fiber bundles linking gray matter (GM) regions that respond to sexual stimulation complement the structural and functional GM alterations [Cantor et al., 2008]. Notably, these WM reductions are also found in hebephilic men, that is, individuals primarily or exclusively sexually attracted by pubescents [Cantor and Blanchard, 2012]. The deficiencies in those fiber bundles have been interpreted as evidence that pedophilia may result from a disconnection within a neural system underlying processing of sexual stimuli [Cantor et al., 2008]. As a consequence of these previous findings, we therefore hypothesized that distinct hotspots of structural anomaly entail a disruption of functional integrity.
In addition to their deviant sexual preference, pedophilic perpetrators also manifest a number of other nonsexual neuropsychological alterations including neurocognitive impairment, non‐right‐handedness, and executive dysfunctions [Blanchard et al., 2007; Cantor et al., 2004; Cantor et al., 2005; Kruger and Schiffer, 2011; Schiffer and Vonlaufen, 2011]. A critical view is warranted in this context, because pedophilic and non‐pedophilic child molesters exhibit specific deficits, respectively [Eastvold et al., 2011; Schiffer and Vonlaufen, 2011; Suchy et al., 2009]. Yet, some alterations may at least partly be explained by factors other than pedophilia [Kruger and Schiffer, 2011]. Irrespective of that, there seems to be consensus that pedophilia is associated with neuropsychological disturbances. Furthermore, there is a high prevalence of mood disorders in pedophilic subjects [Cohen and Galynker, 2002; Raymond et al., 1999]. These nonsexual emotional abnormalities are underlined by findings of altered neural activity during nonerotic emotional stimulation in pedophilic patients [Walter et al., 2007]. Therefore, morphologic aberration underlying neural dysfunction most likely affects not only sexual‐processing‐related but also more general‐purpose brain systems in pedophilia. Importantly, not a single study has so far linked morphometric findings in pedophiles to either their connectivity patterns or functional roles. It thus remains unclear how morphological alterations relate to brain function and might contribute to psychopathology in pedophilia. An important step toward removal of this ambiguity is therefore to pinpoint the physiological neural networks which the candidate regions are embedded in and, moreover, to assess if these functional networks pertain to processing of sexual stimuli. In addition, it is essential in this context to specify the physiological functions (i.e., roles) of the candidate regions. This study hence set out to model functional connectivity (FC) of regions featuring pedophilia‐related neuroanatomical alterations and to delineate their functional characterizations. Using a multimodal approach, we aimed at providing a comprehensive characterization of regions morphologically altered in pedophilic perpetrators by analyzing interactions of their functional connections.
METHODS
Definition of Seed Regions
Seed regions were taken from a recent voxel‐based morphometry study, which identified a set of pedophilia‐related GM alterations [Poeppl et al., 2013] (cf., Fig. 1): (1) Right amygdala, which exhibited volume reduction in three independent samples of pedophilic perpetrators using ROI analyses [Mohnke et al., 2014; Poeppl et al., 2013; Schiltz et al., 2007]. (2) Left DLPFC, whose GM deficits have been found to be correlated with pedosexual interest and sexual offense recidivism [Poeppl et al., 2013] in addition to its aberrant neural activity during visual sexual stimulation in pedophiles [Schiffer et al., 2008a,b; Walter et al., 2007]. (3) Left insular cortex, whose GM volume was found to be reduced in pedophiles [Schiffer et al., 2007], to be inversely correlated with pedosexual interest and sexual recidivism [Poeppl et al., 2013] and to abnormally respond to visual sexual stimulation [Poeppl et al., 2011; Walter et al., 2007]. (4 and 5) Left and right temporoparietal junction that featured the less GM in pedophilic perpetrators, the younger their victims [Poeppl et al., 2013], as well as pedophilia‐related dysfunction in a sexual context [Poeppl et al., 2011; Walter et al., 2007]. (6) Medial orbitofrontal cortex (mOFC), whose GM volume showed the same relationship to victim age as the TPJ [Poeppl et al., 2013], and which was differentially activated in pedophiles during visual sexual stimulation [Schiffer et al., 2008a,b].
Figure 1.
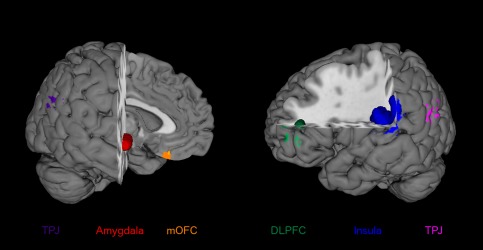
Seed regions. Seed regions were taken from a recent voxel‐based morphometry (VBM) study, which identified a set of pedophilia‐related gray matter (GM) alterations [Poeppl et al., 2013]. The right amygdala exhibited volume reduction in pedophilic perpetrators. GM deficits in the left dorsolateral prefrontal cortex (DLPFC) and left insular cortex were found to be correlated with pedosexual interest and sexual offense recidivism. Left and right temporoparietal junction (TPJ) as well as medial orbitofrontal cortex (mOFC) featured the less GM in pedophilic perpetrators, the younger their victims were. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
We analyzed FC and associated cognitive processes of these morphologically altered regions in a data‐driven approach. This allowed to shed light on the so far unclear relationship between brain structure and function in pedophilia.
Functional Connectivity
Task‐dependent functional connectivity
FC of the six seed regions during task performance was analyzed by means of meta‐analytic connectivity modeling (MACM) [Eickhoff et al., 2011; Laird et al., 2009]. This approach relies on the notion that FC is reflected in the temporal concomitance of activity in spatially distinct brain areas [Friston et al., 1996]. Accordingly, regions that are functionally connected are indicated by above‐chance coactivation in functional neuroimaging studies and vice versa. MACM can quantify FC across large sets of neuroimaging experiments by assessing coherent activation across experiments. It thus delineates potential functional networks that are conjointly recruited by a broad spectrum of diverse tasks. Hence, it has to be noted that task‐constrained FC assessed by MACM is not restricted to sexual stimulus‐driven processing in this study.
Here, whole‐brain co‐activation maps for each voxel of the respective seed region were delineated by capitalizing on the BrainMap database (http://www.brainmap.org) [Fox and Lancaster, 2002; Laird et al., 2011]. BrainMap contains whole‐brain results from functional neuroimaging studies reported in a standard stereotaxic space. We constrained our analysis to fMRI and positron emission tomography experiments in healthy subjects. Experiments investigating age, gender, disease, or drug effects were excluded. Otherwise, we considered all eligible “BrainMap experiments” to enable a data‐driven approach, given that any preselection based on taxonomic categories would have constituted a strong a priori hypothesis about how different tasks involve specific brain networks. These in‐/exclusion criteria yielded ≈7,500 eligible experiments at the time of analysis.
MACM analysis is carried out in a two‐step procedure: First, we identified the pool of all eligible experiments in the BrainMap database that reported at least one activation focus within the respective seed region. Second, activation likelihood estimation (ALE) meta‐analysis was employed to quantitatively summarize that experiment pool in order to compute the connectivity map for each seed.
