Abstract
OBJECTIVES
This study sought to evaluate the effects of beta-blocker withdrawal in acute decompensated heart failure (ADHF).
BACKGROUND
Published reports showed trends for either no harm or increased risk of in-hospital mortality, short-term mortality, and rehospitalization rates in patients admitted for ADHF that discontinued beta-blockers; however, a comprehensive analysis has not been conducted.
METHODS
Relevant studies from January 2000 through January 2015 were identified in the PubMed, EMBASE, and COCHRANE electronic databases. Where appropriate data were available, weighted relative risks were estimated using random-effects meta-analysis techniques.
RESULTS
Five observational studies and 1 randomized clinical trial (n = 2,704 patients who continued beta-blocker therapy and n = 439 patients who discontinued beta-blocker therapy) that reported the short-term effects of beta-blocker withdrawal in ADHF were included in the analyses. In 2 studies, beta-blocker withdrawal significantly increased risk of in-hospital mortality (risk ratio: 3.72; 95% confidence interval [CI]: 1.51 to 9.14). Short-term mortality (relative risk: 1.61; 95% CI: 1.04 to 2.49; 4 studies) and combined short-term rehospitalization or death (relative risk: 1.59; 95% CI: 1.03 to 2.45; 4 studies) were also significantly increased.
CONCLUSIONS
Discontinuation of beta-blockers in patients admitted with ADHF was associated with significantly increased in-hospital mortality, short-term mortality, and the combined endpoint of short-term rehospitalization or mortality. These data suggest beta-blockers should be continued in ADHF patients if their clinical picture allows.
Keywords: acute decompensated heart failure, beta-blockers, meta-analysis
Acute decompensated heart failure (ADHF) presents a major challenge for clinicians with an estimated 1 million admissions in the United States annually (1). Despite major strides in chronic heart failure marked by significant mortality reductions of 33% to 35% with beta-blockers (2–4), 16% to 27% with angiotensin-converting enzyme inhibitors (5,6), 43% with hydralazine/isosorbide dinitrate (7), and 24% to 30% with aldosterone antagonists (8,9), outcomes have changed little in ADHF. In-hospital mortality rates have only modestly decreased from approximately 8% in 1993 to approximately 5% in 2005, and 30-day mortality rates have changed little from 13% in 1993 to 11% in 2006 (10). Simultaneously, 30-day rehospitalization rates have increased from 17% in 1993 (10) to 26.9% in 2009 (11).
Although CIBIS-II (Cardiac Insufficiency Bisoprolol Study II) (3), COPERNICUS (Carvedilol prospective randomized cumulative survival) (4), and MERIT-HF (Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure) (2) showed mortality benefits with beta-blocker treatment in chronic heart failure, the question of how to handle beta- blockers in ADHF remains incompletely answered. Concern about the negative inotropic effects of beta-blockers and thus the potential worsening of hemodynamics leads many physicians to stop beta-blockers in ADHF. However, the evidence supporting such a decision is lacking, and the same concern has been conclusively demonstrated to be misplaced with regard to chronic heart failure management (2–4). A small randomized clinical trial conducted in France showed continuation of beta-blockers did not significantly change inhospital mortality, 3-month rehospitalization, or 3-month mortality rates in ADHF (12). An observational study that assessed outcomes in the Italian Survey for Acute Heart Failure showed increased risk of in-hospital mortality associated with beta-blocker withdrawal (13). Other observational studies showed either no change or reduced short-term mortality, rehospitalization, and combined mortality/rehospitalization rates when beta-blockers were discontinued in ADHF (14–17). To gain a better understanding of how beta-blocker discontinuation affects outcomes in ADHF, we conducted a systematic review and meta-analysis.
METHODS
The present review was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (18).
