Abstract
Effective neuroblastoma (NB) treatments are still limited despite treatment options available today. Therefore, this study attempted to identify novel plant extracts that have anticancer effects. Cytotoxicity and increased intracellular calcium levels were determined using the Sulforhodamine B (SRB) assay and Fluo4-AM (acetoxymethyl) staining and fluorescence microscopy in NB cells in order to screen a library of plant extracts. The current study examined the anticancer effects of a dichloromethane extract from Scrophularia orientalis L. (Scrophulariaceae), a plant that has been used in Traditional Chinese Medicine. This extract contained highly potent agents that significantly reduced cell survival and increased calcium levels in NB cells. Further analysis revealed that cell death induced by this extract was associated with intracellular calcium release, opening of the MPTP, caspase 3- and PARP-cleavage suggesting that this extract induced aberrant calcium signaling that resulted in apoptosis via the mitochondrial pathway. Therefore, agents from Scrophularia orientalis may have the potential to lead to new chemo therapeutic anticancer drugs. Furthermore, targeting intracellular calcium signaling may be a novel strategy to develop more effective treatments for NB.
Keywords: channels, transporters, endoplasmic reticulum, mitochondria
Introduction
Neuroblastoma (NB) is an extra-cranial pediatric cancer. Effective neuroblastoma treatments are still limited despite the advances in modern medicine, and we are in need of novel chemotherapeutic strategies and more effective anticancer agents (1–3). Many drugs used in cancer chemotherapy is plant-derived or based on natural compounds (4–9). A recent study screened a library of 500 plant extracts for anti-proliferative and cytotoxic effects in neuroblastoma cell lines in order to identify new active substances for chemotherapy. The current study examines one extract from Scrophularia orientalis L., as it was shown to have potent anticancer effects in NB cells.
Members of the genus, Scrophularia, are herbaceous flowering plants. There are 200 known species of Scrophularia. Scrophularia species are used in Traditional Chinese Medicine as a component in a formula used to treat arthritis (10–13). It has anti-inflammatory effects and is also a potent analgesic (14–17). Several species of Scrophularia have been found to have anticancer properties (18,19), however, there are no studies documenting the medicinal effects of Scrophularia orientalis L.
In the current study, a Scrophularia orientalis dichloromethane extract was found to have anticancer effects in NB, and was further examined to elucidate these effects. Calcium is a second messenger that regulates many fundamental physiological processes, including tumor progression and apoptosis (20–24). Therefore, we used Fluo4-AM staining and fluorescence microscopy while concurrently performing Sulforhodamine B (SRB) staining on NB cells in order to determine the effect of this extract on intracellular calcium signaling and cell viability, respectively. The dichloromethane extract of Scrophularia orientalis significantly increased intracellular-free calcium levels and reduced NB cell viability. The IC50 for the extract was ~5 μg/ml in NB cells with and without MycN overexpression. The increase in intracellular-free calcium appeared to be mediated by a calcium release mechanism rather than calcium influx.
In addition, the mitochondrial transition pore assay and western blot analysis revealed that cell death is associated with opening of the mitochondrial permeability transition pore (mPTP), and caspase-3 and PARP cleavage in NB cells. The results from the current study suggest that the dichloromethane extract derived from Scrophularia orientalis effectively kills NB cells by inducing apoptosis via calcium release and consequently, the opening of mPTP. The present study indicates that this extract be used as an alternative strategy for treating NB, and may lead to the development of more effective anticancer agents for NB.
Materials and methods
Cell culture
MYCN2 cells, a tetracycline inducible MycN overexpression NB cell line was authenticated by the cell Line Authentication testing Services at Genetica DNA Laboratories (USA) using STR DNA typing to verify each cell line and verify pure cells (no contamination). The cells were maintained in RPMI-1640 (Mediatech, Inc., Manassas, VA, USA) containing 10% (v/v) heat-inactivated fetal bovine serum (FBS) (Atlanta Biologicals, Lawrenceville, GA, USA), and grown at 37°C, 5% CO2, in a 95% humidity.
Chemicals
General chemicals were from VWR (West Chester, PA, USA). Doxorubicin and ionomycin were from Calbiochem (Gibbstown, NJ, USA). The Library of extracts, which included the Scrophularia orientalis extracts, was obtained from Dr Robert Borris.
Preparation of Scrophularia extracts
Samples of Scrophularia orientalis L. were collected in the Sevan Pass between Dilijan and Tsovagyugh, Gegharkunik Province, Armenia, in June 2006. Voucher specimens (Tamanyan 42–2006) have been deposited in the herbaria of the New York Botanical Garden and the Armenian National Academy of Sciences. Fresh samples were air dried and then milled to a coarse powder. A 1 kg (dry weight) portion of each sample was extracted with methanol (3×4 liters) and the solvent removed in vacuo to afford viscous oil. The resulting oil was dispersed in 1 liter of methanol:water (9:1) and extracted with n-hexane (3×1 liters). The depleted hydroalcoholic phase was freed of methanol, dispersed in distilled water (1 liter) and extracted sequentially with dichloromethane (3×1 liter) and water-saturated n-butanol (3×1 liters). The resulting solvent-soluble fractions were individually evaporated to dryness in vacuo, while the residual aqueous phase was freed of solvent and then lyophilized. Extracts and fractions were maintained at −20°C until needed for use.
Calcium assay
MYCN2 cells were washed and incubated with 1 μM Fluo-4 AM, the acetoxymethyl ester form of Fluo-4 (Molecular Probes, Eugene, OR, USA), for 30 min at 37°C in a standard modified Ringer's solution of the following composition (in mM): NaCl 145, KCl 2.8, CsCl 10, CaCl2 2 (or 0), MgCl2 2, glucose 10, Hepes·NaOH 10, pH 7.4, 330 mOsm. For nominally calcium-free experiments 1 mM EGTA was added to the external solution and calcium chloride was omitted. Cells were transferred to 96-well plates at 10,000 cells/well and stimulated as indicated. Epifluorescent measurements were performed using an Operetta High Content Imaging System (PerkinElmer, Santa Clara, CA, USA). Fluorescence intensity was quantified using Harmony (PerkinElmer).
