Abstract
Hereditary hypophosphatemic rickets with hypercalciuria (HHRH) is caused by mutations in SLC34A3, the gene encoding the renal sodium-phosphate co-transporter NaPi-IIc. Despite increased urinary calcium excretion, HHRH is typically not associated with kidney stones prior to treatment. However, here we describe two sisters, who displayed nephrolithiasis or nephrocalcinosis upon presentation. The index patient, II-4, presented with short stature, bone pain, and knee X-rays suggestive of mild rickets at age 8.5 years. Laboratory evaluation showed hypophosphatemia, elevated 1,25(OH) 2 vitamin D levels, and hypercalciuria, later also developing vitamin D deficiency. Her sister, II-6, had a low normal serum phosphorous level, biochemically vitamin D deficiency and no evidence for osteomalacia, but had undergone left nephro-ureterectomy at age 17 because of ureteral stricture secondary to renal calculi. Nucleotide sequence analysis of DNA from II-4 and II-6 revealed a homozygous missense mutation c.586G>A (p.G196R) in SLC34A3/NaPi-IIc. Ultrasonographic examinations prior to treatment showed grade I nephrocalcinosis for II-4, while II-6 had grade I-II nephrocalcinosis in her remaining kidney. Four siblings and the mother were heterozygous carriers of the mutation, but showed no biochemical abnormalities. With oral phosphate supplements, hypophosphatemia and hypercalciuria improved in both homozygous individuals. Renal calcifications that are presumably due to increased urinary calcium excretion can be the presenting finding in homozygous carriers of G196R in SLC34A3/NaPi-IIc, and some or all laboratory features of HHRH may be masked by vitamin D deficiency.
Keywords: nephrocalcinosis, HHRH, Na-PiIIc
Introduction
Hypophosphatemic rickets can be acquired or caused by hereditary conditions leading to renal tubular phosphate-wasting. The most prevalent acquired form is vitamin D-deficiency – still a common cause even in the Western world (Nield et al., 2006) – which leads to PTH-dependent urinary phosphate losses. Other causes include Fanconi’s syndrome secondary to chemotherapeutic agents with tubular toxicity (Drezner, 2003) and tumor-induced osteomalacia (TIO) due to overproduction by the tumors of one of possibly several phosphaturic factors, including fibroblast growth factor 23 (FGF23), FGF7, secreted frizzled-related protein 4 (sFRP4), or matrix extracellular phosphoglycoprotein (MEPE) comprising the C-terminal ASARM motif (Econs and Drezner, 1994, Drezner, 2001, Carpenter et al., 2005).
Hereditary forms of urinary phosphate-wasting include renal tubular abnormalities (i.e. Dent’s syndrome (loss of function mutations in CLCN5, OMIM #300009 (Lloyd et al., 1996)), Fanconi syndrome (unknown molecular defect, OMIM #134600 (Ben-Ishay et al., 1961)), Lowe syndrome (caused by mutation in OCRL1, OMIM #309000 (Leahey et al., 1993)), and syndromes with excess FGF23 production such as X-linked hypophosphatemia (XLH, caused by inactivating mutations in a phosphate-regulating gene with homologies to endopeptidases on the X chromosome (PHEX), OMIM #307800 (HYP-Consortium, 1995)), autosomal-dominant hypophosphatemic rickets (ADHR, caused by activating FGF23 mutations, OMIM #193100 (ADHR consortium, 2000)), and autosomal recessive hypophosphatemia (ARHP, caused by inactivating mutations in dentin matrix protein 1 (DMP1) (Feng et al., 2006, Lorenz-Deperieux et al., 2006)).
Another disorder characterized by renal phosphate-wasting and the development of rickets/osteomalacia is hereditary hypophosphatemic rickets with hypercalciuria (HHRH, OMIM #241530), which was first described by Tieder et al. (Tieder et al., 1985). Inheritance of HHRH follows an autosomal-recessive mode, but only a few kindreds have been reported to date. The hypophosphatemia in patients with HHRH appears to be FGF23-independent (Lorenz-Deperieux et al., 2006), and results in an appropriate elevation of 1,25-(OH)2 vitamin D (1,25-(OH)2-D) levels leading to absorptive hypercalciuria, which distinguishes this disorder from other inherited forms of rickets/osteomalacia such as XLH, ADHR, and ARHP.
Positional cloning approaches recently led to the identification of homozygous or compound heterozygous mutations in SLC34A3 (chromosome 9q34), the gene encoding the renal sodium-phosphate co-transporter NaPi-IIc, as the genetic defect responsible for HHRH (Lorenz-Deperieux et al., 2006, Bergwitz et al., 2006). NaPi-IIc is expressed in the proximal renal tubules and, like the closely related sodium-phosphate co-transporter NaPi-IIa, its expression is regulated by phosphate, PTH, and FGF23 (Miyamoto et al., 2004; Segawa et al., 2007). Interestingly, PTH-dependent internalization of NaPi-IIc appears to be considerably slower (hours) (Segawa et al., 2007) than PTH-dependent internalization of NaPi-IIa (minutes) (Bacic et al., 2006), which may in part explain the unanticipated important role of NaPi-IIc in mammalian phosphate homeostasis (Segawa et al., 2007). Penetrance and expressivity of HHRH appears to be variable since some patients with the homozygous mutation c.228del presented only with hypercalciuria; however, subtle bone changes may have been missed, particularly if the diagnosis of HHRH due to two mutant NaPi-IIc alleles was established only later in life (Bergwitz et al., 2006). Furthermore, some heterozygous carriers of c.228del present with hypercalciuria, one of several laboratory features of HHRH, but without readily detectable bone changes.
