Abstract
Background and aims
Periodontitis is a common chronic adult condition that implicates oxidative damage to gingival tissue, periodontal ligament and alveolar bone. This study aimed at assessing the association between the nitro-oxidative stress and the periodontal tissues destructions in experimental rat periodontitis.
Methods
Periodontitis was induced in 15 male albino rats by repetitive lesions to the gingiva adjacent to the inferior incisors, performed daily, for 16 days. On D1, D3, D6, D8, and D16 the onset and evolution of periodontitis were monitored by clinical and histopathological examinations; blood was collected and serum nitro-oxidative stress was evaluated through total nitrites and nitrates, total oxidative status, total antioxidant capacity, and oxidative stress index.
Results
The results demonstrated that there was a graded and continuous increase in serum levels of total nitrites and nitrates, total oxidative status and oxidative stress index, which was consistent with the severity of periodontal destructions during periodontitis progression. However, total antioxidant capacity was not significantly influenced by the disease progression.
Conclusions
In experimental rat periodontitis, the systemic nitro-oxidative stress was associated with the severity of periodontal destructions assessed clinically and histopathologically. Therefore, systemic nitro-oxidative stress parameters might be used as diagnostic tools in periodontitis.
Keywords: nitro-oxidative stress, experimental periodontitis, pathogenesis, rat
Introduction
Periodontitis is one of the most common oral diseases worldwide, with a prevalence of 50–70% in adults over 30 years [1].
Nowadays it is well known that the complex pathogenesis of periodontitis implicates both the presence of the microbial plaque and the host immune-inflammatory response [2].The main event in the host response against periodontal pathogenic microorganisms is the recruitment and activation of polymorphonuclear neutrophils (PMN) and macrophages [3,4]. Activated PMN produce a large amount of reactive oxygen species (ROS), which lead to local oxidative stress and mediate periodontal tissues destructions [5,6].
Recently, abundant evidence has shown that periodontal inflammation is highly associated with several inflammation-related systemic diseases, such as cardiovascular disease, chronic respiratory diseases, and diabetes mellitus; moreover, the common denominator of all these diseases is the implication of oxidative stress in their pathogenesis [7–11].
However, little is known about the exact mechanism of periodontitis development, including the correspondence between the severity of periodontal destructions and the serum levels of oxidative stress parameters.
It has been hypothesized that oxidative stress arising from periodontal lesions may be an important cause of systemic inflammation. Studies investigating the oxidative stress biomarkers reported high levels in the peripheral blood of periodontitis patients compared with healthy subjects [12,13]. But the dynamic of oxidative stress biomarkers changes during the progression of periodontitis has not been yet assessed.
Firstly, we aimed at assessing the nitro-oxidative damage during the progression of experimental rat periodontitis. Secondly, we investigated the association between the severity of periodontal tissues destructions and serum levels of total nitrites and nitrates (NOx), total oxidative status (TOS), total antioxidant capacity (TAC), and oxidative stress index (OSI).
Materials and methods
Chemicals
Analytical grade chemicals were used exclusively. Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), N-(1-Naphthyl) ethylenediamine dihydrochloride (NEDD), xylenol orange [o-cresosulfonphthalein-3,3-bis(sodium methyliminodiacetate)], ortho dianisidine, vanadium (III) chloride (VCl3), hydrogen peroxide (H2O2), methanol, diethyl ether sulphanilamide (SULF) and ferrous ammonium sulphate were purchased from Sigma-Aldrich (Germany) and Merck (Germany).
Experimental design
The experiment was performed on fifteen adult male Wistar-Bratislava albino rats, weighing between 200 and 250 g that were bred in the Animal Facility of Iuliu Hatieganu University of Medicine and Pharmacy. The rats were kept in a room with controlled temperature (21±1°C) and humidity (50–55%) and a 12h light-12h dark cycle. Animals were fed with standard pellet (Cantacuzino Institute, Bucharest, Romania) basal diet and water ad libitum.
Periodontitis was induced in rats by repetitive acute lesions to the gingival epithelium around the inferior incisors. The rats were anesthetized by intramuscular injection of 50 mg/kg body weight (b.w.) ketamine and 20 mg/kg b.w. xylazine. The lesions were performed with a Gracey curette placed into the gingival sulcus and moved circularly to detach the junctional epithelium from the tooth surface. These interventions were repeated daily, for sixteen days and the progression of the periodontal inflammation was monitored daily (D1 to D16).
On D1, D3, D6, D8, D16, clinical and histopathological examinations were performed, and blood samples were harvested for serum nitro-oxidative stress tests.
The experimental protocol was approved by the Institutional Animal Ethical Committee of the Iuliu Hatieganu University of Medicine and Pharmacy, Cluj-Napoca (approval no. 107/06.03.2015). At completion of the study, rats were euthanized by cervical dislocation.
Clinical examination
Two features were evaluated: the periodontal inflammation and gingival bleeding (GB). Periodontal inflammation was assessed by the aspect of the gingiva: color, consistency and volume. The scores were the followings: 0 – no signs of inflammation; 1 – mild; 2 – moderate; 3 – severe; 4 – periodontitis. GB was assessed upon probing the gingival sulcus with a periodontal probe, and the scores were: (-) – absent or (+) – present; when GB occurred upon gentle removal of the debris on the gingival surface or spontaneously, it was scored with (++) and (+++) respectively.