The ALE algorithm aims to identify areas with a convergence of reported coordinates across experiments that is higher than expected given a random spatial association. Reported foci are treated as centers of 3D Gaussian probability distributions capturing the spatial uncertainty associated with each focus [Eickhoff et al., 2009]. Probabilities of all foci reported of a given experiment were combined for each voxel, yielding a modeled activation (MA) map [Turkeltaub et al., 2012]. Voxelwise ALE scores (union across these MA maps) then quantified the convergence across experiments at each location in the brain. To distinguish “true” from random convergence, ALE scores were compared to an empirical null distribution reflecting a random spatial association among all MA maps. The resulting random‐effects inference focuses on the above‐chance convergence across studies rather than the clustering within a particular study [Eickhoff et al., 2009]. This null hypothesis was derived by computing the distribution that would be obtained when sampling a voxel at random from each of the MA maps and taking the union of these values in the same manner as for the (spatially contingent) voxels in the original analysis [Eickhoff et al., 2012]. The p value of the “true” ALE score was finally given by the proportion of equal or higher values obtained under the null distribution. The resulting nonparametric P values were then assessed at a familywise error (FWE) corrected threshold of P < 0.05 on cluster level (cluster‐forming threshold: P < 0.001 at voxel level) and transformed into z scores for display [Eickhoff et al., 2012].
Task‐independent functional connectivity
Whole‐brain task‐independent FC of the seed regions was assessed using resting‐state correlations in fMRI data from 132 healthy subjects (54 female, mean age: 42.3 years ± 18.08 SD) without any record of neurological or psychiatric disorders taken from a public repository (http://fcon_1000.projects.nitrc.org/indi/pro/nki.html). Participants were instructed to keep their eyes closed and let their minds wander without thinking of anything in particular or falling asleep. Functional whole‐brain images were acquired on a 3T Siemens MAGNETOM Tim Trio scanner using an echo‐planar imaging (EPI) sequence (260 volumes consisting of 38 axial slices with 3.0 mm thickness, TR = 2.5 s, TE = 30 ms, flip angle = 80°, in‐plane resolution = 3.0 × 3.0 mm2). The first four scans were discarded to account for signal saturation. The remaining images were preprocessed using the SPM8 software package (http://www.fil.ion.ucl.ac.uk/spm). Hereby, EPI images were first corrected for movement artifacts by affine registration using a two‐pass procedure. Mean EPI images of each subject were then spatially normalized to the MNI single‐subject template [Holmes et al., 1998] using the “unified segmentation” approach [Ashburner and Friston, 2005]. The ensuing deformation parameters were applied to the individual EPI volumes, which were then smoothed by a 5 mm full width at half maximum Gaussian kernel.
The time‐series data of each individual voxel in the six seed regions were processed as follows [Satterthwaite et al., 2013; zu Eulenburg et al., 2012]: To reduce the likelihood of spurious correlations, variance possibly explained by three nuisance variables was removed: (1) The six motion parameters derived from the image realignment, (2) the first derivative of the realignment parameters, and (3) mean GM, WM, and cerebrospinal fluid signal per time point as obtained by averaging across voxels attributed to the respective tissue class in the SPM8 segmentation. Data were then filtered preserving frequencies between 0.01 and 0.08 Hz, given that meaningful resting‐state correlations will predominantly be found in this frequency range because the blood oxygen level dependent response acts as a low‐pass filter [Biswal et al., 1995; Fox and Raichle, 2007]. The time course of the respective seed was then expressed as the first eigenvariate of its voxels' time courses and compared to time series of all other GM voxels in the entire brain by computing Pearson's correlation coefficients. These coefficients were then transformed into Fisher's z scores and subsequently included in an ANOVA accounting for nonsphericity in the data. Please note that we used the same significance correction procedure for these resting‐state correlations as for the MACM analysis (P < 0.001 at the voxel level, P < 0.05 at the cluster level).
Convergent connectivity and overlap with neural networks for sexual processing
To identify reliable and modality‐independent functional connections of the respective seed regions, we performed a conjunction analysis between the (FWE‐corrected) MACM and (FWE‐corrected) resting state connectivity maps using the strict minimum statistics [Nichols et al., 2005]. Thus, surviving cluster of voxels are functionally associated with a given seed region in task‐constrained (“goal focused”) and task‐unconstrained (“resting”) brain states. Ensuing brain regions hence show congruent FC across idling and task‐focused brain states. In a supplementary analysis, we also identified those regions that were significantly connected with multiple of the seed regions, that is., those regions in which the consensus (task‐constrained and task‐unconstrained) FC maps of more than one seeds‐regions overlapped [Amft et al., 2014].
Pedophilia is a disorder of sexual preference and thus accompanied by altered psychosexual arousal patterns. These should affect both task‐unfocused and task‐focused brain states, since pedophilic preference can become manifest not only in sexually arousing fantasies involving children but also corresponding sexual behavior. We therefore hypothesized that areas showing congruent connectivity with structurally altered brain regions in pedophilia (i.e., the present seed regions) would involve networks for frequently social, but in particular also sexual, processing. In a quantitative coordinate‐based (ALE) and most recent meta‐analysis on functional neuroimaging studies in healthy men [Poeppl et al., 2014], we delineated a neural network for psychosexual arousal as well as a set of brain regions that are deactivated during sexual arousal. To test our hypothesis that FC of pedophilia‐related structural alterations overlaps with neural activity related to sexual processing, we computed conjunction analyses between the connectivity maps (convergent across MACM and resting‐state correlations) of each seed region with (i) the ALE map of activations during psychosexual arousal and (ii) the ALE map of deactivations during sexual arousal. Also here the strict minimum statistics were used [Nichols et al., 2005]. The results for all six seed regions, respectively, were merged into two summary maps, which relate to (i) psychosexual arousal and (ii) deactivations during sexual arousal. Hence, ensuing regions in each summary map are functionally connected to at least one of the seed regions and also part of a meta‐analytically determined network for processing of sexual stimuli.
Anatomical labeling
For macroanatomical labeling, the resulting brain regions were related to the probabilistic Harvard‐Oxford atlas [Desikan et al., 2006] as provided by FSLView v3.1 (http://www.fmrib.ox.ac.uk/fsl/fslview/index.html). For microanatomical labeling, we capitalized on cytoarchitectonic maps of the human brain provided by the SPM Anatomy Toolbox [Eickhoff et al., 2005, 2006b, 2007]. Clusters were thus assigned to the most probable histologically defined area at the respective location. This probabilistic histology‐based anatomical labeling is reported in each respective table. References to details regarding cytoarchitecture are given in the respective table notes.
