Abstract
Background and aims
Visualization of the internal and external root canal morphology is very important for a successful endodontic treatment; however, it seems to be difficult considering the small size of the tooth and the complexity of the root canal system. Film-based or digital conventional radiographic techniques as well as cone beam computed tomography provide limited information on the dental pulp anatomy or have harmful effects. A new non-invasive diagnosis tool is magnetic resonance imaging, due to its ability of imaging both hard and soft tissues. The aim of this study was to demonstrate magnetic resonance imaging to be a useful tool for imaging the anatomic conditions of the external and internal root canal morphology for endodontic purposes.
Methods
The endodontic system of one freshly extracted wisdom tooth, chosen for its well-known anatomical variations, was mechanically shaped using a hybrid technique. After its preparation, the tooth was immersed into a recipient with saline solution and magnetic resonance imaged immediately. A Bruker Biospec magnetic resonance imaging scanner operated at 7.04 Tesla and based on Avance III radio frequency technology was used. InVesalius software was employed for the 3D reconstruction of the tooth scanned volume.
Results
The current ex-vivo experiment shows the accurate 3D volume rendered reconstruction of the internal and external morphology of a human extracted and endodontically treated tooth using a dataset of images acquired by magnetic resonance imaging. The external lingual and vestibular views of the tooth as well as the occlusal view of the pulp chamber, the access cavity, the distal canal opening on the pulp chamber floor, the coronal third of the root canals, the degree of root separation and the apical fusion of the two mesial roots, details of the apical region, root canal curvatures, furcal region and interradicular root grooves could be clearly bordered.
Conclusions
Magnetic resonance imaging offers 3D image datasets with more information than the conventional radiographic techniques. Due to its ability of imaging both hard and soft dental tissues, magnetic resonance imaging can be successfully used as a 3D diagnostic imaging technique in dentistry. When choosing the imaging method, dental clinicians should weight the benefit-risk ratio, taking into account the costs associated to magnetic resonance imaging and the harmful effects of ionizing radiations when cone beam computed tomography or conventional x-ray are used.
Keywords: magnetic resonance imaging, radiographic image enhancement, radiography, tooth morphology, root canal
Background and aims
Imaging techniques have become essential for a successful treatment outcome. Images of the internal and external tooth anatomy are extremely valuable before endodontic treatment initiation, for both pre-visualization and development of the appropriate treatment plan. Visualization of the internal morphology of the tooth seems to be rather difficult considering the small size of the tooth, the pulp chamber volumes ranging from 0.006 mm3 in the case of the lower incisors to 0.068 mm3 in the case of the upper molars [1]. Additionally, the endodontic system has been proven to be very complex, a “straight”, conical canal being considered a utopia [2]. Film-based or digital conventional radiographic techniques have several limitations, such as the two-dimensional nature of the produced images [3], anatomical noise [4] or geometric distortion [5]. Some of these limitations have been overcome by Cone Beam Computed Tomography (CBCT), capable to generate three-dimensional images. However, one of the major concerns regarding the use of CBCT is the ionizing radiation. According to the 2007 Recommendations of the International Commission on Radiological Protection, clinicians must act in agreement with the 3 fundamental principles of radiological protection (justification, optimization, and the application of dose limits) and must keep the dose of the radiation as low as reasonably achievable [6]. The benefits of CBCT must outweigh the risks [6]. The European Society of Endodontology established a set of criteria for the use of CBCT in endodontics [7]. According to this position statement, CBCT may be considered for assessments such as the diagnosis of periapical pathosis with contradictory or nonspecific signs or symptoms, evaluation of complex dento-alveolar trauma or of an extremely complex root canal anatomy [7].
A new alternative to CBCT is magnetic resonance imaging (MRI). MRI technique is non-invasive, which makes it the desired tool for clinical diagnosis, and furthermore, presents the capability of imaging soft tissues. Lately, MRI was successfully used in endodontics [8], orthodontics [9], prosthodontics [10] and diagnosis of dental cavities [11,12]. Normally, soft dental tissues, such as periodontal tissues or the dental pulp, with high water content and long T2 relaxation times, can be accurately visualized with MRI. Special techniques, like Free Induction with Steady State Procession (FISP) have been developed to acquire MRI signals from samples with short T2 relaxation times. Moreover, the usage of this protocol allows the hard dental tissues (enamel and dentin) to be also envisaged. An important feature of MRI is that it does not involve ionizing radiation, being harmless after repetitive examinations.
The aim of this study is to show that MRI is a useful tool for imaging the anatomic conditions of the external and internal root canal morphology for endodontic purposes.