Histopathological examination
On D1, D3, D6, D8 and D16, five rats were sacrificed and the frontal parts of the mandibles were harvested. The specimens were immersed in 10% buffered formalin solution for fixation. The gingiva adjacent to the inferior incisors (including the free gingiva, the interdental papilla and the attached gingiva) was prelevated and submitted to further routine histological processing. The tissues were embedded in paraffin blocks and serial 5μ sections in the bucco-lingual direction were obtained. The histological sections were stained using Goldner’s trichrome technique and examined under an Olympus BX41 light microscope for descriptive evaluation. The following aspects were assessed: the necrosis and ulceration of the gingival epithelium, destruction of the extracellular matrix in the chorion, the types of inflammatory cells and the microcirculatory changes.
Oxidative stress evaluation
Blood was collected by retro-orbital puncture, without anticoagulant; coagulated blood was centrifuged and the serum was submitted to nitro-oxidative stress test. TOS, TAC and NOx were measured in the serum; OSI was also calculated.
Serum samples were filtered through 10-kDd filters (Sartorius AG, Goettingen, Germany) and contaminant proteins were extracted with a 3:1 (v:v) solution of methanol/diethyl ether.
Nitric oxide (NO) synthesis was indirectly determined using the Griess reaction. Nitrate was reduced to nitrite by combining 100 μL of filtered and extracted serum supernatant with 100 μL of 8 mg/mL VCl3; then, the Griess reagents were added, 50 μL of SULF (2%) and 50 μL of NEDD (0.1%). The sample was incubated 30 minutes at 37°C and the absorbance was read at 540 nm. Serum NOx concentration (expressed as nitrite μmol/L) was determined using a sodium nitrite-based curve [14].
TOS was measured using a colorimetric assay that measured the oxidation of ferrous ion to ferric ion in the presence of various reactive oxygen species in an acidic medium [15]. Then, ferric ions were detected by reaction with xylenol orange. Assay measurements were standardized with hydrogen peroxide (H2O2) used as the oxidative species, and the results are expressed in μmol H2O2 Equiv./L.
TAC was measured using a colorimetric assay that monitored the rate of hydroxyl radical production by the Fenton reaction the changes in the absorbance of colored dianisidyl radicals [16]. Upon addition of a serum sample, the antioxidant present in the serum suppressed the oxidative reactions initiated by hydroxyl radicals. Inhibition of dianisidyl oxidation prevented the subsequent colour change, thus measuring the serum TAC. This assay was calibrated using trolox and results were expressed as mmol trolox Equiv/L.
OSI was calculated as the ratio of the TOS to the TAC: OSI (Arbitrary Unit) = TOS (μmol H2O2 Equiv/L) / TAC (mmol trolox Equiv/L) [17].
The spectroscopic measurements were performed using a Jasco V-530 UV-Vis spectrophotometer (Jasco International Co., Ltd., Tokyo, Japan).
Statistical methods
All results were expressed as mean ± standard deviation (SD). Normal distribution was assessed using Shapiro-Wilk test. Statistical comparisons between the moments were made using one-way ANOVA, followed by Tukey’s post hoc test. p-values < 0.05 were regarded as statistically significant. Pearson’s and Spearman’s correlation tests were performed in order to evaluate statistical correlation. Data was analyzed using R 3.0.2 - software environment for statistical computing and graphics.
Results
Clinical and histopathological examination
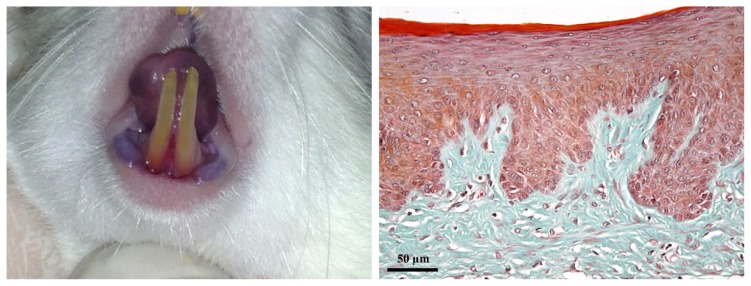
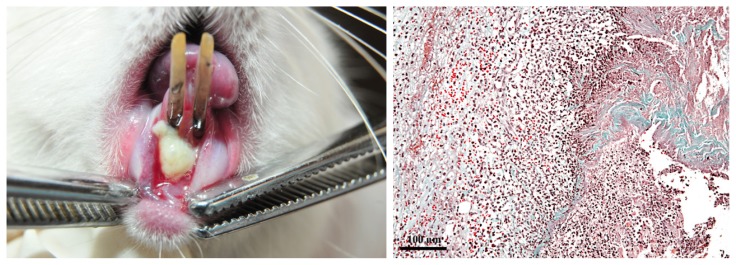
On D1, clinically, gingiva had a normal appearance; periodontal inflammation score = 0, GB: (-). The gingiva was pale pink, with a smooth surface; the gingival sulcus was virtual and gingiva was firmly attached to the cervical zone of the teeth and to alveolar bone. Histologically, the gingival epithelium and lamina propria showed no signs of inflammation (Figure 1).
Figure 1.
Clinical and histological aspect of the gingiva on D1. A: healthy gingiva. B: the gingival stratified squamous parakeratinized epithelium and the lamina propria consisting of connective tissue. (Goldner’s trichromic stain).
