Abstract
Background and aim
In spite of numerous interventions, the control of mineral disturbances remains poor in end-stage renal failure (ESRF) patients. Chronic kidney disease - mineral and bone disorders (CKD-MBD) represent an important cause of mortality and morbidity. The aim of this study is to analyze the relationship between mineral and bone disorders (MBD) and their components impact on all-cause mortality and cardiovascular (CDV) mortality and morbidity in chronic dialysis patients.
Methods
This prospective study was carried out in a cohort of 92 randomly selected patients with ESRF treated with hemodialysis (HD) and peritoneal dialysis (PD). The data regarding demographic and clinical characteristics were recorded, including vascular disease (coronary, cerebral, peripheral). The follow-up lasted 40 months and the final evaluation included the number and causes of deaths, CDV events and disease. Serum Ca, P, ALP, iPTH, albumin, cholesterol, urea and creatinine levels were measured. The plain radiographic films of hands and pelvis evaluated all bone abnormalities suggestive of renal osteodystrophy (ROD) and peripheral vascular calcification (VC).
Results
All-cause annual mortality represented 9.25% in HD and 9.09% in PD patients. The CDV mortality represented almost 44% in HD patients and 66% in PD patients from all deaths. There was a high prevalence of CDV diseases and events. High and low serum P levels were associated with a worse survival rate. Hypercalcaemia was associated with high risk for CDV events in HD patients. In PD patients, the relationship between increased ALP levels and all-cause mortality was significant. Other mineral markers were not predictive of the outcome in the studied patients. In the HD patients the severity of VC was associated with all-cause and CDV mortality, and with CDV events. Male gender, hypercholesterolemia, decreased URR, albumin and creatinine were identified as risk factors for all-cause mortality. The diabetics had higher death rates. Low dialysis efficacy represented a risk factor for mortality and CDV diseases and events. In PD patients, low albumin induced a higher death rate. In PD patients the death rate was similar to HD patients.
Conclusion
All-cause mortality was higher than in general population, but lower than the chronic dialysis patients’ mortality reported in other studies. The death rates in HD and PD patients were similar. VC and serum P levels influenced the outcome in the HD patients – increased the risk for all-cause and CDV mortality, but also for CDV events. ALP levels influenced outcome in PD patients. There were no significant differences between HD and PD patients regarding outcome.
Keywords: mineral and bone disorders, vascular calcification, renalosteodystrophy, mortality, morbidity, cardiovascular events, chronic dialysis
Introduction
Every year, worldwide, millions of people die as a result of renal failure. Although maintenance dialysis prevents death from uremia, patient survival remains an important issue. When kidney function deteriorates, it significantly affects the regulation of calcium (Ca) and phosphorus (P) balance. In spite of numerous interventions, the control of mineral disturbances remained low in end-stage renal failure (ESRF) patients. The most recent guidelines proposed by the “Kidney Disease: Improving Global Outcomes” (KDIGO) foundation introduced the term “chronic kidney disease - mineral and bone disorders” (CKD-MBD), in the attempt to bring together alterations in mineral metabolism markers, renal osteodystrophy (ROD) and vascular or other soft tissue calcifications (VC). Every component of CKD-MBD represents an important cause of mortality and morbidity. These are considered modifiable risk factors [1,2]. Further research is considered still necessary regarding the correlation between mineral markers, bone changes or VC and outcome in ESRF patients. The screening for VC and ROD is not yet recommended, but this subject is a matter of debate. Further work is needed to establish what should be the proper tests for this purpose. Mineral metabolism disturbances occur very early in the course of CKD, they are frequent in dialysis patients, but their control has been poor [3]. Achievement of mineral control is difficult, emphasizing the need to understand the relationship between mineral disturbances and survival to make appropriate treatment recommendations. Large observational studies associated increased Ca, P, ALP or iPTH levels with high mortality risk [4,5]. One of these studies demonstrated that the mineral metabolism risk overcomes the risk induced by anemia or low dialysis efficacy [2]. These results were not consistent in all studies [5]. There is no consensus regarding this issue. Cardiovascular (CDV) diseases are responsible for over 50% of mortality causes in dialysis patients. VC is prevalent in dialysis patients. VC correlated with all-cause and CDV mortality, CDV events and vascular disease [5–7]. ROD manifests as bone pain and deformities and complicates with fractures. Bone fractures have a demonstrated negative impact on mortality in the general population and chronic dialysis patients [8]. There are no available studies about the relationship between ROD diagnosed on radiographs and survival. Mineral metabolism markers, ROD and VC were separately analyzed in relation to morbidity and mortality. According to KDIGO, CKD-MBD is considered an etiopathogenetic entity [9].
Peritoneal dialysis (PD) patients are also affected by these problems. Hypercalcemia influenced survival in PD [10], but relationship between mineral markers, morbidity and mortality was not studied enough. Valvular calcifications were used for CDV risk stratification in PD patients and they can identify the sickest PD patients with the worst clinical outcomes [11]. Extraosseous manifestations, especially VC, represent an important complication for the patients treated by PD [12,13].
The study goal was to analyze the influence of each CKD-MBD component on the outcome of patients with ESRF under dialysis treatment. Outcome was represented by all-cause mortality, CDV mortality, CDV events and diseases. Confounding factors that can modify outcome related to CKD-MBD were analyzed (age, gender, type and duration of dialysis, baseline nephropathy, nutrition -albumin, cholesterol, trygliceride, creatinine; hematological status - Hb, ferritin; inflammation - CRP; dialysis adequacy - urea, URR, spKt/V). General objectives were to assess CKD-MBD influence on all-cause mortality and on CDV mortality and morbidity in chronic dialysis patients.
