Abstract
Importance Scalp reconstructions may be required after tumor resection or trauma. The inherent anatomy of the scalp presents challenges and may limit reconstructive options.
Objective To describe and investigate the scalp rotation flap as a reconstructive technique for complex soft tissue defects.
Design Retrospective case series with a mean follow-up of 13 months.
Setting Tertiary academic center.
Participants A total of 22 patients with large scalp soft tissue defects undergoing scalp rotation flap reconstruction.
Interventions The flap is designed adjacent to the defect and elevated in the subgaleal plane. The flap is rotated into the defect, and a split-thickness skin graft is placed over the donor site periosteum.
Main Outcomes and Measure Data points collected included defect size, operative time, hospital stay, and patient satisfaction with cosmetic outcome.
Results Mean patient age was 71 years. Mean American Society of Anesthesiologist classification was 2.8. Mean defect size was 41 cm2 (range: 7.8–120 cm2), and 19 of 22 defects resulted from a neoplasm resection. Mean operative time was 181 minutes, and mean hospital stay was 2.4 days. There were no intraoperative complications. Three patients with previous radiation therapy had distal flap necrosis. Twenty-one patients (95%) reported an acceptable cosmetic result.
Conclusions and Relevance The scalp rotation flap is an efficient and reliable option for reconstructing complex soft tissue defects. This can be particularly important in patients with significant medical comorbidities who cannot tolerate a lengthy operative procedure.
Keywords: oncology, scalp reconstruction, reconstruction, local flap
Introduction
The incidence of cutaneous malignancies is rising, and sun-exposed regions of the head and neck are at high risk for disease.1 The scalp region of the head and neck, in particular, is a common site involved with skin cancer. The most common tumors involving the scalp include squamous cell cancer, basal cell cancer, and melanoma. Without treatment, these skin cancers can grow radially to involve a significant area of the scalp and vertically, going through soft tissue and periosteum into the cranial bone. Wide local excision of large scalp tumors that extend into and through the periosteum presents both an ablative and reconstructive challenge. Obtaining a negative surgical margin often necessitates removal of a large portion of soft tissue and periosteum. The outer table of the skull and dura must occasionally be removed to obtain clear margins. The resulting defect can be difficult to close due to the poor soft tissue elasticity of the scalp. In addition, skin grafts are unable to survive on exposed bone when the periosteum is removed.
The goals of scalp reconstruction include vascularized soft tissue coverage, acceptable cosmetic appearance, and minimal donor site morbidity. Reconstructive options for scalp defects include local and regional flaps or free tissue transfer. Local flaps have the advantage of good reliability, low donor site morbidity, good color match, and a relatively short operative time. Several techniques to close a defect in the scalp using local tissue including the use of the so-called pinwheel flap or the O-Z closure have been described.2 3 Free tissue transfer has the advantage of reliable vascularized tissue and excellent soft tissue bulk. Anterolateral thigh, radial forearm, and latissimus dorsi free flaps provide excellent soft tissue coverage for the scalp.4 5 6 Free tissue transfer requires longer operative times and donor site morbidity that is not advantageous for patients with a poor underlying health status.7
We describe the use of a local scalp rotational flap for reconstruction of scalp defects that are not amenable to skin graft or simpler methods of tissue rearrangement. The main utility of this flap is for defects that have the pericranium removed and exposed bone. This flap uses the robust vascularity of the scalp in that it can be oriented in any direction relative to the defect. Once the scalp flap is rotated into the defect, the donor site, which has the pericranium preserved, is covered with a skin graft.
Methods
Approval was obtained from the Saint Louis University internal review board. A retrospective medical record review was performed for all patients who underwent reconstruction with a scalp flap at our institution between January 1, 2002, and December 31, 2009. Patient variables included age, preoperative American Society of Anesthesiologists (ASA) classification, total operative time (from skin incision to skin closure), and total length of hospital stay (in days). Additional patient information including sex, pathologic diagnosis, extent of surgical resection, and medical comorbidities were also recorded. Clinical outcomes included medical complications, major and minor wound complications, and donor site complications. Minor wound complications were classified as wound breakdown requiring only dressing changes or a minor local revision procedure for treatment. Major wound complications included hematoma formation, significant wound breakdown, osteomyelitis, or other complications requiring a procedure under general anesthesia.