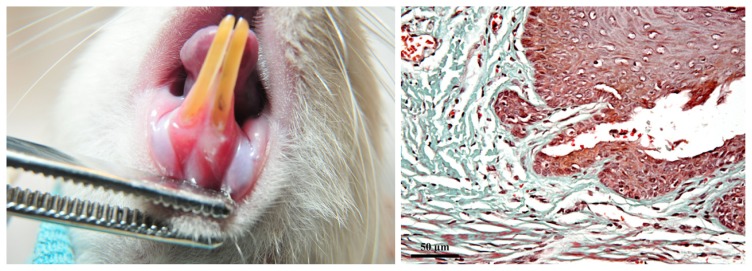
On D3, clinically mild gingivitis was observed; periodontal inflammation score = 1, GB: (+). The gingiva showed discrete oedema and erythema. On the gingival surface, the superficial linear incisions induced experimentally were visible. Histopathologically, the initial lesion was characterized by the following features: epithelium underwent necrosis in the zone where it was detached from the surface of the tooth; in the lamina propria, the inflammatory reaction was characterized by moderate infiltration with PMN, vasodilatation and slight, mainly perivascular oedema (Figure 2).
Figure 2.
Clinical and histological aspect of the gingiva on D3. A: mild gingivitis: discrete inflammation and linear superficial incisions could be observed. B: initial lesion: limited epithelial necrosis, vasodilatation and perivascular oedema in the lamina propria. (Goldner’s trichromic stain).
On D6, clinically moderate gingivitis could be seen; periodontal inflammation score = 2, GB: (+). Gingival oedema and erythema increased, the gingival sulcus was a real and narrow space, as the gingiva was detached from the cervical zone of the teeth. The ulcerations were covered by debris of which detachment during examination caused bleeding. Histopathological examination identified the early lesion: the zone of necrotic epithelium was wider, and the underlying connective tissue was exposed. In the lamina propria, adjacent to the necrotic epithelium, the inflammatory infiltrate with PMN and macrophages was denser; the oedema was diffuse, but more intense in some areas, where the extracellular matrix lysis occurred (Figure 3).
Figure 3.
Clinical and histological aspect of the gingiva on D6. A: moderate gingivitis: gingiva is inflamed and detached from the teeth; the ulcerations were covered by debris. B: early lesion: more extensive epithelial necrosis, diffuse oedema and denser inflammatory infiltrate with PMN and macrophages. (Goldner’s trichromic stain).
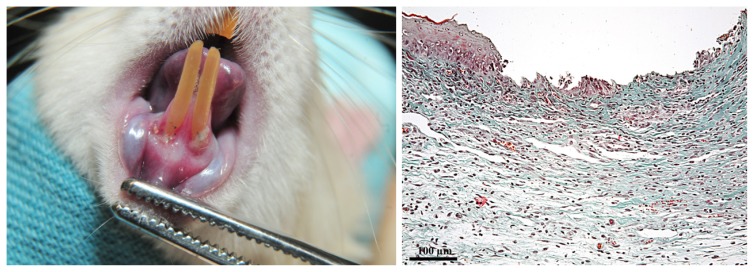
On D8, clinically severe gingivitis was diagnosed; periodontal inflammation score = 3, GB: (++). Gingival erythema and oedema were more intense, the ulceration was deeper, covered by a larger amount of debris. Moreover, the wide gingival detachment and retraction caused the root exposure on a length equal to 1/3 of the clinical crown of the teeth. Histopathologically, the established lesion consisted of necrosis extended both into the epithelium and into the superficial layer of lamina propria. In the profound layers, the areas where the extracellular matrix lysis occurred were occupied by intense oedema and diffuse hemorrhage. The cells in the inflammatory infiltrate were mainly PMN, macrophages and lymphocytes (Figure 4).
Figure 4.
Clinical and histological aspect of the gingiva on D8. A: severe gingivitis: gingival inflammation and oedema were more intense; retraction and dental root exposure could be noticed; larger ulcerations were covered by debris. B: established lesion: areas of necrosis in the epithelium and lamina propria, diffuse oedema and hemorrhage, dense inflammatory infiltrate with PMN, macrophages and lymphocytes. (Goldner’s trichromic stain).
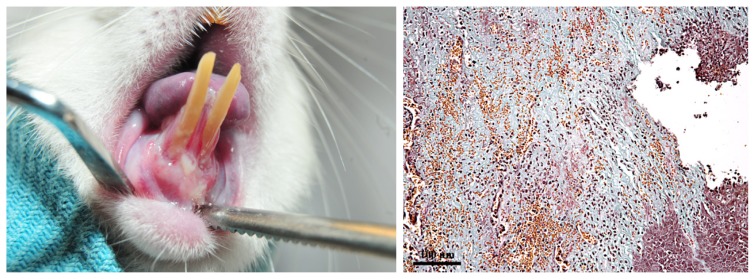
On D16, clinically periodontitis was observed; periodontal inflammation score = 4, GB: (+++). Gingiva was cyanotic, increased in volume, and the increased gingival fragility resulted in spontaneous bleeding. The gingival ulcerations were profound and covered by abundant debris. Moreover, the gingiva was severely detached from the teeth and the root exposure was approximately equal to the clinical crown length. Histopathologically, the advanced lesion was characterized by extensive necrosis of the epithelium. In the lamina propria; the extracellular matrix underwent intense lysis and was replaced by the inflammatory infiltrate with PMN, macrophages and lymphocytes, oedema and hemorrhage; in some areas, necrosis also occurred in the connective tissue. The surface of the lesion was covered by abundant fibrinoid deposits, enclosing numerous PMN, macrophages, lymphocytes and degenerated erythrocytes (Figure 5).