Fluorescence measurements
MYCN2 cells were incubated in a standard modified Ringer's solution of the following composition (in mM): NaCl 145, KCl 2.8, CsCl 10, CaCl2 2 (or 0), MgCl2 2, glucose 10, Hepes·NaOH 10, pH 7.4, 330 mOsm. For nominally calcium-free experiments, 1 mM EGTA was added to the external solution and calcium chloride was omitted. Cells were loaded with fura-2 AM, the acetoxymethyl ester form of fura-2 (Molecular Probes). Cells were perfused with external solutions containing Scrophularia orientalis extract, and cytosolic calcium was measured in individual cells using a Zeiss microscope and monochromatic light source tuned to excite fura-2 fluorescence at 360 and 390 nm for 20 msec each. Emission was detected at 450–550 nm using a photomultiplier.
Sulforhodamine B assay
The SRB colorimetric assay was used to determine cell proliferation following the protocol previously described (8). Briefly, cells were seeded at a density of 10,000 cells/well on a transparent, flat-bottom, 96-well plate and allowed to settle overnight. At the initiation of each experiment (t=0), and after drug treatments, 100 μl of 10% (w/v) TCA were added to each well, incubated for 1 h at 4°C, washed with deionized water, and dried at room temperature. One hundred microliters of 0.057% (w/v) SRB solution were added to each well, incubated for 30 min at room temperature, rinsed four times with 1% (v/v) acetic acid, and allowed to dry at room temperature. Finally, 200 μl of 10 mM Tris base solution (pH 10.5) was added to each well, and after shaking for 5 min at room temperature, the absorbance was measured at 510 nm in a microplate reader. The absorbance at t=0 was compared with the absorbance at the end of the experiment to determine cell growth in treated cells compared with control cells.
Mitochondrial permeability transition pore assay
Treated and untreated NB cells were washed twice in modified Hanks' Balanced Salt Solution (HBSS: sodium bicarbonate, calcium, and magnesium that also included 10 mM HEPES, 2 mM L-glutamine and 100 μM succinate), then labeled with 1.0 μM calcein AM, 200 nM MitoTracker Red CMXRos, 1 μM Hoechst 33342 dye and 1.0 μM CoCl2. Cells were incubated for 15 min at 37°C, 5% CO2, in 95% humidity, then washed in modified HBSS. Ionomycin (1 μM) was used as a positive control for calcium-mediated pore opening. Cells were labelled with Calcein Am and MitoTracker Red. Epifluorescent measurements were performed using an Operetta High Content Imaging System (PerkinElmer). Fluorescence intensity was quantified using Harmony software (PerkinElmer).
Western blot analysis
Cell lysates were prepared in radioim-munoprecipitation assay buffer [20 mmol/l Tris-HCl (pH 7.5), 0.1% (w/v) sodium lauryl sulfate, 0.5% (w/v) sodium deoxycholate, 135 mmol/l NaCl, 1% (v/v) Triton X-100, 10% (v/v) glycerol, 2 mmol/l EDTA] supplemented with Complete protease inhibitor cocktail (Roche Molecular Biochemicals) and phosphatase inhibitors sodium fluoride (20 mmol/l) and sodium vanadate (0.27 mmol/l). Western blot analysis was performed as previously described (20). The total protein concentration was determined using the protein assay dye reagent from Bio-Rad Laboratories. Cell lysates in SDS-sample buffer were boiled for 5 min and equal amounts of total protein were analyzed by 10% SDS-PAGE and western blotting. The antibodies used in this study are mouse monoclonal p53 (1:250) from Santa Cruz Biotechnology; rabbit polyclonal cleaved caspase-3 (1:1,000), rabbit polyclonal cleaved PARP (1:1,000), mouse monoclonal GAPDH (1:1,000), and mouse monoclonal PCNA (1:1000) from Cell Signaling Technology. Proteins were detected using the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NB, USA) and analyzed with Licor Image Studio 2.0 acquisition and analysis software.
Statistical analysis
Results are shown as the mean ± standard deviation. Statistical significance was determined based on Student's t-test. Adjacent to data points in the respective graphs, significant differences were recorded as follows: single asterisk, P<0.05; double asterisk, P<0.01; triple asterisk, P<0.001; no symbol, P>0.05. SRB and FLuo-4 experiments are the number of at least 3, in triplicates. All other experiments are reported as the mean of at least 3.
Results
Scrophularia orientalis extracts reduce NB cell viability
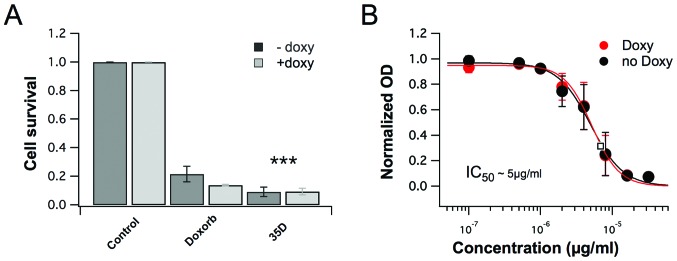
The library of 500 plant extracts was screened for anticancer effects. The results clearly show that the dichloromethane extracts of Scrophularia orientalis significantly decreased viability in NB cells, compared to untreated control cells. In NB cells with non-amplified MYCN gene (NAM), extract at a concentration of 8 μg/ml, reduced cell viability of NB cells by 91.6%, compared to the viability of untreated control cells (Fig. 1A). In NB cells with overexpression of MycN (MOE), the extract reduced cell viability by 92.1% (Fig. 1A). The cytotoxic effects were more potent than that of doxorubicin, which reduced cell viability of NAM and MOE by 79.3% and 84.7%, respectively (Fig. 1A). The IC50 of the extract was determined, and the IC50 of MNA and MOE were 8.33 μg/ml and 8.09 μg/ml, respectively (Fig. 1B). The data suggest that the dichloromethane extract of Scrophularia orientalis has potent anticancer effects and effectively decreases NB cell viability.
