Abstract
This presentation outlines the clinical and imaging characteristics of esthesioneuroblastoma.
Keywords: olfactory neuroblastoma, esthesioneuroblastoma, skull base, imaging
Introduction
Olfactory neuroblastoma, or esthesioneuroblastoma (ENB), is an uncommon malignant tumor of neural crest origin, arising from the olfactory epithelium of the superior nasal cavity. It accounts for ∼ 2 to 6% of the intranasal tumors, with an incidence of ∼ 0.4 per million.1 2 3 The age at presentation is bimodal in distribution in the second and sixth decades of life and without a gender predilection.4 5
Staging
The stage of the disease at the initial presentation is highly predictive of survival, and accurate staging is essential. The Kadish system is the most commonly used approach to classify the anatomical extent of ENB, although other classifications have been proposed.6 7 8 9 The Kadish staging uses three categories, group A, B, and C, as follows: A, tumor confined to the nasal cavity; B, tumor extends into the paranasal sinuses; and C, tumor extends beyond the nasal cavity and paranasal sinuses including involvement of the cribriform plate, skull base, orbit, or intracranial cavity. The modified Kadish staging system includes a fourth stage for patients with nodal or distant metastases.10 This system has been criticized because it requires surgical staging, does not routinely consider metastatic spread, and lacks prognostic value. Additionally, few patients at initial clinical discovery have group A disease if the staging is strictly applied because of a high incidence of ethmoid sinus involvement. Simon et al did not find a single patient in their 20-year experience that presented with stage A disease.5 Similar observations have been reported in other series, and the overall incidence of Kadish A disease is estimated at 5%.11 12 13 14 Modern imaging makes a diagnosis of Kadish A disease less likely.
Despite the inadequacies and attempts to use a TNM classification, the Kadish staging is the most commonly used.8 The Dulguerov staging system uses the TNM classification and includes the imaging data (Table 1).8 Some authors prefer the Dulguerov system because early involvement of the cribriform plate is recognized in the T2 stage, and intracranial but extradural tumors are separated from those with true brain involvement.15
Table 1. Esthesioneuroblastoma staging system as proposed by Dulguerov8 .
| Staging | Description |
|---|---|
| Primary tumor | |
| T1 | Tumor involving the nasal cavity and/or paranasal sinuses (excluding sphenoid), sparing the most superior ethmoid |
| T2 | Tumor involving the nasal cavity and/or paranasal sinuses (including the sphenoid) with extension to or erosion of the cribriform plate |
| T3 | Tumor extending into the orbit or protruding into the anterior cranial fossa, without dural involvement |
| T4 | Tumor involving the brain |
| Lymph nodes | |
| N0 | No cervical lymph node metastasis |
| N1 | Any form of cervical lymph node metastasis |
| Distant metastasis | |
| M0 | No metastases |
| M1 | Distant metastases |
Clinical Presentation
ENB presents with epistaxis, nasal obstruction, decreased olfactory function, diplopia, proptosis, or a combination of these symptoms.6 7 9 16 Local invasion occurs most frequently into the paranasal sinuses, orbits, and anterior cranial fossa. Metastatic disease most frequently involves local lymph nodes, with distant metastasis to the lungs, liver, and bone.1 6 8 The incidence of cervical lymph node metastasis ranges from 20 to 30% and reaches 44% in stage C.2 6 8 17 18 19 Howell et al described a predictable pattern of metastases to the cervical lymph nodes, typically involving level II nodes (93%), with frequent involvement of level I (57%), level III (50%), and retropharyngeal nodes (43%).20
Imaging
Imaging plays a key role in the accurate staging of ENB including both computed tomography (CT) and magnetic resonance imaging (MRI). These imaging modalities should always be included to correctly assess the extent of the disease. CT should be performed with thin slices (1 mm thick) and reformatted in both coronal and sagittal planes. ENB does not have specific CT characteristics, presenting initially as a homogeneous soft tissue mass in the nasal vault. However, CT is essential for evaluation of the osseous involvement of the cribriform plate, fovea ethmoidalis, and lamina papyracea (Fig. 1). Bone remodeling without destruction is not uncommon, due to the indolent growth pattern in some cases. The mass shows moderate and uniform enhancement. Scattered speckled calcifications are occasionally present.21 CT is also useful to assess regional neck and distant metastasis.20 22
Fig. 1.