The procedure for harvesting the scalp rotational flap is as follows. The malignancy is removed with adequate margins depending on the pathology (squamous cell, basal cell, or melanoma). In the patients included in the study, the pericranium was sacrificed to obtain clear margins, and in some cases, the underlying bone was drilled. The diameter of the defect was measured, and a flap with the same diameter was drawn adjacent to the defect (Fig. 1). Rotational flaps must be designed at a slightly greater height to account for their rotational arc. The flap incision is carried to a subgaleal place superficial to the underlying pericranium. The flap is elevated meticulously in the subgaleal plane, being careful to leave intact pericranium (Fig. 2). Any potential redundant tissue at the base of the pedicle is not removed due to concern about compromising flap viability. Once adequate length is obtained, the flap is rotated into the surgical defect. The flap is secured to the tissue surrounding the defect with a two-layer closure. A 0.018-inch split-thickness skin graft (STSG) is harvested from the right or left thigh with a dermatome. The skin graft is then secured to the donor site periosteum, and a compressive bolster is placed (Fig. 3).
Fig. 1.
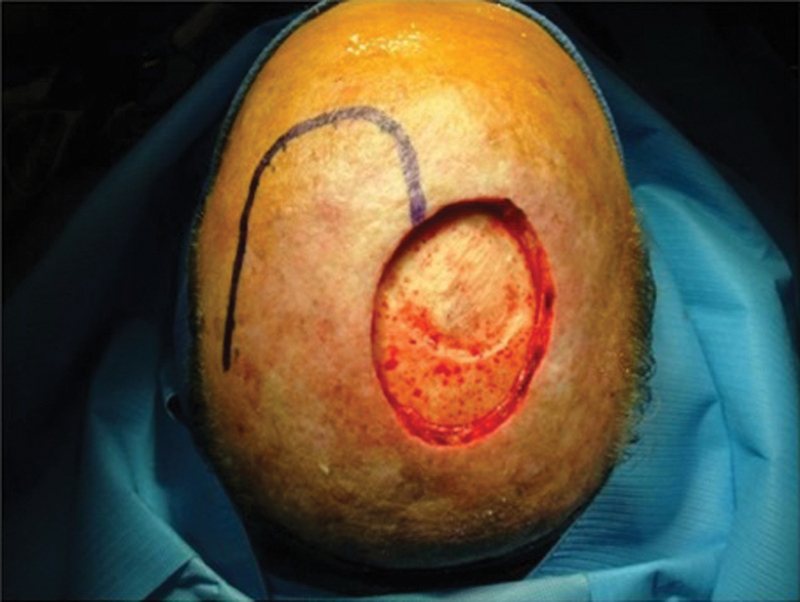
Scalp flap is drawn adjacent to the scalp defect after tumor resection.
Fig. 2.
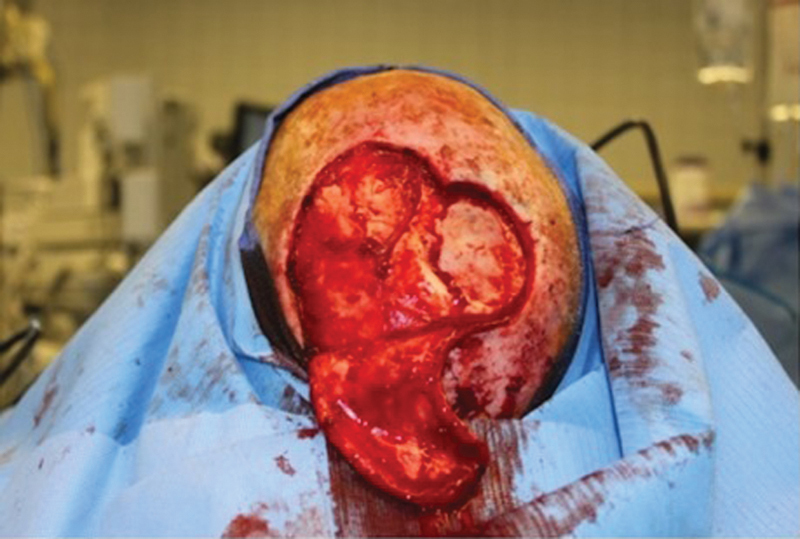
Scalp flap is incised and elevated in a subgaleal plane, ensuring that the pericranium is kept intact.