Patients and methods
This longitudinal, prospective, analytical study was carried out in a cohort of 92 randomly selected patients with ESRD treated by iterative dialysis in Nefromed Dialysis Center Cluj-Napoca. Eligibility criteria were dialysis duration ≥6 months, age >18 years. Exclusion criteria: neoplasm, severe infections or other terminal diseases; parathyroidectomy, previous renal transplantation or previous bone disease, patients who refused the radiographic examination.
All patients underwent conventional (standard bicarbonate) HD, 3 sessions a week, 4 hours a session with synthetic polysulfone membranes. Calcium concentrations in the dialysate were 1.25 mmol/L in 25 pts (30.8%), 1.5 mmol/L in 43 patients (53.1%) and 1.75 mmol/L in 8 patients (9.9%). They received calcium carbonate and acetate as phosphate binders and vitamin D compounds (calcitriol); the total amount of elemental calcium and vitamin D ingested in the previous year were calculated. Blood samples for the biochemical evaluation were drawn prior to the dialysis session.
The CAPD patients had a regimen of 4 changes a day, using Fresenius 1.5% glucose solution with Ca concentration of 1.75 mmol/L in the last 3 months prior to evaluation. Fasting venous blood was collected.
The data regarding demographic and clinical characteristics were recorded, including vascular disease (coronary, cerebral, peripheral). Serum Ca, P, ALP and iPTH levels were measured. The plain radiographic films of the hands and pelvis evaluated all bone abnormalities suggestive of ROD and peripheral VC. Digital, radial, iliac and femoral arteries were examined for VC and a VC score was calculated [5]. More data about the study design and descriptive statistics could be found in previous publications [14,15]. The follow-up lasted 40 months and the final evaluation included: the number and causes of death, cardiovascular events and disease. In deceased patients we calculated survival duration and rate. All-cause and CDV mortality, CDV events, CDV diseases were recorded for each group (HD and PD) and their prevalences were calculated. CKD-MBD impact on all-cause mortality, CDV mortality and events after 40 months of follow-up. Other associated factors were analyzed. The comparison between HD and PD groups regarding outcomes was performed.
Statistical analysis
According to the variable distribution, Student’s t-test or the Mann–Whitney test was used to compare the groups. Fisher’s exact test was applied for proportion comparison. Logistic regression was applied to determine the factors significantly associated with a VC score < 2 or ≥2. Multiple regression analysis was applied between outcome and the potentially associated factors. The 40 months survival rate, according to the VC score, was calculated using the Cox proportional hazard model adjusted for age, sex and diabetes. Mortality was compared stratifying the patients in the two groups according to lab tests, different VC scores and the presence of ROD. We analyzed survival through dichotomial method, comparing characteristics of deceased patients and survivors, using t and Mann-Whitney Rank Sum tests. Cox regression model was used to identify independent variables associated with all-cause (total) deaths, CDV deaths and events. Covariates were age, gender, dialysis duration, presence of diabetes mellitus (DM), serum Ca, P, ALP, iPTH, Hb, albumin, CRP levels, URR, presence of ROD, vascular disease at the begining of the study and VC score. Cox regression estimated the relative risks for deaths and CdV events. Survival analysis was performed with log-rank test, survival curves were represented with Kaplan-Meier curve. Statistical significance threshold was considered p<0.05. Statistical analysis was performed using SPSS 13.0 program. The study was approved by the Ethical Committee of our University and is in accordance with the ethical standards of the Declaration of Helsinki. Each patient signed an informed consent.
Results
Of the total 92 patients, 28 (30.43%) deceased, whichcorresponds to 9.13% annual mortality.
HD group consisted of 35 females and 46 males. There were 10 patients with diabetes mellitus (DM). The treatment received was as follows: 72 patients (88.9%) took calciumsalts and 43 patients (53%) vitamin D compounds. The mean cumulated doses receivedin the previous year were: 311±175.76 g for oral elemental calcium and 31.86±54.66 mcg for vitamin D derivates. No patient received aluminum containing agents. In the CAPD group there were 8 males and 2 diabetics.
1) HD group analysis
After 40 months, 25 (30.86%) of the total of 81 HD patients deceased. Annual all-cause mortality was 9.25%. In 11 patients the causes of death were represented by CDV diseases: myocardial infarction (3 patients), stroke (3 patients), sudden death (2 cases) and cardiorespiratory arrest (3 patients). Other non-CDV causes were sepsis (4 patients), cirrhosis, digestive bleeding, internal haemorrhage, cancers or unknown. CDV mortality was 13.5%. A CDV cause was identified in 44% of deaths. We registered 29 CDV events (35.8%) from which 11 were fatal and 18 were non-fatal. Baseline CDV diseases were_identified in 38 patients (46.91%); 33 patients had coronary, 2 patients had cerebral and 4 patients had peripheral diseases. At the end of the follow-up CDV diseases were identified_in 47 patients (58%); 38 patients had coronary, 10 patients had cerebral and 6 patients had peripheral diseases.
CKD-MBD and other factors associated with all-cause mortality were analyzed after 40 months of follow-up. In the dichotomial analysis, the deceased patients group had a higher mean VC score than the survivors mean VC score (p=0.007). Deceased patients group presented higher number of diabetic patients (p=0.009), serum cholesterol levels (p=0.001) and lower HD vintage (p=0.02), serum albumin (p=0.048), creatinine (p=0.02), spKt/V (p=0.02), URR (p=0.008) (Table I).
Table I.
