Abstract
The esophagus and trachea are tubular organs that initially share a single common lumen in the anterior foregut. Several models have been proposed to explain how this single-lumen developmental intermediate generates two tubular organs. However, new evidence suggests that these models are not comprehensive. I will first briefly review these models and then propose a novel ‘splitting and extension’ model based on our in vitro modeling of the foregut separation process. Signaling molecules (e.g., SHHs, WNTs, BMPs) and transcription factors (e.g., NKX2.1 and SOX2) are critical for the separation of the foregut. Intriguingly, some of these molecules continue to play essential roles during the transition of simple columnar into stratified squamous epithelium in the developing esophagus, and they are also closely involved in epithelial maintenance in the adults. Alterations in the levels of these molecules have been associated with the initiation and progression of several esophageal diseases and cancer in adults.
INTRODUCTION
During development, the anterior portion of the foregut begins as a single lumen that separates into two tubes, the trachea on the ventral side and the esophagus on the dorsal side. Although the exact cellular and molecular mechanisms by which this separation occurs remain largely unknown, multiple models have been proposed (see below and reviews by Que et al.1 and by Billmyre et al.2) (Figure 1). Mouse genetic studies have shown that the dorsal–ventral patterning of certain signaling molecules [e.g., bone morphogenetic proteins (BMPs) and SHH] and transcription factors (e.g., SOX2 and NKX2.1) in the early foregut prior to the separation is crucial for the generation of an intact esophagus and trachea (Figure 2(a)). Disruption of this unique patterning leads to the failure of foregut separation and the formation of a relatively common birth defect (approximately one out of 3500 newborns) known as esophageal atresia with/without tracheoesophageal fistula (EA/TEF) (see review by Jacobs et al.3). Intriguingly, some of the signaling molecules (e.g., BMPs) and transcription factors (e.g., SOX2) involved in foregut development continue to play essential roles for the morphogenesis of the epithelium in the developing esophagus.4,5 More importantly, recent studies suggest that reactivation of relevant signaling pathways (e.g., BMPs) or overexpression of some of the transcription factors are associated with the development of esophageal diseases including gastroesophageal reflux (GERD), Barrett’s esophagus (BE),6,7 and esophageal cancers.8,9 Notably, BE is characterized by the replacement of stratified squamous epithelium which contains multiple layers of cells (click here to link to the description of stratified squamous epithelium, Figure 5(a)) with simple columnar epithelium which is composed of a single layer of columnar cells (click here to link to the description of simple columnar epithelium, Figure 5(b)) at the gastroesophageal junction (GEJ).
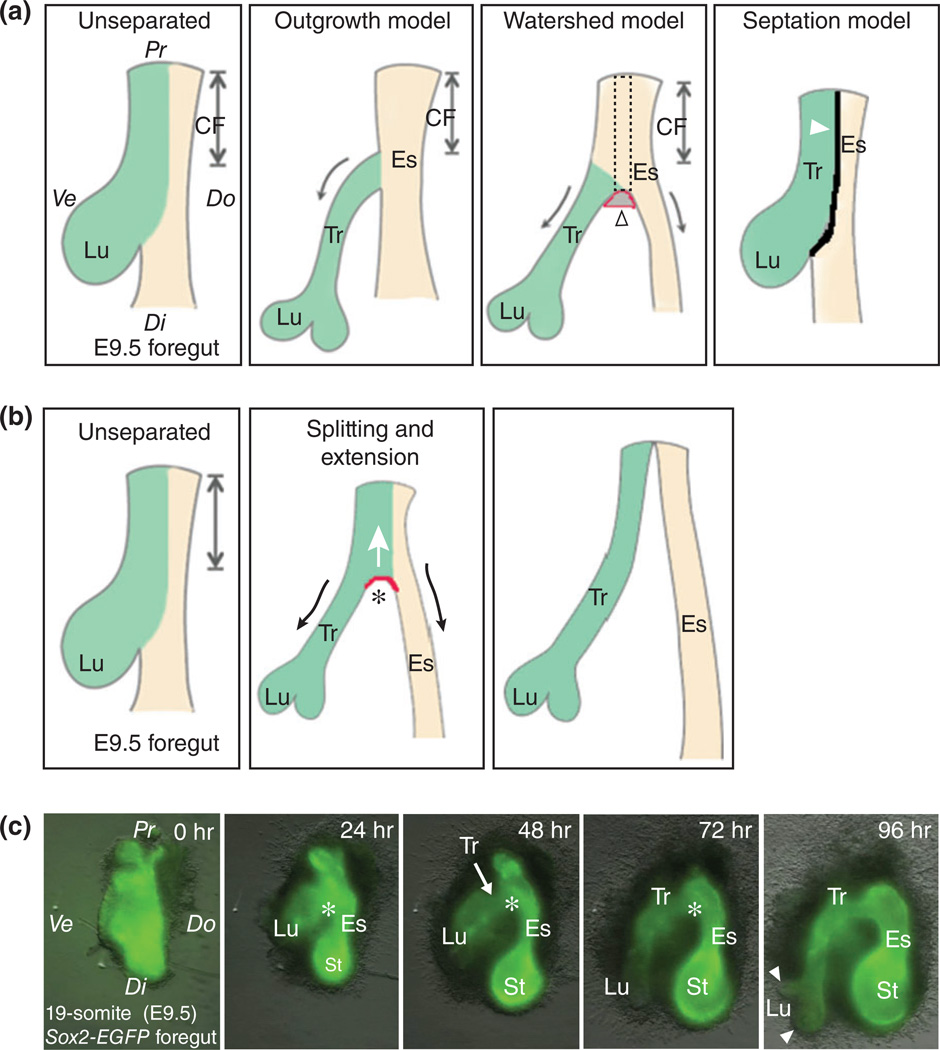
FIGURE 1.
Old and new models of tracheal–esophageal separation. (a) Schematic presentation of three old models of foregut separation: (1) The outgrowth model in which the trachea extends from the common foregut tube as the lung buds grow, while the common foregut tube becomes the esophagus. The arrows indicate the extension of the trachea and esophagus; (2) the watershed model in which both the trachea and esophagus elongate while separated by a mesenchymal septum that serves as a wedge to prevent the extension of the lateral wall at the dorsal–ventral midline. The empty arrowhead indicates hypothetical mesenchymal condensation which has yet to be identified. According to this model, increased proliferation is expected to occur at the ventral and dorsal sides as compared to the midline lateral wall (the dotted rectangle region) of the common foregut; and (3) the septation model in which the epithelial cells at the dorsal–ventral midline make contact across the lumen and fuse to form a septum. The arrowhead indicates the septum. (b) The new model, the ‘splitting and extension model’, proposes that the separation of the trachea and esophagus initiates at the level where the lung grows out and moves rostrally. A saddle-like structure (red arc) moves up and splits the anterior foregut. Meanwhile, the nascent trachea and esophagus extend their lengths as indicated by arrows. This model is based on live-imaging of the cultured anterior foregut which was isolated from E9.5 Sox2-EGFP embryos. (c) Snapshots of the foregut culture that was maintained for 96 h. Note the bottom-up splitting of the foregut and extension of the trachea and esophagus. Also note that the fluorescent intensity of GFP is stronger in the esophagus and stomach than the trachea and lung. See Movie S1 for details. Asterisk indicates the epithelial saddle. Arrowheads indicate lung buds. Abbreviations: Ve, ventral; Do, dorsal; Pr, proximal (rostral); Di, distal (caudal); CF, common foregut; Lu, lung; St, stomach; Es, esophagus; Tr, trachea.