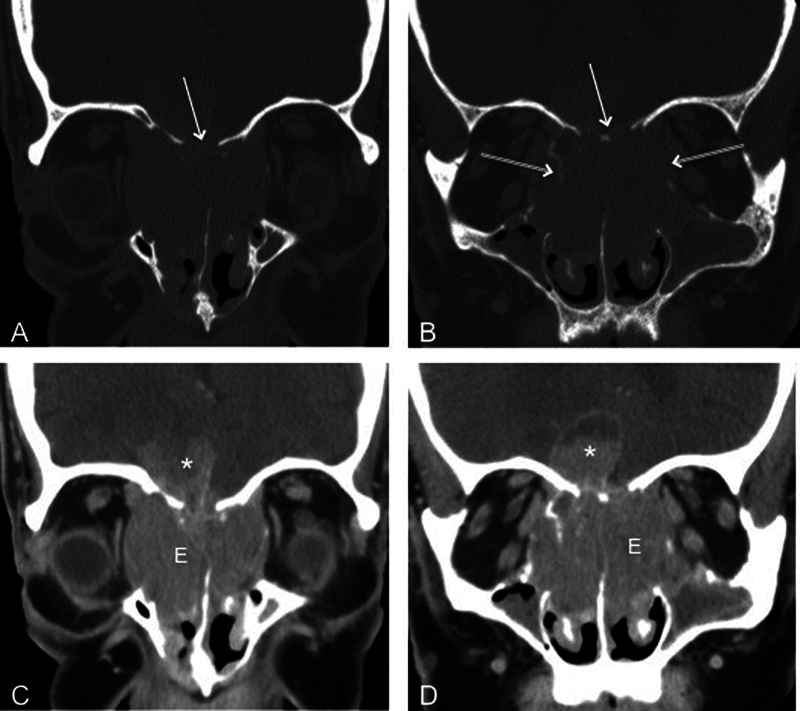
Coronal computed tomography of the face in (A, B) bone and (C, D) soft tissue algorithms demonstrates avidly enhancing esthesioneuroblastoma (E) filling the nasal cavity and extending into orbits and intracranially (asterisk). The mass has eroded the floor of the anterior cranial fossa (single arrow) and lamina papyracea bilaterally (double arrow).
The most important information gained from MRI is differentiating entrapped secretions from neoplasm because CT is unreliable for this distinction (Fig. 2).22 23 MRI is also superior in defining the soft tissue extent and offers a more accurate assessment of suspected intracranial, orbital, or skull base invasion, and perineural spread of tumor.7 22 24 MRI is also excellent at distinguishing dural involvement from parenchymal involvement (Fig. 3).23
Fig. 2.
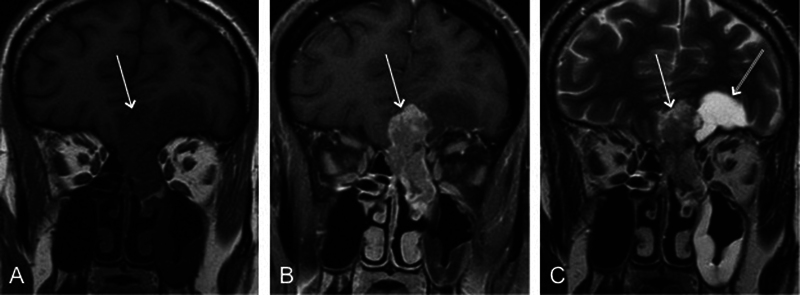
Coronal (A) T1-weighted, (B) contrast-enhanced, and (C) T2-weighted images from magnetic resonance imaging demonstrate a low T1 intensity and avidly enhancing nasal vault mass extending intracranially (arrow). (C) The T2-weighted sequence shows peritumoral cyst (double arrow).
Fig. 3.
