Abstract
Based on animal and ex-vivo experiments, Growth/Differentiation Factor-15 (GDF15, also called Macrophage Inhibitory Cytokine-1, MIC1), a member of the transforming growth factor-beta family, and Matrix Metalloproteinase-9 (MMP9), a member of the matrix metalloprotease family may be potential markers for Lewy body disorders, i.e. Parkinson’s disease with (PDD) and without dementia (PDND) and Lewy body dementia (DLB). GDF15 has a prominent role in development, cell proliferation, differentiation, and repair, whereas MMP9 degrades, as a proteolytic enzyme, components of the extracellular matrix. In this study, cerebrospinal fluid GDF15 and MMP9 levels of 59 PDND, 17 PDD and 23 DLB patients, as well as of 95 controls were determined, and associated with demographic, clinical and biochemical parameters. Our analysis confirmed the already described association of GDF15 levels with age and gender. Corrected GDF15 levels were significantly higher in PDD than in PDND patients, and intermediate in DLB patients. Within Lewy body disorders, GDF15 levels correlated positively with age at onset of Parkinsonism and dementia, Hoehn & Yahr stage and cerebrospinal fluid t-Tau and p-Tau levels, and negatively with the Mini Mental State Examination. Remarkably, it does not relevantly correlate with disease duration. MMP9 was not relevantly associated with any of these parameters. Cerebrospinal GDF15, but not MMP9, may be a potential marker of and in Lewy body disorders.
Introduction
At present, there is no biomarker in blood or cerebrospinal fluid (CSF) available that can differentiate between Lewy body disorders and controls with sufficient accuracy, not to mention the capability of such markers to predict the disease, or correlate with disease progression [1]. Growth/Differentiation Factor-15 (GDF15, identical to Macrophage Inhibitory Cytokine-1, MIC1) [2] and Matrix Metalloproteinase-9 (MMP9) belong to the Transforming growth factor-beta (TGF-beta) superfamily. Members of this family have prominent roles in development, cell proliferation, differentiation, and repair [3]. GDF15 is widely expressed in the central nervous system (CNS) and peripheral nervous system, most prominent in the choroid plexus, and secreted into the cerebrospinal fluid (CSF) [3]. The protein is up-regulated as part of the anti-inflammatory cytokine network within the CNS in response to injury and lesions [4,5], supplementing the roles of other members of the TGF-beta superfamily [6]. Moreover, GDF15 serves as an autocrine regulatory molecule in monocytoid cells, where expression of the protein may be required to limit or inhibit macrophage activation at a later phase [7]. Interestingly, GDF15 has been shown to act as a potent trophic and protective factor for dopaminergic neurons: According to recent results [3], GDF15 seems to be at least as potent as glial derived neurotrophic factor in promoting the survival of dopaminergic neurons, and normalizes motor behaviour. It also protects dopaminergic neurons against iron intoxication [8], induces neuronal survival by activating protein kinase B and glycogen synthase kinase via phosphatidylinositol 3 kinase, and attenuates formation of reactive oxidative species [9]. GDF15 probably acts directly on neurons [3]. Remarkably, GDF15 levels have been associated with cognitive performance and decline. Higher GDF15 levels were shown to be significantly associated with lower global cognition in a cohort of more than 1000 non-demented adults aged 70–90 years [10].
MMP9 is a stress response cytokine [11]. It is capable of degrading stable compounds of the extracellular matrix, as well as growth factors, cytokines, chemokines, cell surface receptors, and serine proteases inhibitors [12–15]. MMP9 is released from astrocytes, neurons and microglia as well as leukocytes and macrophages as an inactive proprotein, which is then activated by extracellular proteinases. MMP9 has been shown to be a potential biomarker for outcome prediction after neuron damage [14], and is particularly induced in neuronal lesions and cerebral ischemia [16]. Moreover, MMP9 might be important in remyelination processes as it facilitates oligodendrocyte regrowth [17]. MMP9 has been found to co-localize with neurofibrillary tangles, senile plaques and the vascular wall in post-mortem Alzheimer brains [18,19]. MMP9 synthesis is e.g. induced by Abeta1-42 peptide [20,21], and vice versa, MMP9 is capable of degrading Abeta1-42 [22].
Based on this knowledge about GDF15 and MMP9 in acute and chronic brain conditions with and without dopaminergic loss and neurodegeneration, we set out to measure CSF levels of these two proteins in a large cohort of Lewy body disorders, i.e. Parkinson’s disease without (PD no dementia, PDND), PD with dementia (PDD) and dementia with Lewy bodies (DLB), to determine their associations with demographical, clinical and routine CSF parameters.
Methods
Ethics Statement
The study was approved by the ethics committee of the Medical Faculty, University of Tuebingen (Germany), and was performed in accordance with the Declaration of Helsinki. All participants, or their next of kin in the case of cognitive impairment (MMSE score of 18 or below), provided their informed written consent.
Study Participants
The study cohort consisted of 200 participants, i.e. 100 control persons and 100 patients with a Lewy body disorder. All samples were collected from donors who were either patients or study participants at the ward and outpatient clinic of the Neurodegenerative Department of the University of Tuebingen, Germany. From all included donors, information about gender, age, age at onset of Parkinsonism, age at onset of dementia, disease duration, Hoehn and Yahr scale (H&Y), Mini Mental State Examination (MMSE) scores and information about medication were collected. Diagnoses of PDND, PDD and DLB as well as of controls (exclusion of a neurological disease according to medical history and clinical examination) were made by movement disorders specialists according to established criteria [23–25]. One PDND patient and five controls were excluded from the final analysis due to missing data (neurodegenerative markers or MMP9 values not available), resulting in data available for final analysis of 99 patients with a Lewy body disorder (59 PDND, 17 PDD, and 23 DLB patients) and 95 controls. Excluded individuals did not relevantly differ from those included with regard to demographic and clinical data. Details are provided in Table 1.