Figure 5.
Clinical and histological aspect of the gingiva on D16. A: periodontitis: gingival cyanosis, ulcerations covered by debris and spontaneous bleeding; extensive gingival retraction and destruction of the underlying alveolar bone and periodontal ligament. B: advanced lesion: the necrotic epithelium was replaced by fibrinoid deposits; in the lamina propria, dense inflammatory infiltrate, oedema and diffuse hemorrhage, and areas of necrosis could be observed. (Goldner’s trichromic stain).
The evaluation of the oxidative stress
We assessed the serum levels of NOx, TOS, TAC and OSI during periodontitis progression (Table I).
Table I.
Comparison between time points during periodontitis progression.
| Moment | NOx (μmol/L) | TOS (μmol Equiv H2O2/L) | TAC (mmol Equiv TROLOX/L) | OSI |
|---|---|---|---|---|
| D1 | 34.111±1.826 | 31.1±1.703 | 1.09±0.001 | 0.285±0.015 |
| D3 | 96.029±2.493 | 39.235±2.508 | 1.0905±0.001 | 0.359±0.023 |
| D6 | 101.95±3.34 | 40.93±1.914 | 1.0902±0.001 | 0.375±0.017 |
| D8 | 101.773±3.234 | 46.354±1.286 | 1.0898±0.001 | 0.425±0.01 |
| D16 | 114.409±6.948 | 44.216±3.962 | 1.0897±0.001 | 0.405±0.036 |
Values are expressed as Mean ± SD of five determinations. NOx = total nitrites and nitrates; TOS = total oxidative status; TAC = total antioxidant capacity; OSI = oxidative stress index, in 5 moments: D1- day 1, D3 – day 3, D6 – day 6, D8 – day 8, D16 – day 16.
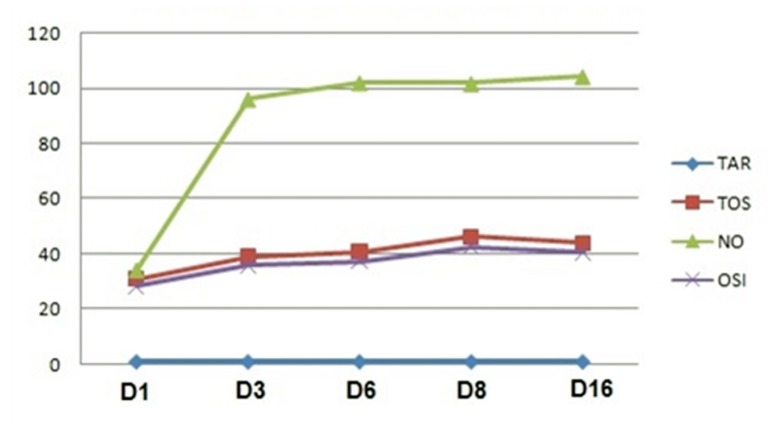
Analyzing the progression of the serum parameters in D1, D3, D6, D8, and D16, excepting TAC which did not change significantly (p>0,05), NOx, TOS and OSI constantly increased: abruptly until D3, and smoothly thereafter (Figure 6).
Figure 6.
Nitro-oxidative stress parameters changes during 16 days of periodontitis progression. NOx = total nitrites and nitrates; TOS = total oxidative status; TAC = total antioxidant capacity; OSI = oxidative stress index, in 5 moments:D1- day 1, D3 – day 3, D6 – day 6, D8 – day 8, D16 – day 16.
NOx serum levels were significantly lower on D1 compared with D3, D6, D8, and D16 (p<0.001). Additionally, NOx serum levels were significantly lower on D3, D6, and D8 compared with D16 (p<0.001).
TOS serum levels were significantly lower on D1 compared with D3 (p < 0.05), D6 (p < 0.05), D8 (p < 0.001), and D16 (p < 0.05). Moreover, TOS serum levels were significantly higher on D8 compared with D3 and D6 (p < 0.05).
OSI serum levels were significantly lower on D1 compared with D3 (p < 0.05), D6 (p < 0.05), D8 (p < 0.001), and D16 (p < 0.05). Additionally, TAC serum levels were significantly higher on D8 compared with D3 and D6 (p < 0.05).
During the 16 experimental days OSI was highly correlated with TOS (r = 0.99).
On D6, NOx was correlated with TOS (r = 0.65), and OSI (r = 0.64).
Discussion
The results of the study proved that there was a good correlation between the clinical, histological and nitro-oxidative stress parameters during the progression of experimental rat periodontitis.
Periodontitis involves complex pathogenic mechanisms that are part of the host immune-inflammatory response to the presence of microbial plaque [18,19]. These mechanisms are characterized by the production of cytokines, enzymes and free radicals in the periodontal tissues, which results in progressive gingival inflammation, periodontal ligament destruction, alveolar bone resorption, and eventually lead to teeth loss [20]. Moreover, the chronic periodontal inflammation is associated with increased systemic nitro-oxidative stress, responsible for numerous disorders such as rheumatoid arthritis, chronic kidney dysfunction, ischemic heart disease, premature births and others [21,22].