Fig. 3.
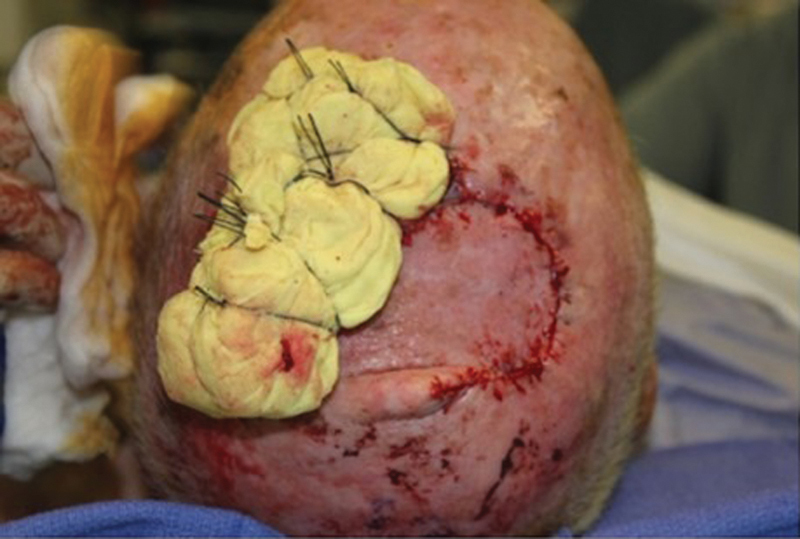
Skin graft placed from thigh donor site and secured with a Xeroform bolster for ∼ 1 week.
Results
The mean patient age at the time of the procedure was 71 years. The mean preoperative ASA classification was 2.8 (standard deviation [SD]: 0.6). The mean operative time was 181 minutes (SD: 120 minutes), and the mean hospital stay was 2.4 days (SD: 2.3 days). Of the 22 defects, 19 were for neoplasm resection. The three most common causes for the scalp defects in our study were squamous cell carcinoma, basal cell carcinoma, and trauma (Fig. 4). The mean defect size was 41 cm2 (range: 7.8–120 cm2) (Fig. 5). There were no intraoperative complications. The skin graft did not survive in one patient and the wound healed secondarily. Three of the patients (14%) had distal flap necrosis; two were treated with local wound care, and one required local flap revision in the operating room for exposed bone. All three of the patients with distal flap necrosis had previous radiation therapy. Twenty-one of the patients (95%) reported an acceptable cosmetic result.
Fig. 4.
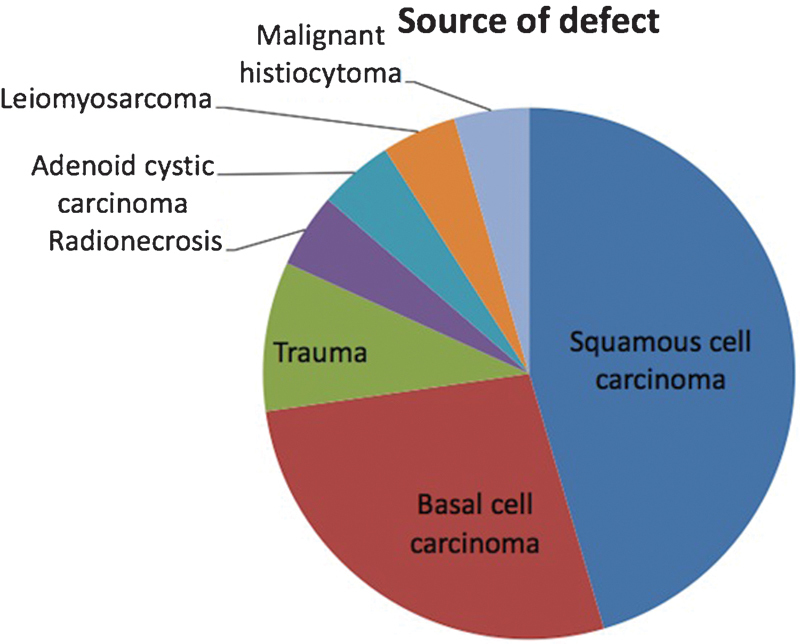
Distribution of scalp pathology in our patient population.