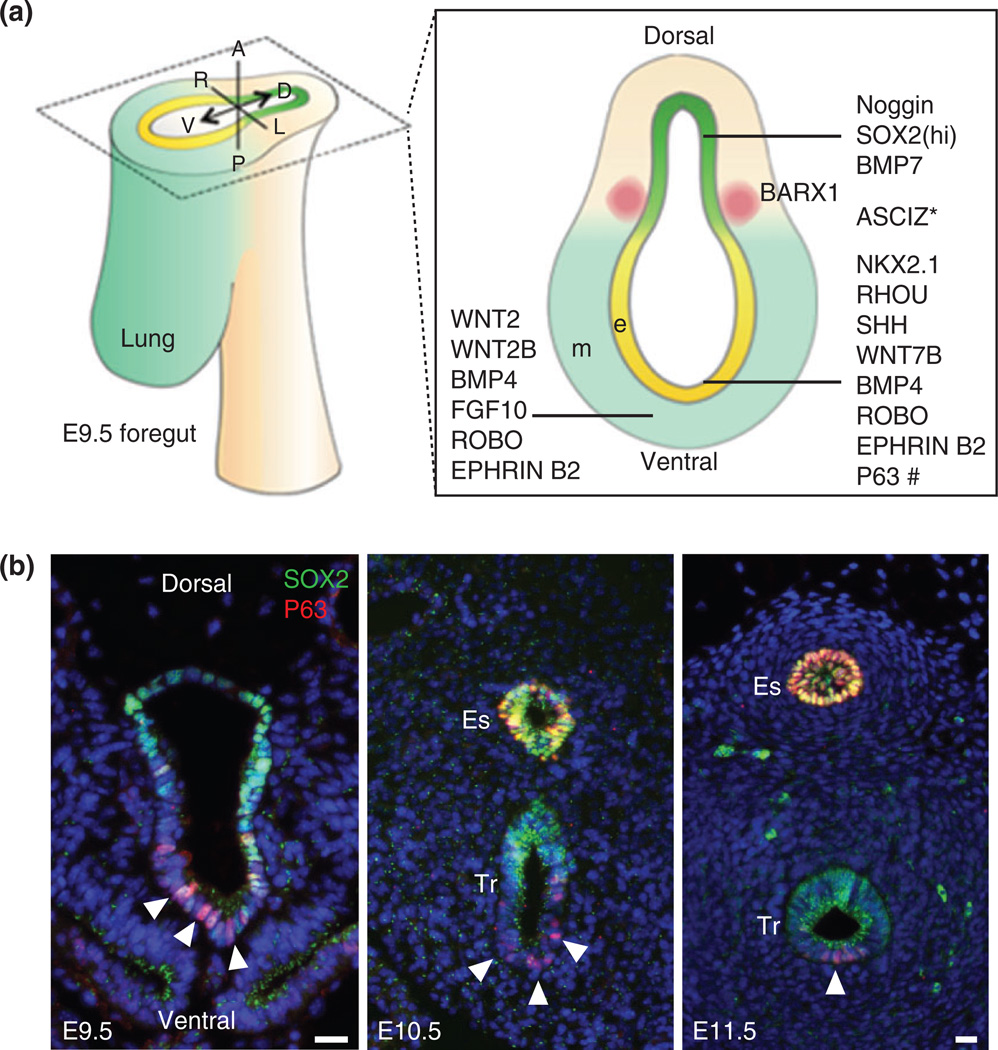
FIGURE 2.
Dorsal–ventral patterning of signaling molecules and transcription factors during the separation of the trachea and esophagus. (a) Schematic diagram of molecules located in the epithelium and mesenchyme of the E9.5 anterior foregut. Note that BARX1 is enriched in the mesenchyme of the tracheoesophageal groove. * The expression pattern of ASCIZ (ATM substrate Chk2-interacting Zn2+-finger protein) remains to be determined. # Trachea-esophageal separation seems normal in the available p63 deletion mutants. (b) Expression of the transcription factors SOX2 and P63 during the separation of the early foregut. Note that P63 is expressed in some of the epithelial cells in the ventral side of the foregut prior to separation (arrowheads). Abbreviation: e, epithelium; m, mesenchyme; Tr, trachea; Es, esophagus. Scale bar: 50 µm.
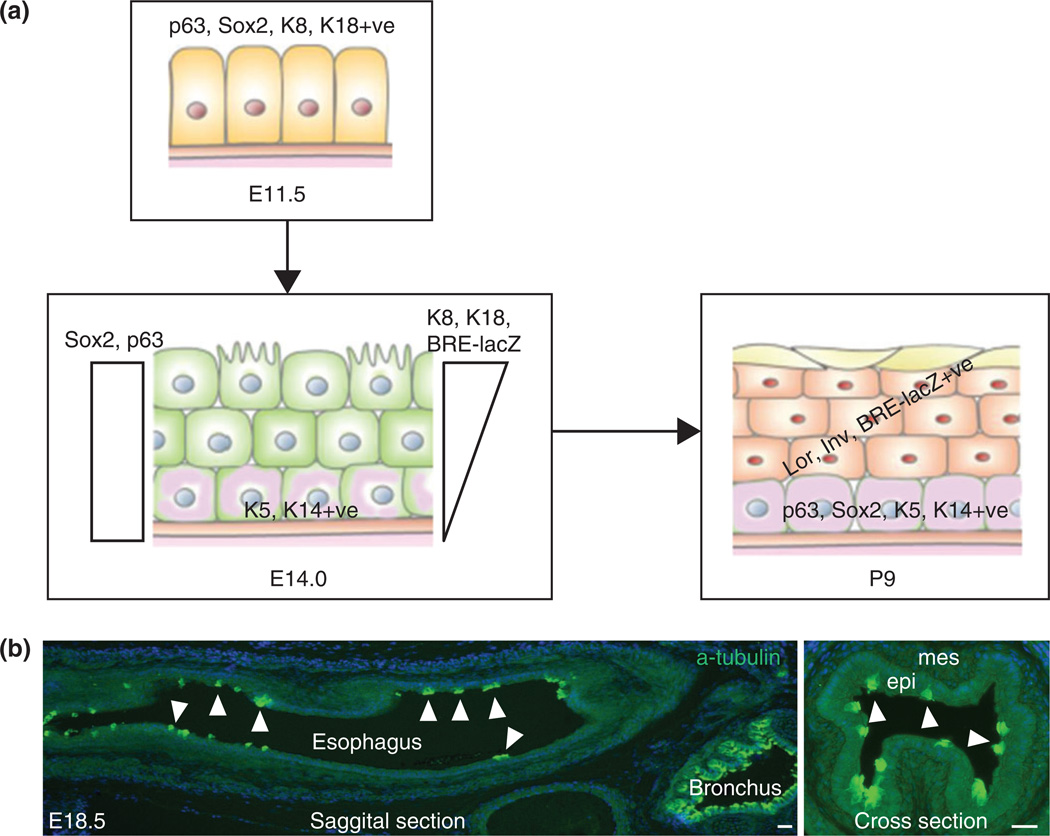
FIGURE 5.