Despite the presence of hypercalciuria, renal calcifications have been described only in a surprisingly small number of HHRH patients and these changes were generally attributed to the incorrect treatment of the disorder with vitamin D analogs (Tieder et al., 1985, Chen et al., 1989, Tieder et al., 1993). Recently, however, stone formation was noticed to be part of the initial presentation in two kindreds (Bergwitz et al., 2006). Further-more, Ishikawa et al. (Ichikawa et al., 2006) described recurrent nephrolithiasis in heterozygous and homozygous carriers of g.2259_2359del, which leads to abnormal splicing and loss-of-function of NaPi-IIc.
Here, we describe two female patients with a homozygous missense mutation in NaPi-IIc (c.586G>A (p.G196R)), but only one of them presented initially with laboratory findings indicative of HHRH, including hypophosphatemia, elevated 1,25-vitamin D, hypercalciuria, and bone changes suggestive of mild rickets; in the other patient, HHRH was masked by vitamin D deficiency. In addition, both affected individuals revealed nephrocalcinosis and nephrolithiasis, providing further evidence that renal stones can be part of the clinical presentation of HHRH.
Materials and Methods
Subjects
For all clinical and genetic studies informed consent was obtained. The index case, II-4, presented with bone pain and short stature at the age of 8.5 years. She is the youngest of 10 children of a consanguineous Turkish-Kurdish family, who had moved at age 2.5 years from Turkey to Germany (Fig. 1). Her previous medical history has been uneventful. Her father (I-2) had died from unrelated causes before the family moved to Germany and history of symptoms suggesting renal stones were absent. II-10 was unavailable for evaluation.
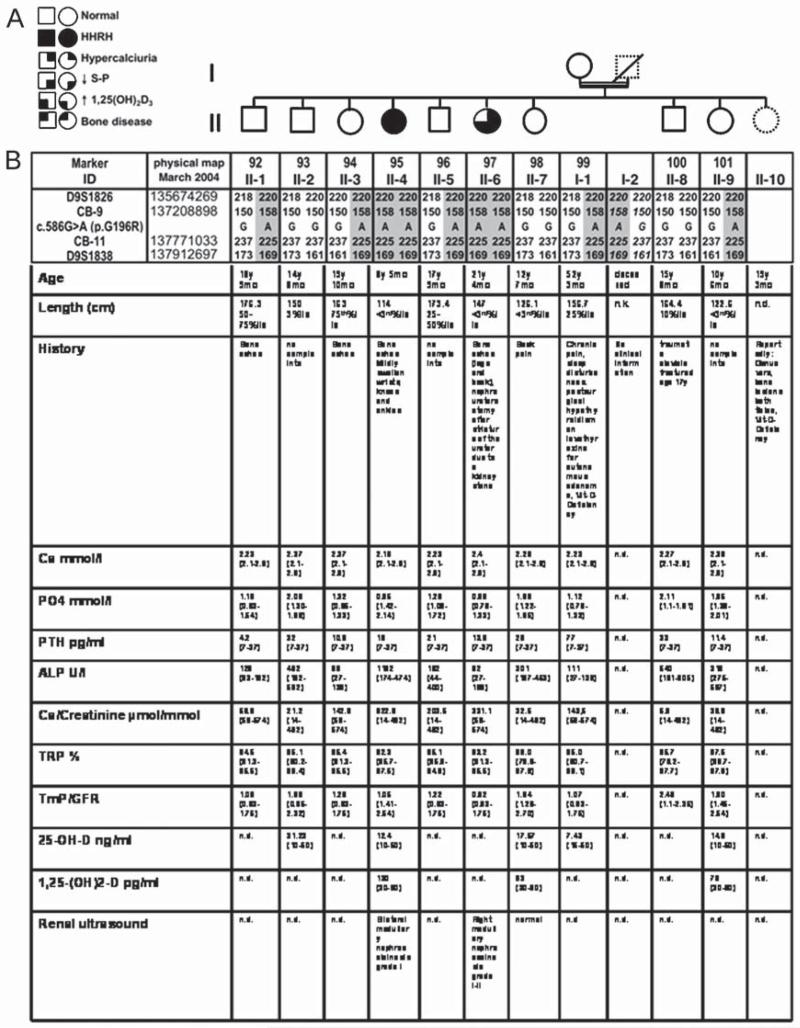
Fig. 1.
Haplotype analysis of the kindred. A: Pedigree: Solid circles or squares indicate individuals, who developed rickets during childhood along with renal phosphate-wasting, hypophosphatemia, and hypercalciuria. Open symbols indicate individuals who were healthy. Samples from individuals with dashed lines were unavailable for genotyping. B: Haplotypes for chromosomal region 9q34 between markers D9S1826 and D9S1838. Alleles for microsatellite markers are designated as bp or coded; the haplotype associated with HHRH is depicted by numbers on gray background. The father’s haplotype is deduced (numbers in italic), no genotyping data are available for II-10; the mutation c.586G>A(p.G196R) was identified by nucleotide sequencing analysis and confirmed on at least two independent PCR products. Clinical and biochemical parameters of the available family members are prior to therapy. Age-related reference ranges in brackets. n.d. = not done.