Functional Characterization
Functional profiling intends to link topographically defined brain regions to corresponding psychological processes. To functionally characterize the seed regions, we made use of the BrainMap meta‐data that contain information on behavioral domain and paradigm class of each neuroimaging experiment included in the database. Behavioral domains describe the mental processes isolated by the statistical contrasts [Fox et al., 2005] and comprise the main categories action, cognition, emotion, interoception, perception, as well as their subcategories. Paradigm classes specify the task employed in the respective neuroimaging studies (see http://www.brainmap.org/scribe/for the complete BrainMap taxonomy). To describe the functional roles of the seed regions, we used a reverse inference approach, which tests the probability of a psychological process being present, given knowledge that a particular brain region is activated [Bzdok et al., 2013]. More precisely, a seed region's functional profile was determined by over‐representation of mental processes (i.e., behavioral domains and paradigm classes) in the experiments activating the respective cluster relative to the entire BrainMap database using a binomial test [Bzdok et al., 2013; Reetz et al., 2012]. The significance threshold was set to P < 0.05, corrected for multiple comparisons using the false discovery rate (FDR).
RESULTS
Functional Connectivity
The analyses of tasked‐based (MACM) and task‐independent (resting‐state correlations) brain‐wide FC revealed largely similar connectivity profiles for the respective seed regions. As a rule of thumb, task‐free FC analyses yielded more liberal activation maps (cf., Supporting Information Tables 1 and 2).
According to the conjunction analyses between both connectivity analyses (cf., Table 1 and Fig. 2), the right amygdala seed was conjointly connected with subcortical regions including bilateral basal ganglia, left claustrum, hypothalamus, bilateral thalamus, left midbrain, and left hippocampus. In contrast, connectivity of the left DLPFC seed was limited to the left cerebral cortex, including the ventro‐ and dorsolateral prefrontal cortices, the dorsomedial prefrontal cortex, anterior insula, superior parietal lobule, and extrastriate cortex. The FC pattern of the left operculoinsular seed comprised both cortical and subcortical areas. Congruent cortical connections were observed with the right DLPFC, posterior medial frontal cortex, bilateral superior temporal gyrus (STG), left postcentral gyrus, and left extrastriate cortex. Subcortical connections were bilaterally localized to putamen and thalamus. While this conjunction analysis demonstrated no regions that were functionally connected with the left TPJ, conjoint connectivity with the right TPJ seed was shown for the medial prefrontal cortex. The latter region held congruent connectivity also with the mOFC seed. The supplementary analysis focusing on overlap between these consensus (task‐based and task‐independent) FC maps identified the putamen (bilateral), thalamus, anterior insula, and DLPFC (left hemisphere, respectively) as those regions connected to more than one seeds‐regions (Supporting Information Table 3).
Table 1.
Brain‐wide functional connectivity of areas featuring pedophilia‐related neuroanatomical alterations
| Connected areas | MNI coordinates | ||||||
|---|---|---|---|---|---|---|---|
| Seed area | Macroanatomical | Cytoarchitectonic | Cluster size (in voxels) | x | y | z | Z score |
| R Amygdala | R Amygdala/Hippocampus | CA/LB/SF | 894 | 26 | −10 | −12 | 8.42 |
| R Ventral striatum | 14 | 8 | −12 | 4.40 | |||
| R Thalamus | 20 | −16 | −4 | 3.95 | |||
| L Amygdala | SF | 684 | −22 | 0 | −12 | 6.24 | |
| L Claustrum | −34 | −14 | −6 | 5.54 | |||
| L Hypothalamus | −10 | −8 | −12 | 4.59 | |||
| L Pallidum/Putamen | −20 | 0 | 6 | 4.00 | |||
| L Hippocampus | CA | −20 | −14 | −22 | 3.80 | ||
| L Midbrain | 17 | −14 | −18 | −8 | 3.45 | ||
| L Thalamus (Th‐Premotor) | −14 | −16 | 0 | 3.29 | |||
| L DLPFC | L DLPFC | 45 | 1209 | −40 | 38 | 8 | 8.31 |
| L Anterior insula | −34 | 28 | 4 | 6.57 | |||
| L DLPFC | 112 | −56 | 12 | 20 | 4.33 | ||
| DMPFC | 87 | 0 | 28 | 46 | 4.43 | ||
| L Peristriate cortex | 75 | −52 | −60 | −4 | 4.43 | ||
| L Superior parietal lobule | 7A/hlP3/7PC | 61 | −32 | −56 | 52 | 4.37 | |
| L VLPFC | 33 | −48 | 18 | −10 | 4.13 | ||
| L DLPFC | 44/45 | 10 | −52 | 20 | 6 | 3.51 | |
| L Insula | L Parietal operculum | OP1/OP4 | 7548 | −46 | −26 | 16 | 8.75 |
| L Anterior insula | −50 | 8 | −2 | 7.69 | |||
| L Postcentral gyrus | 3b/4p | −38 | −26 | 54 | 7.08 | ||
| L Superior temporal gyrus | −56 | −10 | 0 | 6.65 | |||
| L Putamen | −26 | −2 | 6 | 6.11 | |||
| L Insula | Ig1/Ig2 | −34 | 24 | 4 | 5.82 | ||
| R Parietal operculum | OP1/OP4 | 5158 | 52 | −20 | 12 | 8.41 | |
| R Superior temporal gyrus | 62 | −32 | 18 | 7.48 | |||
| R DLPFC/Anterior insula | 44 | 48 | 8 | 2 | 6.83 | ||
| R Putamen | 26 | 4 | 4 | 6.38 | |||
| R pMFC | 6 | 1464 | 0 | 6 | 48 | 8.35 | |
| L Thalamus (Th‐Prefrontal) | 432 | −12 | −18 | 4 | 8.41 | ||
| R Thalamus (Th‐Prefrontal) | 307 | 14 | −16 | 6 | 8.34 | ||
| L Extrastriate cortex | 4 | −50 | −62 | 2 | 3.21 | ||
| L TPJ | L TPJ | PGa/PGp | 1048 | −42 | −56 | 22 | 8.40 |
| R TPJ | R TPJ | PGa/PGp | 561 | 42 | −60 | 22 | 8.24 |
| MPFC | 13 | −6 | 46 | 16 | 3.34 | ||
| MPFC | 1 | 0 | 48 | 12 | 3.14 | ||
| mOFC | mOFC | 333 | 0 | 26 | −24 | 7.12 | |
| MPFC | 120 | −2 | 56 | 12 | 4.87 | ||
Conjunction analyses between (FWE corrected) tasked‐based and task‐independent functional connectivity maps of each seed, respectively. Coordinates (x, y, z) represent peaks within a cluster. For detailed information on cytoarchitectonics and connectivity, see: Amunts et al. [1999] (44, 45), [2005] (CA, LB, SF); Behrens et al. [2003] (Th‐Prefrontal/‐Temporal); Caspers et al. [2006, 2008] (PGa, PGp); Eickhoff et al. [2006a,2006c] (OP1, OP4); Geyer et al. [1996] (4p), [1999, 2000] (3b), [2004] (6); Kurth et al. [2010] (Ig1, Ig2); Scheperjans et al. [2008a,2008b] (7A, 7PC, hlP3).