Comparison between the deceased and survivors groups.
| HD deceased (25 patients) | HD survivors (56 patients) | p | |
|---|---|---|---|
| Age (years) | 58.32±11.61 | 55.94±12.14 | 0.28 |
| HD vintage (months) | 42.20±52.66 | 54.76±46.38 | 0.02 |
| Gender (% males) | 18 (72%) | 28(50%) | 0.10 |
| DM | 8 (32%) | 2(3.57%) | 0.0009 |
| ROD | 20 (80%) | 33 (58.92%) | 0.49 |
| VC score | 4.88±3.12 | 2.66±2.82 | 0.007 |
| Ca in HD solution | 1.42±0.15 | 1.45±0.15 | 0.54 |
| Oral Ca salts | 276.65±175.69 | 325.63±175.37 | 0.26 |
| Vitamin D | 19.52±34.42 | 37.09±60.79 | 0.31 |
| Ca (mg/dl) | 8.36±0.96 | 8.07±1.10 | 0.32 |
| P (mg/dl) | 6.24±2.24 | 5.99±1.52 | 0.79 |
| ALP (U/l) | 311±195.13 | 281.10±175.21 | 0.60 |
| iPTH (pg/ml) | 607.74±649.55 | 612.01±655.50 | 0.84 |
| Creatinine (mg/dl) | 9.47±2.69 | 10.98±2.78 | 0.02 |
| Albumin (mg/dl) | 3.89±0.60 | 4.18±0.33 | 0.04 |
| CRP (mg/dl) | 1.37±1.17 | 1.22±1.45 | 0.10 |
| Cholesterol (mg/dl) | 195.84±48.93 | 163.29±33.9 | 0.001 |
| Trygliceride (mg/dl) | 193.16±214.27 | 139.23±65.47 | 0.22 |
| URR | 64.21±9.20 | 67.98±9.57 | 0.008 |
| spKt/V | 1.26±0.22 | 1.35±0.23 | 0.02 |
| Hb (g/dl) | 10.76±1.25 | 11.25±1.64 | 0.15 |
| Ferritin (ng/ml) | 539±288.59 | 821.54±713.36 | 0.15 |
Legend: HD=hemodilysis; DM=diabetes mellitus; ROD=renal osteodystrophy; VC=vascular calcification; Ca=calcium; P=phosphorus; ALP=alkaline phosphatase; iPTH=intact parathyroid hormone; CRP=C-reactive protein; URR=urea reduction ratio; spKt/V=dialysis adequacy and Hb=hemoglobin.
We compared the number of deaths registered in the group of patients without treatment with calcium salts (3 deaths in 8 untreated patients group) with the number of deaths registered in the group of patients with calcium salts treatment (22 deaths in 73 treated patients) – difference was not statistically significant (p=0.69). The rate of deaths registered in the group of patients not treated with vitamin D (14 deaths in 38 untreated patients) was not statistically different compared with the number of deaths in vitamin D treated patients group (11 deaths in 43 vitamin D treated patients) (p=0.39).
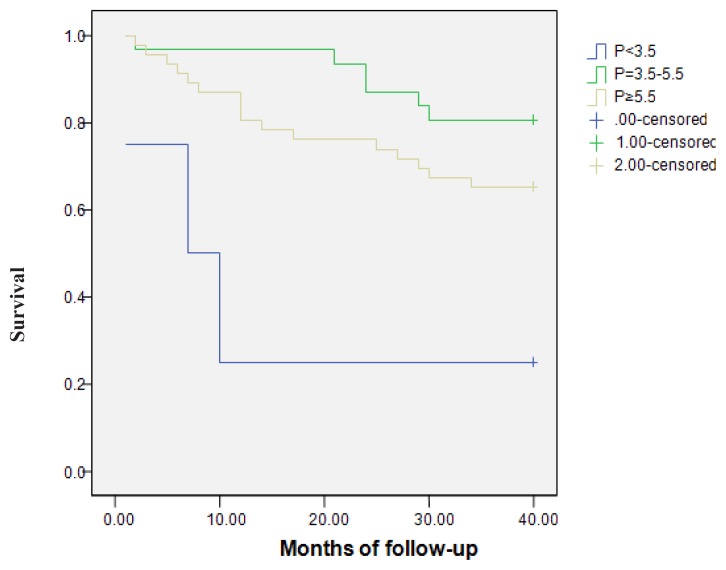
Kaplan-Meier analysis of the relation between mineral markers and survival was performed after a stratification with the cut-off values recommended in K/DOQI guidelines. Survival was compared between groups with low, normal and high Ca, P, iPTH values. All-cause mortality was not influenced by the serum levels of Ca (p=0.67) and iPTH (p=0.54). The serum P levels had a significant influence on all-cause mortality. Three patients (75%) with P<3.5 mg/dl, 6 patients (19.4%) with P=3.5–5.5 mg/dl and 16 patients (34.8%) with P≥5.5 mg/dl died (p=0.003) (Figure 1).
Figure 1.
The relation between phosphorus (P) and all-cause mortality (p=0.003).
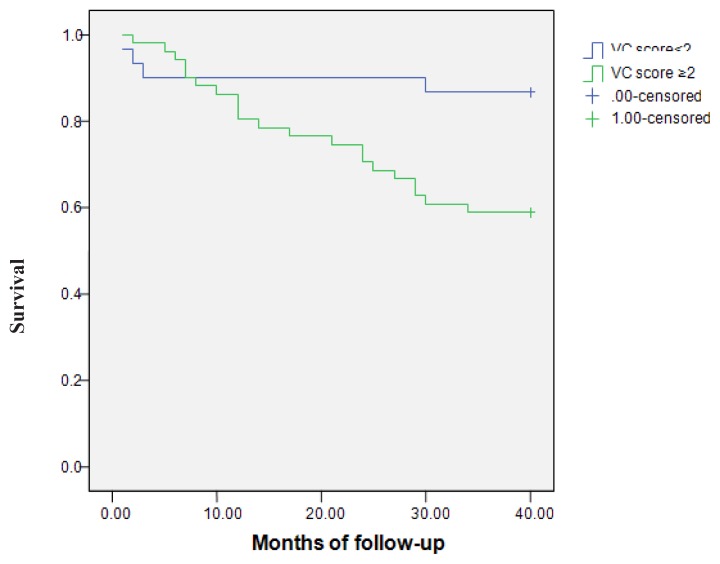
In order to evaluate the relationship between VC severity and all-cause mortality, we created two groups of patients according to VC scores. Since the VC score was ≥2, the VC – all-cause mortality relationship was statistically significant (Figure 2). From the total of 25 deceased patients, 4 patients (13.3%) had a VC score <2 and 21 patients (41.17%) had a VC score ≥2 (p=0.01). The relative risk was 27.83%. Univariate analysis of the relation between ROD and all-cause mortality was not significant (p=0.63). Kaplan-Meier analysis was also not significant (p=0.29).