Figure 1.
Scrophularia orientalis extracts reduce neuroblastoma (NB) cell viability. Cell proliferation was assessed using Sulforhodamine B (SRB) assay in 96-well formats. MYCN2 cells were grown with and without induction of MYCN overexpression for 96 h. Cells were treated with 500 nM doxorubicin and 8 μg/ml of extract 35D for 48 h. Growth inhibition was normalized to untreated cells (A). IC50 value was assessed using SRB assay treating cells with different concentrations of extract. The IC50 value was established at 5 μg/ml for both MYCN uninduced and overexpressed conditions (B).
Scrophularia orientalis extract increases intracellular calcium in NB cells
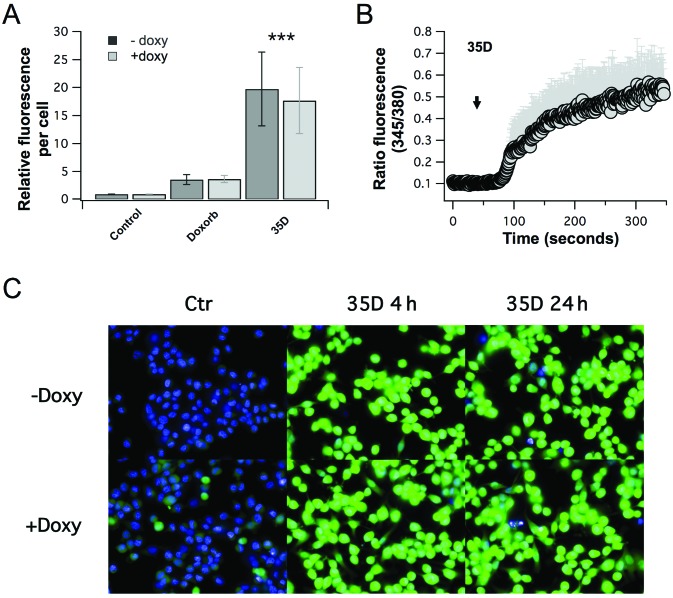
Calcium is a second messenger that plays a fundamental role in a plethora of cellular processes, including cell proliferation and cell death. Therefore, in order to determine the effect of the Scrophularia orientalis extract on calcium, intracellular-free calcium was measured in control, untreated NB cells and NB cells treated with Scrophularia orientalis extract. Treated and untreated cells were loaded with Fluo-4, and relative fluorescence was measured using an Operetta High Content Imaging System. Fig. 2A shows that there was a 20-fold increase in intracellular-free calcium when NAM NB cells were exposed to extract for 24 h, compared to untreated control. Intracellular-free calcium increased by 18-fold when MOE NB cells was exposed to extract, compared to untreated control (Fig. 2A). These changes in intracellular-free calcium was significantly greater than that of doxorubicin which increased intracellular-free calcium by 2.8- and 3.1-fold in NAM and MOE NB cells, respectively (Fig. 2A). Further examination of the calcium inducing effects of the extract showed that external application of extract to MOE NB cells increased intracellular-free calcium steadily after a slight delay of approximately 30–45 sec, whereas no signal was induced by vehicle control (Fig. 2B). This suggests that the extract induces rapid changes in intracellular calcium. Of note, the elevated calcium levels appeared to be sustained, as indicated by a time course analysis of calcium levels after treatment of NB cells with the extract for 4 and 24 h (Fig. 2C). These results clearly show that the Scrophularia dichloromethane extract had significant and distinct effects on calcium signaling in NB cells.
Figure 2.
Scrophularia orientalis extracts increase intracellular calcium in neuroblastoma (NB) cells. MYCN2 cells were grown with and without induction of MYCN overexpression for 96 h. The last 24 h of the experiment cells were treated with 500 nM doxorubicin and 8 μg/ml of extract 35D. Global intracellular calcium level intensity was measured using Fluo-4-am and Perkin Elmer Operetta and normalized per cell (A). For time resolved acquisition of calcium signaling MYCN2 cells were loaded with Fura-2-am. Extract 35D at 8 μg/ml in ringer solution was applied at 40 sec into experiment indicated by black arrow (B). Under same conditions cells were stained with Hoechst 33342 and Fluo-4 and calcium levels were measured after 4 and 24 h (C).
Scrophularia orientalis extracts induce calcium release from intracellular stores and calcium influx
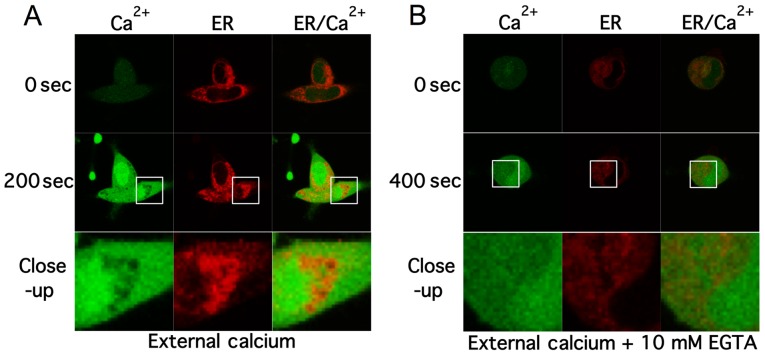
In order to decipher the source of intracellular-free calcium time-lapse confocal experiments were performed. Cells were labelled with Fluo-4 AM and ER-tracker. Confocal images of the 2 channels were acquired every 1.5 sec. Standard extra cellular HBSS buffer was used that contained ~1.3 mM-free calcium and ~800 μM- free magnesium. Baseline fluorescence was measured in cells for 30 sec then cells were perfused with extracellular solution containing Scrophularia extract through a glass application pipette. Upon exposure with extract, intracellular calcium levels started to rise within 40–60 sec and peaked at ~150–200 sec. Fig. 3A shows confocal images of fluo-4 (green) and ER-tracker (red) labelled NB cells pre- and post-application of extract. After 200 sec intracellular calcium rose to peak levels and remained elevated up to 1000 sec, as shown by the increase in fluorescence. Noteworthy, low levels of calcium within the cell co-localized with endoplasmic reticulum (ER) staining, suggesting that the increase in calcium may be due to calcium ER store depletion. Intracellular-free calcium can be mobilized from either transporters in the membrane of organelles, such as the ER, that act as intracellular calcium stores, or the extracellular space through ion channels and transporters. To further investigate the source the Scrophularia extract mediated calcium mobilisation, 10 mM EGTA was added to the HBSS buffer, chelating extracellular-free calcium levels to ~10 nM (Fig. 3B).