DLPFC, dorsolateral prefrontal cortex; DMPFC, dorsomedial prefrontal cortex; L, left; MNI, Montreal Neurological Institute; MPFC, medial prefrontal cortex; pMFC, posterior medial frontal cortex; R, right; TPJ, temporoparietal junction; VLPFC, ventrolateral prefrontal cortex, VMPFC, ventromedial prefrontal cortex.
Figure 2.
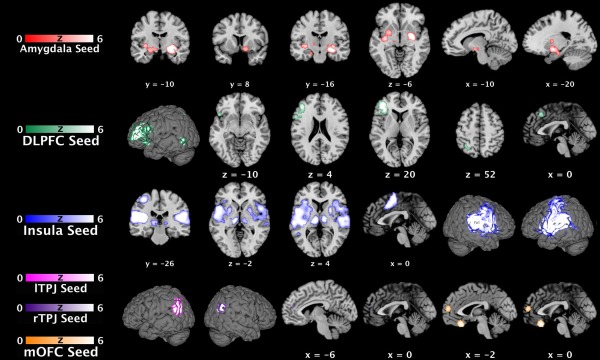
Functional connectivity maps of areas featuring pedophilia‐related neuroanatomical alterations. Conjunction analyses between (FWE corrected) tasked‐based and (FWE corrected) task‐independent functional connectivity maps of each seed, respectively. Sagittal, coronar, and axial brain slices are shown at MNI coordinates (x, y, z). DLPFC, dorsolateral prefrontal cortex; lTPJ, left temporoparietal junction; MNI, Montreal Neurological Institute; mOFC, medial orbitofrontal cortex; rTPJ, right temporoparietal junction. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Comparison (i.e., conjunction analyses) of these connectivity maps of regions featuring pedophilia‐related alterations with a recently meta‐analytically determined network of psychosexual arousal [Poeppl et al., 2014] revealed an intersection in cortico‐thalamo‐limbic brain systems (cf., Table 2 and Fig. 3). More specifically, overlapping regions included the dorsolateral prefrontal, superior parietal, anterior insular, and extrastriate cortices as well as amygdala and midbrain. The analogous analysis concerning regions that are consistently deactivated in sexual arousal showed that these areas overlapped with connectivity maps of areas featuring pedophilia‐related alterations in the left TPJ and right STG.
Table 2.
Topographical overlap between present functional connectivity maps of areas with pedophilia‐related neuroanatomical alterations and previously meta‐analytically determined regions implicated in sexual arousal
| Location | MNI coordinates | Z score | |||||
|---|---|---|---|---|---|---|---|
| Macroanatomical | Cytoarchitectonic | Cluster size (in voxels) | x | y | z | ||
| Overlap with activations in sexual arousal | L Amygdala/Hippocampus | SF/LB/CA | 102 | −22 | −4 | −18 | 4.50 |
| L Superior parietal lobule | 7A/hIP3/7PC | 48 | −32 | −56 | 52 | 4.37 | |
| R Thalamus (Th‐Prefrontal) | 46 | 6 | −16 | 8 | 4.48 | ||
| L Extrastriate cortex | 30 | −50 | −62 | −4 | 4.47 | ||
| R Amygdala | 30 | 26 | −2 | −20 | 3.67 | ||
| L Thalamus (Th‐Prefrontal) | 18 | −4 | −12 | 6 | 3.71 | ||
| R Anterior Insula | 16 | 36 | 22 | 0 | 3.45 | ||
| L Extrastriate cortex | 4 | −50 | −62 | 2 | 3.21 | ||
| Midbrain | 4 | −14 | −18 | −8 | 3.45 | ||
| L DLPFC | 1 | −46 | 10 | 26 | 3.15 | ||
| Overlap with deactivations in sexual arousal | L TPJ | PGa/PGp | 82 | −52 | −56 | 24 | 4.50 |
| R Superior temporal gyrus | 60 | 56 | −6 | −4 | 3.68 | ||
Overall maps of significant overlap between the (FWE corrected) functional connectivity maps of each seed (cf. Table 1) and the previously published coordinate‐based meta‐analysis (ALE) of sexual arousal [Poeppl et al., 2014]. Coordinates (x, y, z) represent peaks within clusters. For detailed information on cytoarchitectonics and connectivity, see: Amunts et al. [2005] (CA, LB, SF); Behrens et al. [2003] (Th‐Prefrontal); Caspers et al. [2006, 2008] (PGa, PGp); Scheperjans et al. [2008a,b] (7A, 7PC, hlP3).
DLPFC, dorsolateral prefrontal cortex; L, left; MNI, Montreal Neurological Institute; R, right; TPJ, temporoparietal junction.
Figure 3.
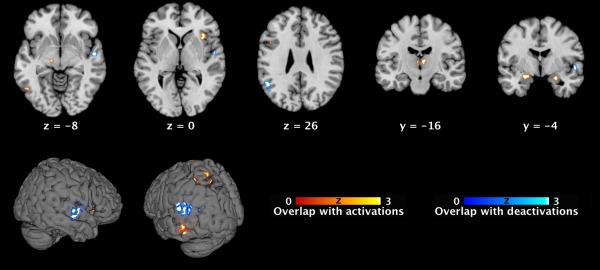
Topographical overlap between present functional connectivity maps of areas with pedophilia‐related neuroanatomical alterations and previously meta‐analytically determined regions implicated in sexual arousal. Conjunction maps of significant overlap between the present (FWE corrected) functional connectivity maps of each seed (cf. Fig. 1) and the previously published (FWE corrected) ALE maps of sexual arousal [Poeppl et al., 2014]. Sagittal, coronar, and axial brain slices are shown at MNI coordinates (x, y, z). ALE, activation likelihood estimation; MNI, Montreal Neurological Institute. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Functional Characterization
We conducted a functional characterization of brain areas featuring morphologic alterations in pedophilia (i.e., of the seed regions) by relating psychological terms to the respective region as registered in the BrainMap database.
The right amygdala seed was significantly associated with the emotional domain, in particular face discrimination tasks. The cluster in left DLPFC in contrast was found to be significantly associated with cognition and perception, specifically working memory and processing of auditory percepts. Corresponding to its rather large size, various associations were identified for the seed cluster in the operculo‐insular cortex. Behavioral domains included perception (somesthetic and auditory), cognition (auditory), and action execution. The corresponding tasks were related to tactile, auditory and pain monitoring and discrimination, auditory comprehension, and overt recitation. The seed clusters in the left and right TPJ exhibited functional congruence. In both hemispheres the TPJ areas were predominantly associated with the cognitive domain, more specifically episodic recall tasks, which inherently require explicit memory retrieval. No significant overrepresentations of behavioral domains or paradigm classes were found for the mOFC cluster (cf., Supporting Information Fig. 1).