SEARCH STRATEGY
Studies were identified by working with a biomedical librarian, and systematically searching the electronic databases EMBASE, PubMed, and COCHRANE from January 2000 through January 2015. We used the following key terms for all databases: acute decompensated heart failure, beta-blockers, mortality, hospitalization, survival analysis, heart failure, and outcomes. Only articles written in English and conducted in human studies were included. Two reviewers (K.W.P. and J.M.N.) retrieved and read the full-text articles from those with an abstract that suggested it was relevant. Results from this step were compared, and any discrepancies resolved through consensus. We also searched the reference lists from the identified articles and www.clinicaltrials.gov to identify other articles.
STUDY SELECTION
To be eligible, studies had to fulfill the following inclusion criteria: 1) randomized controlled trial (RCT) or observational study; 2) compared continuation and withdrawal of beta-blockers; 3) reported at least 1 of the outcomes of in-hospital mortality, rehospitalization, and death rates in short-term (≤6 months after index hospitalization); and 4) reported the estimate of the relative risk (RR) with its corresponding 95% confidence interval (CI) or sufficient data to calculate these measures. Studies reporting different measures of RR, such as the hazard ratio or odds ratio, were included in the meta-analysis.
DATA EXTRACTION
The details extracted independently by 4 reviewers (K.W.P., J.M.N., J.O.T., and S.D.) from each study included the following: primary author's last name, year of publication, type of analysis, patient population, number of patients that continued beta-blocker therapy, number of patients that stopped beta-blocker therapy, outcomes assessed, adjusting variables, and RR estimates with corresponding 95% CIs. If a study reported more than 1 risk estimate, the most completely adjusted estimate was extracted. For those studies where crude data were available, we calculated the RR and CI by reconstructing the contingency tables. Any disagreements were discussed and resolved by consensus.
QUALITY OF STUDIES IN ANALYSIS
Assessment of bias was conducted as described by Downs and Black (19) with two independent reviewers assessing the studies (K.W.P. and J.M.N.). High, medium, and low risks of bias in reporting, external validity, internal validity-bias, internal validity-confounding, and power were quantified as previously described (20).
STATISTICAL ANALYSIS
The natural logarithm of the study-specific RR estimates and their CIs from the observational studies were combined using a random-effects meta-analytic model (21). As a sensitivity analysis, the RCT was included in a separate analysis. I2 statistics were calculated to examine heterogeneity, with a value >50% considered severe heterogeneity. Because of the small number of studies, we did not perform any formal test for publication bias. For p values that were not reported in the original publications, assessment of differences in systolic blood pressure and heart rate were performed from extracted data from Fonarow et al. (16) and Orso et al. (13) using Student t tests. All statistical analyses were conducted with Stata version 13.1 (StataCorp, College Station, Texas).
RESULTS
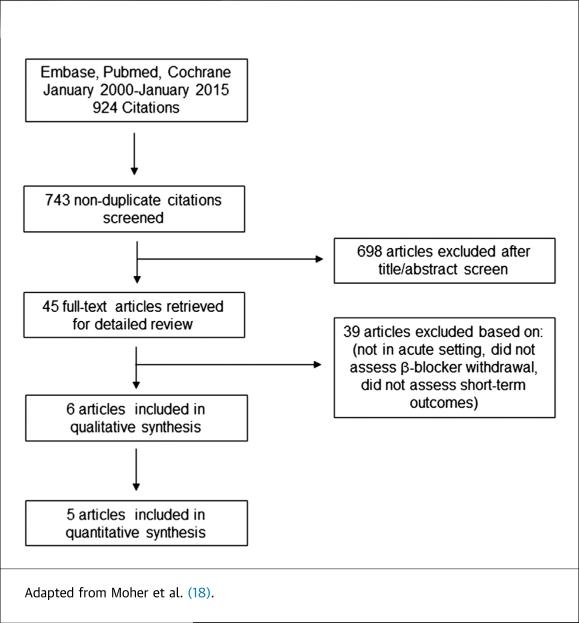
Our literature search revealed 743 nonduplicate articles; 698 articles were excluded after reviewing the title and abstract (Figure 1). After reviewing 45 manuscripts, final analysis was conducted from 1 randomized clinical trial and 5 observational studies (Figure 1), which are summarized in the Online Table 1. Of studies that were amenable to meta-analysis, there were 2,155 patients that continued beta-blocker therapy and 399 patients that discontinued beta-blocker therapy.