Table 1. Demographical, clinical and biochemical data of the included cohorts.
| PDND | PDD | DLB | Controls | p-value | ||
|---|---|---|---|---|---|---|
| Individuals (f/m) [N] | 59 (30/29) | 17 (8/9) | 23 (12/11) | 95 (55/40) | 0.76 | |
| Age [y] | 67 (44–79) | 75 (61–84)§ | 69 (50–82) | 61 (38–79)§#$ | <0.0001 | |
| Aao Parkinsonism [y] | 62 (30–76) | 62 (51–74) | 67 (49–78)§ | - | 0.07 | |
| Duration of Parkinsonism [y] | 4.0 (0.5–21.0) | 10.0 (3.0–21.0)§ | 2 (0.5–13.0)§# | - | <0.0001 | |
| H&Y stage (1–5) | 2 (1–4) | 3 (2–4)§ | 2 (1–4)# | - | 0.004 | |
| H&Y stage (1/2/3/4) [N] | 9/40/7/3 | 0/7/6/4 | 5/8/8/2 | - | 0.0054 | |
| Aao dementia [y] | - | 72 (58–79) | 68 (49–79)# | - | 0.04 | |
| Duration of dementia [y] | - | 3.0 (0.2–5.0) | 2 (0.5–12.0) | - | 0.67 | |
| MMSE (0–30) | 29 (23–30) | 23 (14–26)§ | 20 (10–30)§ | 30 (25–30)§#$ | <0.0001 | |
| LEDD [mg L-Dopa/day] | 225 (0–1333) | 600 (0–1016)§ | 150 (0–700)# | - | <0.0001 | |
| CSF Albumin [mg/L] | 221 (127–391) | 252 (109–440) | 209 (96–427) | 232 (83–575) | 0.98 | |
| CSF Abeta1-42 [pg/mL] | 678 (270–1458) | 478 (213–771)§ | 530 (252–1241)§ | 825 (318–1446)§#$ | 0.00091 | |
| CSF t-Tau [pg/mL] | 176 (25–710) | 225 (68–660) | 279 (77–596)§ | 165 (32–481)$ | 0.151 | |
| CSF p-Tau [pg/mL] | 36 (14–111) | 54 (12–109)§ | 50 (26–86)§ | 36 (13–76)#$ | 0.0461 | |
| CSF GDF15 [pg/mL] | All | 200 (67–467) | 292 (139–571)§ | 235 (101–453)# | 184 (39–461)# | 0.051 |
| f | 198 (67–467) | 285 (139–473) | 209 (101–356)# | 169 (39–371) | 0.131 | |
| m | 202 (113–429) | 296 (204–571)§ | 246 (185–453) | 215 (78–461) | 0.071 | |
| CSF MMP9 [pg/mL] | 50 (15–283) | 47 (23–176) | 42 (9–133) | 46 (11–439) | 0.401 | |
Aao, Age at onset; Abeta1-42, amyloid-beta1-42; CSF, cerebrospinal fluid; DLB, dementia with Lewy bodies; GDF15, growth/differentiation factor 15; f, female; H&Y, Hoehn & Yahr stage; LEDD, Levodopa equivalent daily dose; m, male; MMP9, matrixmetalloproteinase 9; MMSE, Mini Mental State Examination; PDD, Parkinson`s disease demented; PDND, Parkinson`s disease non-demented; p-Tau, phospho-Tau; t-Tau, total Tau.
1 age-corrected p-values
§ versus PDND
# versus PDD
$ versus DLB.
Cerebrospinal Fluid and Serum Collection
CSF and serum collection was performed according to standardized protocols (for details see [26]). In brief, serum and CSF were centrifuged (blood: 2000 g, 4°C, 10 min; CSF: 4000 g, 4°C, 10 min) and stored at -80°C within 60 minutes after collection until analysis. Only samples with normal routine CSF diagnostics were included (leukocytes < 4x106; IgG index CSF/serum < 0.6, CSF albumin levels < 450 mg/l). Erythrocytes counts in CSF were performed using a semi-quantitative approach (level 1, 0–5 erythrocytes/μL; level 2, >5–1000 erythrocytes/μL; level 3, >1000–5000 erythrocytes/μL). As this is a hypothesis-generating study, GDF15 and MMP9 levels were only analysed in CSF. Serum was used for determination of routine laboratory measures, such as the IgG index.
Preparation of GDF15 and MMP9 Antibody-Coupled Microspheres
106 microspheres (Luminex cat L100-C104-16 for GDF15 and cat L100-C156-16 for MMP9) were activated for 20 min in 80 μL 1.38% NaH2PO4*H2O, pH 6.0 supplemented with 10 μL sulfo-NHS (Thermo Fisher, Darmstadt, Germany). Activated microspheres were washed with coupling buffer (1% MES, pH 5.0) by briefly vortexing and centrifugation. 200 μL of monoclonal antibody MAB957 (R&D Systems, Minneapolis, USA; 250 μg IgG/mL in 1% MES, pH 5.0) or 200 μL of monoclonal antibody MAB13458 (Millipore, Darmstadt, Germany; 250 μg IgG/mL in 1% MES, pH 5.0) were added to the microspheres and left to react overnight at room temperature in the dark. The coupled microspheres were centrifuged and washed two times with PBS, 0.05% Tween-20. Microspheres were then resuspended in 500 μL PBS, 0.1% BSA, 0.05% proclin300 and stored at 4°C in the dark until use.