Figure 2.
All-cause mortality– comparison between the groups with a vascular calcifications (VC) score<2 and with VC score≥2. Survival was 86.7% vs 58.8% (log-rank Mantel-Cox =5.956; p=0.015).
Multivariable Cox analysis for all-cause mortality used as covariates age, gender, HD vintage, presence of DM, VC score, presence of ROD, Ca in dialysis solution, oral Ca salts, vitamin D treatment, serum Ca, P, iPTH, ALP, creatinine, Hb, cholesterol, trygliceride, CRP, albumin, ferritin levels, URR, spKt/V baseline renal disease, initial CdV disease. The method was Backward LR stepwise. Variables which remained in the equation were male gender (HR=14.96; 95%CI=2.09–106.98; p=0.007); VC score (HR=1.30; 95% CI=1.05–1.59; p=0.01); P (HR=1.66; 95% CI=1.14–2.43; p=0.008); URR (HR=0.93; 95% CI=0.88–0.98; p=0.01); Albumin (HR=0.27; 95% CI=0.08–0.89; p=0.03); Cholesterol (HR=1.03; 95% CI=1.01–1.04; p<0.001); Creatinine (HR=0.53; 95% CI=0.38–0.72; p<0.001). Male gender, increased VC score, P, Chol and decreased URR, albumin, creatinine represent risk factors for all-cause mortality.
CKD-MBD impact on CDV mortality after 40 months of follow-up. Other factors associated with CDV mortality.
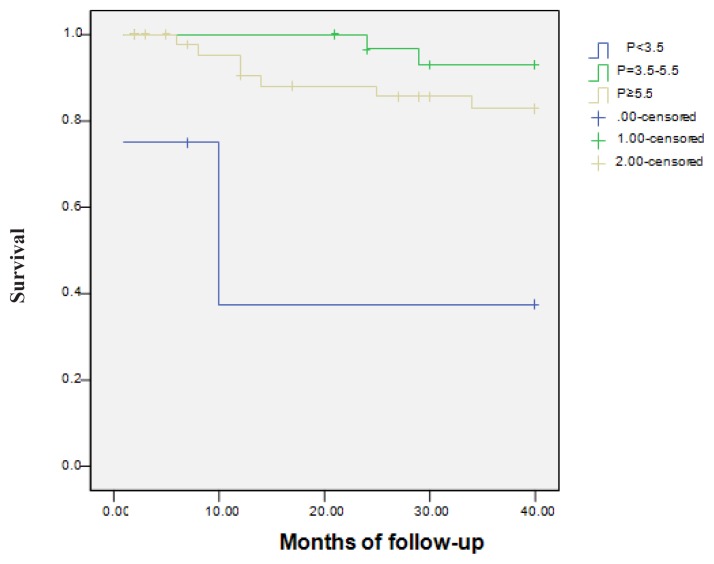
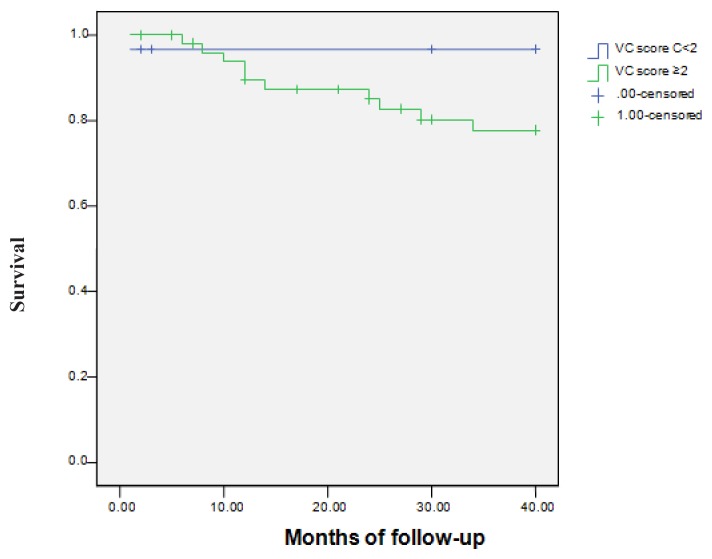
CDV mortality was not influenced by the serum levels of Ca (p=0.09) and iPTH (p=0.26). The serum P levels had a significant influence on CDV mortality. 2 patients (50%) with P<3.5 mg/dl, 2 patients (6.5%) with P=3.5–5.5 mg/dl and 7 patients (15.2%) with P≥5.5 mg/dl died (p=0.001) (Figure 3). The relation between VC severity and CDV mortality was analyzed. One of 30 patients (3.33%) with VC score<2 and 10 of 51 patients (19.60%) with VC score ≥2 died (p=0.04). The relative risk of CDV death was 16.27%. In order to evaluate the relationship between VC severity and CDV mortality, we created two groups of patients according to VC scores. Since the VC score was ≥2, the VC – CDV mortality relationship was statistically significant (Figure 4). Univariate analysis of the relation between ROD and CDV mortality was not significant (p=0.98). Kaplan-Meier analysis was also not significant (p=0.76).
Figure 3.