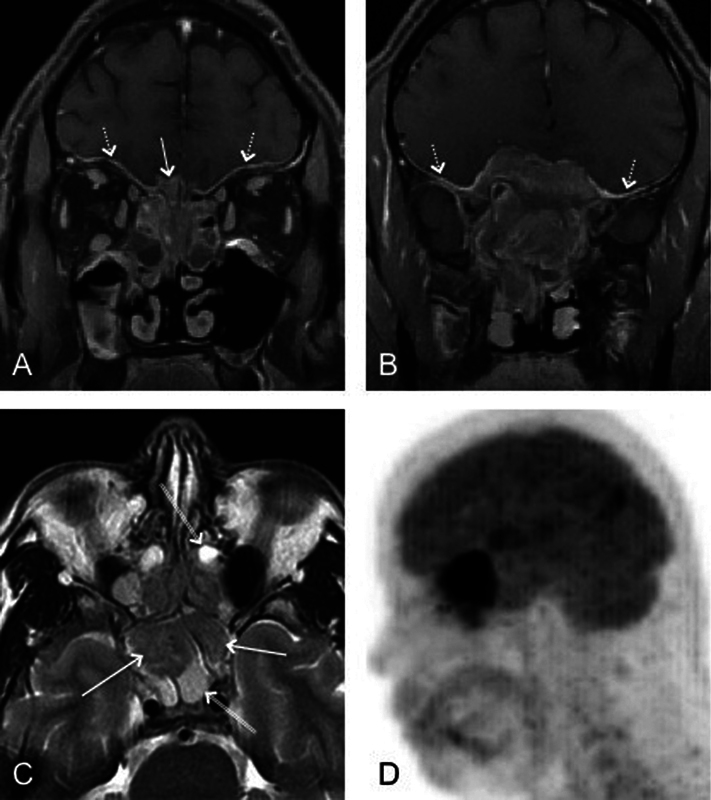
(A, B) Utility of magnetic resonance imaging in the evaluation of dural involvement as demonstrated by coronal fat-saturated contrast-enhanced images (dashed arrow). Intracranial extension fills the right olfactory groove (arrow in A). (C) Axial T2-weighted image shows a clear distinction between low-intensity esthesioneuroblastoma (arrows) and high-intensity trapped secretions (double arrows). (D) Sagittal reformatted image from positron emission tomography shows avid tracer uptake within the nasal vault mass with a large intracranial component.
ENB is usually hypointense to gray matter on T1-weighted images and intermediate to hyperintense on T2-weighted sequences (Fig. 2). The tumor demonstrates avid and homogeneous enhancement except for occasional areas of necrosis or hemorrhage. Trapped secretions in adjacent sinuses are T2 hyperintense and differentiated from the tumor. The presence of intracranial cysts is highly suggestive of, but not diagnostic of ENB. Som et al studied 54 patients who had sinonasal lesions with intracranial extension and found only three patients with intracranial cysts, but all three had ENB.25
Bony destruction can be seen but frequently not as well as with CT. MRI, however, is much more accurate in the evaluation of subtle orbital and intracranial invasion including rare subarachnoid seeding. MRI fat-saturated sequences help distinguish tumor from orbital fat and muscle: a smooth bowing of the tumor–fat interface suggests that the lesion is contained by periorbital fascia, whereas an irregular margin favors frank invasion of the orbit.26 27 However, the definitive diagnosis of invasion of dura and periorbital tissues is possible only at surgery.27
Imaging of the neck in patients with ENB is crucial because neck metastases are found at presentation in 5% of patients.7 Further, it has been estimated that > 23% of patients may eventually develop cervical lymph node metastases.17 One of the most frequent distant metastasis is to bone, with the spine cited as the most common location (86%).28 Cases of asymptomatic bone metastases have been described, and therefore a bone scan should be included in the diagnostic work-up.7
The assessment of recurrent tumor must include both CT and MRI. The imaging characteristics of the recurrent tumor do not differ from its appearance at initial presentation.29 The following protocol for follow-up has been suggested: contrast-enhanced MRI performed 2 to 4 months after completion of all therapy, and then repeated every 4 to 6 months for 5 years and then annually for the patient's lifetime. In addition, an annual chest radiograph should be performed to exclude the presence of metastases.14
Scintigraphic evaluation of ENB has been reported with technetium-99m methylene diphosphonate, indium-111 bleomycin complex, indium-111 octreotide, technetium-99m ethylcysteinate dimer, and radioiodinated metaiodobenzylguanidine.12 30 31 32 More recently, positron emission tomography (PET) with F-18-labeled fluorodeoxyglucose was used in the staging of ENB in a case with ectopic Cushing syndrome.33 PET-CT was also found to be accurate not only in staging but also in the posttherapeutic restaging of ENB.34 While promising, the data on PET and other scintigraphic methods in evaluation of recurrent or residual ENB are very limited.
Finally, because ENB is most often a nonvascular tumor, diagnostic and therapeutic angiographic techniques have no place in the diagnostic work-up of this entity.35
Conclusion
Although the imaging characteristics of ENB are somewhat nonspecific, there are patterns of the disease, such as Kadish grade C, which should strongly suggest this disorder. Metastatic disease, usually regional, occurs frequently with fairly predictable involvement of level II nodes but also level I and III and retropharyngeal nodes that are difficult to assess clinically. Finally, a suggested follow-up scheme is recommended to detect recurrences at the earliest possible time.