Nitro-oxidative stress is induced by an excessive production of ROS [23], associated with increased synthesis of NO by the inducible nitric oxide synthase (iNOS) [24]. The increased production of reactive species might be associated with a decrease in antioxidant capacity. This is why the nitro-oxidative stress should be evaluated by measuring ROS, NO, and TAC [25].
In the present study, in order to assess the severity and progression of experimental rat periodontitis, we employed global nitro-oxidative stress tests. NO synthesis was measured indirectly by the quantification of serum nitrites and nitrates [26], ROS were measured by the assessment of TOS, and antioxidant capacity was assessed by TAC [14–16, 25]. Since the final result depends on the proportion between ROS and the anti-oxidant mechanisms, determination of OSI was necessary [16].
Our study was the first to assess the nitro-oxidative stress parameters as indicators of the progression and severity of periodontal destructions in experimental rat periodontitis. Previous studies evaluated the injury only at the end of the experiment [27,28].
The clinical and histopathological examinations recorded, stage by stage, the structural changes throughout the initiation and progression of gingivitis and periodontitis [29]. These findings were correlated with the serum levels of NOx, TOS, TAC and OSI, which are considered to be markers of the nitro-oxidative stress [30,31] and may be used to stepwise evaluate the progression of the disease [32].
At baseline, the absence of periodontal inflammation was associated with reduced serum levels of NOx, TOS and OSI.
Clinically, on D3, the gingiva showed discrete signs of inflammation, which were consistent with the histopathological changes. Gingival erythema and oedema were the result of the vasodilatation and increased vascular permeability, whereas bleeding was the consequence of capillary fragility. The incisions induced experimentally disrupted the epithelial barrier and initiated the penetration of bacteria into the lamina propria and the consequent recruitment and activation of PMN and macrophages. Serum levels of NOx, TOS and OSI significantly increased compared with D1.
On D6, the gingival erythema and oedema were more intense, due to increased vascular dilatation and permeability. The epithelial ulceration was wider, allowing a higher bacterial invasion into the lamina propria. The inflammatory process extended, as a larger number of inflammatory cells were attracted into the area adjacent to the necrotic epithelium; moreover, the first signs of extracellular matrix lysis occurred. Epithelium detachment at the cervical line of the teeth initiated the periodontal ligament damage. Serum levels of NOx, TOS and OSI were not significantly different from D3, but significantly increased compared with D1.
Furthermore, on D8, destruction of both epithelium and lamina propria intensified, and the necrotic connective tissue was replaced by a dense inflammatory infiltrate and diffuse haemorrhage. The gingival retraction and detachment from the tooth surface suggested the ongoing periodontal ligament damage and alveolar crest resorption. Serum levels of TOS and OSI were significantly increased compared with D6 and D1; moreover, NOx was significantly higher compared with D1.
Finally, on D16, the rats displayed clinical signs of periodontitis: severe gingival inflammation accompanied by cyanosis and spontaneous bleeding. The histopathological features characteristic for advanced lesion associated with periodontitis consisted in: necrosis in the epithelium and lamina propria, lysis of the extracellular matrix, diffuse oedema, haemorrhage and infiltration with lymphocytes, macrophages and neutrophils. Additionally, extensive gingiva detachment and retraction indicated that the supporting tissues also underwent degenerative changes. Since the periodontal ligament and the alveolar bone were affected, the periodontal inflammation was no longer confined to the gingiva, therefore gingivitis developed into periodontitis. NOx significantly increased compared with D8 and D1, whereas TOS and OSI significantly increased compared with D1.
The clinical and histopathological features described in our experimental rat periodontitis were consistent, in evolution, with the specific aspects reported in human chronic periodontitis [33, 34]. On the other hand, our findings regarding the association between periodontitis and the high levels of NOx, TOS and OSI were similar to other experimental and clinical studies [27,28,35,36]
Recent evidence showed a link between the activation of phagocytes (PMN and macrophages) and the pathways leading to periodontal damage [37–39]. Periodontal inflammation is initiated by the chemotactic signals released from the plaque bacteria, which result in the recruitment of PMN into the sulcular epithelium and the gingival crevice [4,6]. As inflammation progresses, a vast number of phagocytes (represented mainly by PMN, but also including macrophages) will be attracted into the periodontal tissues [40]. Our observations indicated that during the experimental rat periodontitis, PMN infiltrate increased constantly.
The key to neutrophil-mediated tissue destructions resides in the homeostatic imbalance caused by three mechanisms associated with PMN response to the oral biofilm: the impaired neutrophils, the hyperactive neutrophils and chronic recruitment and activation of normal neutrophils [41]. The weight of evidence supports two main ideas regarding the neutrophils dysfunction: genetically impaired or defective phenotypes are associated with a predisposition to aggressive periodontitis whereas hyperactive, primed neutrophils may be implicated in chronic periodontitis [41]. Recently, it was suggested that the control of periodontal pathogens can be accomplished through the chronic activation and extended longevity of normal neutrophils over many years, and thus, the increased prevalence of periodontitis with age can be explained [42]. Considering all these, it is important that in our experiment there was a mostly PMN infiltrate, explaining the progressive increase of the nitro-oxidative stress.
Neutrophils are thought to promote oedema due to their interaction with the vascular endothelium during diapedesis and through the production of various mediators, such as: arachidonic acid derivates and chemokines [43]. Additionally, neutrophils release ROS and serine proteases capable of damaging the vascular endothelium [44,45].