Figure 3.
Scrophularia orientalis extracts induce ER calcium store depletion. MYCN2 cells were loaded with fluo-4 and ER-tracker and time-lapse confocal experiments were performed. 35D extract was applied to the cells. The extracellular solution contained 1 mM of calcium. At 200 sec, cytoplasmic calcium levels were elevated and ER localization displays a decrease in calcium levels (close-up) (A). (B) The same experiment was performed. The external solution contained 10 mM EGTA decreasing extracellular-free calcium to ~10 nM. Cytoplasmic calcium levels were elevated after 400 sec and ER localized calcium was decreased.
Scrophularia orientalis extracts induce opening of mitochondrial permeability transition pore (MPTP) in NB cells
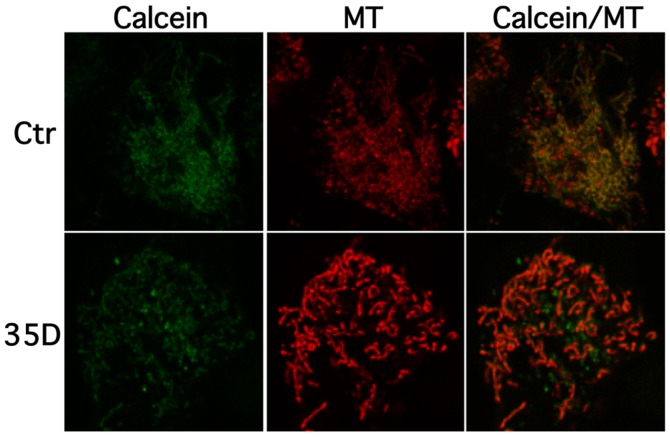
To examine the effect of the Scrophularia dichloromethane extract on loss of mitochondrial membrane integrity, cells were stained with calcein AM cobalt (CoCl2) and mitochondrial dye, and confocal measurements were performed. In untreated healthy cells calcein-AM, CoCl2 are taken up and non-specific esterase activity cleaves and activates calcein. Cytoplasmic calcein is almost entirely quenched by CoCl2 whereas mitochondrial calcein is not accessed by CoCl2 freely leading to an overlap of calcein and mitotracker signal in healthy cells. Upon induction of mitochondrial pore opening CoCl2 enters the organelle leading to quenching and subsequent drop of mitochondrial calcein signal. Fig. 4 shows that treatment of NAM cells with extract decreases mitochondrial calcein co-localisations levels, whereas healthy cells display a clear overlap of calcein (green) and mitochondrial (red) signal. These results suggest that the Scrophularia extract induces loss of mitochondrial membrane integrity, here indicated by calcein and mitochondria distribution.
Figure 4.
Scrophularia orientalis extracts induce opening of the mitochondrial permeability transition pore (MPTP). MYCN2 cells were grown for 48 h. The last 24 h of the experiment cells were treated with 8 μg/ml of extract 35D. Cells were loaded with calcein-AM, CoCl2 and Mitotracker. Opening of MPTP by 35 was measured for co-localization/loss of mitochondrial calcein levels.
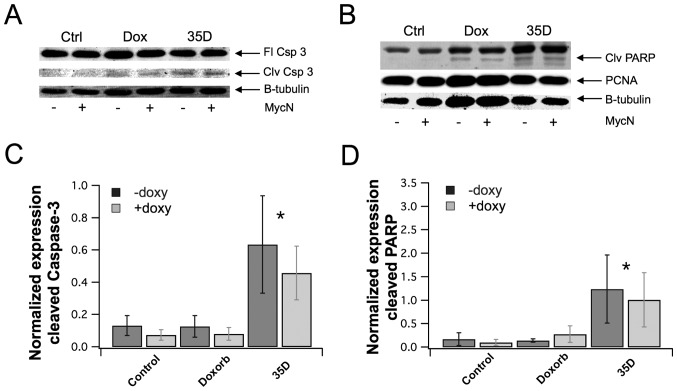
Scrophularia orientalis extract induces apoptosis in NB cells
To determine whether the Scrophularia extract indeed induced mitochondrial calcium overload and subsequently promoted apoptosis, whole cell lysates were prepared from NB cells treated with the extracts or left untreated, and the lysates were analysed by western blot for detection of apoptotic markers, cleaved caspase-3 and cleaved PARP. Fig. 5A and B shows that extract increased the cleavage of caspase-3 and PARP. However, there was no effect on total caspase expression. In addition, there was no effect on proliferation marker, proliferating cell nuclear antigen (PCNA). The band density in each lane of the western blot was quantified, and the level of cleaved caspase-3 and cleaved PARP were normalized to GAPDH expression. The quantification of cleaved caspase-3 showed that the extract significantly increased caspase-3 cleavage by 4.8-fold for NAM and 6.1-fold for MOE, compared to control (Fig. 5C). The quantification of cleaved PARP showed that the extract significantly increased PARP cleavage by 7.2 and 9.9 for NAM and MOE, respectively, compared to control (Fig. 5D). These results suggest that the dichloromethane Scrophularia extract induce mitochondrial calcium overload, opening of the mitochondrial permeability transition pore and promoting apoptosis.