Conversion of simple columnar to stratified squamous epithelium in the developing esophagus. (a) The epithelium in the nascent esophagus is simple columnar and expresses SOX2, P63, K8, and K18. The epithelial stratification starts with the reduction of K8 and K18 and gain of K5 and K14 in the basal layer. Activation of BMP signaling as reported by the BRE-lacZ allele is required for the squamous differentiation of top layers of the epithelium. (b) Ciliated cells detected by a-tubulin labeling, distribute at intervals in the E18.5 esophagus. Ciliated cells can be detected from E13.5 to p3. Arrowheads indicate ciliated cells (light green). Abbreviation: epi, epithelium; mes, mesenchyme. Scale bar: 50 µm.
In this review, I will first overview previous models of foregut separation and propose a novel model based on our in vitro modeling of the separation process. I will then focus on molecules regulating epithelial morphogenesis in the esophagus after it is established from the foregut. I will also briefly discuss how relevant molecules have been involved in the pathogenesis of esophageal diseases in the adult, including the discussion of relevant animal models.
OLD AND NEW MODELS OF TRACHEAL–ESOPHAGEAL SEPARATION
Based on histological characterization of the developing foregut, three models have been proposed previously to explain how the anterior foregut generates the trachea and esophagus (see reviews by Que et al.1 and Billmyre et al.2) (Figure 1(a)). Briefly, they include: (1) the outgrowth model in which the trachea extends from the foregut tube as the lung buds grow, while the common foregut tube anterior to the lung budding sites becomes the esophagus10,11 (see Figure 1(a)); (2) the watershed model in which both the trachea and esophagus elongate while separated by a mesenchymal septum that serves as a wedge to prevent the caudal extension of the lateral wall at the dorsal–ventral midline12 (see Figure 1(a)); and (3) the septation model in which the epithelial cells at the dorsal–ventral midline make contact across the lumen and fuse to form a septum (see Figure 1(a)). The formation of the septum starts from the level where the lung grows out and moves rostrally to separate the common foregut tube into the esophagus and trachea.13
However, recent evidence suggested that none of these models fully account for tracheal–esophageal separation. For example, the outgrowth model predicts that increased proliferation drives the outgrowth of the trachea at the ventral side of the foregut. However, this has not been observed.14 Moreover, according to this model the common foregut tube is destined to become esophagus. However, the expression of the tracheal marker Nkx2.1 is observed in the epithelium of the ventral common foregut.1,5 Similarly, in the watershed model, as the trachea and esophagus extend caudally, increased cellular proliferation is expected to occur in the dorsal and ventral regions as compared to the midline of the lateral walls (Figure 1(a)). However, this has not been detected.14 In addition, one would expect that the common foregut above the mesenchymal wedge would remain undivided if this model holds true. Contrary to this prediction, the length of the undivided foregut becomes shorter and shorter as separation proceeds.14 Although the septation model is well received in the field,12 the predicted septum has not been detected by scanning electron microscopy in the chicken foregut during the separation.15 Notably, an epithelial ‘saddle’ develops at the caudal (distal) end of the anterior foregut when the lung buds form, and this saddle continues to be present at the location where the trachea and esophagus are separating.15 Therefore, it is possible that this saddle moves rostrally to divide the foregut into the trachea and esophagus.
Consistent with this possibility, our live-imaging of actively separating E9.5 Sox2-EGFP foregut (EGFP labels all of the epithelium in the anterior foregut) shows a rostral translocation of a saddle-like structure that splits the anterior foregut into the trachea and esophagus (Figure 1(b) and (c)) (Movie 1). Meanwhile, the extension of both the trachea and esophagus is observed as the saddle-like structure moves up (Figure 1(b) and (c)), suggesting a co-existence of splitting and extension. We therefore call this new model the ‘splitting and extension model’ (Figure 1(b)). Notably, although this might be an artifact of culture condition, mesenchymal cells in this organ culture system migrate away from the epithelium extensively, rather than forming a condensed wedge as proposed in the watershed model, suggesting that the bottom-up movement of the saddle is unlikely driven by the mesenchyme. In other words, it is likely that the separation process is driven by the epithelium.
Additionally, we observed a constriction site spanning a short segment in the epithelial tube at the upper half of the common foregut (Figure 3(a)–(d)). This constriction site coincides with the presence of a stricture in the upper part of the esophagus. Indeed ‘recanalization’ has been thought to be a critical step in the development of the esophagus.16 Therefore it is tempting to postulate this constriction site may serve as a road block when the saddle moves up in some mutants (e.g., NOG−/−), leading to the formation of TEF. How does this happen? Studies of NOG−/− mutants suggested an abnormal detachment of the notochord from the early endoderm that took away endodermal cells, leaving too few epithelial cells for the establishment of the esophagus.1,17,18 It is possible this abnormal delamination affects the restriction site, and movement of the saddle is stopped at this position, leading to the formation of TEF in the NOG−/− mutants (Figure 3(e) and (f)). Alternatively, abnormal dorsal–ventral patterning of signaling molecules and transcription factors may shift the boundary between the future trachea and esophagus as seen in Sox2 hypomorphic and Nkx2.1−/− mutants.5 This D–V boundary shift may also affect the restriction site and block the moving of the saddle. Testing this possibility requires high-resolution 3D reconstruction imaging tools coupled with immunostaining using the territory makers indicated in Figure 2(a) to quantitate the difference at the restriction site between mutants and wildtypes.
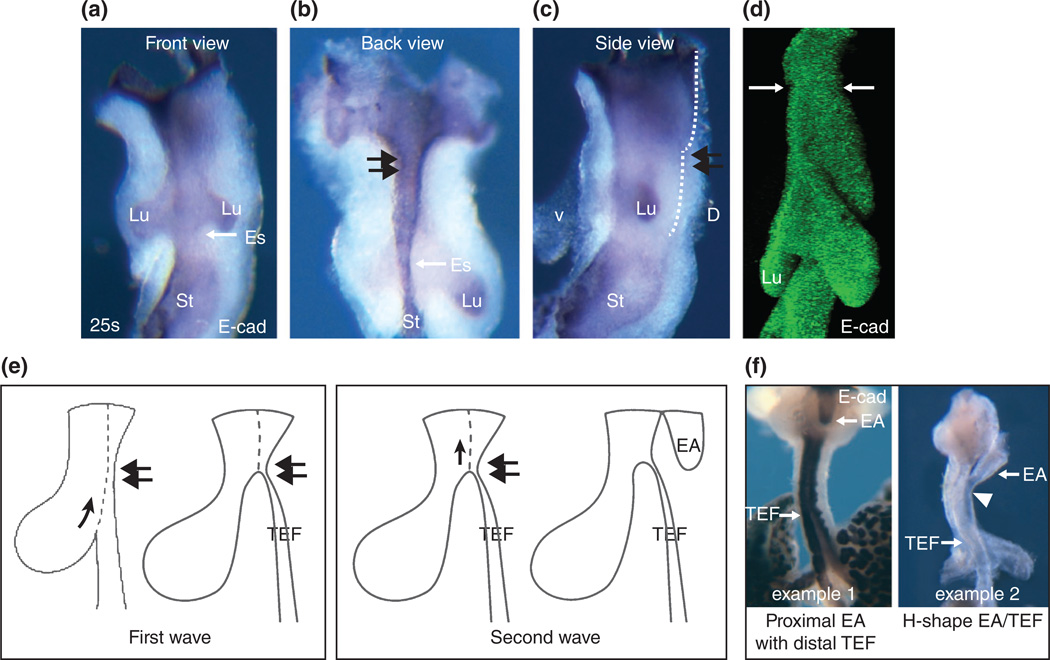
FIGURE 3.