Methods
One freshly extracted wisdom tooth was used in the present study. The molar was chosen due to its well-known complex root canal morphology. An impacted third lower molar was extracted with the patient’s consent to prevent the development of related pathology and well-known complications. The molar was thoroughly debrided immediately after extraction, using manual Gracey curettes. The periodontal ligament was completely removed in order to only keep sound cementum on the external surface of the root. Then, the tooth was stored in saline solution at room temperature (22°C) for 48 hours.
The endodontic system of the tooth was mechanically shaped using a hybrid technique, i.e., gates glidden burrs (VDW, Munich, Germany) for the coronal third of the root canals, rotary instruments for the mid third of the root canal, ProTaper (Dentsply Maillefer, Ballaigues, Switzerland) and manual Kerr files (VDW, Munich, Germany) for the apical one third using a step back technique for a 6% taper. During the mechanical preparation only saline solutions were used for endodontic irrigation, i.e., debris removal, in order to eliminate the chemical effect of the usual irrigant on the root canal wall.
After preparation, the tooth was immersed into a recipient with 0.9% saline solution and MRI scanned immediately, using a Bruker Biospec MRI scanner (Bruker Biospin MRI GmbH, Ettlingen, Germany) with a micro-imaging capability operated at 7.04 Tesla and based on Avance III technology. The 160 mm room temperature bore magnet was fitted with high magnetic field gradient unit BGA 9S HP in order to achieve high resolution. A 40.0 mm Quad Receive radio frequency coil was employed to 3D scan the investigated tooth. The 38.6 × 37.0 × 30.0 mm3 scanned Field of View volume was overlapped by a 512 × 5120 × 256 matrix leading to a 75 μm in plan resolution and 117 μm in volume resolution. The bandwidth of the employed True-FISP protocol, which has a fully balanced gradient waveform, has been set to 50 kHz, while the time of echo and repetition time were adjusted to 6.2 and 12.4 μs, respectively. A low flip angle of 15 was used to image the volume with 0.0 mm interslice distance [13].
Tooth images were acquired using Brukers’s ParaVision® 5.1. software (Bruker Biospin MRI GmbH, Rheinstetten, Germany). Post-acquisition images were used for 3D reconstruction analyses with InVesalius 3.0 Software (Centro de Tecnologia da Informação [CTI] Renato Archer, Brazil. http://www.cti.gov.br/invesalius/). For the 3D reconstruction analysis a mixed, automatic and manually adjusted method was used as this was proven to be the most accurate [14]. The research was performed in agreement with the Declaration of Helsinki.
Results
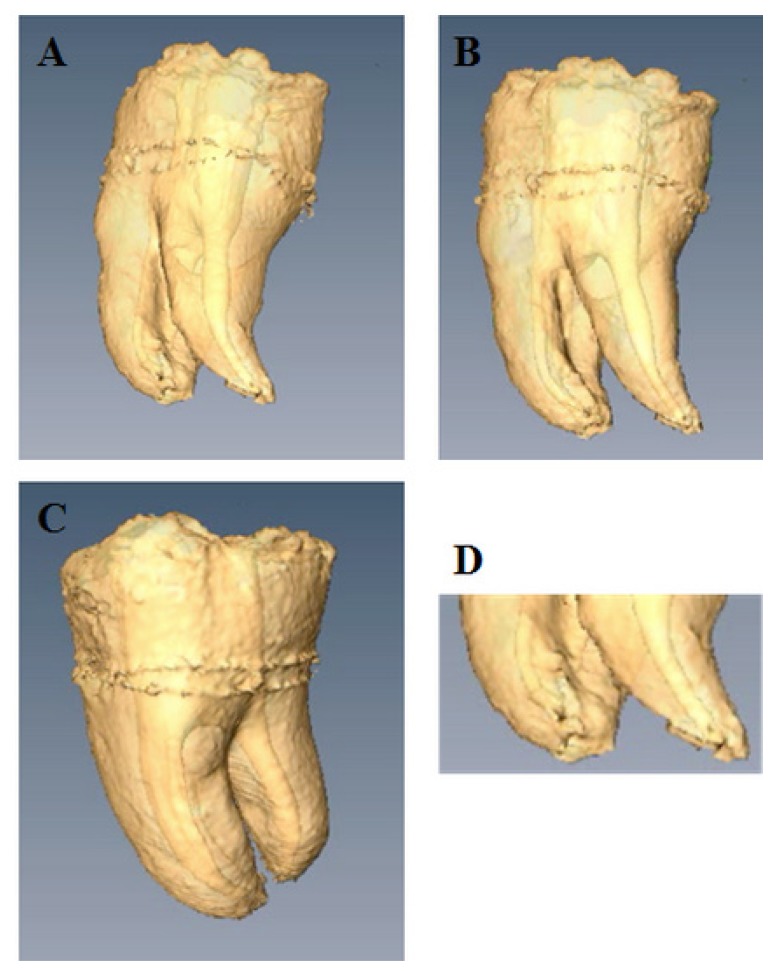
The 3D volume rendered reconstruction of the internal and external morphology of a human extracted and endodontically treated tooth (lower third molar) is presented in Figure 1. Three dimensional rendering and analysis reconstruction of the specimens were obtained with the InVesalius software. The program allowed reconstruction of objects from the stack of acquired 2D DICOM files slices. The reconstructed tooth was a pulp-free endodontically treated wisdom tooth. Accurate images of the human tooth and endodontically shaped root canal morphology provided by MRI were obtained: the external morphology of mesial and distal roots as well as the correspondingly shaped root canals, root canal curvatures, the furcal region, the interradicular root grooves, and details of the apical finishing of the root canal treatment can be clearly seen (Figure 1).