Figure 5.
Scrophularia orientalis extracts induce apoptosis in neuroblastoma (NB) cells. MYCN2 cells were grown with and without induction of MYCN overexpression for 96 h and were treated the last 24 h of the experiment with 500 nM doxorubicin or 8 μg/ml of extract 35D. Whole cell lysates were analyzed by western blotting to determine protein level of cleavage of caspase-3 (A) and PARP (B) as well as PCNA. For quantification of caspase-3 protein levels were normalized on β-tubulin levels (C). For quantification of PARP cleavage fragments were normalized on β-tubulin and cleaved PARP fragment was normalized on full length PARP fragment (D).
Discussion
Scrophularia are herbaceous flowering plants. There are 200 known species of Scrophularia, of which several species have been found to have medicinal effects. For example, Scrophularia is used in Traditional Chinese medicine as a component in a formula used to treat arthritis (25). Previous studies have shown that Scrophularia has anti-inflammatory effects and may also be used as an analgesic (14–17). Several species have been found to have anticancer properties (18,19). However, there are no studies investigating the medicinal effects of Scrophularia orientalis. In the current study, an extract from Scrophularia orientalis which was part of a library of 500 plant extracts that was screened in a previous study was found to have potent anticancer effects in NB cells. The anticancer effects of the Scrophularia orientalis dichloromethane extract were further examined, and the results showed that the extract significantly decreased cell viability of NB cells with and without MycN overexpression. MycN is the protein that is the product of MYCN gene expression. MycN is a transcription factor that contains a C-terminal basic helix-loop-helix zipper motif and an N-terminal transactivation domain. MycN binds to genes that contain the E-box consensus sequence in the promoter (26). MYCN gene amplification is a negative prognostic indicator of NB, and is associated with high-risk and advanced stage NB (27,28).
In addition, MYCN amplification may alter the anticancer effects of chemotherapeutic drugs and/or confer resistance to some treatments (29). Identification of alternative treatments with selectivity for MYCN amplified NB or NB with MycN overexpression would be beneficial for the treatment of advanced stage, high-risk NB. Therefore, this study examined the effect of the dichloromethane Scrophularia orientalis extract in NB cells with and without MycN overexpression. Interestingly, the IC50 of the extracts were similar in NB cells with and without MycN overexpression, suggesting that components of the extracts are effective in NB cells regardless of MycN status.
The Scrophularia extract also altered intracellular calcium regulation. Of note, application of the extracts induced a rapid increase in intracellular calcium. Confocal analysis showed that Scrophularia extract increased intracellular-free calcium through a process that involved calcium release from the ER, when calcium was present in the external media. In the absence of external calcium, exposure of NB cells to Scrophularia extract mobilized intracellular calcium to a lower extent and with a ~200 second delay, compared to calcium mobilization in the presence of external calcium. This suggests that calcium influx, which requires external calcium, is involved in the elevated calcium induced by Scrophularia extract. Also, lower levels of intracellular calcium co-localized with ER marker suggesting that calcium was mobilized, in part, from the ER. Taken together the data suggest that exposure of Scrophularia extract triggers at least two components of calcium mobilisation one being sourced by ER calcium and the other through plasma membrane mediated calcium flux. One of the possibilities in this context would be the induction of store operated calcium entry as one of the main influx pathways (20,21). To confirm the ER-calcium depletion, experiments were also conduced with Rhod-4 that has been reported to have greater access to ER stores confirming the results (data not shown). Further the effect of magnesium was ruled out by using buffer deficient of calcium and magnesium (data not shown).
The increase in cytosolic calcium levels was maintained for over 24 h. This effect may be due to increased or dysregulated activity of calcium release transporters (e.g. IP3 or Ryanodine receptors) or plasma membrane Store-operated calcium channels (Orai) (30–35). Alternatively, activation of transporter proteins that remove cytosolic calcium such as ATPases that transport calcium into intracellular stores (SERCA) or out of the cell (Na/Ca-exchanger, Ca-ATPase), may be hampered (36,37). Inhibition of these processes could lead to the sustained increase in intracellular calcium observed in this study.
Noteworthy, sustained high calcium levels in the cytosol could lead to transport of calcium into the mitochondria. The mitochondria is an interesting organelle. It requires calcium in order to generate energy in the form of adenosine triphosphate (ATP) (38,39). It also serves as a calcium sink and can transiently buffer cytosolic calcium, as a cytoprotective mechanism (40,41). In addition, there are complex signaling between the ER and mitochondria that allows for spatio-temporal patterns of calcium signaling between the two organelles to tightly control cellular function and cell fate (42–47). Transport of calcium from the ER to the mitochondria at mitochondria associated membranes will help to buffer cytosolic calcium levels and induce ATP production. The increased ATP may feed back to the calcium-ATPase pump in the ER membrane (SERCA) to pump calcium back to the ER (48,49). However, alterations in the function of proteins that regulate calcium signaling between these organelles could lead to a sustained increase in mitochondria calcium that could lead to opening of the mitochondria permeability transition pore (mPTP) which eventually leads to activation of the apoptotic pathway calcium (50–52).