FIGURE 1.
Study Flow Diagram
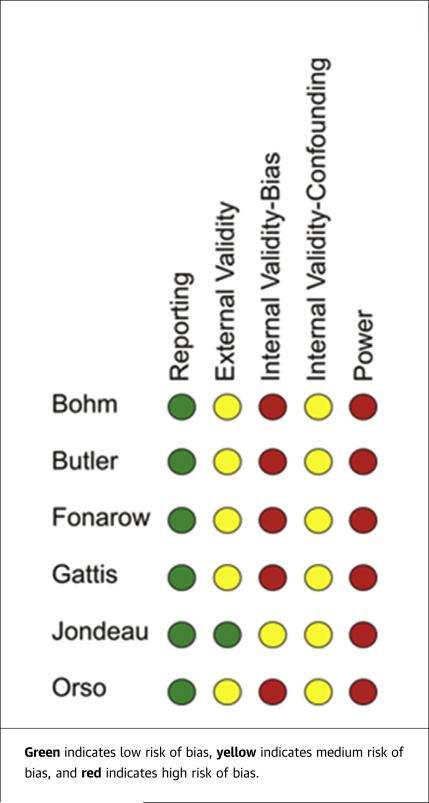
We conducted a bias analysis of all the identified studies and found low risk of reporting bias in all studies; medium risk of external validity bias in Bohm et al. (14), Butler et al. (15), Fonarow et al. (16), Gattis et al. (17), and Orso et al. (13); and low risk of external validity bias in Jondeau et al. (12). High risk of internal validity-bias was found in Bohm, Butler, Fonarow, Gattis, and Orso with medium risk in Jondeau. There was medium risk for internal validity-confounding bias in all studies, and high risk of power bias in all studies (Figure 2).
FIGURE 2.
Risk of Bias Assessment
Heart rate and systolic blood pressures were extracted from these studies to assess for possible differences in disease severity in the retrospective studies and for indications to discontinue beta-blocker therapy. There were no significant differences in systolic blood pressure and heart rate when comparing the groups that either continued or discontinued beta-blocker therapy in any of the studies (Online Table 2). The Bohm et al. (14) study did not report specific numbers for heart rate or systolic blood pressure, but did report there were no differences in rates of hypo-tension or bradycardia between the 2 groups.
IN-HOSPITAL MORTALITY
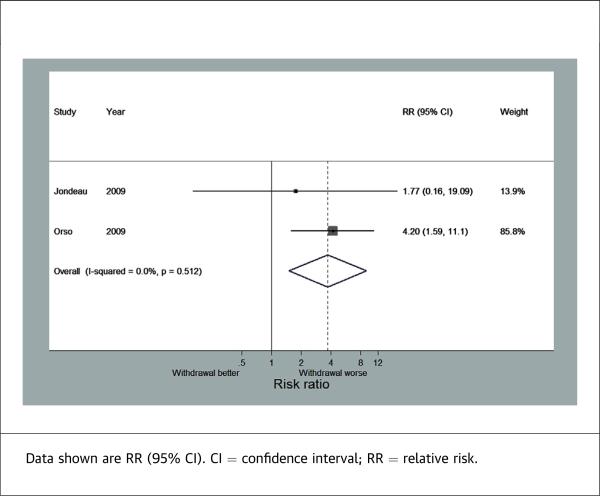
Two of the studies reported in-hospital mortality, which combined consisted of 431 patients that continued beta-blocker therapy and 219 patients that stopped beta-blocker therapy. Discontinuation of beta-blockers was associated with significantly increased risk of in-hospital mortality (RR: 3.72; 95% CI: 1.51 to 9.14) (Figure 3). There was no heterogeneity observed between these 2 studies (I2 = 0%).