Mutational and Haplotype Analysis
SLC34A3, the gene encoding NaPi-IIc, spans ~5 kb of genomic DNA. The entire SLC34A3 gene of the index case II-4 was amplified by PCR (Qiagen PCR kit) in four overlapping fragments as described (Bergwitz et al., 2006). The PCR products were then purified using spin columns (Qiagen) and nucleotide sequence analysis was performed at the Massachusetts General Hospital DNA Sequencing Core Facility using 20 ng/100 bp DNA and nested forward or reverse primers (100 ng each). Multiplex PCR assays for the analysis of c.586G > A (p.G196R) in all subsequent individuals were designed as described (Bergwitz et al., 2006) and performed at the Harvard Partners Center for Genetics and Genomics (Cambridge, MA). Haplotype analysis of the microsatellite markers D9S1826 and D9S1838 (deCODE Genetics (Kong et al., 2002)), and the markers CB-9 and CB-11 (16) was done at the CHGR Genotyping Resource, Center for Human Genetic Research, Massachusetts General Hospital, Boston, MA. Briefly, all DNA fragments (CB-9, CB-11, D9s1826 and D9s1838) were amplified in a 7.5 μl PCR reaction that contained 3 ng of DNA, 2.5 mM MgCl2 (Applied Biosystems), 1 × PCR Buffer II (Applied Biosystems), 0.25 mM dNTPs (Amersham), 0.5 pmol of forward (labeled) and reverse primer, 0.5 U AmpliTaq Gold (Applied Bio-systems). The reactions were cycled as follows: 12 minutes at 95 ° C, 10 cycles of denaturation at 94 ° C for 30 seconds, annealing 58 ° C for 30 seconds and extension at 72 ° C for 1 minute followed by 20 cycles of denaturation at 89 ° C for 30 seconds, annealing 55 ° C for 30 seconds and extension at 72 ° C for 1 minute with a final extension at 72 ° C for 40 minutes. After amplification, the products were run on an Applied Biosystems 3730XL DNA Analyzer along with GeneScan 500Liz as an internal standard and data were analyzed using Applied Biosystems GeneMapper v3.7 software package.
Blood Sample Collection and Clinical Evaluation
The blood samples were taken randomly during the day (not fasting) in the pediatric endocrine outpatient clinic at the University of Lübeck.
The renal ultrasonography was performed with a Phillips HDI 5000 using a 5 MHz sector transducer; this method is sufficiently sensitive to detect even subtle changes of nephrocalcinosis (Weber et al., 1988). Criteria for grades of severity are the distinction in medullary, cortical and global nephrocalcinosis with a subdivision into grades I, IIa, IIb and III regarding the medullary nephrocalcinosis (Hoyer, 1996). Grade I refers to a discrete increase in echogenity of the pyramides, an increase of echogenity in the apex of the pyramides and a loss of cortico-medullary differentiation, grade IIa refers to an increase of echogenity in the outer zone of the pyramides and in the perimedullary cortical zone, while the central zone of the pyramides is not affected and there is no posterior acoustical shadow. Grade IIb refers to a diffuse hyperechogenity of the pyramides overall without posterior acoustical shadow, whereas grade III describes a diffuse hyperechogenity of all pyramides with posterior acoustical shadow.
Radiographic studies were performed in the Department of Radiology at the University Hospital Schleswig-Holstein, Campus Lübeck, Germany.
Calcium, phosphate, creatinine and unfractionated alkaline phosphatase were measured in the Central Laboratory of the University Hospital Schleswig-Holstein, Campus Lübeck, Germany employing the automated Aeroset system (Abbott) using standard procedures.
The following laboratory values were measured in a Pediatric Endocrine Laboratory at the Children’s Hospital, University Hospital Schleswig-Holstein, Campus Lübeck, Germany: 25-hydroxy-vitamin D and 1,25-hydroxy-vitamin D were measured by radio-immunoassay (IDS, Frankfurt am Main, Germany), parathyroid hormone (PTH) was measured by immunoenzymometric assay (IDS, Frankfurt am Main, Germany) and deoxypyridinoline by Metra-DPD-enzymeimmunoassay (Quidel (medical), Hannover, Germany). Bone-specific alkaline phosphatase was measured by standard procedure using the automated Aeroset system (Abbott) after extraction with wheat germ (Sigma, Munich, Germany).
Statistical Analysis
For segregation and linkage analysis, we used Superlink V1.4, a web-based computer program that performs exact genetic linkage analysis with input-output relationships similar to those in standard genetic linkage programs Linkage, Fastlink v4.1, Tlinkage, Vitesse v2.0, and Genehunter v2.1. Superlink can run larger files than previously described programs (http://bioinfo.cs.technion.ac.il/superlink-online/) (Silberstein et al., 2006).
Results
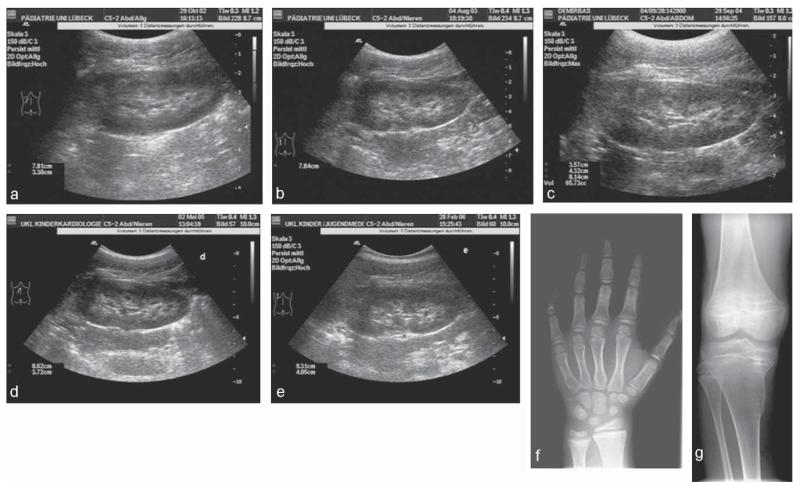
The index case II-4 presented at age 8.5 years with short stature (length 114 cm, < 3rd %ile for Turkish girls) and leg aches that had persisted for one year. Her weight was 19.9 kg (3rd–10th %ile for Turkish girls), head circumference 50.5 cm (10–25th %ile). There were no signs for disproportional short stature with normal measures for the lower body segment (56 cm) and arm span (115 cm). She had mildly swollen knees, wrists and ankles, but no bone deformities or rachitic rosary. Her bone age was delayed by 2 years, X-ray studies of her left hand demonstrated brachymesophalangia, clinodactyly of the 5th digit and slight osteopenia with minimal flaring of the epiphyses, not indicative of severe rickets (Fig. 2). The radiographs of both knees showed flared epiphyses with hypomineralization and absence of trabecular structure, but no bowing (initial x-ray of knees unavailable). A renal ultrasound was suggestive of grade I nephrocalcinosis, according to Hoyer (1996) (Fig. 2).