In the current study, the data suggest that the Scrophularia extract may alter the function of the calcium transporters in the ER and/or mitochondria that may prevent the transport of calcium from mitochondria and cytosol back into the ER or out of the cell, which results in sustained cytosolic and mitochondria calcium and eventually opening of the mPTP. The opening of the mPTP resulted in activation of apoptosis in the NB cells. Western blot analyses showed that the opening of the mPTP consequently induced apoptosis as shown by the increase in both cleaved caspase-3 and cleaved PARP, apoptosis markers. However, the extract did not affect proliferation, as levels of (12) pCNA (proliferating cell nuclear antigen, a proliferation marker) were unchanged when cells were treated with the extract.
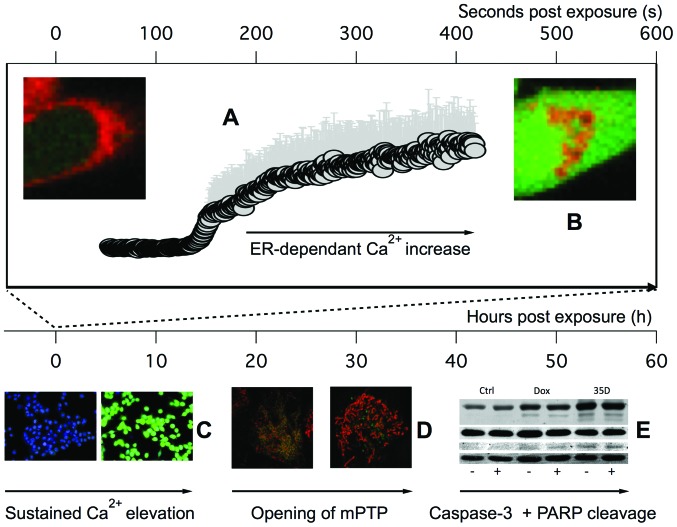
In conclusion, the current study showed that Scrophularia orientalis dichloromethane extract has potent anticancer effects. This extract rapidly increased in intracellular-free calcium and altered calcium handling that led to sustained elevation in intracellular calcium and ultimately, opening of the mitochondria permeability transition pore and apoptosis in NB cells (Fig. 6). Components in the extracts may provide an alternative anticancer treatment for NB, and should be investigated in future studies. This study shows that targeting calcium signaling at the ER and/or mitochondria may be a unique strategy for the development of more effective, and novel drugs for the treatment of NB.
Figure 6.
Timeline of the effects of Scrophularia orientalis extracts on neuroblastoma (NB) cells. Scrophularia orientalis extract is applied to NB cells at t=0 h. Within 40 sec (A), Fura 2AM measurements showed a rapid increase in intracellular-free calcium levels. Within 200 sec, confocal images showed an increase in intracellular calcium and loss of ER calcium (B), in the presence of external calcium. In the absence of external calcium, the ER calcium is still depleted but the rise in intracellular calcium is less than that observed in the presence of external calcium. At 4 h, elevated intracellular calcium is sustained (C). At 24 h, elevated intracellular calcium is sustained, and the opening of the mitochondrial permeability transition pore is observed (D) (loss of calcein AM staining in the mitochondria). Within 48 h, cleavage of caspase and PARP are observed (E). However, there are no changes in cell cycle proteins.
Acknowledgements
This study was supported by the Alex's Lemonade Stand Foundation Young Investigator Award 439744 (D.-L.K), the NIH grants from the National Cancer Institute Mentored Research Scientist Development Award (K01) CA154758, (D.-L.K), and the National Institutes of General Medical Sciences P20GM 103466 (D.-L.K).
References
- 1.Maris JM, Matthay KK. Molecular biology of neuroblastoma. J Clin Oncol. 1999;17:2264–2279. doi: 10.1200/JCO.1999.17.7.2264. [DOI] [PubMed] [Google Scholar]
- 2.Combaret V, Delattre O, Bénard J, Favrot MC. Biological markers for the prognosis of neuroblastoma: Proposal of a method of analysis. Bull Cancer. 1998;85:262–266. (In French) [PubMed] [Google Scholar]
- 3.Park JR, Eggert A, Caron H. Neuroblastoma: Biology, prognosis, and treatment. Hematol Oncol Clin North Am. 2010;24:65–86. doi: 10.1016/j.hoc.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Sawadogo WR, Schumacher M, Teiten MH, Dicato M, Diederich M. Traditional West African pharmacopeia, plants and derived compounds for cancer therapy. Biochem Pharmacol. 2012;84:1225–1240. doi: 10.1016/j.bcp.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 5.Tan W, Lu J, Huang M, Li Y, Chen M, Wu G, Gong J, Zhong Z, Xu Z, Dang Y, et al. Anti-cancer natural products isolated from Chinese medicinal herbs. Chin Med. 2011;6:27. doi: 10.1186/1749-8546-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jo H, Loison F, Hattori H, Silberstein LE, Yu H, Luo HR. Natural product Celastrol destabilizes tubulin heterodimer and facilitates mitotic cell death triggered by microtubule-targeting anti-cancer drugs. PLoS One. 2010;5:e10318. doi: 10.1371/journal.pone.0010318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desai AG, Qazi GN, Ganju RK, El-Tamer M, Singh J, Saxena AK, Bedi YS, Taneja SC, Bhat HK. Medicinal plants and cancer chemoprevention. Curr Drug Metab. 2008;9:581–591. doi: 10.2174/138920008785821657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konkimalla VB, Efferth T. Anti-cancer natural product library from traditional Chinese medicine. Comb Chem High Throughput Screen. 2008;11:7–15. doi: 10.2174/138620708783398368. [DOI] [PubMed] [Google Scholar]
- 9.Mukherjee AK, Basu S, Sarkar N, Ghosh AC. Advances in cancer therapy with plant based natural products. Curr Med Chem. 2001;8:1467–1486. doi: 10.2174/0929867013372094. [DOI] [PubMed] [Google Scholar]
- 10.Zhang LQ, Guo FJ, Wang SC, Li YM. A new triterpenoid tetrasaccharide from the root of Scrophularia ningpoensis. Yao Xue Xue Bao. 2012;47:1358–1362. [PubMed] [Google Scholar]