GDF15 Assay
3000 GDF15 antibody coupled beads (diluted into PBS, 0.1% BSA, 0.05% proclin300) were added per well of a prewetted MultiScreen™ HTS filter plate (Millipore). Wells were then drained using a vacuum manifold holder. 25 μL assay buffer (PBS + 0.1% casein + 0.08% CHAPS + 0.25% octylglucopyranoside [OGP]) and either 75 μL of GDF15 standard in assay buffer (recombinant human GDF15; R&D Systems, cat# 957-GD) or CSF diluted in assay buffer were added and incubated overnight at room temperature on a mini orbital shaker. Wells were drained and washed 3 times with 225 μL wash buffer (PBS, 0.05% Tween-20). A mix of 75 μL assay buffer and 25 μL biotinylated goat anti-GDF15 antibody (R&D Systems, cat# BAF940; 5 ng/well, diluted in assay buffer) was then added and incubated for three h at room temperature on a mini orbital shaker. The filter plate was drained and washed three times with 225 μL wash buffer. 100 μL streptavidin R-Phycoerythrin conjugate (SA-PE)(Thermo Fisher, cat# SA1004-1, lot# 495462A; diluted 1/300 in assay buffer) were added and incubated for one h at room temperature on a mini orbital shaker. Filter plates were then drained and washed three times with 225 μL wash buffer. Finally 100 μL PBS was added per well and incubated for 10 min at room temperature on a mini orbital shaker. The median fluorescent intensity (mfi) in region #104 was then determined for each well within one h using the Luminex 100TM IS System (Luminex Corp, Austin, Texas, USA). Each filter plate contained (i) A duplicate GDF15 standard calibrator curve (10000; 3333.33; 1111.11; 370.37; 123.46; 41.15; 13.72; 4.57; 1.52; 0.51 and 0.17 pg/mL) in assay buffer. A stock of frozen aliquots of the different standard dilutions was used for all measurements; (ii) Two serial dilutions (1/15 and 1/60) of each CSF sample that were prepared in assay buffer prior to analysis. Each dilution was measured in duplicate. Results obtained for the 1/15 and 1/60 dilutions showed very high concordance, so only results of the 1/60 dilution experiments are presented. Results of the 1/15 dilution experiments are provided upon request; and (iii) Blank (assay buffer) in duplicate (for further information see S1 File).
MMP9 Assay
3000 MMP9 antibody coupled beads (diluted in PBS, 0.1% casein) were added per well of a prewetted MultiScreen™ HTS filter plate (Millipore). Wells were then drained using a vacuum manifold holder. 25 μL assay buffer (PBS + 0.1% casein + 0.08% CHAPS + 3% Pluronic F-127) and 75 μL of MMP9 standard in assay buffer (recombinant human MMP9; R&D Systems, cat# 911-MP) or CSF diluted in assay buffer were added and incubated overnight at room temperature on a mini orbital shaker. Wells were drained and washed 3 times with 225 μL wash buffer. A mix of 75 μL assay buffer and 25 μL biotinylated goat anti-MMP9 antibody (R&D Systems, cat# BAF911; 50 ng/well, diluted in assay buffer) was then added and incubated for three h at room temperature on a mini orbital shaker. The filter plate was drained and washed three times with 225 μL wash buffer (PBS, 0.05% Tween-20). 100 μL SA-PE conjugate (Thermo Fisher, cat# SA1004-1, lot# 495462A; diluted 1/300 in assay buffer) were added and incubated for one h at room temperature on a mini orbital shaker. Filter plates were then drained and washed three times with 225 μL wash buffer. Finally 100 μL PBS was added per well and incubated for 10 min at room temperature on a mini orbital shaker. The mfi in region #156 was then determined for each well within one h using the Luminex 100TM IS System. Each plate contained (i) A duplicate MMP9 standard calibrator curve (30,000; 7,500; 1,875; 468.75; 117.19; 29.3; 7.32; 1.83; 0.45; 0.11 and 0.029 pg/mL) in assay buffer. A stock of frozen aliquots of the different standard dilutions was used for all measurements; (ii) Each CSF sample diluted 1/24 in assay buffer and measured in quadruplate; and (iii) Blank (assay buffer) in duplicate (for further information see S1 File).
Data Processing
Concentrations of the CSF samples were calculated using the StatLIA® v3.2 statistical package (Brendan Technologies Inc., Carlsbad, California, USA) using a 4 (GDF15) or 5 (MMP9) parameter logistic curve with POM (power of mean) weighting.
Neurodegenerative Marker Analysis
Neurodegenerative markers (CSF Abeta1-42, total Tau and phospho-Tau) were determined using Innotest® ELISA kits #80324, #80323, or #80317, respectively (Fujirebio Germany GmbH, Hannover, Germany).