The relation between phosphorus (P) and cardiovascular mortality (p=0.001).
Figure 4.
Cardiovascular mortality– comparison between the groups with a vascular calcification (VC) score<2 and with vascular calcification (VC) score≥2 (log-rank Mantel-Cox =4.343; p=0.037). Survival was 86.7% vs 58.8%.
Multivariable Cox analysis of CdV mortality used as covariates age, gender, HD vintage, presence of DM, VC score, presence of ROD, Ca in dialysis solution, oral Ca salts, vitamin D treatment, serum Ca, P, iPTH, ALP, creatinine, Hb, cholesterol, trygliceride, CRP, albumin, ferritin levels, URR, spKt/V baseline renal disease, initial CdV disease. The method was Forward LR stepwise. VC score (HR=1.387; 95.0% CI 1.095–1.757; p=0.007) and URR (HR=0.942; 95.0% CI 0.888–0.999; p=0.046) remained in the ecuation. Increased VC score and decreased URR represent risk factors for CDV mortality.
2) PD group analysis
After 40 months, 3 out of all 11 PD patients deceased. All-cause mortality was 27.27% in 40 months (9.09% per year). Two patients died of CDV causes (1 due to myocardial infarction and 1 due to stroke), which represent 66.66% of death causes. One died of unknown cause. CDV mortality was 18.18%. In PD patients, increased serum ALP levels were associated with increased all-cause mortality (p=0.007).
We compared the group of HD with the group of PD patients regarding characteristics and outcome. In HD patients, serum P, creatinine, albumin and ferritin were significantly higher than in PD patients group. Gender, the presence of DM and ROD were not different (Table II).
Table II.
Comparison between the characteristics of HD and PD groups.
| Characteristics | HD (81 patients) | PD (11 patients) | p |
|---|---|---|---|
| Age (years) | 56.68±12.04 | 56.82±7.80 | 0.97 |
| HD vintage (months) | 50.89±48.43 | 40.09±20.90 | 0.90 |
| VC score | 3.35±3.08 | 4.00±2.72 | 0.44 |
| Ca (mg/dl) | 8.16±1.07 | 8.81±0.71 | 0.053 |
| P (mg/dl) | 6.06±1.77 | 4.92±0.77 | 0.0008 |
| iPTH (pg/ml) | 610.70±649.61 | 309.55±201.8 | 0.28 |
| ALP (U/l) | 290.19±180.76 | 249.27±228.35 | 0.055 |
| Creatinine (mg/dl) | 10.52±2.82 | 8.77±1.83 | 0.02 |
| Hb (g/dl) | 11.10±1.54 | 10.55±2.18 | 0.65 |
| Cholesterol (mg/dl) | 173.46±41.80 | 198.36±40.34 | 0.07 |
| Trygliceride (mg/dl) | 156.09±132.33 | 153.36±43.31 | 0.38 |
| CRP (mg/dl) | 1.27±1.37 | 0.87±0.73 | 0.34 |
| Albumin (g/dl) | 4.09±0.45 | 3.61±0.32 | 0.0003 |
| Ferritin (ng/ml) | 733.26±624.91 | 374.20±571.88 | 0.008 |
| Survival (months) | 28.33±10.34 | 30.27±8.65 | 0.44 |
Legend: HD=hemodialysis; PD=peritoneal dialysis; VC=vascular calcification; Ca=calcium; P=phosphorus; ALP=alkaline phosphatase; iPTH=intact parathyroid hormone; Hb=hemoglobin and CRP=C-reactive protein.
All-cause mortality (P=0.72) and CDV mortality (P=0.65) and events (p=0.37) were similar in both groups.
Discussion
All-cause mortality in our studied chronic dialysis patients group (HD and PD) was increased (9.13% per year) compared to the known data for the general population (5.5% per year), but lower compared to mortality in patients with pre-dialysis CKD (17.7% per year) and chronic dialysis (>20% per year) reported in other studies [16]. One possible explanation for the fact that dialysis patients in the present study had better survival than other patients on dialysis could be that the population was involuntarily selected - of all dialysis patients in our dialysis center, the ones who agreed to undergo X-rays and to participate in the study had a better performance, those harder to mobilize did not participate to the study. Other explanations are the demographic characteristics of the studied population: younger patients, fewer diabetics, different lifestyles, compared with other studies [17]. CDV mortality represented 44% from all causes of mortality, a result which concords with literature data [18]. CDV events and diseases had high prevalence, even higher than in other studies [5]. In several studies, all-cause mortality was associated with increased Ca, P, ALP, iPTH values [2,6], in others was associated with decreased values of these parameters. It is suggested that iPTH relationship with mortality might scored on a J or U-shaped curve [19,20]. Large, observational studies conclude that low and high Ca, P and iPTH values are associated with increased risk of all-cause and CDV mortality [20].
In our study on chronic HD patients, among all mineral markers, only P was associated with all-cause and CDV mortality, in a J-shaped pattern. Association of the risk with hypophosphatemia could be due to malnutrition [2,21,22]. Yet, multivariate analysis showed that hyperphosphatemia could be considered a risk factor for all-cause mortality. This result is concordant with other studies, hyperphosphatemia having the highest impact on mortality among mineral markers [20–22].
Several studies have drawn attention over the association between high serum Ca and CDV morbidity [23,24]. In our research, serum Ca was associated with a higher number of CDV events. Multivariate analysis identified as risk factors for CdV events increased VC score and low URR. Relationship between VC and CDV events was demonstrated by other studies also [2,5].
Advanced age was a risk factor for the presence of CDV disease at the beginning of the study. For the presence of CDV disease at the end of the study, the risk factors were the advanced age and low URR. Age is a recognized CDV risk factor, which is why it is called traditional. Another risk factor for CdV disease at the end of follow-up was ineffective dialysis. Although other researches demonstrated a link between MBD and CDV morbidity assessed through CDV hospitalizations [5], in our study, MBD (MM, VC and ROD) did not have a significant relationship with CDV disease at the beginning and the end of the study.