- 11.Chen C, Duan LN, Zhou XL, Chen BL, Fu CX. Molecular authentication of geo-authentic Scrophularia ningpoensis. J Zhejiang Univ Sci B. 2011;12:393–398. doi: 10.1631/jzus.B1000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang CG, Shang YJ, Zhang J, Zhang JR, Li WJ, Jiao BH. Hypouricemic effects of phenylpropanoid glycosides acteoside of Scrophularia ningpoensis on serum uric acid levels in potassium oxonate-pretreated mice. Am J Chin Med. 2008;36:149–157. doi: 10.1142/S0192415X08005667. [DOI] [PubMed] [Google Scholar]
- 13.Wang S, Zheng Z, Weng Y, Yu Y, Zhang D, Fan W, Dai R, Hu Z. Angiogenesis and anti-angiogenesis activity of Chinese medicinal herbal extracts. Life Sci. 2004;74:2467–2478. doi: 10.1016/j.lfs.2003.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad M, Muhammad N, Mehjabeen, Jahan N, Alam SM, Ahmad M, Obaidullah Biological screening of Scrophularia nodosa extract and its fractions. Pak J Pharm Sci. 2012;25:307–313. [PubMed] [Google Scholar]
- 15.García D, Fernández A, Sáenz T, Ahumada C. Anti-inflammatory effects of different extracts and harpagoside isolated from Scrophularia frutescens L. Farmaco. 1996;51:443–446. [PubMed] [Google Scholar]
- 16.Giner RM, Villalba ML, Recio MC, Máñez S, Cerdá-Nicolás M, Ríos J. Anti-inflammatory glycoterpenoids from Scrophularia auriculata. Eur J Pharmacol. 2000;389:243–252. doi: 10.1016/S0014-2999(99)00846-8. [DOI] [PubMed] [Google Scholar]
- 17.Fernández MA, Sáenz MT, García MD. Anti-inflammatory activity in rats and mice of phenolic acids isolated from Scrophularia frutescens. J Pharm Pharmacol. 1998;50:1183–1186. doi: 10.1111/j.2042-7158.1998.tb03332.x. [DOI] [PubMed] [Google Scholar]
- 18.Giessrigl B, Yazici G, Teichmann M, Kopf S, Ghassemi S, Atanasov AG, Dirsch VM, Grusch M, Jäger W, Ozmen A, et al. Effects of Scrophularia extracts on tumor cell proliferation, death and intravasation through lymphoendothelial cell barriers. Int J Oncol. 2012;40:2063–2074. doi: 10.3892/ijo.2012.1388. [DOI] [PubMed] [Google Scholar]
- 19.Ardeshiry Lajimi A, Rezaie-Tavirani M, Mortazavi SA, Barzegar M, Moghadamnia SH, Rezaee MB. Study of anti-cancer property of Scrophularia striata extract on the human astrocytoma cell line (1321) Iran J Pharm Res. 2010;9:403–410. [PMC free article] [PubMed] [Google Scholar]
- 20.Qu B, Al-Ansary D, Kummerow C, Hoth M, Schwarz EC. ORAI-mediated calcium influx in T cell proliferation, apoptosis and tolerance. Cell Calcium. 2011;50:261–269. doi: 10.1016/j.ceca.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 21.Liu H, Hughes JD, Rollins S, Chen B, Perkins E. Calcium entry via ORAI1 regulates glioblastoma cell proliferation and apoptosis. Exp Mol Pathol. 2011;91:753–760. doi: 10.1016/j.yexmp.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Huang W, Lu C, Wu Y, Ouyang S, Chen Y. T-type calcium channel antagonists, mibefradil and NNC-55-0396 inhibit cell proliferation and induce cell apoptosis in leukemia cell lines. J Exp Clin Cancer Res. 2015;34:54. doi: 10.1186/s13046-015-0171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi DL, Jang SJ, Cho S, Choi HE, Rim HK, Lee KT, Lee JY. Inhibition of cellular proliferation and induction of apoptosis in human lung adenocarcinoma A549 cells by T-type calcium channel antagonist. Bioorg Med Chem Lett. 2014;24:1565–1570. doi: 10.1016/j.bmcl.2014.01.071. [DOI] [PubMed] [Google Scholar]
- 24.Ohkubo T, Yamazaki J. T-type voltage-activated calcium channel Cav3.1, but not Cav3.2, is involved in the inhibition of proliferation and apoptosis in MCF-7 human breast cancer cells. Int J Oncol. 2012;41:267–275. doi: 10.3892/ijo.2012.1422. [DOI] [PubMed] [Google Scholar]
- 25.Zhu T, Zhang L, Ling S, Duan J, Qian F, Li Y, Xu JW. Scropolioside B inhibits IL-1β and cytokines expression through NF-κB and inflammasome NLRP3 pathways. Mediators Inflamm. 2014;2014:819053. doi: 10.1155/2014/819053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy DM, Buckley PG, Bryan K, Das S, Alcock L, Foley NH, Prenter S, Bray I, Watters KM, Higgins D, et al. Global MYCN transcription factor binding analysis in neuroblastoma reveals association with distinct E-box motifs and regions of DNA hypermethylation. PLoS One. 2009;4:e8154. doi: 10.1371/journal.pone.0008154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan HS, Gallie BL, DeBoer G, Haddad G, Ikegaki N, Dimitroulakos J, Yeger H, Ling V. MYCN protein expression as a predictor of neuroblastoma prognosis. Clin Cancer Res. 1997;3:1699–1706. [PubMed] [Google Scholar]
- 28.Tonini GP, Boni L, Pession A, Rogers D, Iolascon A, Basso G, Cordero di Montezemolo L, Casale F, Pession A, Perri P, et al. MYCN oncogene amplification in neuroblastoma is associated with worse prognosis, except in stage 4s: The Italian experience with 295 children. J Clin Oncol. 1997;15:85–93. doi: 10.1200/JCO.1997.15.1.85. [DOI] [PubMed] [Google Scholar]
- 29.Gogolin S, Dreidax D, Becker G, Ehemann V, Schwab M, Westermann F. MYCN/MYC-mediated drug resistance mechanisms in neuroblastoma. Int J Clin Pharmacol Ther. 2010;48:489–491. doi: 10.5414/CPP48489. [DOI] [PubMed] [Google Scholar]