Constriction site in the upper common foregut tube and its implication in the formation of EA/TEF. (a–c) The front, back and side views of the E9.5 (25 somite stage) foregut stained with E-cadherin antibody to visualize the epithelium. The double arrows indicate the constriction site (a short segment). (d) 3-D reconstruction of the epithelial tube of the E9.5 foregut stained with E-cadherin. Note the constriction site at the upper half of the tube (arrows). (e) EA/TEF formation model: Two waves of bottom-up movement of the epithelial saddle during the formation of EA/TEF in genetic mutants (e.g., Nog−/−). The first wave of movement stops at the constriction site (double arrows) and leads to the formation of TEF. The second wave starts right above the constriction site and leads to the formation of EA. (f) The most common EA/TEF is proximal EA with distal TEF as illustrated in some Nog−/− mutants (example 1). TEF can be detached from the trachea and becomes the isolated TEF. Similarly, EA/TEF can be linked to the trachea with a common tube, leading to the formation of H-shape EA/TEF as seen in a limited number of Nog−/− mutants (example 2).
How does EA develop? It is likely that there is a second wave of bottom-up movement of a new saddle right above the constriction site in the mutants, leading to the formation of EA (Figure 3(e) and (f)). Intriguingly, in all of the presentations of EA/TEF, the separation of the top common foregut appears normal which leads to the complete separation the trachea and esophagus at the proximal end (Figure 3(e) and (f)). The resulting anterior part of the esophagus is the EA. This raises the possibility that the second wave of bottom-up movement of the new saddle is independent of the original one (to see pictures of various EA/TEF follow this Link).
Many questions arise from this new tracheal–esophageal separation model. For example, what drives the movement of the saddle? Does the saddle act as a zipper to bring together epithelial cells from opposite sides of the common foregut wall? What are the underlying cellular and molecular mechanisms? How does the dorsal–ventral patterning of signaling molecules and transcription factors integrate into this model? Answers to these questions will provide us with an entirely new perspective on early foregut morphogenesis as well as mechanistic insights into the pathobiology of foregut malformations including EA/TEF.
OVERVIEW OF MORPHOGENETIC PROCESSES IN THE DEVELOPING ESOPHAGUS
When the esophagus is established from the anterior foregut at around embryonic (E) day 11 (4–5 weeks of human gestation period), the epithelial tube is enclosed by layers of mesenchymal cells. Extensive proliferation and differentiation occurs in these two compartments. In the case of the mesenchyme, it eventually gives rise to the muscularis mucosa (a thin layer of smooth muscle) and the muscularis externa, with networks of blood vessels and nerves running throughout. The muscularis externa includes the inner circular and outer longitudinal layers of muscles (Figure 4). In humans, the upper third of the esophagus is skeletal muscle and the lower third, including the lower esophageal sphincter (LES), is smooth muscle, while the middle third is a mixture of both muscle types. By contrast, the muscularis externa in the adult mouse is composed of skeletal muscle with the exception of a short, broad segment below the diaphragm, including the LES, which is composed of smooth muscle.19 Characterization studies of mouse and human embryonic esophagus have suggested that the esophageal musculature begins as a smooth muscle-lined tube, with subsequent conversion to varying proportions of striated muscle in a cranial-caudal direction.20,21 Although controversy remains regarding the cellular origin of this striated muscle, the use of genetic mouse models has revealed that the homeobox transcription factors Foxp1 and Foxp2 are important for striated muscle development. Mutants lacking Foxp2 in a Foxp1 heterozygous background completely lose the striated muscle.22 In addition, Hoxc4 has also been implicated in muscle development, as removal of Hoxc4 leads to disorganized muscle layers over an extensive region in the mutant esophagus.16 These findings have been discussed in our previous review3 along with other mesenchyme-derived tissues.
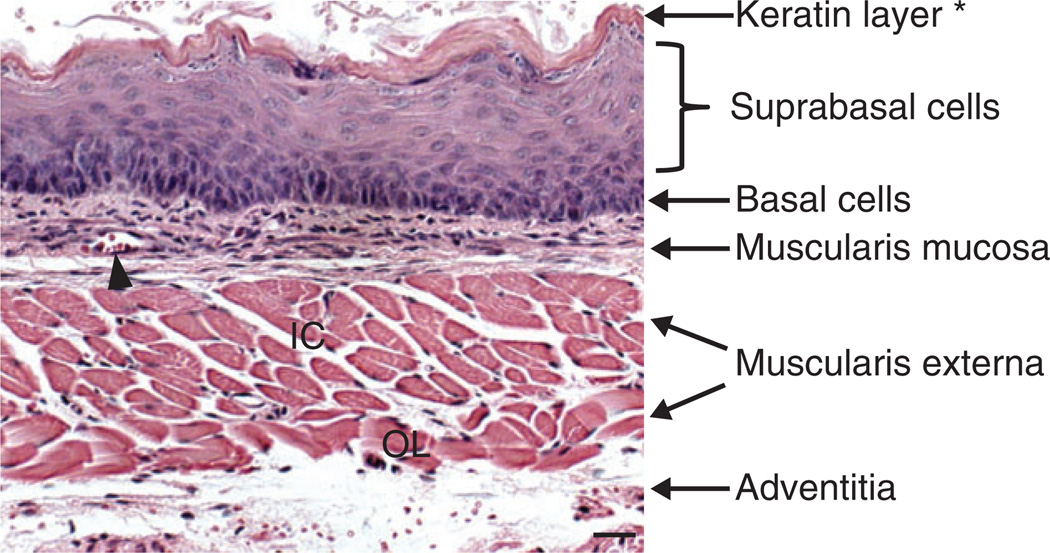
FIGURE 4.
Histology of the adult mouse esophagus. The stratified squamous epithelium consists of undifferentiated basal and differentiated suprabasal cells. Note that the epithelium is covered with a layer of keratin, which is not present in the human esophagus. The mesenchyme is composed of multiple layers of muscles (muscularis mucosa and muscularis externa which can be further divided into inner circular and outer longitudinal muscles). The esophagus is wrapped by the tunica adventitia, a layer of loose fibrous tissue. The arrowhead indicates a blood vessel. Abbreviation: IC, inner circular; OL, outer longitudinal. Scale bar: 50 µm.
The epithelium begins as simple columnar which contains a single layer of cell, Figure 5(b) in the nascent esophagus of both humans and mice and is gradually replaced by a stratified (multiple-layered) squamous epithelium composed of basal progenitor cells at the bottom layer and differentiated cells in the topmost layers (click here to link to the description of stratified squamous epithelium, Figure 5(a)). Histologically, the epithelium remains simple columnar in the human embryonic esophagus up until 8 weeks, at which time a subpopulation of cells become ciliated. The number of ciliated cells decreases starting from the 17th week and is completely lost when a non-keratinized stratified squamous epithelium is fully formed at birth.23 Notably, ciliated cells reappear upon long-term inflammation in GERD patients and persist in the form of multilayered epithelium, a pathological condition considered as a precursor of Barrett’s esophagus.24–26 In addition, during human embryonic esophageal development, residual islands of columnar epithelium remain along the longitudinal axis of the human esophagus and grow into the mesenchyme to form submucosal glands (SMG). The epithelium lining the SMGs remains simple columnar and the ducts of the glands are lined by cuboidal cells which later transition into stratified, squamous epithelium as they open to the lumen.27 Some species, including rat and mouse, do not have SMGs in the esophagus. Furthermore, there are some additional histological and structural differences between humans and rodents (Table 1). That being said, the conversion of simple columnar into stratified squamous epithelium occurs in both the human and rodent esophagus28 and studies of rodent models have provided important insights into our understanding of the morphogenesis of human esophageal epithelium.