FIGURE 3.
Forest Plot for In-Hospital Mortality
SHORT-TERM MORTALITY
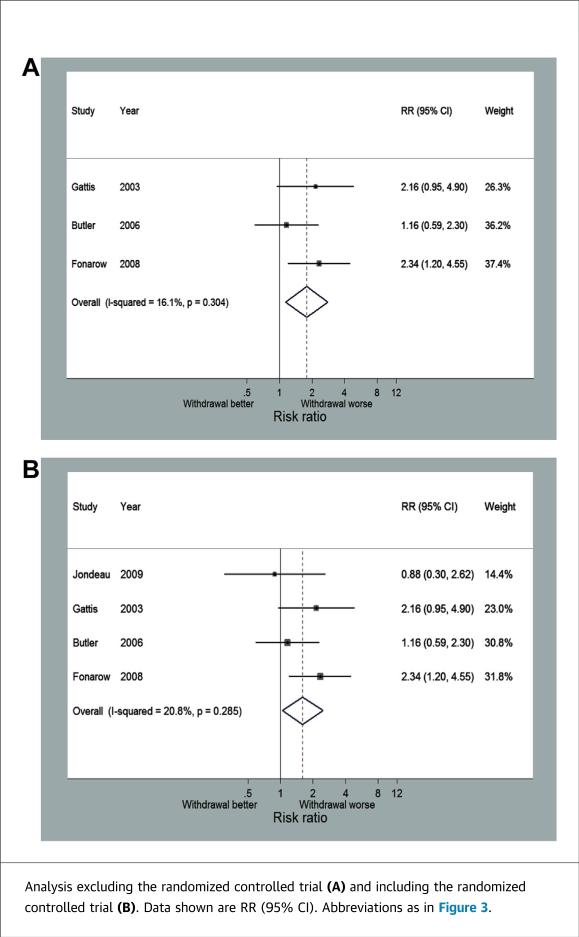
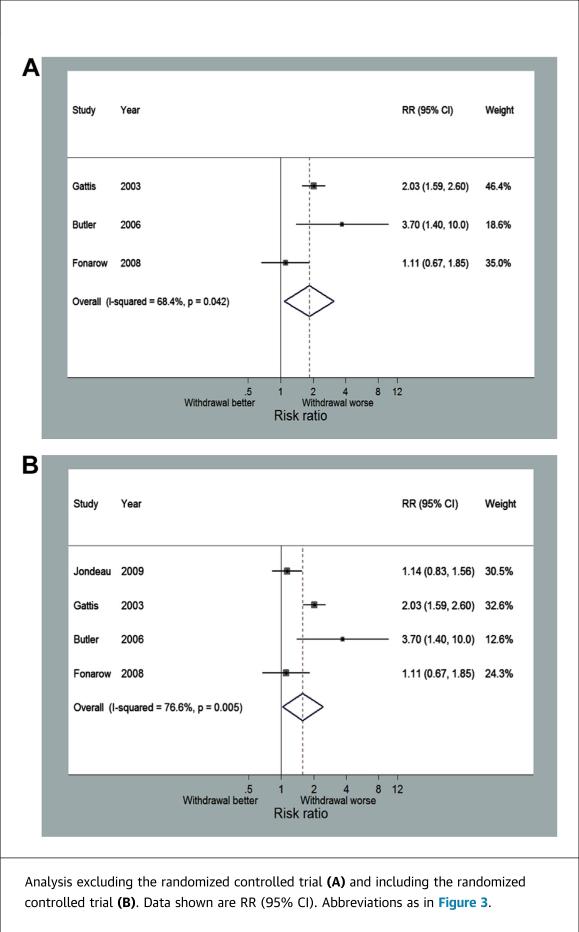
Four studies, including the RCT, reported short-term mortality with follow-up ranging from 60 to 180 days. Across the 3 observational studies, there were 1,724 patients that continued beta-blocker therapy and 180 patients that discontinued beta-blockers. Discontinuation of beta-blockers was associated with a significantly increased risk of death (RR: 1.78; 95% CI: 1.13 to 2.79) (Figure 4A). There was no significant variability between these 3 studies (I2 = 16.1%). There was no difference between this pooled estimate and the RCT estimate, and moreover, the combined estimate minimally changed with the addition of the RCT (RR: 1.61; 95% CI: 1.04 to 2.49) (Figure 4B).