References
- 1.Broich G Pagliari A Ottaviani F Esthesioneuroblastoma: a general review of the cases published since the discovery of the tumour in 1924 Anticancer Res 199717(4A):2683–2706. [PubMed] [Google Scholar]
- 2.Jethanamest D, Morris L G, Sikora A G, Kutler D I. Esthesioneuroblastoma: a population-based analysis of survival and prognostic factors. Arch Otolaryngol Head Neck Surg. 2007;133(3):276–280. doi: 10.1001/archotol.133.3.276. [DOI] [PubMed] [Google Scholar]
- 3.Thompson L D. Olfactory neuroblastoma. Head Neck Pathol. 2009;3(3):252–259. doi: 10.1007/s12105-009-0125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elkon D, Hightower S I, Lim M L, Cantrell R W, Constable W C. Esthesioneuroblastoma. Cancer. 1979;44(3):1087–1094. doi: 10.1002/1097-0142(197909)44:3<1087::aid-cncr2820440343>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 5.Simon J H, Zhen W, McCulloch T M. et al. Esthesioneuroblastoma: the University of Iowa experience 1978–1998. Laryngoscope. 2001;111(3):488–493. doi: 10.1097/00005537-200103000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Kadish S, Goodman M, Wang C C. Olfactory neuroblastoma. A clinical analysis of 17 cases. Cancer. 1976;37(3):1571–1576. doi: 10.1002/1097-0142(197603)37:3<1571::aid-cncr2820370347>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 7.Bradley P J, Jones N S, Robertson I. Diagnosis and management of esthesioneuroblastoma. Curr Opin Otolaryngol Head Neck Surg. 2003;11(2):112–118. doi: 10.1097/00020840-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Dulguerov P, Calcaterra T. Esthesioneuroblastoma: the UCLA experience 1970–1990. Laryngoscope. 1992;102(8):843–849. doi: 10.1288/00005537-199208000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Dulguerov P, Allal A S, Calcaterra T C. Esthesioneuroblastoma: a meta-analysis and review. Lancet Oncol. 2001;2(11):683–690. doi: 10.1016/S1470-2045(01)00558-7. [DOI] [PubMed] [Google Scholar]
- 10.Morita A Ebersold M J Olsen K D Foote R L Lewis J E Quast L M Esthesioneuroblastoma: prognosis and management Neurosurgery 1993325706–714.; discussion 714–715 [DOI] [PubMed] [Google Scholar]
- 11.Polin R S, Sheehan J P, Chenelle A G. et al. The role of preoperative adjuvant treatment in the management of esthesioneuroblastoma: the University of Virginia experience. Neurosurgery. 1998;42(5):1029–1037. doi: 10.1097/00006123-199805000-00045. [DOI] [PubMed] [Google Scholar]
- 12.Jekunen A P, Kairemo K J, Ramsay H A, Kajanti M J. Imaging of olfactory neuroblastoma by In-111 bleomycin complex. Clin Nucl Med. 1996;21(2):129–131. doi: 10.1097/00003072-199602000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Jekunen A P, Kairemo K JA, Lehtonen H P, Kajanti M J. Treatment of olfactory neuroblastoma. A report of 11 cases. Am J Clin Oncol. 1996;19(4):375–378. doi: 10.1097/00000421-199608000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Slevin N J, Irwin C JR, Banerjee S S, Gupta N K, Farrington W T. Olfactory neural tumours—the role of external beam radiotherapy. J Laryngol Otol. 1996;110(11):1012–1016. [PubMed] [Google Scholar]
- 15.Girod D, Hanna E, Marentette L. Esthesioneuroblastoma. Head Neck. 2001;23(6):500–505. doi: 10.1002/hed.1067. [DOI] [PubMed] [Google Scholar]
- 16.Olsen K D, DeSanto L W. Olfactory neuroblastoma. Biologic and clinical behavior. Arch Otolaryngol. 1983;109(12):797–802. doi: 10.1001/archotol.1983.00800260019005. [DOI] [PubMed] [Google Scholar]
- 17.Rinaldo A, Ferlito A, Shaha A R, Wei W I, Lund V J. Esthesioneuroblastoma and cervical lymph node metastases: clinical and therapeutic implications. Acta Otolaryngol. 2002;122(2):215–221. doi: 10.1080/00016480252814261. [DOI] [PubMed] [Google Scholar]