Among CKD-MBD in HD patients, VC had the most consistent influence on outcome– all-cause and CDV mortality and CDV events. VC were extensively studied in the last years [25,26]. VC had been associated with mortality, regardless of imagistic test used for detection [27]. Radiograph had proofed to be useful in detecting VC [23,28]. The results of our research are in concordance with these results. Some studies demonstrated that VC can predict the CDV mortality risk in non-renal population [27,29], but, especially in patients with renal failure on HD treatment [30,31] and on PD [11,12]. Several studies demonstrated that VC represent a link between renal and CDV diseases [17,32,33]. VC can predict ischemic coronary disease and CDV events in general population and in renal population [34,35], but our study did not detect a significant relationship between VC presence and CDV diseases.
The main complication of ROD is represented by fractures, which is a risk factor for mortality in dialysis patients [36,37]. In this study, there was no evidence that ROD was a prognostic factor in HD patients.
Other risk factors for all-cause mortality were male gender, high cholesterol and low levels of albumin, creatinine and URR. Malnutrition (lower albumin and creatinine) is another risk factor for mortality; inverse epidemiology was not confirmed [19] regarding cholesterol values. The explanation could be that high cholesterol does not have a protective effect in dialysis patients and only low cholesterol is associated with malnutrition - inflammation syndrome. Low HD adequacy was a risk factor for all-cause and CDV mortality. Paradoxically, all-cause mortality was higher in patients with short HD vintage – those who started HD treatment in the last 5 years (especially in the last 2 years) were sicker because of increasing the access to the treatment. Ten years or more ago, the patients who could benefit of chronic dialysis were carefully selected because of low availability of dialysis. They were young and without comorbidities. The presence of DM was detected as another mortality risk factor, similar to other studies [18,38]. Mortality in patients treated with Ca salts or vitamin D compounds was not significantly different comparing with untreated HD patients.
In PD patients, all-cause mortality was similar to that of HD patients and CDV death was the main cause. Increased ALP levels were predictive factors for mortality in chronic HD patients [39], but there are no relevant studies yet regarding this link in PD patients. All-cause mortality was higher in patients with increased ALP levels and decreased albumin levels. Some authors detected associations hypocalcemia and CDV morbidity and between hyperphosphatemia and the CDV mortality in PD patients. Also, the CDV calcifications had been recognized as risk factors for all-cause and CDV mortality [12]. In our study, mineral markers, VC and ROD did not influence the outcome in PD patients.
The groups of HD and PD patients were compared. Serum P, creatinine, albumin and ferritin levels were significantly higher in HD patients than PD patients. Similar results were communicated by other research groups also [40,41]. Increased P and creatinine levels seem to be the consequence of the anuria and the intermitency of HD prescription. Decreased serum albumin levels are a sign of protein malnutrition which is more prevalent in this category of patients. The other parameters were not significantly different between the two groups. The modality of dialysis was associated with an increased risk of all-cause and CDV mortality, in some studies for PD [42], in others for HD [43]. In our study there were no significant differences regarding all-cause and CDV mortalities or CDV events and diseases between HD and PD groups, corresponding with the results of other studies [44,45].
There are some limitations of the present study that should be considered. The PD patients group was too small and any conclusions or comparisons with the HD group restrain us to generalize the results. X-ray examination cannot detect details about the extent of VC, as more accurate techniques would.
Conclusions
All-cause mortality in the studied group (HD and PD) was higher than in the general population, but lower than mortality reported in other studies for dialysis patients. Mortality rates in HD and DP patients were similar. CDV mortality represented the main cause of death in HD and DP patients. Hyperphosphatemia can predict the risk for all-cause mortality in HD patients. Hypercalcemia is associated with increased risk for CDV events in HD patients. VC severity is correlated with clinical outcomes and it represents a risk factor for all-cause mortality, CDV mortality and CDV events in HD patients. In PD patients, increased ALP was associated with all-cause mortality. VC did not influence prognosis in PD patients. There were no significant differences between all-cause and CDV mortality, CDV events and disease comparing HD and PD groups.
Acknowledgements
We are indebted to the patients who participated in this study.
This paper was published under the frame of European Social Found, Human Resources Development Operational Program 2007–2013, project no POSDRU/159/1.5/138776.