Fig. 2.
Ultrasound of the left kidney and X-rays of individual II-4 homozygous for the G196R mutation. Panels a to e show the renal ultrasound (a: age 8; 10 years, b: age 9; 8 years, c: age 10; 9 years, d: age 11; 5 years, e: age 12; 2 years). Panel a was taken before initiation of therapy and demonstrates slight nephrocalcinosis corresponding to grade I, nephrocalcinosis worsens slightly in due course to grade IIa (panels b to e). Panel f demonstrates the X-ray of the hand before therapy (age 8; 5 years) with a bone age of 6; 6 years (Greulich and Pyle) and shows slight flaring and mild osteopenia apart from brachymesophalangia. Panel g shows the X-ray of the right knee (age 11; 5 years) with epiphyseal flaring, numerous transverse striations in the metaphysis and Erlenmeyer flask deformities in the metaphysic.
Laboratory values (Table 1) of II-4 at the time of initial presentation revealed a low phosphate level of 0.95 mmol/l (normal range for this age: 1.42–2.14 mmol/l) due to renal phosphate-wasting; TmP/GFR was low at 1.05 (normal range: 1.42–2.54), an elevated unfractionated alkaline phosphatase (1162 U/l; normal range: 174–474 U/l) and an elevated bone-specific alkaline phosphatase (1078 U/l; normal range: 78–394 U/l)), elevated 1,25-(OH)2-vitamin D of 130 pg/ml (normal range: 30–90 pg/ml), and elevated urinary calcium excretion (622.6 μmol calcium/mmol creatinine; normal range: 14–492 μmol calcium/mmol creatinine). PTH, calcium and 25-OH-vitamin D were normal, thus excluding vitamin D deficient rickets at this time. Her other laboratory findings were normal, including serum bicarbonate, normal liver enzymes and normal complete blood counts and there were no signs of a generalized tubulopathy i.e. glycosuria and aminoaciduria. All available family members were screened biochemically at this time showing no signs for hypophosphataemic rickets. The mother’s laboratory findings were consistent with secondary hyperparathyroidism due to vitamin D deficiency (Fig. 1B).
Table 1.
Clinical course of both homozygous patients II-4 and II-6. Reference ranges in parenthesis, n.d. = not done, abnormal values in bold
| Clinical course | II-4 | II-4 | II-4 | II-4 | II-4 | II-6 | II-6 | II-6 |
|---|---|---|---|---|---|---|---|---|
| 8.5 years prior to therapy | 9.5 years | 10.5 years | 11.5 years | 12.5 years* | age 21 years prior to therapy |
age 25 years* prior to therapy |
age 25.3 years | |
| Ca mmol/l | 2.18(2.1–2.6) | 2.23(2.1–2.6) | 2.27 (2.1–2.6) | 2.22(2.1–2.6) | 2.26 (2.1–2.6) | 2.4 (2.1–2.6) | 2.4(2.1–2.6) | 2.51 (2.1–2.6) |
|
| ||||||||
| PO4mmol/l | 0.95(1.42–2.14) | 0.81(0.9–1.75) | 0.9(0.9–1.75) | 0.9(0.9–1.75) | 0.63(0.9–1.75) | 0.89(0.79–1.33) | 0.56(0.85–1.45) | 0.92(0.85–1.45) |
|
| ||||||||
| PTH pg/ml | 16(7–37) | 21(7–37) | 12(7–37) | 37(7–37) | 48(7–37) | 13.6(7–37) | 25.7(7–37) | 10,74(7–37) |
|
| ||||||||
| ALP U/l | 1162(174–474) | 824(40–370) | 893(40–370) | 1383(40–370) | 1038(40–370) | 92(27–189) | 83(44–155) | 71 (44–155) |
|
| ||||||||
| TRP % | 92.3(85.7–97.5) | 92.8(83.5–97.2) | 91.4(88.7–97.3) | 73.1(79.9–97.9) | 71.8(76.2–97.7) | 93.5(81.3–95.5) | 92.5(81.3–95.5) | n.d. |
|
| ||||||||
| TmP/GFR | 1.05(1.41–2.54) | 0.88(1.52–2.39) | 0.93(1.42–2.48) | 0.66(1.27–2.65) | 0.45(1.1–2.35) | 0.82(0.63–1.75) | 0.60(0.66–1.55) | n.d. |
|
| ||||||||
| 25-OH-D pg/ml | 12.4(10–50) | n.d. | 7.9(10–50) | 8.1(10–50) | 5.4(10–50) | n.d. | 7.9(10–50) | 10.2(10–50) |
|
| ||||||||
| 1,25-(OH)2-D ng/ml | 130(30–90) | n.d. | 112.9(20–60) | 65.4(20–60) | 20.4 (20–60) | n.d. | 29(20–60) | 54.8 (20–60) |
|
| ||||||||
|
Ca/Creatinine
μmol/mmol |
622.6(14–492) | 615.2(14–492) | 1131.5(14–492) | 496.4(14–492) | 503.0(14–492) | 331.1(59–574) | 595.7(59–574) | n.d. |
|
| ||||||||
|
Deoxypyrdinoline/
Creatinine nmol/mmol |
57.5(12–51) | 27.0(12–51) | n.d. | 62.9(12–51) | 44.2(5–41) | n.d. | n.d. | n.d. |
|
| ||||||||
| X-ray findings | brachymesophalangia, clinodac- tyly of DV, slight osteopenia and subtle flaring, not indicative of severe rickets, bone age 6 years 6 months, both knees: reduced bone density and structure, epiphyseal flaring, transverse sclerotic striations in the meta- physic of tibiae and femura |