- 18.Monroe A T, Hinerman R W, Amdur R J, Morris C G, Mendenhall W M. Radiation therapy for esthesioneuroblastoma: rationale for elective neck irradiation. Head Neck. 2003;25(7):529–534. doi: 10.1002/hed.10247. [DOI] [PubMed] [Google Scholar]
- 19.Davis R E, Weissler M C. Esthesioneuroblastoma and neck metastasis. Head Neck. 1992;14(6):477–482. doi: 10.1002/hed.2880140610. [DOI] [PubMed] [Google Scholar]
- 20.Howell M C, Branstetter B F IV, Snyderman C H. Patterns of regional spread for esthesioneuroblastoma. AJNR Am J Neuroradiol. 2011;32(5):929–933. doi: 10.3174/ajnr.A2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manelfe C, Bonafé A, Fabre P, Pessey J J. Computed tomography in olfactory neuroblastoma: one case of esthesioneuroepithelioma and four cases of esthesioneuroblastoma. J Comput Assist Tomogr. 1978;2(4):412–420. [PubMed] [Google Scholar]
- 22.Ahmad A Branstetter B F IV CT versus MR: still a tough decision Otolaryngol Clin North Am 20084111–22., v [DOI] [PubMed] [Google Scholar]
- 23.Branstetter B F IV Weissman J L Role of MR and CT in the paranasal sinuses Otolaryngol Clin North Am 20053861279–1299., x [DOI] [PubMed] [Google Scholar]
- 24.Ling F TK, Kountakis S E. Advances in imaging of the paranasal sinuses. Curr Allergy Asthma Rep. 2006;6(6):502–507. doi: 10.1007/s11882-006-0028-1. [DOI] [PubMed] [Google Scholar]
- 25.Som P M, Lidov M, Brandwein M, Catalano P, Biller H F. Sinonasal esthesioneuroblastoma with intracranial extension: marginal tumor cysts as a diagnostic MR finding. AJNR Am J Neuroradiol. 1994;15(7):1259–1262. [PMC free article] [PubMed] [Google Scholar]
- 26.Sievers K W, Greess H, Baum U, Dobritz M, Lenz M. Paranasal sinuses and nasopharynx CT and MRI. Eur J Radiol. 2000;33(3):185–202. doi: 10.1016/s0720-048x(99)00142-4. [DOI] [PubMed] [Google Scholar]
- 27.Dulguerov P, Allal A S. Nasal and paranasal sinus carcinoma: how can we continue to make progress? Curr Opin Otolaryngol Head Neck Surg. 2006;14(2):67–72. doi: 10.1097/01.moo.0000193177.62074.fd. [DOI] [PubMed] [Google Scholar]
- 28.Koka V N, Julieron M, Bourhis J. et al. Aesthesioneuroblastoma. J Laryngol Otol. 1998;112(7):628–633. doi: 10.1017/s0022215100141295. [DOI] [PubMed] [Google Scholar]
- 29.Pickuth D, Heywang-Köbrunner S H. Imaging of recurrent esthesioneuroblastoma. Br J Radiol. 1999;72(863):1052–1057. doi: 10.1259/bjr.72.863.10700820. [DOI] [PubMed] [Google Scholar]
- 30.Shanley D J, Buckner A B. Esthesioneuroblastoma demonstrated on bone scan. Correlation with CT and MRI. Clin Nucl Med. 1992;17(3):231–232. doi: 10.1097/00003072-199203000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Bustillo A, Telischi F, Weed D. et al. Octreotide scintigraphy in the head and neck. Laryngoscope. 2004;114(3):434–440. doi: 10.1097/00005537-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Prado G L, Itabashi Y, Noda H, Miura H, Mariya Y, Abe Y. Olfactory neuroblastoma visualized by Technetium-99m-ECD SPECT. Radiat Med. 2001;19(5):267–270. [PubMed] [Google Scholar]
- 33.Yu J, Koch C A, Patsalides A. et al. Ectopic Cushing's syndrome caused by an esthesioneuroblastoma. Endocr Pract. 2004;10(2):119–124. doi: 10.4158/EP.10.2.119. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen B D, Roarke M C, Nelson K D, Chong B W. F-18 FDG PET/CT staging and posttherapeutic assessment of esthesioneuroblastoma. Clin Nucl Med. 2006;31(3):172–174. doi: 10.1097/01.rlu.0000200735.55296.73. [DOI] [PubMed] [Google Scholar]
- 35.Rosengren J E, Jing B S, Wallace S, Danziger J. Radiographic features of olfactory neuroblastoma. AJR Am J Roentgenol. 1979;132(6):945–948. doi: 10.2214/ajr.132.6.945. [DOI] [PubMed] [Google Scholar]