TABLE 1.
Comparison of Human and Mouse Esophagus
| Length (adult) |
Epithelial Thickness |
Keratin Layer |
Submucosal Gland |
Forestomach Epithelium |
|
|---|---|---|---|---|---|
| Mouse | 1.0–1.5 cm | 3–5 cells | Yes | No | Stratified squamous |
| Human | 18–26 cm | 20–30 cells | No | Yes | Simple columnar |
DIRECT CONVERSION OF SIMPLE COLUMNAR TO STRATIFIED SQUAMOUS EPITHELIUM IN THE EARLY MOUSE ESOPHAGUS
Histological characterization of the mouse embryonic esophagus suggests that the stratified squamous epithelium is directly converted from simple columnar cells.29 The simple epithelium lining the nascent mouse esophagus at E11.5 expresses high levels of columnar markers cytokeratin 8 and 18 (K8, K18). These epithelial cells also express high levels of the transcription factors P63 and Sox24 (Figure 5(a)). When stratification begins, the bottom (basal) layer of the epithelium expresses lower levels of K8 and K18, meanwhile gaining the expression of the squamous cell markers K5 and K14.29 Throughout the stratification process the topmost layers of the epithelium maintain high levels of K8 and K18 until birth and completely lose their expression shortly thereafter. Although the top layers of cells maintain high levels K8 and K18 during the stratification, the squamous differentiation markers Involucrin and Loricrin can be detected starting from approximately E14.54 (Figure 5(a)). Similar to the human counterpart, some of the epithelial cells facing the lumen become ciliated at this stage, before disappearing shortly after birth30 (Figure 5(a) and (b)).
The brief overlap in the expression of K8/K18 with K5/K14 in the basal layer suggests that there is a direct conversion of simple columnar epithelium into squamous epithelium. This is further supported by an in vitro labeling experiment in which a plasmid construct containing K14-GFP was used to report the expression of K14 in the embryonic esophagus in an organ culture system.29 Consistent with in vivo immunostaining results, all epithelial cells initially express high levels of K8 but no GFP can be detected. After being cultured for 5 days, GFP expression can be detected in some of the K8 positive cells in the basal layer, supporting a direct conversion of K8+ ve into K14+ ve cells during the esophageal development.29
THE BMP SIGNALING PATHWAY PLAYS A TWO-STAGE ROLE IN THE MORPHOGENESIS OF THE ESOPHAGEAL EPITHELIUM
The BMP signaling pathway has been shown to control the development of many organs including the lung.31 Nevertheless, we have only recently begun to understand its role in the developing esophagus. When the esophagus is established from the early mouse foregut, the BMP ligand BMP7 and its inhibitor Noggin are enriched in the epithelium at the dorsal side of the unseparated foregut and maintain this expression pattern in the epithelium (Figure 5(a)). The presence of Noggin at this stage is critical for epithelial stratification. Deletion of Noggin leads to the ectopic formation of glandular units that are lined with secretory simple columnar epithelium.4 Notably, glandular units are not present in the normal mouse esophagus at any stages of development (Table 1). Consistently, ectopic activation of BMP signaling using the Shh-Cre mouse line combined with a conditional mouse line that harbors a constitutively activated Bmp receptor Ia knockin allele into the Rosa26 locus (Rosa26loxp-stop-loxp-caBmpr1a) also leads to the failure of the formation of the stratified epithelium. Shh-Cre activity can be detected in all of the foregut epithelium from E9.0, prior to the establishment of the esophagus. The simple columnar epithelium in the esophagus of Shh-Cre; Rosa26loxp-stop-loxp-caBmpr1a mutants expresses high levels of K8 and K18 but not K5 and K14.4 These findings suggest that suppressed BMP signaling in the nascent esophagus is not only critical for the initiation of the epithelial stratification but also plays important roles in the specification of the progenitor cells.
As the levels of Noggin decrease, activation of BMP signaling in the E14.5 esophagus can be detected in the top layers of the stratified epithelium using the BMP signaling reporter allele BRE-lacZ.4 Meanwhile, the expression of the progenitor markers SOX2 and P63 is decreased in these cells and accompanied by increased expression of the differentiation markers Loricrin and Involucrin. In addition, inactivation of BMP signaling blocks the differentiation of epithelial cells in the Shh-Cre; Bmpr1aloxp/loxp mutants and all cells maintain high levels of P63 and SOX2.4 These findings suggest that activation of BMP signaling at approximately E14.5 is required for the differentiation of suprabasal cells in the developing esophagus.
Taken together, both the BMP gain- and loss-of-function studies suggest that BMP signaling has stage-dependent roles in the epithelial morphogenesis of the developing esophagus. Noggin-mediated suppression of BMP signaling allows the initiation of stratification at E11.5, whereas BMP activation at approximately E14.5 is required for the squamous differentiation of suprabasal cells. Moreover, our recent study revealed that the Wnt signaling pathway is active in the epithelium of the developing mouse esophagus and forestomach which is also lined by the stratified squamous epithelium,3 but whether it is involved in epithelial morphogenesis remains to be elucidated.
THE ROLES FOR P63, SOX2, AND NRF2/KEAP1 IN THE MORPHOGENESIS OF ESOPHAGEAL EPITHELIUM
Some of the transcription factors that exhibit dorsal–ventral patterning continue to play essential roles in the conversion of simple columnar to stratified epithelium (Figure 2(a), 5(a)).
SOX2
SOX2 belongs to the B1 subgroup of the SOX [sex determining region Y-related HMG (High Mobility Group) Box] family of transcription factors.32 SOX2 is critical for the self-renewal of embryonic stem cells and lineage specification of progenitor cells in multiple tissues including the taste bud, retina and trachea.33–35 Embryos deficient in both copies of the Sox2 gene exhibit embryonic lethality shortly after implantation (approximately E6.0).36 However, embryos with a null allele (Sox2EGFP) and a hypomorphic allele (Sox2COND)35 survive until birth.5 Approximately 60% of Sox2EGFP/COND hypomorphic mutants develop EA/TEF,5 suggesting that SOX2 is critical for the separation of the anterior foregut into the esophagus and trachea. In the remaining mutants, the esophagus has clearly separated from the trachea but the epithelial cells lining the esophagus are disorganized. In the bottom third of the E18.5 esophagus, the epithelium remains simple columnar and secretes a large amount of mucin.5 The BMP pathway has been shown to negatively regulate the levels of SOX2 during the separation of the anterior foregut.37 We also found that the activation of BMP signaling in the E14.5 epithelium is correlated with the squamous differentiation of progenitor cells concomitant with reductions in SOX2 protein levels.4 It will be interesting to determine whether BMP also directly regulates the transcription of the Sox2 gene during epithelial morphogenesis in the developing esophagus.