left hand at age 9 years 7 months, transparent corticalis of metacarpal I–V brachymesophalan- gia, clinodactily of DV, bone age 8 years 10 months |
X-ray at age 10 years 9 months: cupping of the metaphysis of the ulna, brachymesophalangia of DV, bone age 10 years, both knees at age 10 years 9 months, no epiphyseal flaring, trans- verse sclerotic striation in the metaphysis |
right knee at 11 years 5 months: Erlenmeyer flask deformity of the proximal meta- physic of tibia and fibula, numerous transverse sclerotic striations |
n.d. | n.d. | thickened cortical bone structure and ulnar deviation of digitae II and III in the proximal interphalangeal joints |
n.d. |
|
| ||||||||
| Renal ultrasound | bilateral medullary nephrocalci- nosis grade I |
bilateral medullary nephrocalcinosis grade IIa |
bilateral medullary nephrocalcinosis IIa |
bilateral medullary nephrocalcinosis grade IIa |
medullary nephrocalcinosis grade IIa, |
n.d. | right medullary nephrocalcinosis, grade I–II |
right medullary nephrocalcinosis, grade I–II |
age of genetic diagnosis
Although these laboratory findings were not entirely consistent with the diagnosis of XLH, therapy was first initiated with calcitriol 0.25 μg/day (11.6 ng/kg/day) and phosphorus 500 mg/day (23 mg/kg/day). The dose of oral phosphate was subsequently increased to 500 mg phosphorus twice daily (46 mg/kg/day). Approximately 6 months later nephrocalcinosis stage IIa (according to Hoyer, 1996) was detected by repeat renal ultrasound and persistent hypophosphatemia suggested poor compliance with her phosphate supplements (Fig. 2). Although nephrocalcinosis has been observed during calcitriol therapy in patients with XLH, the concurrent hypercalciuria raised the suspicion for HHRH, particularly since in the meantime nucleotide sequence analysis of the exons and flanking regions of PHEX (XLH) and FGF23 (ADHR) had revealed no mutation and heterozygous SNPs excluded large deletions for both genomic regions (data not shown). Similarly, a MIBG-skeletal scintigraphy and octreotide-scans to search for tumor-induced osteomalacia were negative; her ophthalmologic evaluation was within normal limits and the urine was negative for glucose and protein and the excretion of amino acids was normal, thus excluding a generalized tubulopathy (i.e. Dent’s disease, Fanconi syndrome, cystinosis). While 24-hr urine phosphate excretions to confirm non-compliance were unavailable, treatment under close clinical observation ultimately normalized her serum phosphorous level and decreased her alkaline phosphatase supporting the suspicion of poor compliance. Since her hypercalciuria worsened with calcitriol treatment, this medication was discontinued at age 9 years and 8 months, but therapy with phosphorus at 50 mg/kg/day was continued. However, outpatient compliance remained poor explaining her persistent hypophosphatemia and elevation of alkaline phosphatase. At 11 years 5 months, she was found to have genua vara (intercondylar distance of 7 cm) and severe muscular weakness now limited her gait. Radiographic studies of the knees showed osteomalacia with genua vara (Fig. 2g). Her laboratory studies showed development of vitamin D deficiency from age 10.5 years, but treatment with 1 000 U Vitamin D per day was only initiated at age 12.5 years. Interestingly, at this time her initial hypercalciuria and the 1,25-(OH)2-D levels had improved in the context of vitamin D deficiency. Her urine studies now indicated significant hyperaminoaciduria (threonine 47.0 mmol/mol creatinine (8–28), serine 95.0 mmol/mol creatinine (23–69), asparagine 66.0 mmol/mol creatinine (< 24), glutamine 151.0 mmol/mol creatinine (20–112), glycine 845.0 mmol/mol creatinine (64–236), alanine 88.0 mmol/mol creatinine (17–65), ß-alanine 21.0 mmol/mol creatinine ( <4), 1-meth-histidine 243 mmol/mol creatinine ( <40), carnosine 40.0 mmol/mol creatinine ( <10), all other amino acids were within normal limits)) and because of microalbuminuria (albumine 31 g/g creatinine ( <25)), treatment with an ACE-inhibitor (enalapril 2.5 mg/day) was initiated.
At age 12.5 sequence analysis of the gene encoding NaPi-IIc became available and a homozygous mutation c.586G > A (p.G196R) was identified, which confirmed the clinical diagnosis of HHRH.