Fig. 5.
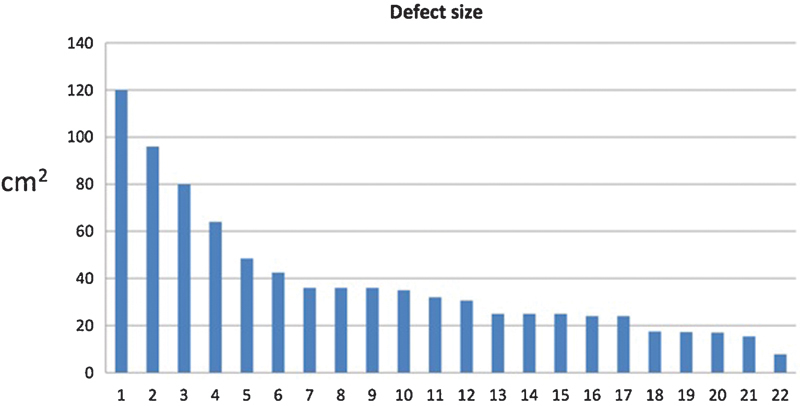
Distribution of average scalp defect size after resection in our patient population.
Comment
The rising incidence of cutaneous neoplasms predicts an increased need for reconstruction of complex scalp wounds. In this series, the technique was effective for reconstructing defects after oncologic surgery, but the technique was also successful for trauma and osteoradionecrosis. The scalp rotation flap could conceivably be used for burns or other patients unsuitable for free tissue transfer.
Reconstructing a scalp defect presents several unique challenges. Limited elasticity of the scalp precludes primary closure of moderate-size defects. Decreased elasticity also prevents mobility of local flaps for closure of large defects. Another potential challenge is that when periosteum is removed with the tumor to obtain clear margins, a skin graft may not be used over the defect because survival is improbable on exposed bone.
The main reconstructive options pertaining to the scalp include healing by secondary intention, primary closure, skin grafting, local flap coverage, and finally, free flap reconstruction.8 Healing by secondary intention is often the simplest approach to scalp closure. Ideal candidates include patients with an intact pericranium, patients who require tumor surveillance, and patients with significant medical comorbidities. Given enough time, the scalp naturally heals through the process of granulation tissue formation, cicatricial scar contracture, and reepithelialization. The disadvantages of secondary intention healing include the time for healing, alopecia, and the overall inferior cosmetic result. Of note, when a scalp defect heals thorough this process, the resulting scar is 60% of the initial wound size through the process of contraction.9 In the patient population discussed in our study, secondary wound healing was not utilized because vascularized tissue must underlie the defect, and in our cohort, the periosteum was removed leaving exposed bone.
The restrictive tissue of the scalp makes primary closure a difficult reconstructive option. The main advantage of primary closure is its simplicity; however, a significant amount of undermining may be required to bring the scalp tissue together. On the scalp, defects < 3 cm are more amenable to primary closure.10 Given that the mean defect size in our study was 41 cm2, primary closure was a suboptimal option in our group of patients. In defects approaching 3 cm, a vertical closure may be preferred due to a dehiscence of the frontalis muscle at the anterior extension of the galeal midline raphe that can facilitate better tissue advancement. The scalp is usually closed in two or three layers when primary closure is used. Judicious use of galeatomies may be attempted to recruit extra tissue for a tension-free closure.
Skin grafting is a common technique for reconstruction of larger scalp defects. The main advantage of skin grafting is the short operative time for patients with medical comorbidities and the high risk of prolonged general anesthesia. An important secondary advantage is that using a skin graft allows surveillance of a wound in post-oncologic reconstruction, which is imperative in high-risk pathologies (melanoma, sclerosing basal cell carcinoma).11 The donor site morbidity for a skin graft is low. Graft survival requires a well-vascularized wound bed via soft tissue or intact periosteum. In our study, the scalp defects were devoid of periosteum, precluding the use of skin graft reconstruction.