SOX2 is enriched in the basal progenitor cells of the stratified epithelium in both human and mouse adult esophagus.8 Genetic ablation of the Sox2 gene leads to reduced self-renewal of the progenitor cells in organoid culture in which esophageal basal cells isolated from the esophagi of Sox2-CreER;Sox2CreER/loxp mice mutants were used and they were treated with Tamoxifen in vitro.38 Nevertheless, it remains unknown whether Sox2 is also required for the maintenance of the basal progenitor cells in vivo as in other adult tissues. Intriguingly, reduction in the protein levels of SOX2 and increased BMP signaling activity has been associated with the development of Barrett’s esophagus, although the underlying mechanisms remain unknown.6,39 On the other hand, increased SOX2 protein levels and Sox2 gene amplification have been found in approximately 30% of squamous cell carcinomas.9 When Sox2 is conditionally overexpressed in basal cells using the K5 promoter driven CreER (K5-CreER) mouse line,40 the progenitor cell populations are expanded due to decreased differentiation and increased proliferation.6 The combination of Sox2 overexpression with inflammatory signaling can further transform basal progenitor cells, resulting in the formation of poorly differentiated squamous cell carcinoma.6
P63
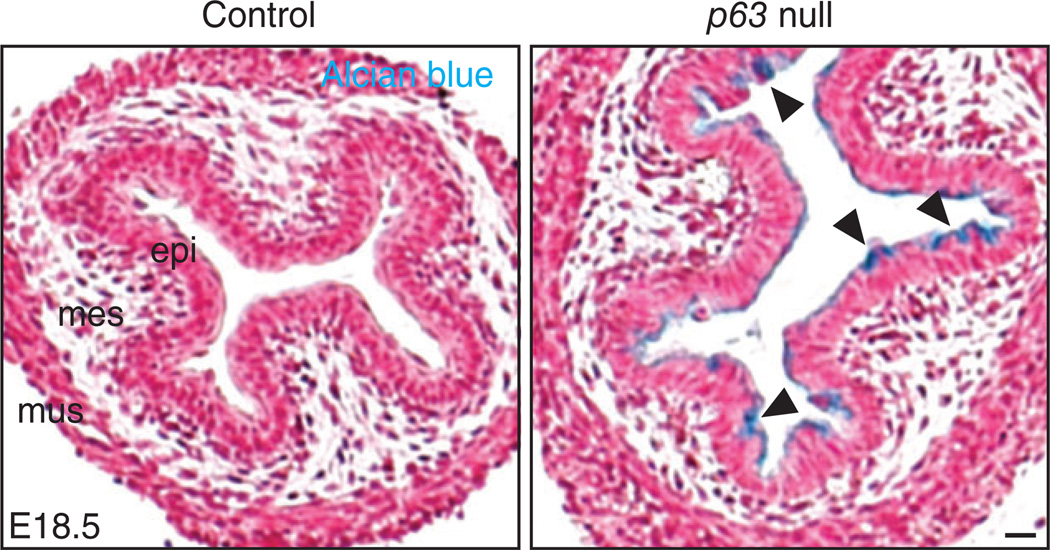
Trp-63 (P63) is a member of the P53 protein family which includes three transcription factors: P63, P53, and P73. P63 has two isoforms, TAP63 and ΔNP63 which have distinct functions in tissue development.41 For example, TAP63 plays important roles in oocyte development42 whereas ΔNP63 regulates the morphogenesis of the stratified epithelium in the skin and esophagus.30,43 Mice lacking P63 have truncated limbs and fail to form the stratified epithelium in both the skin and esophagus. The mutants die of dehydration soon after birth.44 When examined at birth, the epithelium in the mutant esophagus remains simple columnar with multiple cilia on the apical surface and K14 is not expressed in these cells.30 These findings suggest P63 deletion blocks the conversion of simple columnar cells into a squamous cell fate. In a separate study, Wang et al. found the simple columnar epithelium in the forestomach expresses genes that mark mucous-producing cells. Interestingly, some of these genes, e.g., Muc4 and Agr2, are actively transcribed in Barrett’s esophagus.45 We also found that the simple columnar epithelium lining the E18.5 P63−/− esophagus stains positively by Alcian blue at pH2.5 (Figure 6), indicative of mucin secretion, suggesting that P63 not only regulates epithelial stratification but also controls cell fate decisions. Notably, reduction in the protein levels of P63 has been found in human Barrett’s biopsies.30,45
FIGURE 6.
Deletion of p63 blocks the conversion of simple columnar to stratified squamous epithelium in the developing esophagus. The epithelium remains simple columnar and ciliated, and it produces glycoproteins (Alcian blue positive as indicated by the arrowheads) Scale bar: 50 µm.
A recent study demonstrates that P63 forms a complex with SOX2 and co-localizes at genetic loci to regulate common downstream target genes (e.g., ETV4) in human esophageal squamous cell lines.46 It will be interesting to determine whether a similar regulatory mechanism exists in the developing esophagus given that both Sox2 hypomorphic and P63 null mutants develop mucus metaplasia.5,45 In addition, P63 is also enriched in basal progenitor cells in the adult esophagus.8 Questions remain regarding its role in the maintenance of these progenitor cells.
The NRF2/KEAP1 complex
A balanced intracellular level of reactive oxygen species (ROS) is critical for cellular differentiation and maintenance. There is evidence that low, nontoxic levels of ROS contribute to stem/progenitor homeostasis in several tissues. For example, low levels of ROS in the hematopoietic system are correlated with stem cell quiescence, whereas high levels of ROS drive stem cells into cell cycling and lead to stem cell exhaustion.47 The level of intracellular ROS is monitored by the transcription factor Nuclear factor erythroid 2-related factor 2 (NRF2), which is negatively regulated by KEAP1. Intriguingly, deletion of Keap1 leads to hyperkeratosis, characterized by excessive squamous differentiation of basal progenitor cells in the developing esophagus and forestomach.48,49 Complementary removal of Nrf2 in a Keap1 null background rescues the hyperkeratosis phenotype.35 In addition, simultaneous deletion of MafF and MafG, two downstream targets of NRF2, is also able to rescue the excessive differentiation of progenitor cells.50
A recent study showed that NRF2 activation is associated with the pathogenesis of GERD and its deficiency impairs the maintenance of barrier function of the epithelium in the adult mouse esophagus.51 These findings raise several interesting questions: How is NRF2 activated during the normal differentiation of progenitor cells? Is there any crosstalk between BMP signaling and NRF2 activation? These questions await appropriate animal models and in vitro assays to address.
SUMMARY AND CONCLUSION
Compared to other organs, we are only beginning to understand the molecular mechanisms underlying the development of the esophagus. The BMP signaling pathway plays stage-dependent roles during the epithelial morphogenesis and it regulates both proliferation and differentiation of the epithelium in the developing esophagus. The transcription factors SOX2 and P63 are also essential for generating a stratified squamous epithelium. In addition, NRF2/KEAP1 interaction is also important for maintaining intra-cellular ROS levels critical for sustaining the balance of self-renewal and differentiation of the epithelial progenitor cells. Importantly, some of these signaling pathways and transcription factors have been associated with esophageal diseases in adults.
Nevertheless, many questions remain. For example, is the abnormal level of the relevant molecule a driver or passenger during disease initiation and progression? How do the relevant signaling pathways and transcription factors regulate the stratification process at a cellular level? Ciliated cells in human embryonic esophagus have been associated with the formation of the submucosal glands, but the mouse esophagus has no such gland in this tissue. Then what is the role for these ciliated cells transiently present in the developing mouse esophagus? Establishment of relevant disease models will help shed light on these questions. In addition, Hoxc4 null mutants not only have defects in muscle organization but also have defects in the epithelium such that it is hyperplastic and occludes the lumen.16 Nevertheless, how this gene regulates epithelial morphogenesis has yet to be characterized.
