Abstract
Objective Chordomas are locally aggressive, highly recurrent tumors requiring adjuvant radiotherapy following resection for successful management. We retrospectively reviewed patients treated for intracranial chordomas with adjuvant stereotactic radiosurgery (SRS) and stereotactic radiation therapy (SRT).
Methods A total of 57 patients underwent 83 treatments at the UCLA Medical Center between February 1990 and August 2011. Mean follow-up was 57.8 months. Mean tumor diameter was 3.36 cm. Overall, 8 and 34 patients received adjuvant SRS and SRT, and the mean maximal dose of radiation therapy was 1783.3 cGy and 6339 cGy, respectively.
Results Overall rate of recurrence was 51.8%, and 1- and 5-year progression-free survival (PFS) was 88.2% and 35.2%, respectively. Gross total resection was achieved in 30.9% of patients. Adjuvant radiotherapy improved outcomes following subtotal resection (5-year PFS 62.5% versus 20.1%; p = 0.036). SRS and SRT produced comparable rates of tumor control (p = 0.28). Higher dose SRT (> 6,000 cGy) (p = 0.013) and younger age (< 45 years) (p = 0.03) was associated with improved rates of tumor control.
Conclusion Adjuvant radiotherapy is critical following subtotal resection of intracranial chordomas. Adjuvant SRT and SRS were safe and improved PFS following subtotal resection. Higher total doses of SRT and younger patient age were associated with improved rates of tumor control.
Keywords: chordoma, radiosurgery, radiation therapy, surgery, prognostic factors
Introduction
Chordomas are rare slow-growing tumors originating from the notochord associated with the sacrococcygeal region, skull base, and vertebral column of the sagittal midline axis. They are characterized as locally aggressive lesions with high rates of recurrence but have a low rate of metastasis.1 2 3 4 5 6 7 8 Skull base chordomas pose a unique treatment challenge due to tumor location, large tumor burden on presentation due to an indolent course, involvement of bony structures, and impingement on sensitive neurovascular structures. Surgery plays a critical role in the treatment of chordomas, and aggressive resection with wide margins remains the most significant predictor for improved survival and recurrence-free survival in sacral-, spinal-, and skull-based chordomas.9 10 11 12 13 14 15 16
En bloc resection may not always be feasible for skull base chordomas, and approach and extent of surgical resection largely depends on tumor location and surgeon preference.12 17 Resection with or without adjuvant radiation therapy (RT) is not curative because chordomas demonstrate high rates of recurrence, 51% within the present study, regardless of treatment modalities. Recurrence remains high with subtotal resection, and despite advances in surgical technique, radical excision has only been attainable in ∼ 50% of cases of sacral tumors, with lower rates reported for skull base and spinal chordomas.9 12 18 19 RT plays an important role in therapy because chordomas are not surgically curable tumors due to high rates of recurrence.20 Advances in radiotherapy techniques capable of delivering higher doses of irradiation, such as stereotactic radiosurgery (SRS) and stereotactic radiation therapy (SRT), have been important in advancing the role of RT following surgery. Given the increased risk for local progression following incomplete resection, radiotherapy has been utilized as an adjuvant therapy to decrease rates of recurrence. In addition to photon-based therapies, proton beam therapy has also been applied to the treatment of skull-based chordomas.21 22 Advantages of proton beam therapy include enhanced dose delivery to targeted areas with steep radiation falloff beyond the margins of the target.
The available literature contains many series aiming to elucidate significant prognostic factors for survival including age < 40 years, tumor pathology, total resection, and adjuvant radiation.2 3 23 Dosing and timing of radiation must be controlled to limit complications of exposure to the pituitary stalk, optic apparatus, brainstem, and surrounding radiation-sensitive structures.24 25 Although radiation treatment has been associated with good tumor control rates, the rarity of the disease leaves the optimal factors for radiation unknown. Additionally, the details of optimal use of RT are unclear due to a heterogeneous body of studies with differing timing, radiation modalities, and dosing.1 26 27 28 29 30 31 The goal of this study is to report a large and modern institutional experience in the treatment of intracranial chordomas to examine the role of photon-based adjuvant RT following surgical resection and identify factors that predict tumor progression following treatment.
Methods and Materials
Patient Selection
Between February 1990 and August 2011, 57 patients were evaluated and treated for intracranial chordomas at the University of California Los Angeles (UCLA) Medical Center. Only patients with histologically confirmed intracranial chordomas were included. Patients who were unable to complete a full course of RT, underwent previous surgical resection or RT prior to initial evaluation at the UCLA Medical Center, or lacked adequate postoperative follow-up were excluded. Patients were divided into groups based on the extent of resection (gross total resection [GTR] or subtotal resection [STR]) and use of adjuvant RT. Those receiving RT were further divided into those receiving SRS or SRT. Data on patients, treatments, and outcomes were collected and retrospectively reviewed from electronic medical records. This study was approved by the institutional review board and the Office of the Human Research Protection Program at the UCLA.
Surgery
Initial treatment for all patients consisted of surgery either alone or surgery followed by adjuvant RT. There were a total of 81 surgeries with or without adjuvant RT, comprising 57 initial surgeries and 24 repeat surgeries for recurrent disease. Of the 57 patients undergoing treatment for initial disease, 31 received surgery only and 26 received surgery with subsequent SRS or SRT for the initial treatment. Of the 24 repeat surgeries for recurrent tumors, 15 were for first-time recurrences, 7 were for second, and 2 were for third. Although maximal safe resection was the goal for all surgeries, GTR was achieved in 25 resections with the remaining 56 received STR.