The described technique is a local flap reconstruction. Advantages of local flaps include good color, texture, and depth match. The three main patterns in local flap reconstruction include advancement, rotation, and transposition. Advancement flaps have a limited role in scalp reconstruction due to the limited elasticity in the scalp and the multidirectional lines of tension provided by the galea. Transposition flaps involve transferring tissue over or under intervening tissue or structures. As in advancement flaps, the lack of tissue laxity hinders the ability to transpose tissue. Transposition flaps for scalp reconstruction include the temporoparietal flap, the temporoparietal-occipital (Juri flap), and the parietal temporal postauricular vertical flap.12 Rotational flaps, as described here, are the most practical pattern for local flap reconstruction of the scalp, particularly if the defect is > 3 cm.10 Large widely based flaps are preferred over smaller combinations of several rotational flaps because of increased reliability and fewer scars. On the scalp, these flaps can be based anteriorly (supratrochlear artery), laterally (superficial temporal artery), or posteriorly (occipital artery). Significant subgaleal undermining combined with a small “back-cut” toward the pedicle is often required to obtain sufficient rotation to cover a larger defect. When designing a larger scalp flap, attention must be paid to the anterior, temporal, and posterior hairline. Inattention to flap design around the hairline could cause a noticeable distortion of the hairline and a poor cosmetic result.
Due to the unique shape of the scalp, longer flap lengths may be required compared with rotational flaps on flat surfaces. Larger defects on the scalp require an arc incision that is up to six times the greatest diameter of the defect.12 As mentioned, galeal-relaxing incisions may be made in an effort to reduce wound tension. These galeal incisions are typically made parallel to the scalp arterial supply and must be performed conservatively to preserve blood flow. One study found that galeal incisions decreased wound tension by as much as 40%.13 Intraoperative tissue expansion using Foley catheters is also a reliable technique that can help produce less tension around the scalp wound. In addition, preoperative tissue expanders are commonly used to provide both mechanical and biological creep and effectively provide additional tissue, which because of the expansion process has improved vascularity. Potential complications of tissue expansion include infection, expander exposure, and flap ischemia. The disadvantage of larger, widely based flaps, as used in our cohort, is that the donor site requires a skin graft.
Free tissue transfer is an excellent reconstructive option for large scalp defects, > 120 cm2, and complex defects that involve exposed vital structures. Both muscle and fasciocutaneous flaps have been described for scalp reconstruction including the latissimus dorsi, rectus abdominis, radial forearm flap, scapula, and anterolateral thigh flap.14 The latissimus dorsi flap with an STSG is classically described for large defects due to the amount of donor tissue available, a long pedicle, and low donor site morbidity. The main disadvantages of free flap reconstruction include long operative time, anesthetic risk to the patient, and the possibility of flap failure that may require another free flap harvest and anastomosis.15
We report a series of 22 patients who received a scalp rotational flap that resulted in a successful, safe, and cosmetically acceptable result. The robust blood supply in the scalp allows the scalp rotational flap to be harvested from any direction, provided there was no previous surgery or radiation to the area. The rotation of the scalp flap, in combination with an STSG to the donor site, is relatively simple and results in less operative time and donor site morbidity compared with regional or free flap techniques.