Much of our knowledge about the esophageal epithelial morphogenesis is indeed borrowed from the study of the epidermis, an organ also lined with the stratified squamous epithelium. Interestingly, Notch signaling has been shown to regulate P63 during epithelial maturation in the skin.52 Whether a similar regulatory mechanism exists in the developing esophagus remains unknown. Moreover, members of Grainyhead-like (GRHL) family have been shown to regulate epithelial differentiation in the skin and trachea52,53 but their roles in the esophagus remain to be determined. The other question that has not been addressed with mouse models is the mechanisms regulating the morphogenesis of the submucosal gland. With the advent of new genome editing technology (e.g., Cas9-Crispr54), one would be able to address this issue by developing genetically engineered animal models that normally have submucosal glands (e.g., pig27).
Supplementary Material
Acknowledgments
I thank members of our laboratory and Dr. Brigid Hogan at Duke University and Drs. Douglas Portman and Dirk Bohmann in the University of Rochester for critical reading of the manuscript and helpful discussion. I am particularly grateful to Ms. Wei-Yao Ku for her assistance with the figures. Research in the Que laboratory is partly supported by R01DK100342, NYSTEM C029555 and March of Dimes Research Grant 1-FY14-283.
Footnotes
Conflict of interest: The author has declared no conflicts of interest for this article.
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Que J, Choi M, Ziel JW, Klingensmith J, Hogan BL. Morphogenesis of the trachea and esophagus: current players and new roles for noggin and Bmps. Differentiation. 2006;74:422–437. doi: 10.1111/j.1432-0436.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- 2.Billmyre KK, Hutson M, Klingensmith J. One shall become two: Separation of the esophagus and trachea from the common foregut tube. Dev Dyn. 2014 doi: 10.1002/dvdy.24219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobs IJ, Ku WY, Que J. Genetic and cellular mechanisms regulating anterior foregut and esophageal development. Dev Biol. 2012;369:54–64. doi: 10.1016/j.ydbio.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez P, Da Silva S, Oxburgh L, Wang F, Hogan BL, Que J. BMP signaling in the development of the mouse esophagus and forestomach. Development. 2010;137:4171–4176. doi: 10.1242/dev.056077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Que J, Okubo T, Goldenring JR, Nam KT, Kurotani R, Morrisey EE, Taranova O, Pevny LH, Hogan BL. Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development. 2007;134:2521–2531. doi: 10.1242/dev.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milano F, van Baal JW, Buttar NS, Rygiel AM, de Kort F, DeMars CJ, Rosmolen WD, Bergman JJ, Vam J, Wang KK, et al. Bone morphogenetic protein 4 expressed in esophagitis induces a columnar phenotype in esophageal squamous cells. Gastroenterology. 2007;132:2412–2421. doi: 10.1053/j.gastro.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 7.Wang DH, Clemons NJ, Miyashita T, Dupuy AJ, Zhang W, Szczepny A, Corcoran-Schwartz IM, Wilburn DL, Montgomery EA, Wang JS, et al. Aberrant epithelial-mesenchymal Hedgehog signaling characterizes Barrett’s metaplasia. Gastroenterology. 2010;138:1810–1822. doi: 10.1053/j.gastro.2010.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu K, Jiang M, Lu Y, Chen H, Sun J, Wu S, Ku W-Y, Nakagawa H, Kita Y, Natsugoe S, et al. Sox2 cooperates with inflammation-mediated Stat3 activation in the malignant transformation of foregut basal progenitor cells. Cell Stem Cell. 2013;12:304–315. doi: 10.1016/j.stem.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, Kim SY, Wardwell L, Tamayo P, Gat-Viks I, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009;41:1238–1242. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Rahilly R, Muller F. Respiratory and alimentary relations in staged human embryos. New embryological data and congenital anomalies. Ann Otol Rhinol Laryngol. 1984;93:421–429. doi: 10.1177/000348948409300501. [DOI] [PubMed] [Google Scholar]
- 11.Zaw-Tun HA. The tracheo-esophageal septum—fact or fantasy? Origin and development of the respiratory primordium and esophagus. Acta Anat (Basel) 1982;114:1–21. [PubMed] [Google Scholar]
- 12.Sasaki T, Kusafuka T, Okada A. Analysis of the development of normal foregut and tracheoesophageal fistula in an adriamycin rat model using three-dimensional image reconstruction. Surg Today. 2001;31:133–139. doi: 10.1007/s005950170197. [DOI] [PubMed] [Google Scholar]
- 13.Qi BQ, Beasley SW. Stages of normal tracheo-bronchial development in rat embryos: resolution of a controversy. Dev Growth Differ. 2000;42:145–153. doi: 10.1046/j.1440-169x.2000.00488.x. [DOI] [PubMed] [Google Scholar]
- 14.Ioannides AS, Massa V, Ferraro E, Cecconi F, Spitz L, Henderson DJ, Copp AJ. Foregut separation and tracheo-oesophageal malformations: the role of tracheal outgrowth, dorso-ventral patterning and programmed cell death. Dev Biol. 2010;337:351–362. doi: 10.1016/j.ydbio.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metzger R, Wachowiak R, Kluth D. Embryology of the early foregut. Semin Pediatr Surg. 2011;20:136–144. doi: 10.1053/j.sempedsurg.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Boulet AM, Capecchi MR. Targeted disruption of hoxc-4 causes esophageal defects and vertebral transformations. Dev Biol. 1996;177:232–249. doi: 10.1006/dbio.1996.0159. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Litingtung Y, Ten Dijke P, Chiang C. Aberrant Bmp signaling and notochord delamination in the pathogenesis of esophageal atresia. Dev Dyn. 2007;236:746–754. doi: 10.1002/dvdy.21075. [DOI] [PubMed] [Google Scholar]
- 18.Fausett SR, Brunet LJ, Klingensmith J. BMP antagonism by Noggin is required in presumptive notochord cells for mammalian foregut morphogenesis. Dev Biol. 2014;391:111–124. doi: 10.1016/j.ydbio.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Samarasinghe DD. Some observations on the innervation of the striated muscle in the mouse oesophagus—an electron microscopy study. J Anat. 1972;112:173–184. [PMC free article] [PubMed] [Google Scholar]
- 20.Rishniw M, Xin HB, Deng KY, Kotlikoff MI. Skeletal myogenesis in the mouse esophagus does not occur through transdifferentiation. Genesis. 2003;36:81–82. doi: 10.1002/gene.10198. [DOI] [PubMed] [Google Scholar]
- 21.Katori Y, Cho BH, Song CH, Fujimiya M, Murakami G, Kawase T. Smooth-to-striated muscle transition in human esophagus: an immunohistochemical study using fetal and adult materials. Ann Anat. 2010;192:33–41. doi: 10.1016/j.aanat.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Shu W, Lu MM, Zhang Y, Tucker PW, Zhou D, Morrisey EE. Foxp2 and Foxp1 cooperatively regulate lung and esophagus development. Development. 2007;134:1991–2000. doi: 10.1242/dev.02846. [DOI] [PubMed] [Google Scholar]