Data Analysis
Data were analysed with JMP software (Version 11.2.0; SAS Institute Inc., Cary, North Carolina, USA). Demographic and clinical data are presented with median and range / confidence interval (CI). Comparison of GDF15 or MMP9 levels with clinical and routine biochemical parameters were performed using Pearson correlation. Based on results of previous studies (e.g. [27–29]) and on correlations performed in this study indicating at least a weak association of age with GDF15, MMP9, CSF Abeta1-42, t-Tau and p-Tau, all calculations including one of these parameters were corrected for age with a logistic regression model. Median GDF15 levels in controls were 16.0% lower for females as compared to males (p = 0.03), therefore GDF15 was calculated separately for females and males. We also checked for potentially relevant differences of CSF albumin levels and erythrocytes/μL CSF between controls and patients with a Lewy body disorder, which was not the case (p = 0.87 and p = 0.70, respectively). As these variables did not show relevant differences between cohorts, we did not include them as covariables in our analyses. Due to the exploratory nature of the study, differences were considered significant at uncorrected p < 0.05.
Results
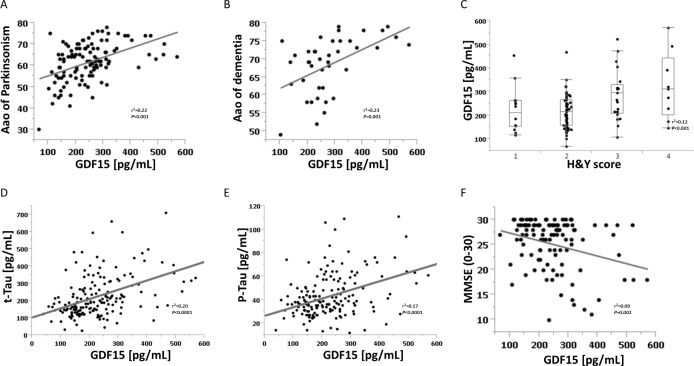
Patients with a Lewy body disorder had higher CSF GDF15 levels, compared to controls (235 versus 184 pg/mL, p < 0.0001). Among patients, age- and gender-corrected median CSF GDF15 levels in the PDD subgroup were 18% higher than in the DLB subgroup (95% CI 6–24%), 16% higher than in the PDND subgroup (6–27%), and 17% higher than the control group (7–28%). In the overall Lewy body disorder cohort, GDF15 was positively correlated with age at onset of Parkinsonism, age at onset of dementia, H&Y stage, and with the neurodegenerative markers t-Tau and p-Tau. CSF GDF15 was negatively associated with the MMSE score (Fig 1). It was not significantly associated with any other parameter comprised in the analysis, including disease duration of Parkinsonism (r2 = 0.03, p = 0.11) and levodopa equivalent daily dose (LEDD, r2 = 0.02, p = 0.19; Tables 1 and 2).
Fig 1. Significant correlations of cerebrospinal fluid GDF15 levels with clinical and routine neurodegenerative markers in Lewy body disorders.
(A) GDF15 and age of onset of Parkinsonism. (B) GDF15 and age of onset of dementia. (C) GDF15 and Hoehn and Yahr score. (D) GDF15 and t-Tau. (E) GDF15 and p-Tau. (F) GDF15 and Mini Mental State Examination. Aao, age at onset; CSF, cerebrospinal fluid; GDF15, growth differentiation factor 15; H&Y, Hoehn and Yahr score; MMSE, Mini Mental State Examination; p-Tau, phospho-Tau; r2, correlation coefficient; t-Tau, total-Tau.
Table 2. Correlation coefficients (r2) of CSF GDF15 and MMP9 and demographic, clinical and neurodegenerative parameters.
| PDND | PDD | DLB | Controls | |||
|---|---|---|---|---|---|---|
| GDF15 | All | Gender | 0.00 | 0.07 | 0.29** | 0.08** |
| Age | 0.36*** | 0.38** | 0.15 | 0.45*** | ||
| Aao Parkinsonism | 0.29*** | 0.19 | 0.16 | - | ||
| Duration of Parkinsonism | 0.00 | 0.06 | 0.00 | - | ||
| H&Y stage | 0.06 | 0.38** | 0.00 | - | ||
| Aao dementia | - | 0.30* | 0.13 | - | ||
| Duration of dementia | - | 0.21 | 0.00 | - | ||
| MMSE | 0.01 | 0.26* | 0.00 | 0.01 | ||
| LEDD | 0.07* | 0.18 | 0.03 | - | ||
| CSF Abeta1-42 | 0.041 | 0.011 | 0.291* | 0.081 | ||
| CSF t-Tau | 0.491*** | 0.121 | 0.301 | 0.041 | ||
| CSF p-Tau | 0.431*** | 0.081 | 0.191 | 0.011 | ||
| f | Age | 0.39*** | 0.61* | 0.34* | 0.49*** | |
| Aao Parkinsonism | 0.40*** | 0.47 | 0.30 | - | ||
| Duration of Parkinsonism | 0.09 | 0.04 | 0.01 | - | ||
| H&Y stage total | 0.03 | 0.23 | 0.04 | - | ||
| Aao dementia | - | 0.51* | 0.37* | - | ||
| Duration of dementia | - | 0.75** | 0.02 | - | ||
| MMSE | 0.01 | 0.26 | 0.23 | 0.00 | ||
| LEDD | 0.11 | 0.34 | 0.07 | - | ||
| CSF Abeta1-42 | 0.171 | 0.921** | 0.101 | 0.171 | ||
| CSF t-Tau | 0.681*** | 0.051 | 0.401 | 0.091 | ||
| CSF p-Tau | 0.741*** | 0.011 | 0.191 | 0.101 | ||
| m | Age | 0.33** | 0.32 | 0.23 | 0.45*** | |
| Aao Parkinsonism | 0.19* | 0.12 | 0.20 | - | ||
| Duration of Parkinsonism | 0.12 | 0.08 | 0.02 | - | ||
| H&Y stage total | 0.13 | 0.44 | 0.01 | - | ||
| Aao dementia | - | 0.24 | 0.16 | - | ||
| Duration of dementia | - | 0.06 | 0.00 | - | ||
| MMSE | 0.05 | 0.20 | 0.13 | 0.06 | ||
| LEDD | 0.04 | 0.09 | 0.00 | - | ||
| CSF Abeta1-42 | 0.011 | 0.021 | 0.481 | 0.031 | ||