FIGURE 4.
Forest Plot for Short-Term Mortality
SHORT-TERM REHOSPITALIZATION OR MORTALITY
Four of the studies reviewed including the RCT reported a combined endpoint of short-term rehospitalization or mortality with follow-up ranging from 60 to 180 days. A total of 1,724 patients continued beta-blocker therapy and 180 patients discontinued beta-blocker therapy across the 3 observational studies. Discontinuation of beta-blockers was associated with an increased risk of the combined endpoint of rehospitalization or mortality (RR: 1.84; 95% CI: 1.08 to 3.1) (Figure 5A). There was significant heterogeneity between these 3 studies (I2 = 68%). There was no difference between the combined effect in the observational studies and the RCT estimate, and there was no significant change in the pooled estimate when the RCT was included (RR: 1.59; 95% CI: 1.03 to 2.45) (Figure 5B).
FIGURE 5.
Forest Plot for Combined Short-Term Mortality or Rehospitalization
BETA-BLOCKERS AND INOTROPE USAGE
Our search identified 2 studies that examined the effects of beta-blocker withdrawal in patients treated with inotropes. In the Gattis et al. (17) study, discontinuation of beta-blockers in the milrinone-treated group was associated with an odds ratio of 6.48 (95% CI: 1.75 to 23.98) for death at 60 days and 1.47 (95% CI: 0.56 to 3.90) for death or hospitalization at 60 days in an un-adjusted assessment. Although not amenable to meta-analysis, the Bohm et al. (14) study examined the effects of beta-blocker withdrawal in patients treated with either dobutamine or levosimendan and showed a nonsignificant reduction in risk of death at 31 days (hazard ratio: 0.49; 95% CI: 0.086 to 2.77) and 180 days (hazard ratio: 0.64; 95% CI: 0.29 to 1.46) in an adjusted model when beta-blockers were continued.
DISCUSSION
Because of the small number of studies available, we combined the only randomized clinical trial with observational studies in our meta-analysis. One concern with this approach is that the retrospective studies may be biased on the basis of their retrospective design. However, the Jondeau et al. (12) study showed a trend toward reductions for inhospital mortality and in short-term mortality or rehospitalization with continuation of beta-blockers, which was in agreement with the retrospective studies. Perhaps the Jondeau et al. (12) study was underpowered to detect any differences with only 145 patients in the trial. Furthermore, bias assessment showed the retrospective studies had similar bias profiles when compared with the Jondeau study, indicating the studies were comparable in quality and thus amenable to meta-analysis.
Although only 2 studies specifically examined the effects of beta-blocker withdrawal in patients treated with inotropes, there was a trend toward reduction in short-term death or rehospitalization in the group that continued beta-blockers. A possible explanation for the improvements in outcomes was the antiar-rhythmic effects of beta-blockers, which is of particular importance because inotropes are proarrhythmic. Unfortunately, the Bohm et al. (14) and Gattis et al. (17) studies did not report arrhythmia-related events during hospitalization or following discharge. Nonetheless, these results suggest that continuation of beta-blockers in patients treated with milrinone but not likely dobutamine may reduce short-term adverse events, because beta-blockers reduce the positive hemodynamic effects of dobutamine (22).