Our cohort of patients was relatively advanced in age (mean age: 71 years), and the average ASA classification was high (mean: 2.8). This patient population is typical for patients who present with a scalp neoplasm of significant size due to cumulative ultraviolet light exposure and multiple medical comorbidities. Previously published studies have suggested that older patients with significant medical comorbidities may be at higher risk for medical or surgical complications if the anesthesia time exceeds 10 hours.16 In addition, other factors that have been mentioned as leading to increased complications include the requirement for blood transfusions and coexisting liver disease.17 For our group of patients, the mean operative time was 181 minutes, significantly less time than required for free flap reconstruction. In addition, the mean length of hospital stay was 2.4 days, minimizing patient risk for postoperative complications that are common in the hospital setting. Of the 22 patients who underwent a scalp rotation flap, none of them experienced intraoperative complications. Three had minor wound complications that were distal flap necrosis, likely due to previous radiation therapy. A common complication in scalp reconstruction is alopecia. In our patient cohort, alopecia was not a complication because the patients in the study had significant hair loss and defects that were not located around a hairline (anterior/posterior/temporal). We do believe this technique can be used in a patient with hair present, but rotation of hairline skin may cause anatomical distortion. Overall, both the frequency and severity of complications compare favorably with what has been described for other head and neck reconstructive options.18
Despite the success of this scalp rotational flap in our population, this technique does have limitations. The color and texture after an STSG heals in the flap donor site may not match the surrounding tissue. There is a distinct height discrepancy between the donor site and adjacent scalp. In addition, a standing cone frequently occurs at the base of the scalp flap. It is not advised to excise this redundant tissue because the flap is designed so the vascular supply goes through the flap base. This standing cone decreases with time, and nearly all patients reported an acceptable cosmetic outcome (Fig. 6). Three of our patients who underwent previous radiation therapy experienced distal flap necrosis that was left to heal by secondary intention; this shows that radiation has a significant effect even on the robust blood supply in the scalp.
Fig. 6.
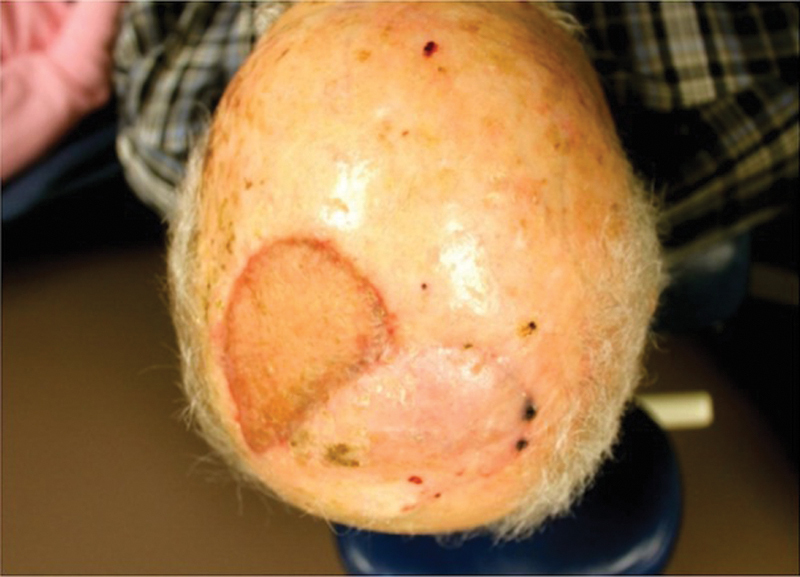
Postoperative view of scalp flap and healed split-thickness skin graft.
Finally, this is a retrospective observational study that does not compare the outcomes of patients receiving the scalp rotation flap with other comparable reconstructive techniques including regional or free flap reconstruction. We believe this could be the subject of future prospective studies. It is reasonable, however, to suggest that patients receiving a scalp rotation flap experience less time under general anesthesia, a shorter hospital stay, and a reduction in donor site morbidity compared with patients undergoing more complex regional or free flap reconstructions.
Conclusions
In patients with large soft tissue scalp defects, the reconstruction can be challenging due to unforgiving soft tissue elasticity. The growing population of patients with cutaneous neoplasms tends to be older and with more medical comorbidities; therefore, expeditious and less morbid reconstructive options may be desired. This retrospective observational study provides evidence that a rotational scalp flap is a reliable and safe reconstructive option for medium size complex scalp defects.