- 23.Menard D. Morphological studies of the developing human esophageal epithelium. Microsc Res Tech. 1995;31:215–225. doi: 10.1002/jemt.1070310305. [DOI] [PubMed] [Google Scholar]
- 24.Takubo K, Vieth M, Honma N, Izumiyama N, Sawabe M, Arai T, Iwakiri K, Kammori M, Mafune K. Ciliated surface in the esophagogastric junction zone: a precursor of Barrett’s mucosa or ciliated pseudostratified metaplasia? Am J Surg Pathol. 2005;29:211–217. doi: 10.1097/01.pas.0000149705.66592.ee. [DOI] [PubMed] [Google Scholar]
- 25.Rubio CA, Aberg B, Stemmermann G. Ciliated cells in papillary adenocarcinomas of Barrett’s esophagus. Acta Cytol. 1992;36:65–68. [PubMed] [Google Scholar]
- 26.Shields HM, Rosenberg SJ, Zwas FR, Ransil BJ, Lembo AJ, Odze R. Prospective evaluation of multilayered epithelium in Barrett’s esophagus. Am J Gastroenterol. 2001;96:3268–3273. doi: 10.1111/j.1572-0241.2001.05324.x. [DOI] [PubMed] [Google Scholar]
- 27.Long JD, Orlando RC. Esophageal submucosal glands: structure and function. Am J Gastroenterol. 1999;94:2818–2824. doi: 10.1111/j.1572-0241.1999.1422_b.x. [DOI] [PubMed] [Google Scholar]
- 28.Raymond C, Anne V, Millane G. Development of esophageal epithelium in the fetal and neonatal mouse. Anat Rec. 1991;230:225–234. doi: 10.1002/ar.1092300210. [DOI] [PubMed] [Google Scholar]
- 29.Yu WY, Slack JM, Tosh D. Conversion of columnar to stratified squamous epithelium in the developing mouse oesophagus. Dev Biol. 2005;284:157–170. doi: 10.1016/j.ydbio.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 30.Daniely Y, Liao G, Dixon D, Linnoila RI, Lori A, Randell SH, Oren M, Jetten AM. Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. Am J Physiol Cell Physiol. 2004;287:C171–C181. doi: 10.1152/ajpcell.00226.2003. [DOI] [PubMed] [Google Scholar]
- 31.Weaver M, Dunn NR, Hogan BL. Bmp4 and Fgf10 play opposing roles during lung bud morphogenesis. Development. 2000;127:2695–2704. doi: 10.1242/dev.127.12.2695. [DOI] [PubMed] [Google Scholar]
- 32.Liu K, Lin B, Zhao M, Yang X, Chen M, Gao A, Liu F, Que J, Lan X. The multiple roles for Sox2 in stem cell maintenance and tumorigenesis. Cell Signal. 2013;25:1264–1271. doi: 10.1016/j.cellsig.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Que J, Luo X, Schwartz RJ, Hogan BL. Multiple roles for Sox2 in the developing and adult mouse trachea. Development. 2009;136:1899–1907. doi: 10.1242/dev.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okubo T, Pevny LH, Hogan BL. Sox2 is required for development of taste bud sensory cells. Genes Dev. 2006;20:2654–2659. doi: 10.1101/gad.1457106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taranova OV, Magness ST, Fagan BM, Wu Y, Surzenko N, Hutton SR, Pevny LH. SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev. 2006;20:1187–1202. doi: 10.1101/gad.1407906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Domyan ET, Ferretti E, Throckmorton K, Mishina Y, Nicolis SK, Sun X. Signaling through BMP receptors promotes respiratory identity in the foregut via repression of Sox2. Development. 2011;138:971–981. doi: 10.1242/dev.053694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeWard AD, Cramer J, Lagasse E. Cellular heterogeneity in the mouse esophagus implicates the presence of a nonquiescent epithelial stem cell population. Cell Rep. 2014;9:701–711. doi: 10.1016/j.celrep.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen X, Qin R, Liu B, Ma Y, Su Y, Yang CS, Glickman JN, Odze RD, Shaheen NJ. Multilayered epithelium in a rat model and human Barrett’s esophagus: similar expression patterns of transcription factors and differentiation markers. BMC Gastroenterol. 2008;8:1. doi: 10.1186/1471-230X-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Candi E, Dinsdale D, Rufini A, Salomoni P, Knight RA, Mueller M. TAp63 and [Delta]Np63 in cancer and epidermal development. Cell Cycle. 2007;6:274–285. doi: 10.4161/cc.6.3.3797. [DOI] [PubMed] [Google Scholar]
- 42.Laurikkala J, Mikkola ML, James M, Tummers M, Mills AA, Thesleff I. p63 regulates multiple signalling pathways required for ectodermal organogenesis and differentiation. Development. 2006;133:1553–1563. doi: 10.1242/dev.02325. [DOI] [PubMed] [Google Scholar]
- 43.Shalom-Feuerstein R, Lena AM, Zhou H, De La Forest DS, Van Bokhoven H, Candi E. [Delta]Np63 is an ectodermal gatekeeper of epidermal morphogenesis. Cell Death Differ. 2011;18:887–896. doi: 10.1038/cdd.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- 45.Wang X, Ouyang H, Yamamoto Y, Kumar PA, Wei TS, Dagher R, Vincent M, Lu X, Bellizzi AM, Ho KY, et al. Residual embryonic cells as precursors of a Barrett’s-like metaplasia. Cell. 2011;145:1023–1035. doi: 10.1016/j.cell.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watanabe H, Ma Q, Peng S, Adelmant G, Swain D, Song W, Fox C, Francis JM, Pedamallu CS, DeLuca DS, et al. SOX2 and p63 colocalize at genetic loci in squamous cell carcinomas. J Clin Invest. 2014;124:1636–1645. doi: 10.1172/JCI71545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wakabayashi N, Itoh K, Wakabayashi J, Motohashi H, Noda S, Takahashi S, Imakado S, Kotsuji T, Otsuka F, Roop DR, et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet. 2003;35:238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- 49.Chen H, Li J, Li H, Hu Y, Tevebaugh W, Yamamoto M, Que J, Chen X. Transcript Profiling Identifies Dynamic Gene Expression Patterns and an Important Role for Nrf2/Keap1 Pathway in the Developing Mouse Esophagus. PLoS One. 2012;7:e36504. doi: 10.1371/journal.pone.0036504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen H, Fang Y, Li W, Orlando RC, Shaheen N, Chen XL. NFkB and Nrf2 in esophageal epithelial barrier function. Tissue Barriers. 2013;1:e27463. doi: 10.4161/tisb.27463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen H, Hu Y, Fang Y, Djukic Z, Yamamoto M, Shaheen NJ, Orlando RC, Chen X. Nrf2 deficiency impairs the barrier function of mouse oesophageal epithelium. Gut. 2014;63:711–719. doi: 10.1136/gutjnl-2012-303731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nguyen BC, Lefort K, Mandinova A, Antonini D, Devgan V, Della Gatta G, Koster MI, Zhang Z, Wang J, Tommasi di Vignano A, et al. Cross-regulation between Notch and p63 in keratinocyte commitment to differentiation. Genes Dev. 2006;20:1028–1042. doi: 10.1101/gad.1406006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boglev Y, Wilanowski T, Caddy J, Parekh V, Auden A, Darido C, Hislop NR, Cangkrama M, Ting SB, Jane SM. The unique and cooperative roles of the Grainy head-like transcription factors in epidermal development reflect unexpected target gene specificity. Dev Biol. 2011;349:512–522. doi: 10.1016/j.ydbio.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 54.Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
FURTHER READING
- Kuo B, Urma D. Esophagus – anatomy and development. [Accessed May 16, 2006];GI Motility online. 2006 Available at: http://www.nature.com/gimo/contents/pt1/full/gimo6.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.