The mechanism by which continuation of beta-blockers resulted in improvements in short-term outcomes may have been explained by increased beta-blocker use following hospitalization. Jondeau et al. (12) reported a significant reduction in 3-month post-discharge beta-blocker therapy in the group that discontinued therapy during hospitalization (76% vs. 90%). Similarly, the Fonarow et al. (16) study showed a reduction in beta-blocker usage in the discontinuation group at the 60- to 90-day follow-up (56.5% vs. 93.6%). The IMPACT-HF study also reported that the rate of beta-blocker use at 60 days after discharge was 91% when initiated before discharge, in contrast to 73% when initiated post-discharge, highlighting the inertia that can follow decisions made in the inpatient setting (23). Taken together, these data suggest discontinuation of beta-blockers during hospitalization results in reduced beta-blocker usage at follow-up, which is likely to contribute to poorer long-term outcomes.
Another explanation for the beneficial effects of beta-blocker therapy in ADHF could have been that the patients that stopped beta-blockers were end-stage heart failure patients with poor hemodynamics. This seems less likely because there were no reported significant differences in systolic blood pressure in any of the studies (Online Table 2), and blood pressure was an adjusting variable in all of the retrospective studies (Online Table 1). Moreover, the Butler study showed discontinuation of beta-blockers was not associated with differences in invasive hemodynamics because right atrial pressure (13 ± 7 mm Hg vs. 12 ± 10 mm Hg), wedge pressure (25 ± 9 mm Hg vs. 23 ± 9 mm Hg), and cardiac index (2.0 ± 0.6 l/min/m2 vs. 2.1 ± 0.6 l/min/m2) were not significantly different between the 2 groups. However, we cannot exclude the possibility that patients may have decompensated during hospitalization and beta-blocker therapy was stopped because of severity of illness.
Although our study examined the effects of continuation versus stopping beta-blockers in ADHF, another strategy often used is reduction of beta-blocker dose. Although not specifically investigated in our study, we did come across 1 paper that showed that reduction of beta-blocker dose during ADHF was associated with increased 5-year mortality when compared with no dose change and survival was the same as those that discontinued beta-blockers (24). Future studies assessing beta-blocker dose changes in ADHF may be useful to further define how to handle beta-blockers in ADHF.
STUDY LIMITATIONS
First, this analysis contained retrospective data that are subject to bias. To minimize the risk of bias, we used the most adjusted data, but the Butler et al. (15) study did not report adjusted values for short-term mortality and the Gattis et al. (17) study did not report adjusted values so we used the reported crude data to calculate effect estimates. Another limitation is that some of the studies had different timing in endpoints, which may have contributed to some variability in the meta-analysis. However, studies that had longer duration of follow-up had higher risks of adverse event, which was consistent with a retrospective analysis of the COMET trial that showed that as time from hospitalization increased, the difference in outcomes between those that continued beta-blocker and those that discontinued beta-blockers became more apparent (24).
CONCLUSIONS
Our meta-analysis showed that continuation of beta-blockers in ADHF was associated with significant reductions in risk of in-hospital mortality, short-term mortality, and short-term combined rehospitalization or death. These data suggest that beta-blockers should be continued in ADHF if the clinical situation allows in an attempt to reduce adverse outcomes.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: ADHF is a major problem facing clinicians, and our data suggest that continuation of beta-blockers in ADHF reduces adverse events in the short-term.
TRANSLATIONAL OUTLOOK: Additional clinical studies are needed to determine if beta-blocker dose should be reduced or unchanged in ADHF to further define the optimal treatment for this patient population.
ACKNOWLEDGMENT
The authors thank Amy Claus-sen for assistance with the literature search.
ABBREVIATIONS AND ACRONYMS
- ADHF
acute decompensated heart failure
- CI
confidence interval
- RCT
randomized controlled trial
- RR
relative risk
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
APPENDIX For supplemental tables, please see the online version of this article.