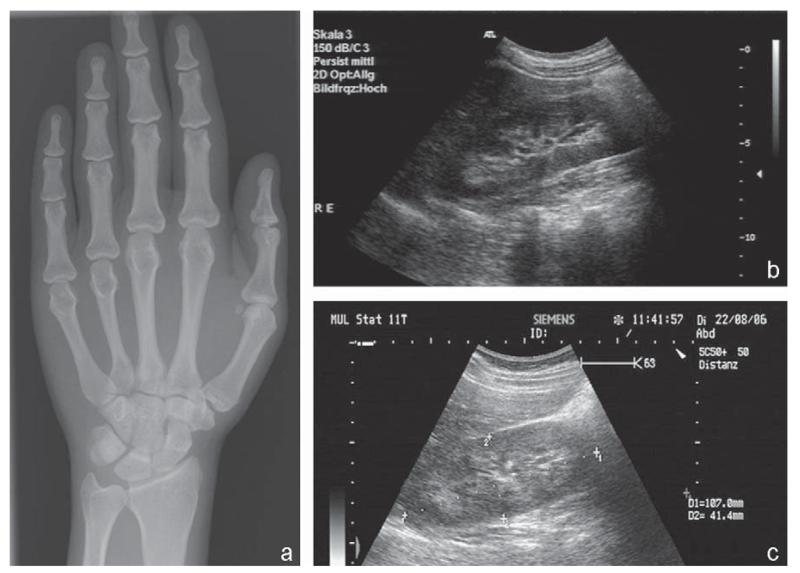
At this time all available family members were investigated genetically, including the mother of the index case II-4 and 8 of her 9 siblings. The mother was found to be heterozygous for p.G196R, as were four of her siblings. The DNA of the deceased father and of one, possibly affected, sister are unavailable. Haplotype analysis was performed to deduce the paternal allele, which was consistent with consanguinity for both parents. None of the heterozygous carriers showed evidence for hypophosphatemia, hypercalciuria, or rickets (Fig. 1B). Interestingly, one sister (II-6) was homozygous for p.G196R; however, due to normophosphatemia and normal renal calcium and phosphate excretion upon initial evaluation at age 21 (Fig. 1B), her diagnosis had been missed. Retrospectively, however, II-6 had many features consistent with HHRH. At age 17.5 years she had under-gone left nephroureterctomy after stricture of the ureter due to a kidney stone, she had bone pain throughout her childhood and adolescence, and her adult height is below the 3rd percentile for Turkish girls. Her laboratory evaluation at age 25 (Table 1) – prompted by our genetic studies – now showed hypophosphatemia (0.56 mmol/l, normal range: 0.85–1.45 mmol/l) and mild hypercalciuria (595.74 μmol calcium/mmol creatinine; normal range: 59–574 μmol calcium/mmol) with decreased transport maximum for phosphate (0.60; normal range: 1.52–2.39), while her alkaline phosphatase, PTH and 1,25-(OH)2-vitamin D levels were within normal limits. The 25-OH-vitamin D level was below the normal range (7.9 pg/ml; normal range: 10–50) indicative of mild vitamin D deficiency. Radiographic studies of her left hand revealed no signs of osteomalacia (hypomineralization or absence of the trabecular structure), but thickened cortical bone structure and ulnar deviation of digits II and III in the proximal interphalangeal joints (Fig. 3). A renal ultra-sound of the remaining right kidney showed medullary nephrocalcinosis grade I-II (according to Hoyer et al., (23)) (Fig. 3). Treatment with elemental phosphorus at 1600 mg/day (32 mg/kg/day) in four divided doses was initiated. The first evaluation after three months of treatment continued to show medullary nephrocalcinosis grade I-II (Fig. 3) and the laboratory values showed no changes apart from a normalized serum phosphate (0.92 mmol/l; normal: 0.85–1.45 mmol/l) and a 25-OH vitamin-D level within the lower end of the normal range whereas the 1,25-(OH)2 vitamin-D level was now within the upper end of the normal range (Table 1).
Fig. 3.
Ultrasound and X-ray of individual II-6. Panel a shows the X-ray of the left hand at age 25 years with cortical thickening and ulnar deviation in the interphalangeal joints Dig II and III. Panel b depicts the ultrasound of the remaining right kidney at age 25 years before initiation of therapy, showing slight medullary nephrocalcinosis. Panel c demonstrates the ultrasound of the right kidney after 2 months of therapy with phosphorus continuing to show medullary nephrocalcinosis.
Assuming a recessive disease model with 99% penetrance and a frequency of the disease-causing mutant gene of 0.00001%, a lod score of 2.3 (theta 0) was calculated for this kindred, which is suggestive for linkage of HHRH and kidney stones to p.G196R.
Discussion
We here describe a large sibship of Turkish-Kurdish background with two siblings affected by HHRH, who both carry a homozygous missense mutation in SLC34A3/NaPi-IIc (c.586G > A (p.G196R)). The mutation co-segregates with the disease pheno-type in our kindred, making it likely that the homozygous p.G196R mutation causes HHRH. Most previously described patients are compound heterozygous for NaPi-IIc mutations and heterozygosity for p.G196R was previously reported in two unrelated compound heterozygous individuals with HHRH (Lorenz-Deperieux et al., 2006, Bergwitz et al., 2006). We here describe the clinical characterization of the first kindred in which affected individuals are homozygous for p.G196R. Haplo-type analysis of all available family members is consistent with consanguinity and, furthermore, indicates that our kindred is unrelated to the previously reported kindreds A and C (Bergwitz et al., 2006). G196 is an amino acid residue that is evolutionarily conserved in this sodium-phosphate transporter and that was previously shown to be a determinant of stoichiometry in the murine ortholog (Bacconi et al., 2005).
The diagnosis of HHRH was initially missed in II-6 because her serum phosphorous level as well as urinary phosphate and calcium excretion were normal at the age of 21 years when the entire family was screened for laboratory abnormalities; furthermore both affected individuals, II-4 and II-6, presented with a relatively mild skeletal phenotype. Although we cannot completely exclude that subtle bone changes in II-4 and II-6 were present earlier in life, it appears plausible that p.G196R causes only a mild form of HHRH, possibly due to residual activity of the co-transporter. Consistent with this conclusion idiopathic hypercalciuria, which can be observed in heterozygous carriers of different NaPi-IIc mutations (Bergwitz et al., 2006), was absent in their heterozygous mother and their four heterozygous siblings; however, the absence of hypercalciuria could have also been related to co-existing vitamin D deficiency. Furthermore, genetic background may be contributing since relatives in our previously reported kindreds A and C, who are heterozygous for p.G196R showed mild hypophosphatemia, elevated 1,25-(OH)2 vitamin D levels, and hypercalciuria (Bergwitz et al., 2006).