In attempt to eliminate pathogens, host defence cells release various factors, which can broadly be included in two categories: enzymes and oxidative burst products [46]. Even though these factors are effective in destroying bacteria, increased levels of enzymes cause substantial damage to the periodontal tissues, whereas high levels of free radicals generate oxidative stress [6,47,48].
In vitro studies demonstrated that free radicals released by neutrophils and macrophages are capable to damage resident cells and to degrade the main constituents of extracellular matrix in the periodontal tissues. Intracellulary, ROS induce lipid peroxidation through activation of cyclooxygenases and lipooxygenases [35], and DNA damage through strain breaks and hydroxylations [31]. In glycosaminoglycans, free radicals induce chains depolymerisation, whereas in proteoglycans, they modify the amino acid functional groups leading to core protein fragmentation [49].
Moreover, ROS cause oxidation of enzymes [46], stimulate release of proinflammatory cytokines from macrophages by activating Nuclear Factor κB (NF-κB) and are also involved in alveolar bone resorption [50,51,46].
In our study, the clinical and histopathological dynamic of periodontitis was correlated with the variations in the serum levels of NOx, TOS and OSI: higher nitro-oxidative stress was associated with more severe gingival injury. Our results were consistent with other studies [12, 13,52,53].
After the first interventions on the gingiva, there was an important increase in serum levels of NOx, TOS and OSI, suggesting the initiation of local and systemic nitro-oxidative stress. Additionally, in the following days, these parameters were significantly higher compared with the baseline, proving the role of nitro-oxidative stress in the pathogenesis of experimental rat periodontitis.
The comparison between the days showed that NOx, TOS and OSI progressively increased; occasionally, there were significant differences between consequent observations, made after a period of two days (D1 - D3 and D6 - D8), but in most cases, the significant increases could be observed when there were longer periods of time between examinations (D3 - D8, D6 - D16 and D8 - D16).
At completion of the study, the most severe periodontal destructions were consistent with the highest serum levels of nitro-oxidative stress parameters. However, TAC was not influenced by the progression of the periodontal inflammation. These findings were different from the observations of a number of clinical studies that reported lower serum TAC in periodontitis patients compared with the healthy controls [46,54,55]. A recent meta-analysis by Liu et al. discussed serum levels of oxidative biomarkers and indicated that TAC, MDA (malondialdehyde), and NO were strongly associated with periodontitis [36], whereas SOD levels were not different from healthy subjects. Data reported by most studies showed that TAC levels were lower, whereas NO and MDA levels were significantly higher in peripheral blood of periodontitis patients [12,13,54,55,56].
Our results showed a correlation between the tissue damage and NOx, TOS and OSI, suggesting that these nitro-oxidative stress markers could be used as diagnostic tools for periodontitis. However, further studies are needed in order to validate the relationship between periodontitis and systemic oxidative stress.
Conclusions
In experimental rat periodontitis, the severity of periodontal damage assessed by clinical and histopathological examinations was correlated with the serum levels of nitro-oxidative stress parameters.
Serum levels of NOx, TOS and OSI steadily increased throughout the progression of experimental rat periodontitis, but TAC was not significantly influenced by the disease progression.
Nitro-oxidative stress parameters might be used as diagnostic tools in periodontitis.
Acknowledgement
This paper was published under the frame of European Social Found Human Resources Development Operational Programme 2007–2013, project no. POSDRU/159/1.5/S/138776.
References
- 1.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. CDC Periodontal Disease Surveillance workgroup: James Beck (University of North Carolina, Chapel Hill, USA), Gordon Douglass (Past President, American Academy of Periodontology), Roy Page (University of Washin. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91(10):914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 2.Preshaw PM. Host response modulation in periodontics. Periodontol 2000. 2008;48:92–110. doi: 10.1111/j.1600-0757.2008.00252.x. [DOI] [PubMed] [Google Scholar]
- 3.Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott DA, Krauss J. Neutrophils in periodontal inflammation. Front Oral Biol. 2012;15:56–83. doi: 10.1159/000329672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Çanakçi CF, Çiçek Y, Çanakçi V. Reactive oxygen species and human inflammatory periodontal diseases. Biochemistry (Mosc) 2005;70(6):619–628. doi: 10.1007/s10541-005-0161-9. [DOI] [PubMed] [Google Scholar]
- 6.Chapple IL, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000. 2007;43(1):160–232. doi: 10.1111/j.1600-0757.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 7.Ying Ouyang X, Mei Xiao W, Chu Y, Ying Zhou S. Influence of periodontal intervention therapy on risk of cardiovascular disease. Periodontol 2000. 2011;56(1):227–257. doi: 10.1111/j.1600-0757.2010.00368.x. [DOI] [PubMed] [Google Scholar]