REFERENCES
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353:2001–7. [PubMed] [Google Scholar]
- 3.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 4.Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–8. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 5.The CONSENSUS trial study group Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316:1429–35. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 6.The SOLVD investigators Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 7.Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049–57. doi: 10.1056/NEJMoa042934. [DOI] [PubMed] [Google Scholar]
- 8.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study investigators. N Engl J Med. 1999;341:709–17. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 9.Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 10.Gheorghiade M, Vaduganathan M, Fonarow GC, Bonow RO. Rehospitalization for heart failure: problems and perspectives. J Am Coll Cardiol. 2013;61:391–403. doi: 10.1016/j.jacc.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 11.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–28. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 12.Jondeau G, Neuder Y, Eicher JC, et al. B-CONVINCED: Beta-blocker CONtinuation vs. INterruption in patients with congestive heart failure hospitalizED for a decompensation episode. Eur Heart J. 2009;30:2186–92. doi: 10.1093/eurheartj/ehp323. [DOI] [PubMed] [Google Scholar]
- 13.Orso F, Baldasseroni S, Fabbri G, et al. Role of beta-blockers in patients admitted for worsening heart failure in a real world setting: data from the Italian survey on acute heart failure. Eur J Heart Fail. 2009;11:77–84. doi: 10.1093/eurjhf/hfn008. [DOI] [PubMed] [Google Scholar]
- 14.Bohm M, Link A, Cai D, et al. Beneficial association of beta-blocker therapy on recovery from severe acute heart failure treatment: data from the survival of patients with acute heart failure in need of intravenous inotropic support trial. Crit Care Med. 2011;39:940–4. doi: 10.1097/CCM.0b013e31820a91ed. [DOI] [PubMed] [Google Scholar]
- 15.Butler J, Young JB, Abraham WT, et al. Beta-blocker use and outcomes among hospitalized heart failure patients. J Am Coll Cardiol. 2006;47:2462–9. doi: 10.1016/j.jacc.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 16.Fonarow GC, Abraham WT, Albert NM, et al. Influence of beta-blocker continuation or withdrawal on outcomes in patients hospitalized with heart failure: findings from the OPTIMIZE-HF program. J Am Coll Cardiol. 2008;52:190–9. doi: 10.1016/j.jacc.2008.03.048. [DOI] [PubMed] [Google Scholar]
- 17.Gattis WA, O'Connor CM, Leimberger JD, Felker GM, Adams KF, Gheorghiade M. Clinical outcomes in patients on beta-blocker therapy admitted with worsening chronic heart failure. Am J Cardiol. 2003;91:169–74. doi: 10.1016/s0002-9149(02)03104-1. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG, for the PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–84. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galvagno SM, Jr., Thomas S, Stephens C, et al. Helicopter emergency medical services for adults with major trauma. Cochrane Database Syst Rev. 2013;3:CD009228. doi: 10.1002/14651858.CD009228.pub2. [DOI] [PubMed] [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Metra M, Nodari S, D'Aloia A, et al. Beta-blocker therapy influences the hemodynamic response to inotropic agents in patients with heart failure: a randomized comparison of dobutamine and enoximone before and after chronic treatment with metoprolol or carvedilol. J Am Coll Cardiol. 2002;40:1248–58. doi: 10.1016/s0735-1097(02)02134-4. [DOI] [PubMed] [Google Scholar]
- 23.Gattis WA, O'Connor CM, Gallup DS, Hasselblad V, Gheorghiade M, for the IMPACT-HF Investigators and Coordinators Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: results of the initiation management predischarge: process for assessment of carvedilol therapy in heart failure (IMPACT-HF) trial. J Am Coll Cardiol. 2004;43:1534–41. doi: 10.1016/j.jacc.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 24.Metra M, Torp-Pedersen C, Cleland JG, et al. Should beta-blocker therapy be reduced or withdrawn after an episode of decompensated heart failure? Results from COMET. Eur J Heart Fail. 2007;9:901–9. doi: 10.1016/j.ejheart.2007.05.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.