Figure 1.
3D volume reconstruction of the internal and the external morphology of an endodontically treated third molar.
A. Distal-vestibular view of the distal root and root canal shaped at a 6% taper. An edifying image of the prepared root canal provided by MRI. A clear view upon the irregular shape of the prepared distal root canal, meaning that a part of the complex anatomy of the distal root canal was left untouched, i.e., not cleaned by the mechanical shaping files.
B. Vestibular view of the external morphology of mesio vestibular and distal roots and the correspondingly shaped root canals. Root canal curvatures, the furcal region, the interradicular root grooves can be clearly seen.
C. Proximal (mesial) view. Note the degree of root separation and the apical fusion of the two mesial roots.
D. Detail of the apical part of the three roots and root canals. The root canal openings (portal of exits), the apical finishing of the root canal treatment can be viewed with great accuracy.
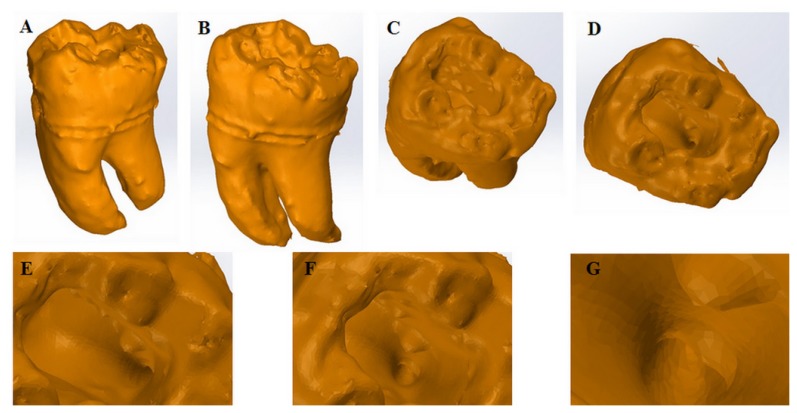
The hard tissue reconstruction by 3D volume rendered filled surface is presented in Figure 2. The external lingual and vestibular side of the tooth as well as the occlusal view of the pulp chamber, the access cavity, the distal canal opening on the pulp chamber floor, the distal root canal opening and the inside of the coronal third of the root canal were clearly bordered.
Figure 2.
3D volume rendered filled surface reconstruction.
A. External Lingual view;
B. External Vestibular view;
C. Occlusal view of the pulp chamber;
D. Access Cavity;
E. View of the distal canal opening, on the pulp chamber floor;
F. Distal Root canal opening (close-up);
G. View inside the coronal third of the root canal.
The model can be rotated by 360° in all directions. Coloring and lightening effects can be added to the desired structures. Virtual models can be made semi-transparent, some parts of main interest can be magnified or enhanced. Accurate morphometric measurements can be performed on the 2D images or on the 3D models. Basically all the features that are facilitated by a CBCT reconstruction software are made possible.
Discussion
Our study showed that with the aid of high-resolution MRI highly accurate data of the external and internal morphology of the tooth could be acquired. MRI offers 3D image datasets with more information as compared to conventional radiographic techniques. For example, Sustercic et al. showed that a fourth canal of a second molar, smaller and originating from the same place as the mesio-lingual canal, cannot be detected on horizontal slices across the molar but becomes visible when a sequence of volume rendered 3D images are acquired [15].