DISCUSSION
Alterations of brain structure and function in individuals diagnosed with pedophilia have so far been documented in few and in independent neuroimaging studies. Here, previously established structural alterations were first linked to their corresponding connectivity profiles. These largely overlapped with neural networks implicated in sexual processing in a set of cortical, thalamic, and limbic regions. We subsequently delineated the functional role of brain regions with pedophilia‐related structural disturbances, which mainly pertained to nonsexual cognitive, emotional, and executive processes. The combination of these connectional and functional characterizations may account for the sexual and nonsexual psychopathology in pedophilia.
Functional Connectivity and Its Relationship to Sexual Processing
We determined topographical overlap between the seed regions' congruent connectivity maps and the neural networks for processing of sexual stimuli (i.e., ALE maps of a recent neuroimaging meta‐analysis [Poeppl et al., 2014]). This suggests that structural brain alterations in pedophilic individuals may entail alterations of (neural) sexual arousal patterns through aberrant FC, although the specific mechanism remains elusive due to the complexity of this aspect of sexual behavior and of the corresponding neural network. However, at least parts of this network responding to visual sexual stimuli in healthy men seem to be active also in pedophiles when viewing pedosexual stimuli [Mohnke et al., 2014]. Yet, abnormal brain activation patterns in pedophiles during visual sexual stimulation have been documented by fMRI studies and are regarded as the neural correlates underlying sexual attraction to children [Habermeyer et al., 2013a; Poeppl et al., 2011; Sartorius et al., 2008; Schiffer et al., 2008a,b; Stoléru et al., 2013; Walter et al., 2007]. In addition, this neurobiological endophenotype may potentially be informative to be further developed into a biomarker allowing for observer‐independent diagnosis of pedophilia [Ponseti et al., 2012]. However, caution is warranted in this regard because preliminary results are based on admitting pedophiles and the use of neuroimaging to identify men who are unwilling to reveal their sexual interests is a complicated ethical question.
More specifically, we observed overlap in a set of regions including the left extrastriate cortex, superior parietal lobule, DLPFC, and hippocampus. In the context of sexual arousal, the superior parietal lobule most likely modulates neural activity in the extrastriate cortex by top‐down signals [Culham and Kanwisher, 2001; Kastner et al., 1999]. The latter in turn is known to attentionally enhance visual processing of salient stimuli such as sexual content [Kastner et al., 1999; Poeppl et al., 2014]. Attention modulation during sexual arousal mediated by the superior parietal lobule may be triggered by functional coupling between the DLPFC and hippocampus, since both regions are crucial for reward‐based and memory‐guided categorization of visual stimuli [DeGutis and D'Esposito, 2007; Freedman et al., 2001; Pan et al., 2008]. In this context, they have been assigned to the cognitive component of a neurophenomenological model of sexual arousal [Stoléru et al., 2012]. We also found overlap in putative dopaminergic pathways, including the amygdala, thalamus, and midbrain. Neural activity in amygdala and thalamus during sexual arousal is associated with a general feeling of pleasure [Walter et al., 2008], possibly induced by dopamine release in the midbrain, which projects to both thalamus and amygdala [Haber and Knutson, 2010]. It has further been proposed that these regions trigger the sexual‐cognitive processes by relevance detection and affective evaluation of sexual stimuli [Poeppl et al., 2014] and therefore relate to the motivational and emotional components of sexual arousal [Stoléru, 2014]. Equally related to these components is the anterior insula [Stoléru et al., 2012], where we also noted overlap. The insula is believed to constitute a key node of the so‐called salience network [Seeley et al., 2007] and has been assumed to integrate aroused states of mind and body into the awareness of sexual arousal [Craig, 2010; Poeppl et al., 2014]. Finally, the conjunction analyses revealed overlap in the left TPJ and right STG. These regions, however, are consistently deactivated during sexual arousal. This has been interpreted as concomitant impairment of metacognitive and self‐reflexive processing (TPJ) as well as release of intrinsic inhibition (STG) [Poeppl et al., 2014; Stoléru, 2014].
Intriguingly, brain regions showing structural alterations in pedophiles are functionally connected to regions that are critically involved in processing of sexual stimuli. Hence, possibly altered connectivity among these regions may entail disturbed emotional evaluation (amygdala, thalamus) and subsequent miscategorization (DLPFC, hippocampus) of children as sexually relevant stimuli. In line with this notion, pedophilic subjects indeed recruit similar networks during sexual excitement induced by pedosexual stimuli as teleiophilic (i.e., nonpedophilic) subjects in response to adult sexual content [Poeppl et al., 2011; Schiffer et al., 2008b]. In contrast, pedophilic perpetrators, compared with nonpedophilic men, differentially activate DLPFC, hippocampus, thalamus, amygdala as well as superior parietal lobule and STG, when viewing child or adult stimuli, respectively [Poeppl et al., 2011; Sartorius et al., 2008; Schiffer et al., 2008a,b; Walter et al., 2007]. Taking the conjunction across present and previous findings, one might speculate that an (altered) structure‐connectivity‐function sequence constitutes the neural substrate underlying pedosexual interests. We assessed physiological connections of structures with morphologic alterations in pedophilic perpetrators and can therefore make no definite statements on dys‐connectivity. However, the notion of an altered structure‐connectivity‐function sequence would be in line with preliminary research suggesting that pedophilia results from a partial disconnection within a neural network for recognizing sexually relevant stimuli [Cantor et al., 2008]. More precisely, the present results indicate functionally dysconnectivity within brain regions that serve to identify sexually relevant stimuli. This confirms dysconnectivity hypothesis proposed by Cantor et al. [2008], as evidenced by decreased WM (i.e., structural) connectivity.
Functional Characterization and Contribution to Nonsexual Psychopathology
We functionally characterized the seed regions in a data‐driven fashion using the BrainMap database to assess whether structural brain alterations could account for known behavioral and neuropsychological abnormalities in pedophilia. The seed in the amygdala was consistently associated with emotional discrimination tasks, including emotional evaluation of faces. This is noteworthy since brain networks subserving sexual and facial attraction show an abnormal tuning to sexually immature faces in pedophiles [Ponseti et al., 2014]. Importantly, long‐term changes of neural amygdala response in the context of sexual behavior depend on prior sexual experience [Stark, 2005]. The amygdala has therefore been regarded as a structure of particular importance for normal sexual maturation, which is likely to be impaired in pedophiles [Schiltz et al., 2007; Stark, 2005]. Interestingly, the amygdala is critically involved in the conditioning of sexual arousal [Klucken et al., 2009]. Although conditioning by itself cannot sufficiently explain the development of pedophilia [Seto, 2009], a coincidence with often‐suspected early neurodevelopmental perturbations [Cantor et al., 2008; Cohen et al., 2002; Schiltz et al., 2007], specifically affecting the amygdala, may significantly contribute to its etiopathology. Moreover, stronger activation and possibly relevance of the amygdala in men than in women during sexual conditioning [Klucken et al., 2009] may not only account for the relative proneness to sexual conditioning but consequently also for susceptibility for pedophilia, and even more generally for paraphilias in men [Krueger and Kaplan, 2001]. In sum, the significant association of the amygdala seed with emotional facial discrimination tasks suggests an aberrant emotional evaluation of personal features, most likely also concerning sexual characteristics, in pedophiles. This dysfunction may be most relevant during the period of sexual maturation but also affect postpubertal sexual behavior.