| CSF t-Tau | 0.351* | 0.621 | 0.411 | 0.021 | ||
| CSF p-Tau | 0.201 | 0.451 | 0.461 | 0.001 | ||
| MMP9 | Gender | 0.00 | 0.05 | 0.08 | 0.01 | |
| Age | 0.02 | 0.01 | 0.07 | 0.01 | ||
| Aao Parkinsonism | 0.00 | 0.05 | 0.05 | - | ||
| Duration of Parkinsonis | 0.01 | 0.04 | 0.01 | - | ||
| H&Y stage total | 0.02 | 0.00 | 0.07 | - | ||
| Aao dementia | - | 0.01 | 0.05 | - | ||
| Duration of dementia | - | 0.04 | 0.02 | - | ||
| MMSE | 0.00 | 0.00 | 0.06 | 0.00 | ||
| LEDD | 0.02 | 0.11 | 0.00 | - | ||
| CSF Abeta1-42 | 0.001 | 0.001 | 0.011 | 0.091 | ||
| CSF t-Tau | 0.101 | 0.161 | 0.211 | 0.021 | ||
| CSF p-Tau | 0.131 | 0.141 | 0.151 | 0.011 | ||
Pearson correlation and a logistic regression model including age as a covariate 1 were used to calculate the extent of correlation between both, growth differentiation factor 15 (GDF15) and matrix metalloproteinase 9 (MMP9), with demographic and clinical parameters of Lewy body disorder subcohorts. The correlation coefficient (r2) is presented. Aao, Age at onset; Abeta1-42, Amyloid-beta1-42; CSF, cerebrospinal fluid; DLB, dementia with Lewy bodies; f, female; H&Y, Hoehn & Yahr stage; LEDD, Levodopa equivalent daily dose; m, male; MMSE, Mini Mental State Examination; PDD, Parkinson`s disease demented; PDND, Parkinson`s disease non-demented; p-Tau, phospho-Tau; t-Tau, total Tau.
* p < 0.05
** p < 0.01
*** p < 0.001.
CSF MMP9 was not significantly associated with any of the included demographic, clinical and routine neurochemical parameters. CSF GDF15 and MMP9 levels did not relevantly correlate. Detailed results structured by included subcohorts are shown in Table 2.
Discussion
The results of this study indicate that CSF GDF15 but not MMP9 has potential to (i) differentiate Lewy body disorder patients from controls, (ii) show severity of the disease independent of disease duration, and (iii) even detect prodromal phases of the disorder. Specifically the relatively close associations of GDF15 with t-Tau and p-Tau levels are remarkable. T-Tau is associated with neuronal damage and death [30], and p-Tau with axonal pathology [31]. Both processes [32] as well as alpha-synuclein pathology in general [33,34] have been repeatedly associated with inflammation and remodelling, which makes it suggestive that GDF15 is indeed involved in dopaminergic cell death and Lewy body pathology, not only in animal models (e.g. [3,35–38]) but also in humans.
CSF GDF15 levels were comparably low in controls and PDND patients (who had a relatively short disease duration of 4.5 years, mild motor signs and good cognitive function), intermediate in DLB patients (who had relatively good motor, but poor cognitive functioning), and highest in PDD patients (who had most regularly poor motor and poor cognitive functions). Consistent with this observation, disease severity parameters (H&Y and MMSE) were accordingly correlated with GDF15 levels. Hence, comparable to Tau levels, GDF15 levels seem to be directly associated with severity and co-occurrence of symptoms in Lewy body disorders, rather than with specific subtypes and with “simple” disease duration. This observation is, indirectly and outside the Lewy body field, confirmed by studies with initially healthy older adults and patients with brain tumours, which found higher levels of GDF15 associated with increased decline in global cognition, executive function, memory, and processing speed, and increased mortality [10,39].
Although this is a cross-sectional analysis, our results suggest that CSF GDF15 levels have even a potential to detect prodromal stages of Lewy body disorders. In our study, GDF15 levels were higher in control males than in females. If we assume that higher levels indicate more disease “activity”, GDF15 may serve as a surrogate marker for Lewy body disorder even in prodromal disease stages, as risk for Lewy body disorders is higher in males than in females [40]. Interestingly, a recent study [41] observed higher baseline levels of biomarkers indicative of inflammation and remodelling in males compared to females, suggesting higher biological activity in these pathophysiological pathways in males compared to females. Moreover, GDF15 levels were positively and closely correlated with age at onset of Parkinsonism and dementia. In other words, the higher the CSF GDF15 levels, the later occurred the disease itself, and cognitive deterioration within the disease which is closely associated with the disorder.