References
- 1.Tentori F, Wang M, Bieber BA, Karaboyas A, Li Y, Jacobson SH, et al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am Soc Nephrol. 2015;10(1):98–109. doi: 10.2215/CJN.12941213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 3.Stevens LA, Djurdjev O, Cardew S, Cameron EC, Levin A. Calcium, phosphate, and parathyroid hormone levels in combination and as a function of dialysis duration predict mortality: evidence for the complexity of the association between mineral metabolism and outcomes. J Am Soc Nephrol. 2004;15:770–779. doi: 10.1097/01.asn.0000113243.24155.2f. [DOI] [PubMed] [Google Scholar]
- 4.Jean G, Bresson E, Terrat JC, Vanel T, Hurot JM, Lorriaux C, et al. Peripheral vascular calcification in long-haemodialysis patients: associated factors and survival consequences. Nephrol Dial Transplant. 2009;4(3):948–955. doi: 10.1093/ndt/gfn571. [DOI] [PubMed] [Google Scholar]
- 5.Adragao T, Pires A, Lucas C, Birne R, Magalhaes L, Gonçalves M, et al. A simple vascular calcification score predicts cardiovascular risk in haemodialysis patients. Nephrol Dial Transplant. 2004;19:1480–1488. doi: 10.1093/ndt/gfh217. [DOI] [PubMed] [Google Scholar]
- 6.Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK. Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001;12:2131–2138. doi: 10.1681/ASN.V12102131. [DOI] [PubMed] [Google Scholar]
- 7.Covic A, Kanbay M, Voroneanu L, Turgut F, Serban DN, Serban IL, et al. Vascular calcification in chronic kidney disease. Clin Sci (Lond) 2010;119(3):111–121. doi: 10.1042/CS20090631. [DOI] [PubMed] [Google Scholar]
- 8.Noto-Kadou-Kaza B, Teuwafeu DG, Sabi KA, Zenasni N, Amekoudi EY, Tsevi CM, et al. Chutes chez l’hémodialysé: incidence et facteurs de risque. Néphrologie & Thérapeutique. 2015;11(4):246–249. doi: 10.1016/j.nephro.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2009;(113):S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 10.Khawar O, Mehrotra R, Duong U, Kovesdy C, Shapiro M, McCallister C, et al. Serum Calcium and Survival in a Large USA Cohort of Chronic Peritoneal Dialysis Patients. Perit Dial Int. 2007;27(3):S11. [Google Scholar]
- 11.Ammirati AL, Dalboni MA, Cendoroglo M, Draibe SA, Santos RD, Miname M, et al. The progression and impact of vascular calcification in peritoneal dialysis patients. Perit Dial Int. 2007;27(3):340–346. [PubMed] [Google Scholar]
- 12.Wang AY, Wang M, Woo J, Lam CW, Li PK, Lui SF, et al. Cardiac valve calcification as an important predictor for all-cause mortality and cardiovascular mortality in long-term peritoneal dialysis patients: a prospective study. J Am Soc Nephrol. 2003;14(1):159–168. doi: 10.1097/01.asn.0000038685.95946.83. [DOI] [PubMed] [Google Scholar]
- 13.Wang AY, Lam CW, Chan IH, Wang M, Lui SF, Sanderson JE. Long-term mortality and cardiovascular risk stratification of peritoneal dialysis patients using a combination of inflammation and calcification markers. Nephrol Dial Transplant. 2009;24(12):3826–3833. doi: 10.1093/ndt/gfp325. [DOI] [PubMed] [Google Scholar]
- 14.Moldovan D, Rusu C, Patiu I, Racasan S, Orasan R, Kacso I, et al. Could the serum parathormone be a predictive marker for peripheral vascular calcifications in chronic dialysis patients? Experience of a single center in Transylvania. Acta Endocrinologica (Buc) 2010;6(1):43–55. [Google Scholar]
- 15.Moldovan D, Moldovan I, Rusu C, Racasan S, Patiu IM, Brumboiu A, et al. Vascular calcifications and renal osteodystrophy in chronic hemodialysis patients: what is the relationship between them? Int Urol Nephrol. 2011;43(4):1179–1186. doi: 10.1007/s11255-010-9841-5. [DOI] [PubMed] [Google Scholar]
- 16.Collins AJ, Foley RN, Gilbertson DT, Chen SC. United States Renal Data System public health surveillance of chronic kidney disease and end-stage renal disease. Kidney Intl Suppl (2011) 2015;5(1):2–7. doi: 10.1038/kisup.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlieper G, Schurgers L, Brandenburg V, Reutlingsperger C, Floege J. Vascular calcification in chronic kidney disease: an update. Nephrol Dial Transplant. 2015;31:31–39. doi: 10.1093/ndt/gfv111. [DOI] [PubMed] [Google Scholar]
- 18.Ortiz A, Covic A, Fliser D, Fouque D, Goldsmith D, Kanbay M, et al. Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet. 2014;383(9931):1831–1843. doi: 10.1016/S0140-6736(14)60384-6. [DOI] [PubMed] [Google Scholar]
- 19.Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63(3):793–808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 20.Naves-Diaz M, Passlick-Deetjen J, Guinsburg A, Marelli C, Fernández-Martín JF, Rodríguez-Puyol D, et al. Calcium, phosphorus, PTH and death rates in a large sample of dialysis patients from Latin America. The CORES Study. Nephrol Dial Transplant. 2011;26(6):1938–1947. doi: 10.1093/ndt/gfq304. [DOI] [PubMed] [Google Scholar]
- 21.Floege J, Kim J, Ireland E, Chazot C, Drueke T, de Francisco A, et al. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant. 2011;26(6):1948–1955. doi: 10.1093/ndt/gfq219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foley RN. Phosphate levels and cardiovascular disease in the general population. Clin J Am Soc Nephrol. 2009;4(6):1136–1139. doi: 10.2215/CJN.01660309. [DOI] [PubMed] [Google Scholar]
- 23.Covic A, Kothawala P, Bernal M, Robbins S, Chalian A, Goldsmith D. Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of all-cause mortality, cardiovascular mortality and cardiovascular events in chronic kidney disease. Nephrol Dial Transplant. 2009;24(5):1506–1523. doi: 10.1093/ndt/gfn613. [DOI] [PubMed] [Google Scholar]