- 8.Loukides S, Bakakos P, Kostikas K. Oxidative stress in patients with COPD. Curr Drug Targets. 2011;12(4):469–477. doi: 10.2174/138945011794751573. [DOI] [PubMed] [Google Scholar]
- 9.Stadler K. Oxidative stress in diabetes. Adv Exp Med Biol. 2012;771:272–287. doi: 10.1007/978-1-4614-5441-0_21. [DOI] [PubMed] [Google Scholar]
- 10.Lee R, Margaritis M, Channon KM, Antoniades C. Evaluating oxidative stress in human cardiovascular disease: methodological aspects and considerations. Curr Med Chem. 2012;19(16):2504–2520. doi: 10.2174/092986712800493057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuder RM, Petrache I. Pathogenesis of chronic obstructive pulmonary disease. J Clin Invest. 2012;122(8):2749–2755. doi: 10.1172/JCI60324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei D, Zhang XL, Wang YZ, Yang CX, Chen G. Lipid peroxidation levels, total oxidant status and superoxide dismutase in serum, saliva and gingival crevicular fluid in chronic periodontitis patients before and after periodontal therapy. Aust Dent J. 2010;55(1):70–78. doi: 10.1111/j.1834-7819.2009.01123.x. [DOI] [PubMed] [Google Scholar]
- 13.Trivedi S, Lal N, Mahdi AA, Mittal M, Singh B, Pandey S. Evaluation of antioxidant enzymes activity and malondialdehyde levels in patients with chronic periodontitis and diabetes mellitus. J Periodontol. 2014;85(5):713–720. doi: 10.1902/jop.2013.130066. [DOI] [PubMed] [Google Scholar]
- 14.Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 15.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37:112–119. doi: 10.1016/j.clinbiochem.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Harma M, Harma M, Erel O. Increased oxidative stress in patients with hydatidiform mole. Swiss Med Wkly. 2003;133:563–566. doi: 10.4414/smw.2003.10397. [DOI] [PubMed] [Google Scholar]
- 18.Kirkwood KL, Cirelli JA, Rogers JE, Giannobile WV. Novel host response therapeutic approaches to treat periodontal diseases. Periodontol 2000. 2007;43:294–315. doi: 10.1111/j.1600-0757.2006.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gulati M, Anand V, Govila V, Jain N. Host modulation therapy: An indispensable part of perioceutics. J Indian Soc Periodontol. 2014;18(3):282–288. doi: 10.4103/0972-124X.134559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deo V, Bhongade ML. Pathogenesis of periodontitis: role of cytokines in host response. Dent Today. 2010;29(9):60–2. 64–66. quiz 68–69. [PubMed] [Google Scholar]
- 21.D’Aiuto F, Nibali L, Parkar M, Patel K, Suvan J, Donos N. Oxidative stress, systemic inflammation, and severe periodontitis. J Dent Res. 2010;89(11):1241–1246. doi: 10.1177/0022034510375830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Oyinloye BE, Adenowo AF, Kappo AP. Reactive oxygen species, apoptosis, antimicrobial peptides and human inflammatory diseases. Pharmaceuticals (Basel) 2015;8(2):151–175. doi: 10.3390/ph8020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harasym J, Oledzki R. Effect of fruit and vegetable antioxidants on total antioxidant capacity of blood plasma. Nutrition. 2014;30(5):511–517. doi: 10.1016/j.nut.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 25.Dahiya P, Kamal R, Gupta R, Bhardwaj R, Chaudhary K, Kaur S. Reactive oxygen species in periodontitis. J Indian Soc Periodontol. 2013;17(4):411–416. doi: 10.4103/0972-124X.118306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Sa Siqueira MA, Fischer RG, da Silva Figueredo CM, Brunini TM, Mendes-Ribeiro AC. Nitric oxide and oral diseases: can we talk about it? Cardiovasc Hematol Agents Med Chem. 2010;8(2):104–112. doi: 10.2174/187152510791170942. [DOI] [PubMed] [Google Scholar]
- 27.Culic C, Parvu AE, Alb SF, Alb C, Pop A. Effect of cimetidine on nitro-oxidative stress in a rat model of periodontitis. Clujul Med. 2014;87(3):177–181. doi: 10.15386/cjmed-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conea S, Parvu AE, Taulescu MA, Vlase L. Effects of Eryngium planum and Eryngium campestre extracts on ligature-induced rat periodontitis. Digest Journal of Nanomaterials and Biostructures. 2015;10(2):693–704. [Google Scholar]
- 29.Boşca AB, Ilea A, Şovrea AS, Constantin AM, Ruxanda F, Rus V, et al. An Experimental Model for Inducing Periodontal Pathology in Rat: Histopathological and Enzymatic Aspects. Bull UASVM Vet Med. 2014;71(2):507–508. [Google Scholar]
- 30.Akpinar A, Toker H, Ozdemir H, Bostanci V, Aydin H. The effects of non-surgical periodontal therapy on oxidant and anti-oxidant status in smokers with chronic periodontitis. Arch Oral Biol. 2013;58(6):717–723. doi: 10.1016/j.archoralbio.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Borges I, Jr, Moreira EA, Filho DW, De Oliveira TB, da Silva MB, Fröde TS. Proinflammatory and oxidative stress markers in patients with periodontal disease. Mediators Inflamm. 2007;2007:45794. doi: 10.1155/2007/45794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghiselli A, Serafini M, Natella F, Scaccini C. Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radic Biol Med. 2000;29(11):1106–1114. doi: 10.1016/s0891-5849(00)00394-4. [DOI] [PubMed] [Google Scholar]