Taken together, CSF GDF15 levels show surprisingly high association values with relevant clinical, demographic and biochemical parameters of Lewy body disorder, with negative associations with severity of symptoms. We therefore hypothesize that GDF15 as a central member of neuroprotective mechanisms is induced by brain injury [42], such as neurodegeneration. These mechanisms obviously include further anti-inflammatory species and neurotrophic factors. In a recent study using multiplex panels, we observed increased levels of such species and factors (note that we could not determine GDF15 levels due to technical issues) in PD patients compared to controls [43]. Moreover, it may well be that a subtype of PD / DLB exists that is particularly influenced by these anti-inflammatory species and neurotrophic factors: We found that PD patients with a LRRK2 mutation who displayed a diffuse-malignant phenotype [44], showed higher levels compared to PD LRRK2 patients with the much more benign pure motor phenotype [45].
Use of MMP inhibitors in Parkinson’s disease might show great promise as the death of dopaminergic neurons seems to be associated with release of MMPs by the activated cells around them [46], making the definition of useful outcome parameters inevitable. However, CSF MMP9 levels between patients with Lewy body disorder and controls did not differ significantly in our analyses, indicating that this parameter does not have a high potential as trait marker for the disease. Moreover, none of the demographic, clinical and neurochemical markers showed significant correlations with CSF MMP9 levels, arguing against the potential of CSF MMP9 as state and predictive marker of the disease. Therefore, even if MMP inhibitors will be tested in Lewy body disorders, the usefulness of at least CSF MMP9 as an outcome parameter remains unclear at present.
The study has limitations. First, we measured a cross-sectional sample, and, particularly as the results indicate that CSF GDF15 is associated with severity of disease, longitudinal information will be necessary to clarify the specific role of the protein with respect to prediction and evaluation of disease severity in Lewy body disorders. Second, due to the complex collection process, cohort sizes are relatively small, and a second study using independent samples is certainly necessary to validate our results. The strength of the study is the examination of biofluid samples from one biobank with highly standardized and fast sample processing and storage procedures, including only material from patients and controls evaluated by movement disorder specialists.
In conclusion, CSF levels of GDF15, but not MMP9, are associated with occurrence and severity in Lewy body disorders. CSF GDF15 may therefore serve as an interesting parameter for, e.g. a panel that aims at differentiating Lewy body disorders from controls, and for progression studies in this arena. Moreover, the direct correlation of CSF GDF15 with age at onset of Parkinsonism and dementia as well as the gender difference of the parameter suggests that GDF15 in CSF has some potential to detect prodromal phases of the disease.
Supporting Information
(DOCX)
Acknowledgments
We thank Evelyn Dubois, Elke Stransky and Siegfried Ebner (Clinical-chemical Laboratory of the Department of Neurology, University Hospital Tuebingen, Tuebingen, Germany) for technical assistance in CSF analysis. We thank all participants in this study. Samples were obtained from the Neuro-Biobank of the University of Tuebingen, Tuebingen, Germany (http://www.hih-tuebingen.de/nd/biobank/for-researchers/). This biobank is supported by the Hertie Institute and the DZNE. We acknowledge support by Deutsche Forschungsgemeinschaft and Open Access Publishing Fund of Tuebingen University, Tuebingen, Germany.
Data Availability
Due to ethical restrictions imposed by the Ethics Committee of the Medical Faculty of the University of Tuebingen related to approved patient consent procedure and protecting patient privacy, all relevant data are available upon request (walter.maetzler@uni-tuebingen.de).
Funding Statement
This work was supported by Deutsche Forschungsgemeinschaft and Open Access Publishing Fund of Tuebingen University, Tuebingen, Germany. Fujirebio Europe NV provided support in the form of salaries for two authors [WD, VH]. The specific roles of these authors are articulated in the ‘author contributions’ section. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mielke MM, Maetzler W. A “bird”s eye’ view on the current status and potential benefits of blood biomarkers for Parkinson's disease. Biomarkers in medicine. 2014;8: 225–7. 10.2217/bmm.13.139 [DOI] [PubMed] [Google Scholar]
- 2.Böttner M, Laaff M, Schechinger B, Rappold G, Unsicker K, Suter-Crazzolara C. Characterization of the rat, mouse, and human genes of growth/differentiation factor-15/macrophage inhibiting cytokine-1 (GDF-15/MIC-1). Gene. 1999;237: 105–111. [DOI] [PubMed] [Google Scholar]
- 3.Strelau J, Sullivan A, Böttner M, Lingor P, Falkenstein E, Suter-Crazzolara C, et al. Growth/differentiation factor-15/macrophage inhibitory cytokine-1 is a novel trophic factor for midbrain dopaminergic neurons in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20: 8597–8603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breit SN, Johnen H, Cook AD, Tsai VWW, Mohammad MG, Kuffner T, et al. The TGF-β superfamily cytokine, MIC-1/GDF15: a pleotrophic cytokine with roles in inflammation, cancer and metabolism. Growth factors (Chur, Switzerland). 2011;29: 187–195. [DOI] [PubMed] [Google Scholar]