- 30.Guo RW, Huang L. New insights into the activation mechanism of store-operated calcium channels: Roles of STIM and Orai. J Zhejiang Univ Sci B. 2008;9:591–601. doi: 10.1631/jzus.B0820042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peel SE, Liu B, Hall IP. ORAI and store-operated calcium influx in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2008;38:744–749. doi: 10.1165/rcmb.2007-0395OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hewavitharana T, Deng X, Soboloff J, Gill DL. Role of STIM and Orai proteins in the store-operated calcium signaling pathway. Cell Calcium. 2007;42:173–182. doi: 10.1016/j.ceca.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A, et al. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carafoli E, Guerini D. Molecular and cellular biology of plasma membrane calcium ATPase. Trends Cardiovasc Med. 1993;3:177–184. doi: 10.1016/1050-1738(93)90003-O. [DOI] [PubMed] [Google Scholar]
- 37.Newton T, Black JP, Butler J, Lee AG, Chad J, East JM. Sarco/endoplasmic-reticulum calcium ATPase SERCA1 is maintained in the endoplasmic reticulum by a retrieval signal located between residues 1 and 211. Biochem J. 2003;371:775–782. doi: 10.1042/bj20021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martínez F, Uribe A, Espinosa-García MT, Flores-Herrera O, García-Pérez C, Milán R. Calcium modulates the ATP and ADP hydrolysis in human placental mitochondria. Int J Biochem Cell Biol. 2002;34:992–1003. doi: 10.1016/S1357-2725(02)00020-1. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka J, Kono Y, Shimahara Y, Sato T, Jones RT, Cowley RA, Trump BF. A study of oxidative phosphorylative activity and calcium-induced respiration of rat liver mitochondria following living Escherichia coli injection. Adv Shock Res. 1982;7:77–90. [PubMed] [Google Scholar]
- 40.García-Sancho J. The coupling of plasma membrane calcium entry to calcium uptake by endoplasmic reticulum and mitochondria. J Physiol. 2014;592:261–268. doi: 10.1113/jphysiol.2013.255661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santo-Domingo J, Demaurex N. Calcium uptake mechanisms of mitochondria. Biochim Biophys Acta. 2010;1797:907–912. doi: 10.1016/j.bbabio.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Patergnani S, Suski JM, Agnoletto C, Bononi A, Bonora M, De Marchi E, Giorgi C, Marchi S, Missiroli S, Poletti F, et al. Calcium signaling around Mitochondria Associated Membranes (MAMs) Cell Commun Signal. 2011;9:19. doi: 10.1186/1478-811X-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smaili SS, Hsu YT, Carvalho AC, Rosenstock TR, Sharpe JC, Youle RJ. Mitochondria, calcium and pro-apoptotic proteins as mediators in cell death signaling. Braz J Med Biol Res. 2003;36:183–190. doi: 10.1590/S0100-879X2003000200004. [DOI] [PubMed] [Google Scholar]
- 44.Ermak G, Davies KJ. Calcium and oxidative stress: From cell signaling to cell death. Mol Immunol. 2002;38:713–721. doi: 10.1016/S0161-5890(01)00108-0. [DOI] [PubMed] [Google Scholar]
- 45.Lewis A, Hayashi T, Su TP, Betenbaugh MJ. Bcl-2 family in inter-organelle modulation of calcium signaling; roles in bioen-ergetics and cell survival. J Bioenerg Biomembr. 2014;46:1–15. doi: 10.1007/s10863-013-9527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Annunziata I, d'Azzo A. Interorganellar membrane microdomains: Dynamic platforms in the control of calcium signaling and apoptosis. Cells. 2013;2:574–590. doi: 10.3390/cells2030574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krebs J, Agellon LB, Michalak M. Ca(2+) homeostasis and endoplasmic reticulum (ER) stress: An integrated view of calcium signaling. Biochem Biophys Res Commun. 2015;460:114–121. doi: 10.1016/j.bbrc.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 48.Balaban RS. The role of Ca(2+) signaling in the coordination of mitochondrial ATP production with cardiac work. Biochim Biophys Acta. 2009;1787:1334–1341. doi: 10.1016/j.bbabio.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malli R, Frieden M, Trenker M, Graier WF. The role of mitochondria for Ca2+ refilling of the endoplasmic reticulum. J Biol Chem. 2005;280:12114–12122. doi: 10.1074/jbc.M409353200. [DOI] [PubMed] [Google Scholar]
- 50.Dahlem YA, Wolf G, Siemen D, Horn TF. Combined modulation of the mitochondrial ATP-dependent potassium channel and the permeability transition pore causes prolongation of the biphasic calcium dynamics. Cell Calcium. 2006;39:387–400. doi: 10.1016/j.ceca.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 51.Deniaud A, Sharaf el Dein O, Maillier E, Poncet D, Kroemer G, Lemaire C, Brenner C. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene. 2008;27:285–299. doi: 10.1038/sj.onc.1210638. [DOI] [PubMed] [Google Scholar]
- 52.Ma X, Tian X, Huang X, Yan F, Qiao D. Resveratrol-induced mitochondrial dysfunction and apoptosis are associated with Ca2+ and mCICR-mediated MPT activation in HepG2 cells. Mol Cell Biochem. 2007;302:99–109. doi: 10.1007/s11010-007-9431-8. [DOI] [PubMed] [Google Scholar]