II-4 initially had mild initial nephrocalcinosis, which worsened on erroneous treatment with calcitriol. This complication, which has previously been recognized (Chen et al., 1989, Tieder et al., 1993), re-emphasizes that calcitriol is not usually indicated for the treatment of HHRH. However, her sister II-6 presented with stones and nephrocalcinosis I-II prior to the initiation of therapy, Together with one other recent description of nephrolithiasis prior to initiation of treatment with vitamin D analogs (Ichikawa et al., 2006), our findings thus emphasize further that HHRH can present with nephrolithiasis and nephrocalcinosis, and that NaPi-IIc mutations should be considered as a rare differential diagnosis in patients with these renal findings.
Four other homozygous mutations have been described (c.228del (15), c.905delC and c.1058G > T (p.R353L) (Feng et al., 2006)), and g.2259–2359del (Ichikawa et al., 2006); In addition to p.G196R, only g.2259_2359del was found be associated with nephrolithiasis or nephrocalcinosis, which could indicate that only certain NaPi-IIc mutations lead to renal calcifications.
The absence of hypophosphatemia and hypercalciuria in the initial evaluation of II-6 also illustrates that repeated measurements of blood and urine samples are necessary to establish the biochemical diagnosis and that renal ultrasounds should be performed in all family members of individuals to clinically exclude the diagnosis HHRH, even if no biochemical abnormalities in serum or urine are detected. Clinical and laboratory re-evaluation of II-6 at age 25 – prompted by our genetic findings – now revealed some features of HHRH in the setting of vitamin D deficiency, including hypophosphatemia and hypercalciuria. Like-wise, II-4 developed severe bone changes consistent with rickets only when she became vitamin D deficient at age 10.5 years (as defined by 25-vitamin D levels below 10 pg/ml). Furthermore, vitamin D deficiency led to near-normalization of the urinary calcium excretion and serum 1,25-OH vitamin D levels in II-4, illustrating that vitamin D deficiency can mask the biochemical features of HHRH.
Both affected individuals, II-4 and II-6, are now being treated with phosphate alone, which would be expected to reduce their hypercalciuria and to prevent progression of their nephrocalcinosis, or even to reverse these findings. FGF23 levels are normal or low in patients affected by HHRH, which is different from other FGF23-dependent phosphate-wasting disorders. Increasing serum phosphate levels should there-fore suppress the renal 1-alpha hydroxylase leading to a reduction in 1,25 (OH)2 levels and thus a normalization of urinary calcium excretion. However, II-4 has thus far failed to significantly improve her nephrocalcinosis and she continues to show an elevated urinary calcium excretion, raising the concern of non-compliance with her phosphate supplementation.
The hand radiographs of both II-4 and II-6 showed brachymesophalangia and clinodactyly of D5, or D2 and D3, respectively, but it remains uncertain whether these findings are related to the identified NaPi-IIc mutation. NaPi-IIc does not appear to be expressed in the skeleton and it is unlikely that these isolated changes are related to hypophosphatemia alone.
We conclude that the homozygous missense mutation p.G196R in NaPi-IIc leads to a form of HHRH with a relatively mild skeletal phenotype and some heterozygous carriers of this mutation failed to show obvious laboratory abnormalities. Nephrocalcinosis or nephrolithiasis can be the first symptoms of HHRH; and NaPi-IIc mutations thus need to be considered in the differential diagnosis when such kidney findings are observed. Our longitudinal follow-up data illustrate that the laboratory and radio-graphic manifestations of HHRH can vary considerably depending the vitamin D status, since our index patient II-4 developed rachitic changes only in the setting of vitamin D deficiency. Finally, vitamin D deficiency can mask some of the characteristic laboratory findings of HHRH; vitamin D levels and renal ultra-sonographic examinations thus should be obtained before excluding HHRH in relatives of individuals affected by HHRH.
Acknowledgments
This work was funded in part by the Else-Kröhner-Fresenius Foundation (to OH) and the National Kidney Foundation (to CB). The authors are grateful for the technical assistance of Claudia Havel for molecular analysis of the PHEX gene.
Footnotes
Disclosure/Conflicts of Interest: The authors have no disclosures.
References
- 1.ADHR Consortium. White KE, Evans WE, O’Riordan JLH, Speer MC, Econs MJ, Lorenz-Depiereux B, Grabowski M, Mettinger T, Strom TM. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 2.Bacconi A, Virkki LV, Biber J, Murer H, Forster IC. Renouncing electroneutrality is not free of charge: switching on electrogenicity in a Na+-coupled phosphate cotransporter. Proc Nat Acad Sci (USA) 2005;102(35):12606–12611. doi: 10.1073/pnas.0505882102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacic D, Lehir M, Biber J, Kaissling B, Murer H, Wagner CA. The renal Na+/phosphate cotransporter NaPi-IIa is internalized via the receptor-mediated endocytic route in response to parathyroid hormone. Kidney Internl. 2006;69(3):495–503. doi: 10.1038/sj.ki.5000148. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Ishay D, Dreyfuss F, Ullmann TD. Fanconi syndrome with hypouricemia in an adult: family study. Am J Med. 1961;31:793–800. doi: 10.1016/0002-9343(61)90163-2. [DOI] [PubMed] [Google Scholar]