- 5.Johnen H, Kuffner T, Brown DA, Wu BJ, Stocker R, Breit SN. Increased expression of the TGF-b superfamily cytokine MIC-1/GDF15 protects ApoE(-/-) mice from the development of atherosclerosis. Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology. 2012;21: 499–505. [DOI] [PubMed] [Google Scholar]
- 6.Schober A, Böttner M, Strelau J, Kinscherf R, Bonaterra GA, Barth M, et al. Expression of growth differentiation factor-15/ macrophage inhibitory cytokine-1 (GDF-15/MIC-1) in the perinatal, adult, and injured rat brain. The Journal of comparative neurology. 2001;439: 32–45. [DOI] [PubMed] [Google Scholar]
- 7.Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proceedings of the National Academy of Sciences of the United States of America. 1997;94: 11514–11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lingor P, Unsicker K, Krieglstein K. Midbrain dopaminergic neurons are protected from radical induced damage by GDF-5 application. Short communication. Journal of neural transmission (Vienna, Austria : 1996). 1999;106: 139–144. [DOI] [PubMed] [Google Scholar]
- 9.Subramaniam S, Strelau J, Unsicker K. Growth differentiation factor-15 prevents low potassium-induced cell death of cerebellar granule neurons by differential regulation of Akt and ERK pathways. The Journal of biological chemistry. 2003;278: 8904–8912. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs T, Trollor JN, Crawford J, Brown DA, Baune BT, Samaras K, et al. Macrophage inhibitory cytokine-1 is associated with cognitive impairment and predicts cognitive decline—the Sydney Memory and Aging Study. Aging cell. 2013;12: 882–889. 10.1111/acel.12116 [DOI] [PubMed] [Google Scholar]
- 11.Wiklund FE, Bennet AM, Magnusson PKE, Eriksson UK, Lindmark F, Wu L, et al. Macrophage inhibitory cytokine-1 (MIC-1/GDF15): a new marker of all-cause mortality. Aging cell. 2010;9: 1057–1064. 10.1111/j.1474-9726.2010.00629.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorenzl S, Buerger K, Hampel H, Beal MF. Profiles of matrix metalloproteinases and their inhibitors in plasma of patients with dementia. International psychogeriatrics / IPA. 2008;20: 67–76. [DOI] [PubMed] [Google Scholar]
- 13.Kaczmarek L. Mmp-9 inhibitors in the brain: can old bullets shoot new targets? Current pharmaceutical design. 2013;19: 1085–1089. [DOI] [PubMed] [Google Scholar]
- 14.Liu C-L, Chen C-C, Lee H-C, Cho D-Y. Matrix metalloproteinase-9 in the ventricular cerebrospinal fluid correlated with the prognosis of traumatic brain injury. Turkish neurosurgery. 2014;24: 363–368. 10.5137/1019-5149.JTN.8551-13.0 [DOI] [PubMed] [Google Scholar]
- 15.Van den Steen PE, Dubois B, Nelissen I, Rudd PM, Dwek RA, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9). Critical reviews in biochemistry and molecular biology. 2002;37: 375–536. [DOI] [PubMed] [Google Scholar]
- 16.Schindowski K, von Bohlen und Halbach O, Strelau J, Ridder DA, Herrmann O, Schober A, et al. Regulation of GDF-15, a distant TGF-β superfamily member, in a mouse model of cerebral ischemia. Cell and tissue research. 2011;343: 399–409. 10.1007/s00441-010-1090-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsen PH, Wells JE, Stallcup WB, Opdenakker G, Yong VW. Matrix metalloproteinase-9 facilitates remyelination in part by processing the inhibitory NG2 proteoglycan. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23: 11127–11135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asahina M, Yoshiyama Y, Hattori T. Expression of matrix metalloproteinase-9 and urinary-type plasminogen activator in Alzheimer’s disease brain. Clinical neuropathology. 2001;20: 60–63. [PubMed] [Google Scholar]
- 19.Backstrom JR, Lim GP, Cullen MJ, Tökés ZA. Matrix metalloproteinase-9 (MMP-9) is synthesized in neurons of the human hippocampus and is capable of degrading the amyloid-beta peptide (1–40). The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16: 7910–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deb S, Wenjun Zhang J, Gottschall PE. Beta-amyloid induces the production of active, matrix-degrading proteases in cultured rat astrocytes. Brain research. 2003;970: 205–13. [DOI] [PubMed] [Google Scholar]
- 21.Nagele RG, Wegiel J, Venkataraman V, Imaki H, Wang K- C, Wegiel J. Contribution of glial cells to the development of amyloid plaques in Alzheimer’s disease. Neurobiology of aging. 2004;25: 663–74. [DOI] [PubMed] [Google Scholar]
- 22.Yan P, Hu X, Song H, Yin K, Bateman RJ, Cirrito JR, et al. Matrix metalloproteinase-9 degrades amyloid-beta fibrils in vitro and compact plaques in situ. The Journal of biological chemistry. 2006;281: 24566–24574. [DOI] [PubMed] [Google Scholar]
- 23.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. Journal of neurology, neurosurgery, and psychiatry. 1992;55: 181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2007;22: 1689–707; quiz 1837. [DOI] [PubMed] [Google Scholar]
- 25.McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65: 1863–1872. [DOI] [PubMed] [Google Scholar]
- 26.Maetzler W, Schmid SP, Wurster I, Liepelt I, Gaenslen A, Gasser T, et al. Reduced but not oxidized cerebrospinal fluid glutathione levels are lowered in Lewy body diseases. Movement disorders : official journal of the Movement Disorder Society. 2011;26: 176–81. [DOI] [PubMed] [Google Scholar]