Further functional profiling associated the seeds with perceptual (DLPFC, insula) and memory‐related (DLPFC, left/right TPJ) processes as well as action execution (insula). While, to the best of our knowledge, no studies exist that investigated perceptual abnormalities in pedophilia, evidence for impaired neurocognitive, and executive functions has been provided repeatedly. Patients with pedophilia show deficits in working memory and more specifically recall memory tests [Cantor et al., 2004; Tost et al., 2004], which accurately matches the above‐chance association of working memory and episodic recall tasks with the DLPFC and left TPJ seed, respectively. Related to this, pedophilia has also been reported to be linked to lowered processing speed [Kruger and Schiffer, 2011; Suchy et al., 2009], verbal deficits [Cohen et al., 2002; Joyal et al., 2007], and reduced general IQ [Blanchard et al., 2007; Cantor et al., 2004; Kruger and Schiffer, 2011], measures that correlate with GM in both bilateral TPJ and left DLPFC [Haier et al., 2005]. Moreover, failure to deactivate the TPJ during response inhibition has been observed in pedophiles [Habermeyer et al., 2013b]. Remarkably, there was no significant functional attribution of behavioral domains or paradigms to the mOFC seed. However, OFC dysfunction has been assumed to account for impaired executive functions, particularly response inhibition, in pedophilia [Schiffer and Vonlaufen, 2011]. This assumption is in line with the correlation of our seed's anatomical features with executive functions in healthy subjects [Takeuchi et al., 2013] and its structural alteration in pedophilia [Poeppl et al., 2013]. Moreover, these morphology‐based inhibitory deficits in pedophiles should also impact the sexual domain, given the mOFC's contribution to tonic inhibition of sexual arousal [Stoléru, 2014]. In contrast, the insula seed was consistently associated with “action execution” in the BrainMap database. Hence, the morphological alteration of this region seems to contribute to the well‐known executive deficits, most notably in sustained and response inhibition, in pedophiles [Joyal et al., 2007; Schiffer and Vonlaufen, 2011; Suchy et al., 2009; Tost et al., 2004]. This causal connection is strengthened by the FC of the insula seed with key structures for cognitive and motor control such as inferior frontal gyrus, supplementary motor area, midcingulate cortex, thalamus, basal ganglia, and cerebellum (cf. Table 1) [Cieslik et al., 2013; Hoffstaedter et al., 2014].
CONCLUSIONS
In summary, we delineated FC of regions featuring pedophilia‐related structural brain changes. We thereby found that these were connected to key areas for processing of sexual stimuli. Moreover, we demonstrated that the functional implications of morphologically altered brain regions in pedophilia relate to nonsexual emotional as well as to cognitive and executive functions known to be impaired in pedophiles. Our results suggest that structural brain alterations affect neural networks for sexual processing through disrupted FC and account for pertinent affective and neurocognitive impairment in pedophilia. These findings coherently link brain structure and function as well as sexual and nonsexual psychopathology in pedophilia.
Supporting information
Supporting Information
Supporting Information Figure 1
REFERENCES
- Amft M, Bzdok D, Laird AR, Fox PT, Schilbach L, Eickhoff SB (2014): Definition and characterization of an extended social‐affective default network. Brain Struct Funct 220:1031–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Bürgel U, Mohlberg H, Uylings HB, Zilles K (1999): Broca's region revisited: Cytoarchitecture and intersubject variability. J Comp Neurol 412:319–341. [DOI] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K (2005): Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: Intersubject variability and probability maps. Anat Embryol (Berl) 210:343–352. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2005): Unified segmentation. Neuroimage 26:839–851. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Johansen‐Berg H, Woolrich MW, Smith SM, Wheeler‐Kingshott CAM, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM (2003): Non‐invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 6:750–757. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34:537–541. [DOI] [PubMed] [Google Scholar]
- Blanchard R, Kolla NJ, Cantor JM, Klassen PE, Dickey R, Kuban ME, Blak T (2007): IQ, handedness, and pedophilia in adult male patients stratified by referral source. Sex Abuse 19:285–309. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Langner R, Schilbach L, Jakobs O, Roski C, Caspers S, Laird AR, Fox PT, Zilles K, Eickhoff SB (2013): Characterization of the temporo‐parietal junction by combining data‐driven parcellation, complementary connectivity analyses, and functional decoding. Neuroimage 81:381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor JM, Blanchard R (2012): White matter volumes in pedophiles, hebephiles, and teleiophiles. Arch Sex Behav 41:749–752. [DOI] [PubMed] [Google Scholar]
- Cantor JM, Blanchard R, Christensen BK, Dickey R, Klassen PE, Beckstead AL, Blak T, Kuban ME (2004): Intelligence, memory, and handedness in pedophilia. Neuropsychology 18:3–14. [DOI] [PubMed] [Google Scholar]
- Cantor JM, Klassen PE, Dickey R, Christensen BK, Kuban ME, Blak T, Williams NS, Blanchard R (2005): Handedness in pedophilia and hebephilia. Arch Sex Behav 34:447–459. [DOI] [PubMed] [Google Scholar]
- Cantor JM, Kabani N, Christensen BK, Zipursky RB, Barbaree HE, Dickey R, Klassen PE, Mikulis DJ, Kuban ME, Blak T, Richards BA, Hanratty MK, Blanchard R (2008): Cerebral white matter deficiencies in pedophilic men. J Psychiatr Res 42:167–183. [DOI] [PubMed] [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K (2006): The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. Neuroimage 33:430–448. [DOI] [PubMed] [Google Scholar]
- Caspers S, Eickhoff SB, Geyer S, Scheperjans F, Mohlberg H, Zilles K, Amunts K (2008): The human inferior parietal lobule in stereotaxic space. Brain Struct Funct 212:481–495. [DOI] [PubMed] [Google Scholar]
- Cieslik EC, Zilles K, Caspers S, Roski C, Kellermann TS, Jakobs O, Langner R, Laird AR, Fox PT, Eickhoff SB (2013): Is there “one” DLPFC in cognitive action control? Evidence for heterogeneity from co‐activation‐based parcellation. Cereb Cortex 23:2677–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LJ, Galynker II (2002): Clinical features of pedophilia and implications for treatment. J Psychiatr Pract 8:276–289. [DOI] [PubMed] [Google Scholar]
- Cohen LJ, Nikiforov K, Gans S, Poznansky O, McGeoch P, Weaver C, King EG, Cullen K, Galynker I (2002): Heterosexual male perpetrators of childhood sexual abuse: A preliminary neuropsychiatric model. Psychiatr Q 73:313–336. [DOI] [PubMed] [Google Scholar]