Due to its ability of imaging both hard and soft dental tissues, MRI can be successfully used as a 3D diagnostic imaging technique [12], being feasible for visualization of the teeth and of the periodontal anatomy [16]. In this study, we have used this ability to visualize the internal and external anatomy of the tooth.
Desiccation of the teeth might lead to the appearance of artefacts, as void spaces might be produced at the dentin-pulp interface [15]. To prevent desiccation during imaging, the tooth was immersed in saline immediately after extraction and during MRI scanning.
For in vivo measurements, special attention should be given to the influence of dental materials on dental MRI. Many factors may affect the strength of artefacts: magnetic field strength, echo time, pulse sequence, imaging plane, image resolution and the strength of the related gradient field, the amount and the shape of the dental material as well as the distance between the scanned object and the material [17]. Tymofiyeva and co-workers published a classification of the dental materials based on their compatibility with dental MRI at 1.5 T [18]. According to their classification, AH Plus resin, 3M ESPE composites (Seefeld, Germany), glass ionomer cement, gutta-percha, and zirconium dioxide are compatible materials, meaning that the material can be present in the tooth of interest. Amalgam, Ivoclar Vivadent composites (Ellwangen, Germany), gold alloys, gold-ceramic crowns, titanium alloys, and nickel-titanium alloy orthodontic wires should not be present in the tooth of interest, nor in its neighbors and antagonists. Cobalt-chrome alloys as well as stainless steel orthodontic wires and brackets are non-compatible materials that can produce strong image distortions even if they are located far from the imaging region (they should not be present in the mouth at the moment of imaging) [18].
Compared to in vitro experiments, where the scanning time can be extended to increase the resolution of the images, a special attention should be given to the optimization of the used imaging sequence in order to reduce the scanning time when used in vivo. Tymofiyeva et al. assessed the feasibility of dental MRI for 3D visualization and quantification of cavities [19]. The measurements were performed in vivo on a clinical whole-body system of 1.5 T and images of 300 × 300 × 300 μm3 resolution were acquired after 8 minutes of scanning [19].
For a better accuracy of the in vivo experiments, an intraoral dental radiofrequency coil, for both, excitation and acquisition, as presented by Idiyatullin and co-workers, should be used [20]. A drawback in the wide use of MRI in clinical practice are the high costs associated with the MRI equipment in general and cryogenics fluid(s) in particular, besides the software and training of qualified personnel. However, the detailed information regarding tooth anatomy cannot be obtained with the x-ray projection technique routinely used in dentistry. When choosing the imaging method, dental clinicians should weight the benefit/risk considering the costs associated to MRI and the harmful effects of radiations when x-ray-based techniques are used.
Conclusions
Although x-ray imaging has been successfully used as a diagnostic tool in dental pathology, its lack of accuracy, exposure to ionizing radiation and its associated risk of cancer [21] prompted the search for a new direction. Our study, within the limits of in vitro use of the method, showed that high resolution MRI enables a clear view of the tooth and root canal anatomy. By acquisition of 3D images with a high spatial resolution of both soft and hard dental tissues, dental MRI could be used for accurate visualization of the tooth morphology, from which endodontic and other dental treatments could benefit. Compared to conventional radiographic techniques, MRI is less invasive and the highly accurate MRI 3D image datasets provide more information. Future studies should be conducted for the in vivo use of this method that would represent a major finding in the benefit of both patients and dental clinicians.
Acknowledgments
This paper was published under the frame of European Social Fund, Human Resources Development Operational Programme 2007–2013, project no. POSDRU/159/1.5/S/138776.