- 27.Sapojnikova N, Kartvelishvili T, Asatiani N, Zinkevich V, Kalandadze I, Gugutsidze D, et al. Correlation between MMP-9 and extracellular cytokine HMGB1 in prediction of human ischemic stroke outcome. Biochimica et biophysica acta. 2014;1842: 1379–84. 10.1016/j.bbadis.2014.04.031 [DOI] [PubMed] [Google Scholar]
- 28.Brown DA, Stephan C, Ward RL, Law M, Hunter M, Bauskin AR, et al. Measurement of serum levels of macrophage inhibitory cytokine 1 combined with prostate-specific antigen improves prostate cancer diagnosis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12: 89–96. [DOI] [PubMed] [Google Scholar]
- 29.El-Sharkawi F, El Sabah M, Hassan Z, Khaled H. The biochemical value of urinary metalloproteinases 3 and 9 in diagnosis and prognosis of bladder cancer in Egypt. Journal of biomedical science. 2014;21: 72 10.1186/s12929-014-0072-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schraen-Maschke S, Sergeant N, Dhaenens C-M, Bombois S, Deramecourt V, Caillet-Boudin M-L, et al. Tau as a biomarker of neurodegenerative diseases. Biomarkers in medicine. 2008;2: 363–84. 10.2217/17520363.2.4.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maetzler W, Tian Y, Baur SM, Gauger T, Odoj B, Schmid B, et al. Serum and Cerebrospinal Fluid Levels of Transthyretin in Lewy Body Disorders with and without Dementia. PLoS ONE. 2012;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meraz-Ríos MA, Toral-Rios D, Franco-Bocanegra D, Villeda-Hernández J, Campos-Peña V. Inflammatory process in Alzheimer’s Disease. Frontiers in integrative neuroscience. 2013;7: 59 10.3389/fnint.2013.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roodveldt C, Christodoulou J, Dobson CM. Immunological features of alpha-synuclein in Parkinson’s disease. Journal of cellular and molecular medicine. 2008;12: 1820–9. 10.1111/j.1582-4934.2008.00450.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao H-M, Zhang F, Zhou H, Kam W, Wilson B, Hong J-S. Neuroinflammation and α-synuclein dysfunction potentiate each other, driving chronic progression of neurodegeneration in a mouse model of Parkinson’s disease. Environmental health perspectives. 2011;119: 807–14. 10.1289/ehp.1003013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strelau J, Strzelczyk A, Rusu P, Bendner G, Wiese S, Diella F, et al. Progressive postnatal motoneuron loss in mice lacking GDF-15. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29: 13640–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krieglstein K, Strelau J, Schober A, Sullivan A, Unsicker K. TGF-beta and the regulation of neuron survival and death. Journal of physiology, Paris. 96: 25–30. [DOI] [PubMed] [Google Scholar]
- 37.Charalambous P, Wang X, Thanos S, Schober A, Unsicker K. Regulation and effects of GDF-15 in the retina following optic nerve crush. Cell and tissue research. 2013;353: 1–8. 10.1007/s00441-013-1634-6 [DOI] [PubMed] [Google Scholar]
- 38.Strelau J, Schober A, Sullivan A, Schilling L, Unsicker K. Growth/differentiation factor-15 (GDF-15), a novel member of the TGF-beta superfamily, promotes survival of lesioned mesencephalic dopaminergic neurons in vitro and in vivo and is induced in neurons following cortical lesioning. Journal of neural transmission Supplementum. 2003; 197–203. [DOI] [PubMed] [Google Scholar]
- 39.Shnaper S, Desbaillets I, Brown DA, Murat A, Migliavacca E, Schluep M, et al. Elevated levels of MIC-1/GDF15 in the cerebrospinal fluid of patients are associated with glioblastoma and worse outcome. International journal of cancer Journal international du cancer. 2009;125: 2624–2630. 10.1002/ijc.24639 [DOI] [PubMed] [Google Scholar]
- 40.Nelson PT, Schmitt FA, Jicha GA, Kryscio RJ, Abner EL, Smith CD, et al. Association between male gender and cortical Lewy body pathology in large autopsy series. Journal of neurology. 2010;257: 1875–81. 10.1007/s00415-010-5630-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer S, van der Meer P, van Deursen VM, Jaarsma T, van Veldhuisen DJ, van der Wal MHL, et al. Neurohormonal and clinical sex differences in heart failure. European heart journal. 2013;34: 2538–2547. 10.1093/eurheartj/eht152 [DOI] [PubMed] [Google Scholar]
- 42.Yi M- H, Zhang E, Baek H, Kim S, Shin N, Kang JW, et al. Growth Differentiation Factor 15 Expression in Astrocytes After Excitotoxic Lesion in the Mouse Hippocampus. Experimental neurobiology. 2015;24: 133–8. 10.5607/en.2015.24.2.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brockmann K, Apel A, Schulte C, Schneiderhan-Marra N, Pont-Sunyer C, Vilas D, et al. Inflammatory Profile in LRRK2-associated prodromal and clinical PD. in revision. [DOI] [PMC free article] [PubMed]
- 44.Fereshtehnejad S-M, Romenets SR, Anang JBM, Latreille V, Gagnon J-F, Postuma RB. New Clinical Subtypes of Parkinson Disease and Their Longitudinal Progression: A Prospective Cohort Comparison With Other Phenotypes. JAMA neurology. 2015;72: 863–73. 10.1001/jamaneurol.2015.0703 [DOI] [PubMed] [Google Scholar]
- 45.Brockmann K, Schulte C, Schneiderhan-Marra N, Apel A, Pont-Sunyer C, Vilas D, et al. Inflammatory profile discriminates clinical subtypes in LRRK2-associated PD. in revision. [DOI] [PubMed]
- 46.Rosenberg GA. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. The Lancet Neurology. 2009;8: 205–216. 10.1016/S1474-4422(09)70016-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
Due to ethical restrictions imposed by the Ethics Committee of the Medical Faculty of the University of Tuebingen related to approved patient consent procedure and protecting patient privacy, all relevant data are available upon request (walter.maetzler@uni-tuebingen.de).