- 33.Lindhe J, Lang NP, Karring T. Clinical Periodontology and Implant Dentistry. 5th ed. Oxford: Blackwell Publishing; 2008. [Google Scholar]
- 34.Soames JV, Southam JC. Oral Pathology. Oxford University Press; 2007. [Google Scholar]
- 35.Akalin FA, Baltacioğlu E, Alver A, Karabulut E. Lipid peroxidation levels and total oxidant status in serum, saliva and gingival crevicular fluid in patients with chronic periodontitis. J Clin Periodontol. 2007;34(7):558–565. doi: 10.1111/j.1600-051X.2007.01091.x. [DOI] [PubMed] [Google Scholar]
- 36.Liu Z, Liu Y, Song Y, Zhang X, Wang S, Wang Z. Systemic oxidative stress biomarkers in chronic periodontitis: a meta-analysis. Dis Markers. 2014;2000:931083. doi: 10.1155/2014/931083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kantarci A, Oyaizu K, van Dyke TE. Neutrophil-mediated tissue injury in periodontal disease pathogenesis: findings from localized aggressive periodontitis. J Periodontol. 2003;74(1):66–75. doi: 10.1902/jop.2003.74.1.66. [DOI] [PubMed] [Google Scholar]
- 38.Katsuragi H, Ohtake M, Kurasawa I, Saito K. Intracellular production and extracellular release of oxygen radicals by PMNs and oxidative stress on PMNs during phagocytosis of periodontopathic bacteria. Odontology. 2003;91(1):13–18. doi: 10.1007/s10266-003-0022-1. [DOI] [PubMed] [Google Scholar]
- 39.Sakallioğlu U, Aliyev E, Eren Z, Akşimşek G, Keskiner I, Yavuz Ü. Reactive oxygen species scavenging activity during periodontal mucoperiosteal healing: an experimental study in dogs. Arch Oral Biol. 2005;50(12):1040–1046. doi: 10.1016/j.archoralbio.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 40.Page RC, Schroeder HE. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Invest. 1976;34(3):235–249. [PubMed] [Google Scholar]
- 41.Ryder MI. Comparison of neutrophil functions in aggressive and chronic periodontitis. Periodontol 2000. 2010;53:124–137. doi: 10.1111/j.1600-0757.2009.00327.x. [DOI] [PubMed] [Google Scholar]
- 42.Hajishengallis G. Too old to fight? Aging and its toll on innate immunity. Mol Oral Microbiol. 2010;25(1):25–37. doi: 10.1111/j.2041-1014.2009.00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DiStasi MR, Ley K. Opening the flood-gates: how neutrophil-endothelial interactions regulate permeability. Trends Immunol. 2009;30(11):547–556. doi: 10.1016/j.it.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lindbom L. Regulation of vascular permeability by neutrophils in acute inflammation. Chem Immunol Allergy. 2003;83:146–166. doi: 10.1159/000071559. [DOI] [PubMed] [Google Scholar]
- 45.Nakatani K, Takeshita S, Tsujimoto H, Kawamura Y, Sekine I. Inhibitory effect of serine protease inhibitors on neutrophil-mediated endothelial cell injury. J Leukoc Biol. 2001;69(2):241–247. [PubMed] [Google Scholar]
- 46.Chapple IL. Role of free radicals and antioxidants in the pathogenesis of the inflammatory periodontal diseases. Clin Mol Pathol. 1996;49(5):M247–M255. doi: 10.1136/mp.49.5.m247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pendyala G, Thomas B, Kumari S. The challenge of antioxidants to free radicals in periodontitis. J Indian Soc Periodontol. 2008;12(3):79–83. doi: 10.4103/0972-124X.44100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boşca AB, Miclăuş V, Raţiu C, Melincovici C. Matrix Metalloproteinase-8 – a salivary diagnostic biomarker related to soft tissue distruction in chronic periodontitis. Ann RSCB. 2012;17:251–257. [Google Scholar]
- 49.Waddington RJ, Moseley R, Embery G. Reactive oxygen species: a potential role in the pathogenesis of periodontal diseases. Oral Dis. 2000;6(3):138–151. doi: 10.1111/j.1601-0825.2000.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 50.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford, UK: Clarendon Press; 1998. pp. 617–783. [Google Scholar]
- 51.Wang ML, Hauschka PV, Tuan RS, Steinbeck MJ. Exposure to particles stimulates superoxide production by human THP-1 macrophages and avian HD-11EM osteoclasts activated by tumor necrosis factor-α and PMA. J Arthroplasty. 2002;17(3):335–346. doi: 10.1054/arth.2002.30416. [DOI] [PubMed] [Google Scholar]
- 52.Wadhwa D, Bey A, Hasija M, Moin S, Kumar A, Aman S, et al. Determination of levels of nitric oxide in smoker and nonsmoker patients with chronic periodontitis. J Periodontal Implant Sci. 2013;43(5):215–220. doi: 10.5051/jpis.2013.43.5.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sundar NM, Krishnan V, Krishnaraj S, Hemalatha VT, Alam MN. Comparison of the salivary and the serum nitric oxide levels in chronic and aggressive periodontitis: a biochemical study. J Clin Diagn Res. 2013;7(6):1223–1227. doi: 10.7860/JCDR/2013/5386.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brock GR, Butterworth CJ, Matthews JB, Chapple IL. Local and systemic total antioxidant capacity in periodontitis and health. J Clin Periodontol. 2004;31:515–521. doi: 10.1111/j.1600-051X.2004.00509.x. [DOI] [PubMed] [Google Scholar]
- 55.Abou Sulaiman AE, Shehadeh RM. Assessment of total antioxidant capacity and the use of vitamin C in the treatment of non-smokers with chronic periodontitis. J Periodontol. 2010;81(11):1547–1554. doi: 10.1902/jop.2010.100173. [DOI] [PubMed] [Google Scholar]
- 56.Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metabol Cardiovasc Dis. 2005;15(4):316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]