- 24.Paloian NJ, Giachelli CM. A current understanding of vascular calcification in CKD. Am J Physiol Renal Physiol. 2014;307(8):F891–F900. doi: 10.1152/ajprenal.00163.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang Y, Ginsberg C, Sugatani T, Monier-Faugere MC, Malluche H, Hruska KA. Early chronic kidney disease–mineral bone disorder stimulates vascular calcification. Kidney Int. 2014;85(1):142–150. doi: 10.1038/ki.2013.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson T, Shields KJ, Barinas-Mitchell E, Newman A, Sutton-Tyrrell K. Calcified carotid artery plaques predict cardiovascular outcomes in the elderly. J Hypertens. 2015;33(4):810–817. doi: 10.1097/HJH.0000000000000488. [DOI] [PubMed] [Google Scholar]
- 27.Lanzer P, Boehm M, Sorribas V, Thiriet M, Janzen J, Zeller T, et al. Medial vascular calcification revisited: review and perspectives. Eur Heart J. 2014;35(23):1515–1525. doi: 10.1093/eurheartj/ehu163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kansu Ö, Özbek M, Avcu N, Gençtoy G, Kansu H, Turgan C. The prevalence of carotid artery calcification on the panoramic radiographs of patients with renal disease. Dentomaxillofac Radiol. 2005;34(1):16–19. doi: 10.1259/dmfr/72474954. [DOI] [PubMed] [Google Scholar]
- 29.Juutilainen A, Lehto S, Suhonen M, Ronnemaa T, Laakso M. Thoracoabdominal calcifications predict cardiovascular disease mortality in type 2 diabetic and nondiabetic subjects: 18-year follow-up study. Diabetes Care. 2010;33(3):583–585. doi: 10.2337/dc09-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jean G, Mayor B, Deleaval P, Lorriaux C, Hurot JM, Bresson E, et al. Vascular Calcification Progression Is an Independent Predictor of Mortality in Patients on Haemodialysis. Nephron. 2015;130(3):169–174. doi: 10.1159/000431288. [DOI] [PubMed] [Google Scholar]
- 31.Scialla JJ, Kao WL, Crainiceanu C, Sozio SM, Oberai PC, Shafi T, et al. Biomarkers of vascular calcification and mortality in patients with ESRD. Clin J Am Soc Nephrol. 2014;9(4):745–755. doi: 10.2215/CJN.05450513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zoccali C, London G. Con: vascular calcification is a surrogate marker, but not the cause of ongoing vascular disease, and it is not a treatment target in chronic kidney disease. Nephrol Dial Transplant. 2015;30(3):352–357. doi: 10.1093/ndt/gfv021. [DOI] [PubMed] [Google Scholar]
- 33.Salam S, Paggiosi M, Eastell R, Khwaja A. Peripheral vascular calcification and bone changes in advanced chronic kidney disease using high resolution peripheral quantitative computed tomography (HR-pQCT) Nephrology Dialysis Transplantation. 2015;30(suppl 3):iii286–iii287. [Google Scholar]
- 34.Kiu Weber CI, Duchateau-Nguyen G, Solier C, Schell-Steven A, Hermosilla R, Nogoceke E, et al. Cardiovascular risk markers associated with arterial calcification in patients with chronic kidney disease Stages 3 and 4. Clin Kidney J. 2014;7(2):167–173. doi: 10.1093/ckj/sfu017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Afsar B, Turkmen K, Covic A, Kanbay M. An update on coronary artery disease and chronic kidney disease. Int J Nephrol. 2014 doi: 10.1155/2014/767424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuo CH, Hsieh TC, Wang CH, Chou CL, Lai YH, Chen YY, et al. Increased risks of mortality and atherosclerotic complications in incident hemodialysis patients subsequently with bone fractures: a nationwide case-matched cohort study. PloS One. 2015;10(4):e0121705. doi: 10.1371/journal.pone.0121705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin JC, Liang WM. Mortality and complications after hip fracture among elderly patients undergoing hemodialysis. BMC Nephrol. 2015;16:100. doi: 10.1186/s12882-015-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernández-Martín JL, Martínez-Camblor P, Dionisi MP, Floege J, Ketteler M, London G, et al. Improvement of mineral and bone metabolism markers is associated with better survival in haemodialysis patients: the COSMOS study. Nephrol Dial Transplant. 2015;30(9):1542–1551. doi: 10.1093/ndt/gfv099. [DOI] [PubMed] [Google Scholar]
- 39.Regidor DL, Kovesdy CP, Mehrotra R, Rambod M, Jing J, McAllister CJ, et al. Serum alkaline phosphatase predicts mortality among maintenance hemodialysis patients. J Am Soc Nephrol. 2008;19:2193–2203. doi: 10.1681/ASN.2008010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rao R, Ansell D, Gilg JA, Davies SJ, Lamb EJ, Tomson CR. Effect of change in renal replacement therapy modality on laboratory variables: a cohort study from the UK Renal Registry. Nephrol Dial Transplant. 2009;24(9):2877–2882. doi: 10.1093/ndt/gfp163. [DOI] [PubMed] [Google Scholar]
- 41.Moon SJ, Kim DK, Chang JH, Kim CH, Kim HW, Park SY, et al. The impact of dialysis modality on skin hyperpigmentation in haemodialysis patients. Nephrol Dial Transplant. 2009;24(9):2803–2809. doi: 10.1093/ndt/gfp143. [DOI] [PubMed] [Google Scholar]
- 42.Johnson DW, Dent H, Hawley CM, McDonald SP, Rosman JB, Brown FG, et al. Association of dialysis modality and cardiovascular mortality in incident dialysis patients. Clin J Am Soc Nephrol. 2009;4:1620–1628. doi: 10.2215/CJN.01750309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weinhandl ED, Foley RN, Gilbertson DT, Arneson TJ, Snyder JJ, Collins AJ. Propensity-matched mortality comparison of incident hemodialysis and peritoneal dialysis patients. J Am Soc Nephrol. 2010;21:499–506. doi: 10.1681/ASN.2009060635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Termorshuizen F, Korevaar JC, Dekker FW, Van Manen JG, Boeschoten EW, Krediet RT, et al. Hemodialysis and peritoneal dialysis: comparison of adjusted mortality rates according to the duration of dialysis: analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis 2. J Am Soc Nephrol. 2003;14(11):2851–2860. doi: 10.1097/01.asn.0000091585.45723.9e. [DOI] [PubMed] [Google Scholar]
- 45.Lee CC, Sun CY, Wu MS. Long-term modality-related mortality analysis in incident dialysis patients. Perit Dial Int. 2009;29(2):182–190. [PubMed] [Google Scholar]