- Craig ADB (2010): The sentient self. Brain Struct Funct 214:563–577. [DOI] [PubMed] [Google Scholar]
- Culham JC, Kanwisher NG (2001): Neuroimaging of cognitive functions in human parietal cortex. Curr Opin Neurobiol 11:157–163. [DOI] [PubMed] [Google Scholar]
- DeGutis J, D'Esposito M (2007): Distinct mechanisms in visual category learning. Cogn Affect Behav Neurosci 7:251–259. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006): An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31:968–980. [DOI] [PubMed] [Google Scholar]
- Eastvold A, Suchy Y, Strassberg D (2011): Executive function profiles of pedophilic and nonpedophilic child molesters. J Int Neuropsychol Soc 17:295–307. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K (2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25:1325–1335. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Amunts K, Mohlberg H, Zilles K (2006a): The human parietal operculum. II. Stereotaxic maps and correlation with functional imaging results. Cereb Cortex 16:268–279. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K (2006b): Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage 32:570–582. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Schleicher A, Zilles K, Amunts K (2006c): The human parietal operculum. I. Cytoarchitectonic mapping of subdivisions. Cereb Cortex 16:254–267. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras M‐H, Evans AC, Zilles K, Amunts K (2007): Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage 36:511–521. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT (2009): Coordinate‐based activation likelihood estimation meta‐analysis of neuroimaging data: a random‐effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 30:2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Roski C, Caspers S, Zilles K, Fox PT (2011): Co‐activation patterns distinguish cortical modules, their connectivity and functional differentiation. Neuroimage 57:938–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT (2012): Activation likelihood estimation meta‐analysis revisited. Neuroimage 59:2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME (2007): Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8:700–711. [DOI] [PubMed] [Google Scholar]
- Fox PT, Lancaster JL (2002): Opinion: Mapping context and content: the BrainMap model. Nat Rev Neurosci 3:319–321. [DOI] [PubMed] [Google Scholar]
- Fox PT, Laird AR, Fox SP, Fox PM, Uecker AM, Crank M, Koenig SF, Lancaster JL (2005): BrainMap taxonomy of experimental design: Description and evaluation. Hum Brain Mapp 25:185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DJ, Riesenhuber M, Poggio T, Miller EK (2001): Categorical representation of visual stimuli in the primate prefrontal cortex. Science 291:312–316. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Fletcher P, Liddle PF, Frackowiak RS (1996): Functional topography: Multidimensional scaling and functional connectivity in the brain. Cereb Cortex 6:156–164. [DOI] [PubMed] [Google Scholar]
- Geyer S (2004): The microstructural border between the motor and the cognitive domain in the human cerebral cortex. Adv Anat Embryol Cell Biol 174:I–VIII, 1–89. [DOI] [PubMed] [Google Scholar]
- Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Bürgel U, Klingberg T, Larsson J, Zilles K, Roland PE (1996): Two different areas within the primary motor cortex of man. Nature 382:805–807. [DOI] [PubMed] [Google Scholar]
- Geyer S, Schleicher A, Zilles K (1999): Areas 3a, 3b, and 1 of human primary somatosensory cortex. Neuroimage 10:63–83. [DOI] [PubMed] [Google Scholar]
- Geyer S, Schormann T, Mohlberg H, Zilles K (2000): Areas 3a, 3b, and 1 of human primary somatosensory cortex. Part 2. Spatial normalization to standard anatomical space. Neuroimage 11:684–696. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B (2010): The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 35:4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermeyer B, Esposito F, Händel N, Lemoine P, Klarhöfer M, Mager R, Dittmann V, Seifritz E, Graf M (2013a): Immediate processing of erotic stimuli in paedophilia and controls: A case control study. BMC Psychiatry 13:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermeyer B, Esposito F, Händel N, Lemoine P, Kuhl HC, Klarhöfer M, Mager R, Mokros A, Dittmann V, Seifritz E, Graf M (2013b): Response inhibition in pedophilia: An fMRI pilot study. Neuropsychobiology 68:228–237. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Jung RE, Yeo RA, Head K, Alkire MT (2005): The neuroanatomy of general intelligence: Sex matters. Neuroimage 25:320–7. [DOI] [PubMed] [Google Scholar]
- Hoffstaedter F, Grefkes C, Caspers S, Roski C, Palomero‐Gallagher N, Laird AR, Fox PT, Eickhoff SB (2014): The role of anterior midcingulate cortex in cognitive motor control: Evidence from functional connectivity analyses. Hum Brain Mapp 35:2741–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC (1998): Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr 22:324–333. [DOI] [PubMed] [Google Scholar]
- Joyal CC, Black DN, Dassylva B (2007): The neuropsychology and neurology of sexual deviance: A review and pilot study. Sex Abuse 19:155–73. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG (1999): Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron 22:751–761. [DOI] [PubMed] [Google Scholar]
- Klucken T, Schweckendiek J, Merz CJ, Tabbert K, Walter B, Kagerer S, Vaitl D, Stark R (2009): Neural activations of the acquisition of conditioned sexual arousal: Effects of contingency awareness and sex. J Sex Med 6:3071–3085. [DOI] [PubMed] [Google Scholar]
- Krueger RB, Kaplan MS (2001): The paraphilic and hypersexual disorders: an overview. J Psychiatr Pract 7:391–403. [DOI] [PubMed] [Google Scholar]
- Kruger THC, Schiffer B (2011): Neurocognitive and personality factors in homo‐ and heterosexual pedophiles and controls. J Sex Med 8:1650–1659. [DOI] [PubMed] [Google Scholar]
- Kurth F, Eickhoff SB, Schleicher A, Hoemke L, Zilles K, Amunts K (2010): Cytoarchitecture and probabilistic maps of the human posterior insular cortex. Cereb Cortex 20:1448–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT (2009): Investigating the functional heterogeneity of the default mode network using coordinate‐based meta‐analytic modeling. J Neurosci 29:14496−1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Fox PM, Uecker AM, Ray KL, Saenz JJ, McKay DR, Bzdok D, Laird RW, Robinson JL, Turner JA, Turkeltaub PE, Lancaster JL, Fox PT (2011): The BrainMap strategy for standardization, sharing, and meta‐analysis of neuroimaging data. BMC Res Notes 4:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez M, Shapira JS (2011): Pedophilic behavior from brain disease. J Sex Med 8:1092–1100. [DOI] [PubMed] [Google Scholar]
- Mohnke S, Müller S, Amelung T, Krüger THC, Ponseti J, Schiffer B, Walter M, Beier KM, Walter H (2014): Brain alterations in paedophilia: A critical review. Prog Neurobiol 122:1–23. [DOI] [PubMed] [Google Scholar]