Note
The principal investigator (D.C.) had access to all of the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Rogers H W Coldiron B M Analysis of skin cancer treatment and costs in the United States Medicare population, 1996–2008 Dermatol Surg 201339(1 Pt 1):35–42. [DOI] [PubMed] [Google Scholar]
- 2.Vecchione T R, Griffith L. Closure of scalp defects by using multiple flaps in a pinwheel design. Plast Reconstr Surg. 1978;62(1):74–77. doi: 10.1097/00006534-197807000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Arpey C J. New York, NY: McGraw-Hill; 1997. Random-pattern flaps; p. 222. [Google Scholar]
- 4.Bo B, Qun Y, Zheming P, Haitao X, Tianyi L. Reconstruction scalp defects after malignant tumor resection with anterolateral thigh flaps. J Craniofac Surg. 2011;22(6):2208–2211. doi: 10.1097/SCS.0b013e318231fdb2. [DOI] [PubMed] [Google Scholar]
- 5.Sweeny L, Eby B, Magnuson J S, Carroll W R, Rosenthal E L. Reconstruction of scalp defects with the radial forearm free flap. Head Neck Oncol. 2012;4(4):21. doi: 10.1186/1758-3284-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hierner R, van Loon J, Goffin J, van Calenbergh F. Free latissimus dorsi flap transfer for subtotal scalp and cranium defect reconstruction: report of 7 cases. Microsurgery. 2007;27(5):425–428. doi: 10.1002/micr.20386. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka K, Sakuraba M, Miyamoto S. et al. Analysis of operative mortality and post-operative lethal complications after head and neck reconstruction with free tissue transfer. Jpn J Clin Oncol. 2011;41(6):758–763. doi: 10.1093/jjco/hyr046. [DOI] [PubMed] [Google Scholar]
- 8.Leedy J E, Janis J E, Rohrich R J. Reconstruction of acquired scalp defects: an algorithmic approach. Plast Reconstr Surg. 2005;116(4):54e–72e. doi: 10.1097/01.prs.0000179188.25019.6c. [DOI] [PubMed] [Google Scholar]
- 9.Becker G D, Adams L A, Levin B C. Secondary intention healing of exposed scalp and forehead bone after Mohs surgery. Otolaryngol Head Neck Surg. 1999;121(6):751–754. doi: 10.1053/hn.1999.v121.a98216. [DOI] [PubMed] [Google Scholar]
- 10.Tolhurst D E Carstens M H Greco R J Hurwitz D J The surgical anatomy of the scalp Plast Reconstr Surg 1991874603–612.; discussion 613–614 [PubMed] [Google Scholar]
- 11.Angelos P C, Downs B W. Options for the management of forehead and scalp defects. Facial Plast Surg Clin North Am. 2009;17(3):379–393. doi: 10.1016/j.fsc.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Earnest L M Byrne P J Scalp reconstruction Facial Plast Surg Clin North Am 2005132345–353., vii [DOI] [PubMed] [Google Scholar]
- 13.Raposio E, Santi P L, Nordström R E. Serial scalp reductions: a biomechanical approach. Dermatol Surg. 1999;25(3):210–214. doi: 10.1046/j.1524-4725.1999.08047.x. [DOI] [PubMed] [Google Scholar]
- 14.Temple C L Ross D C Scalp and forehead reconstruction Clin Plast Surg 2005323377–390., vi–vii [DOI] [PubMed] [Google Scholar]
- 15.Beasley N J, Gilbert R W, Gullane P J, Brown D H, Irish J C, Neligan P C. Scalp and forehead reconstruction using free revascularized tissue transfer. Arch Facial Plast Surg. 2004;6(1):16–20. doi: 10.1001/archfaci.6.1.16. [DOI] [PubMed] [Google Scholar]
- 16.Singh B, Cordeiro P G, Santamaria E, Shaha A R, Pfister D G, Shah J P. Factors associated with complications in microvascular reconstruction of head and neck defects. Plast Reconstr Surg. 1999;103(2):403–411. doi: 10.1097/00006534-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Farwell D G, Reilly D F, Weymuller E A Jr, Greenberg D L, Staiger T O, Futran N A. Predictors of perioperative complications in head and neck patients. Arch Otolaryngol Head Neck Surg. 2002;128(5):505–511. doi: 10.1001/archotol.128.5.505. [DOI] [PubMed] [Google Scholar]
- 18.Suh J D, Sercarz J A, Abemayor E. et al. Analysis of outcome and complications in 400 cases of microvascular head and neck reconstruction. Arch Otolaryngol Head Neck Surg. 2004;130(8):962–966. doi: 10.1001/archotol.130.8.962. [DOI] [PubMed] [Google Scholar]