References
- 1.Schroeder H. Orale Strukturbiologie. Stuttgart/New York: 1987. [Google Scholar]
- 2.Vertucci FJ. Root canal anatomy of the human permanent teeth. Oral Surg Oral Med Oral Pathol. 1984;58:589–599. doi: 10.1016/0030-4220(84)90085-9. [DOI] [PubMed] [Google Scholar]
- 3.Velvart P, Hecker H, Tillinger G. Detection of the apical lesion and the mandibular canal in conventional radiography and computed tomography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:682–688. doi: 10.1067/moe.2001.118904. [DOI] [PubMed] [Google Scholar]
- 4.Paurazas SB, Geist JR, Pink FE, Hoen MM, Steiman HR. Comparison of diagnostic accuracy of digital imaging by using CCD and CMOS-APS sensors with E-speed film in the detection of periapical bony lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:356–362. doi: 10.1016/s1079-2104(00)70102-8. [DOI] [PubMed] [Google Scholar]
- 5.Forsberg J, Halse A. Radiographic simulation of a periapical lesion comparing the paralleling and the bisecting-angle techniques. Int Endod J. 1994;27:133–138. doi: 10.1111/j.1365-2591.1994.tb00242.x. [DOI] [PubMed] [Google Scholar]
- 6.The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP. 2007;37:1–332. doi: 10.1016/j.icrp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Patel S, Durack C, Abella F, Roig M, Shemesh H, Lambrechts P, et al. European Society of Endodontology position statement: the use of CBCT in endodontics. Int Endod J. 2014;47:502–504. doi: 10.1111/iej.12267. [DOI] [PubMed] [Google Scholar]
- 8.Kress B, Buhl Y, Anders L, Stippich C, Palm F, Bahren W, et al. Quantitative analysis of MRI signal intensity as a tool for evaluating tooth pulp vitality. Dentomaxillofac Radiol. 2004;33:241–244. doi: 10.1259/dmfr/33063878. [DOI] [PubMed] [Google Scholar]
- 9.Tymofiyeva O, Rottner K, Jakob PM, Richter EJ, Proff P. Three-dimensional localization of impacted teeth using magnetic resonance imaging. Clin Oral Investig. 2010;14:169–176. doi: 10.1007/s00784-009-0277-1. [DOI] [PubMed] [Google Scholar]
- 10.Tymofiyeva O, Schmid F, von Kienlin M, Breuer FA, Rottner K, Boldt J, et al. On precise localization of boundaries between extended uniform objects in MRI: tooth imaging as an example. MAGMA. 2011;24:19–28. doi: 10.1007/s10334-010-0229-4. [DOI] [PubMed] [Google Scholar]
- 11.Bracher AK, Hofmann C, Bornstedt A, Boujraf S, Hell E, Ulrici J, et al. Feasibility of ultra-short echo time (UTE) magnetic resonance imaging for identification of carious lesions. Magn Reson Med. 2011;66:538–545. doi: 10.1002/mrm.22828. [DOI] [PubMed] [Google Scholar]
- 12.Idiyatullin D, Corum C, Moeller S, Prasad HS, Garwood M, Nixdorf DR. Dental MRI: making the invisible visible. J Endod. 2011;37:745–752. doi: 10.1016/j.joen.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hargreaves BA. Rapid gradient-echo imaging. J Magn Reson Imaging. 2012;36:1300–1313. doi: 10.1002/jmri.23742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forst D, Nijjar S, Flores-Mir C, Carey J, Secanell M, Lagravere M. Comparison of in vivo 3D cone-beam computed tomography tooth volume measurement protocols. Prog Orthod. 2014;15:69. doi: 10.1186/s40510-014-0069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sustercic D, Sersa I. Human tooth pulp anatomy visualization by 3D magnetic resonance microscopy. Radiol Oncol. 2012;46:1–7. doi: 10.2478/v10019-012-0018-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaudino C, Cosgarea R, Heiland S, Csernus R, Beomonte Zobel B, Pham M, et al. MR-Imaging of teeth and periodontal apparatus: An experimental study comparing high-resolution MRI with MDCT and CBCT. Eur Radiol. 2011;21:2575–2583. doi: 10.1007/s00330-011-2209-0. [DOI] [PubMed] [Google Scholar]
- 17.Schenck JF. The role of magnetic susceptibility in magnetic resonance imaging: MRI magnetic compatibility of the first and second kinds. Med Phys. 1996;23:815–850. doi: 10.1118/1.597854. [DOI] [PubMed] [Google Scholar]
- 18.Tymofiyeva O, Vaegler S, Rottner K, Boldt J, Hopfgartner AJ, Proff PC, et al. Influence of dental materials on dental MRI. Dentomaxillofac Radiol. 2013;42:20120271. doi: 10.1259/dmfr.20120271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tymofiyeva O, Boldt J, Rottner K, Schmid F, Richter EJ, Jakob PM. High-resolution 3D magnetic resonance imaging and quantification of carious lesions and dental pulp in vivo. MAGMA. 2009;22:365–374. doi: 10.1007/s10334-009-0188-9. [DOI] [PubMed] [Google Scholar]
- 20.Idiyatullin D, Corum CA, Nixdorf DR, Garwood M. Intraoral approach for imaging teeth using the transverse B1 field components of an occlusally oriented loop coil. Magn Reson Med. 2014;72:160–165. doi: 10.1002/mrm.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Longstreth WT, Jr, Phillips LE, Drangsholt M, Koepsell TD, Custer BS, Gehrels JA, et al. Dental X-ray and the risk of intracranial meningioma: a population-based case-control study. Cancer. 2004;100:1026–1034. doi: 10.1002/cncr.20036. [DOI] [PubMed] [Google Scholar]