- Mokros A, Osterheider M, Nitschke J (2012): [Pedophilia. Prevalence, etiology, and diagnostics]. Nervenarzt 83:355–8. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline J‐B (2005): Valid conjunction inference with the minimum statistic. Neuroimage 25:653–60. [DOI] [PubMed] [Google Scholar]
- Pan X, Sawa K, Tsuda I, Tsukada M, Sakagami M (2008): Reward prediction based on stimulus categorization in primate lateral prefrontal cortex. Nat Neurosci 11:703–712. [DOI] [PubMed] [Google Scholar]
- Poeppl TB, Nitschke J, Dombert B, Santtila P, Greenlee MW, Osterheider M, Mokros A (2011): Functional cortical and subcortical abnormalities in pedophilia: A combined study using a choice reaction time task and fMRI. J Sex Med 8:1660–1674. [DOI] [PubMed] [Google Scholar]
- Poeppl TB, Nitschke J, Santtila P, Schecklmann M, Langguth B, Greenlee MW, Osterheider M, Mokros A (2013): Association between brain structure and phenotypic characteristics in pedophilia. J Psychiatr Res 47:678–685. [DOI] [PubMed] [Google Scholar]
- Poeppl TB, Langguth B, Laird AR, Eickhoff SB (2014): The functional neuroanatomy of male psychosexual and physiosexual arousal: A quantitative meta‐analysis. Hum Brain Mapp 35:1404–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponseti J, Granert O, Jansen O, Wolff S, Beier K, Neutze J, Deuschl G, Mehdorn H, Siebner H, Bosinski H (2012): Assessment of pedophilia using hemodynamic brain response to sexual stimuli. Arch Gen Psychiatry 69:187–194. [DOI] [PubMed] [Google Scholar]
- Ponseti J, Granert O, van Eimeren T, Jansen O, Wolff S, Beier K, Deuschl G, Bosinski H, Siebner H (2014): Human face processing is tuned to sexual age preferences. Biol Lett 10:20140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond NC, Coleman E, Ohlerking F, Christenson GA, Miner M (1999): Psychiatric comorbidity in pedophilic sex offenders. Am J Psychiatry 156:786–788. [DOI] [PubMed] [Google Scholar]
- Reetz K, Dogan I, Rolfs A, Binkofski F, Schulz JB, Laird AR, Fox PT, Eickhoff SB (2012): Investigating function and connectivity of morphometric findings—exemplified on cerebellar atrophy in spinocerebellar ataxia 17 (SCA17). Neuroimage 62:1354–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorius A, Ruf M, Kief C, Demirakca T, Bailer J, Ende G, Henn FA, Meyer‐Lindenberg A, Dressing H (2008): Abnormal amygdala activation profile in pedophilia. Eur Arch Psychiatry Clin Neurosci 258:271–277. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE, Wolf DH (2013): An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting‐state functional connectivity data. Neuroimage 64:240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F, Eickhoff SB, Hömke L, Mohlberg H, Hermann K, Amunts K, Zilles K (2008a): Probabilistic maps, morphometry, and variability of cytoarchitectonic areas in the human superior parietal cortex. Cereb Cortex 18:2141–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F, Hermann K, Eickhoff SB, Amunts K, Schleicher A, Zilles K (2008b): Observer‐independent cytoarchitectonic mapping of the human superior parietal cortex. Cereb Cortex 18:846–867. [DOI] [PubMed] [Google Scholar]
- Schiffer B, Vonlaufen C (2011): Executive dysfunctions in pedophilic and nonpedophilic child molesters. J Sex Med 8:1975–1984. [DOI] [PubMed] [Google Scholar]
- Schiffer B, Krueger T, Paul T, de Greiff A, Forsting M, Leygraf N, Schedlowski M, Gizewski E (2008a): Brain response to visual sexual stimuli in homosexual pedophiles. J Psychiatry Neurosci 33:23–33. [PMC free article] [PubMed] [Google Scholar]
- Schiffer B, Paul T, Gizewski E, Forsting M, Leygraf N, Schedlowski M, Kruger THC (2008b): Functional brain correlates of heterosexual paedophilia. Neuroimage 41:80–91. [DOI] [PubMed] [Google Scholar]
- Schiffer B, Peschel T, Paul T, Gizewski E, Forsting M, Leygraf N, Schedlowski M, Krueger THC (2007): Structural brain abnormalities in the frontostriatal system and cerebellum in pedophilia. J Psychiatr Res 41:753–762. [DOI] [PubMed] [Google Scholar]
- Schiltz K, Witzel J, Northoff G, Zierhut K, Gubka U, Fellmann H, Kaufmann J, Tempelmann C, Wiebking C, Bogerts B (2007): Brain pathology in pedophilic offenders: evidence of volume reduction in the right amygdala and related diencephalic structures. Arch Gen Psychiatry 64:737–746. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto MC (2009): Pedophilia. Annu Rev Clin Psychol 5:391–407. [DOI] [PubMed] [Google Scholar]
- Stark CP (2005): Behavioral effects of stimulation of the medial amygdala in the male rat are modified by prior sexual experience. J Gen Psychol 132:207–24. [DOI] [PubMed] [Google Scholar]
- Stoléru S (2014): Reading the Freudian theory of sexual drives from a functional neuroimaging perspective. Front Hum Neurosci 8:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoléru S, Fonteille V, Cornélis C, Joyal C, Moulier V (2012): Functional neuroimaging studies of sexual arousal and orgasm in healthy men and women: A review and meta‐analysis. Neurosci Biobehav Rev 36:1481–1509. [DOI] [PubMed] [Google Scholar]
- Stoléru S, Fonteille V, Moulier V (2013): Corrélats neuraux de l'attirance sexuelle pédophile. Ann Médico‐psychologiques, Rev Psychiatr 171:444–448. [Google Scholar]
- Suchy Y, Whittaker JW, Strassberg DS, Eastvold A (2009): Neurocognitive differences between pedophilic and nonpedophilic child molesters. J Int Neuropsychol Soc 15:248–257. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R (2013): Brain structures associated with executive functions during everyday events in a non‐clinical sample. Brain Struct Funct 218:1017–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, Vollmert C, Brassen S, Schmitt A, Dressing H, Braus DF (2004): Pedophilia: Neuropsychological evidence encouraging a brain network perspective. Med Hypotheses 63:528–531. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P (2012): Minimizing within‐experiment and within‐group effects in activation likelihood estimation meta‐analyses. Hum Brain Mapp 33:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Witzel J, Wiebking C, Gubka U, Rotte M, Schiltz K, Bermpohl F, Tempelmann C, Bogerts B, Heinze HJ, Northoff G (2007): Pedophilia is linked to reduced activation in hypothalamus and lateral prefrontal cortex during visual erotic stimulation. Biol Psychiatry 62:698–701. [DOI] [PubMed] [Google Scholar]
- Walter M, Bermpohl F, Mouras H, Schiltz K, Tempelmann C, Rotte M, Heinze HJ, Bogerts B, Northoff G (2008): Distinguishing specific sexual and general emotional effects in fMRI‐subcortical and cortical arousal during erotic picture viewing. Neuroimage 40:1482–1494. [DOI] [PubMed] [Google Scholar]
- zu Eulenburg P, Caspers S, Roski C, Eickhoff SB (2012): Meta‐analytical definition and functional connectivity of the human vestibular cortex. Neuroimage 60:162–169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information Figure 1