- 5.Bergwitz C, Roslin NM, Tieder M, Loredo-Osti JC, Bastepe M, Abu-Zahra H, Frappier D, Burkett K, Carpenter TO, Anderson D, Garabédian M, Sermet I, Fujiwara M, Morgan K, Tenenhouse HS, Jüppner H. SLC34A3 Mutations in patients with hereditary hypophosphataemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am J Hum Genet. 2006;78(2):179–192. doi: 10.1086/499409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpenter TO, Ellis BK, Insogna KL, Philbrick WM, Sterpka J, Shimkets R. Fibroblast growth factor 7: an inhibitor of phosphate transport derived from oncogenic osteomalacia-causing tumors. J Clin Endocrinol Metab. 2005;90(2):1012–1020. doi: 10.1210/jc.2004-0357. [DOI] [PubMed] [Google Scholar]
- 7.Chen C, Carpenter T, Steg N, Baron R, Anast C. Hypercalciuric hypophosphatemic rickets, mineral balance, bone histomorphometry, and therapeutic implications of hypercalciuria. Pediatrics. 1989;84(2):276–280. [PubMed] [Google Scholar]
- 8.Drezner MK. Hypophosphatemic rickets. Endocr Develop. 2003;6:126–155. doi: 10.1159/000072774. [DOI] [PubMed] [Google Scholar]
- 9.Drezner MK. Tumor-induced osteomalacia. Rev Endocr Metab Disord. 2001;2(2):175–186. doi: 10.1023/a:1010006811394. [DOI] [PubMed] [Google Scholar]
- 10.Econs MJ, Drezner MK. Tumor-induced osteomalacia–unveiling a new hormone. N Engl J Med. 1994;330(23):1679–1681. doi: 10.1056/NEJM199406093302310. [DOI] [PubMed] [Google Scholar]
- 11.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38(11):1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoyer PF, Hofmann V, Deeg KH, Hoyer PF, Thieme . Ultraschalldiagnostik in Pädiatrie und Kinderchirurgie Lehrbuch und Atlas. 2. New York: 1996. [Google Scholar]
- 13.HYP-Consortium A gene (PHEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat Genet. 1995;11:130–136. doi: 10.1038/ng1095-130. [DOI] [PubMed] [Google Scholar]
- 14.Ichikawa S, Sorenson AH, Imel EA, Friedman NE, Gertner JM, Econs MJ. Intronic deletions in the SLC34a3 gene cause hereditary hypophosphatemic rickets with hypercalciuria. J Clin Endocrinol Metab. 2006;91(10):4022–4027. doi: 10.1210/jc.2005-2840. [DOI] [PubMed] [Google Scholar]
- 15.Kong A, Gudbjartson DF, Sainz J, Jonsdottir GM, Gudjonsson SA, Richardson B, Sigurdardottir S, Barnard J, Hallbeck B, Masson G, Shlien A, Palsson ST, Frigge ML, Thorgeirsson TE, Gulcher JR, Stefansson K. A high-resolution recombinant map of the human genome. Nat Genet. 2002;31:241–247. doi: 10.1038/ng917. [DOI] [PubMed] [Google Scholar]
- 16.Leahey AM, Charnas LR, Nussbaum RL. Nonsense mutations in the OCRL-1 gene in patients with the oculocerebrorenal syndrome of Lowe. Hum Molec Genet. 1993;2(4):461–463. doi: 10.1093/hmg/2.4.461. [DOI] [PubMed] [Google Scholar]
- 17.Lloyd SE, Pearce SH, Fisher SE, Steinmeyer K, Schwappach B, Scheinman SJ, Harding B, Bolino A, Devoto M, Goodyer P, Rigden SP, Wrong O, Jentsch TJ, Craig IW, Thakker RV. A common molecular basis for three inherited kidney stone diseases. Nature. 1996;379(6564):445–449. doi: 10.1038/379445a0. [DOI] [PubMed] [Google Scholar]
- 18.Lorenz-Deperieux B, Benet-Pages A, Eckstein G, Tenenbaum-Rakover Y, Wagenstaller J, Tiosano D, Gershoni-Baruch R, Albers N, Schnabel D, Hochberg Z, Strom TM. Hereditary hypophosphataemic rickets with hypercalciuria is caused by mutations in the sodium-phosphate cotransporter gene SLC43A3. Am J Hum Genet. 2006;78(2):193–201. doi: 10.1086/499410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyamoto K, Segawa H, Ito M, Kuwahata M. Physiological regulation of renal sodium-dependent phosphate cotransporters. Jpn J Physiol. 2004;54(2):93–102. doi: 10.2170/jjphysiol.54.93. [DOI] [PubMed] [Google Scholar]
- 20.Nield LS, Majahan P, Joshi A, Kamat D. Rickets: Not a disease of the past. Am Fam Phys. 2006;74:619–626. [PubMed] [Google Scholar]
- 21.Segawa H, Yamanaka S, Onitsuka A, Tomoe Y, Kuwahata M, Ito M, Taketani Y, Miyamoto K. Parathyroid hormone dependent endocytosis of renal type IIc Na/Pi cotransporter. Am J Physiol Renal Physiol. 2007;292(1):F395–403. doi: 10.1152/ajprenal.00100.2006. Epub 2006 Sep 19. [DOI] [PubMed] [Google Scholar]
- 22.Silberstein M, Tzemach A, Dovgolevsky N, Fishelson M, Schuster A, Geiger D. Online system for faster multipoint linkage analysis via parallel execution on thousands of personal computers. Am J Hum Genet. 2006;78(6):922–935. doi: 10.1086/504158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tieder M, Blonder J, Strauss S, Shaked U, Maor J, Gabizon D, Manor H, Sela BA. Hyperoxaluria is not a cause of nephrocalcinosis in phosphate-treated patients with hereditary hypophosphatemic rickets. Nephron. 1993;64(4):526–531. doi: 10.1159/000187395. [DOI] [PubMed] [Google Scholar]
- 24.Tieder M, Modai D, Samuel R, Arie R, Halabe A, Bab I, Gabizon D, Liberman UA. Hereditary hypophosphatemic rickets with hypercalciuria. N Engl J Med. 1985;312(10):611–617. doi: 10.1056/NEJM198503073121003. [DOI] [PubMed] [Google Scholar]
- 25.Weber G, Cazuffi MA, Frisone F, de Angelis N, Pasolini D, Tomaselli V, Chiumello G. Nephrocalcinosis in children and adolescents: sonographic evaluation during long-term treatment with 1,25-dihydroxycholecalciferol. Child Nephrol Urol. 1988-1989;9(5):273–276. [PubMed] [Google Scholar]