Radiation Therapy
Treatment planning for RT procedures was performed with 1.5-T magnetic resonance imaging (MRI) and computed tomography (CT) scans. CT-MRI fusion was used for the three-dimensional calculation of the target. Isodose treatment plans were created with Brainlab iPlan software (Westchester, Illinois, United States). Final SRS plans were determined by the neurosurgeon, radiation oncologist, and physicist. The 6-MV Novalis linear accelerator (LINAC) (Novalis, Heimstetten, Germany) was used to deliver the radiation. Those receiving SRS or SRT wore a fitted thermoplastic face mask (U-PLAST, BrainLAB, Munich, Germany).
A total of 42 therapies included RT. Of the 81 surgical resections, 40 were followed by either adjuvant SRT or SRS. Two additional patients received SRS alone for the treatment of a recurrent tumor. A total of eight patients received SRS; five had received STR, one had received GTR, and two did not have surgery. Of these patients, five had received some form of RT prior to SRS. In six patients, the average prescribed dose was 1783.3 cGy (range: 1,400 to 2,000 cGy) to the 90% isodose line (IDL) for an average maximal dose of 1974 cGy (range: 1,555 to 2,200 cGy) delivered to the tumor. In two patients, the prescribed doses were 2,000 and 2,500 cGy to the 50% IDL, for a maximal dose of 4,000 and 5,000 cGy delivered to the tumor. Mean number of isocenters was 1.8 (range: 1–4). Thirty-four patients received adjuvant SRT, of which 30 and 4 had received STR and GTR, respectively. Of these patients, four had received some form of RT prior to SRS. Mean maximal dose was 6339 cGy (range: 4500–8272 cGy) over 25 to 42 fractions delivered to the 90% IDL.
Data Collection and Statistical Analysis
Kaplan–Meier log–rank analysis was utilized to evaluate progression-free survival (PFS). The Fisher exact test was used to compare proportions when appropriate. Threshold for significance was determined by a two-tailed p value < 0.05. The primary end point evaluated was tumor recurrence that was confirmed on imaging on successive postoperative follow-ups. Tumor size is defined by the largest diameter measured radiographically in the anteroposterior, transverse, or craniocaudal axis. Prognostic factors influencing rates of tumor recurrence following RT were evaluated through univariate analysis of the subgroup based on patient demographics, tumor characteristics, and treatment parameters.
Results
Patient Features
Overall, there were 24 females and 33 males, with a mean age of 44.6 years (range: 3–74 years) at the first surgery and a mean follow-up of 57.8 months. Mean ages for those in the surgery-only group and in the surgery with adjuvant RT group were 42.7 years (range: 3–74 years) and 52.53 years (5–76 years), respectively (Table 1). Mean length of follow-up was 59 and 56.9 months for those receiving surgery only and those receiving adjuvant RT, respectively. Differences in sex, age, and length of follow-up were not statistically significant between the surgery-only and surgery with radiotherapy groups. There were no differences in sex, age, and follow-up between the SRS and SRT groups.
Table 1. Summary of patient demographics and outcomes.
| Total | SRS | SRT | |
|---|---|---|---|
| No. of patients | 42 | 8 | 34 |
| Sex | |||
| Female | 18 | 3 | 15 |
| Male | 24 | 5 | 19 |
| Mean age, y | 52.5 | 57.3 | 51.4 |
| Surgery | |||
| GTR | 5 | 1 | 4 |
| STR | 34 | 5 | 29 |
| Radiation only | 3 | 2 | 1 |
| Follow-up, mo | 60.5 | 48.3 | 63.3 |
| PFS, % | |||
| 1 y | 97.4 | 100 | 97 |
| 5 y | 37 | 20 | 41 |
| Recurrences | 24 | 5 | 19 |
| Histology | |||
| Chondroid | 9 | 1 | 8 |
| Classic | 33 | 7 | 26 |
Abbreviations: GTR, gross total resection; PFS, progression-free survival; SRS, stereotactic radiosurgery; SRT, stereotactic radiotherapy; STR, subtotal resection.
Except for three incidentally diagnosed chordomas, all patients presented with neurologic symptoms, most commonly involving cranial nerve dysfunction. Diplopia and visual impairment was present in 26 (47.3%) and 10 (18.2%) patients on initial evaluation and represented the most common presenting complaints. Hypacusis, headache, trigeminal signs, and dysphonia were each present in four patients (7.3%). Anosmia, oculomotor nerve palsy, and dysphagia were each present in three (5.5%). Less common symptoms included neck pain, tinnitus, and dizziness.
Tumor Features
Histologic subtypes, determined by pathology evaluation following initial resection, included 39 (68.4%) patients with typical chordoma and 18 (31.6%) with chondroid chordoma. Average tumor size was 3.2 cm (range: 1–8 cm). Mean tumor size in the surgery-only and surgery with RT group was 3.1 and 3.35 cm, respectively. Mean tumor volume was 27.18 cc (range: 1.64–107.7 cc). Most were clival (75.4%) and petroclival (7%) chordomas. Other tumor locations included sella (5.2%), skull base (3.5%), petrous bone (3.5%), posterior fossa (1.7%), sphenoid sinus (1.7%), and basion of the skull (1.7%). Differences in histologic subtypes and tumor size were not statistically significant between the surgery-only and surgery with RT groups.
Treatment
For all cases, including all treatment strategies for both primary and recurrent tumors, there were 43 of 83 (51.8%) recurrences at last clinical follow-up. The 1- and 5-year PFS was 88.2% and 35.2%, respectively(Fig. 1). Mean time to recurrence was 47 months. GTR was achieved in 22 of 57 patients (36.6%) for resection of primary tumors, and 3 of 24 patients (12.5%) for resection of recurrent chordomas (p = 0.0043).
Fig. 1.
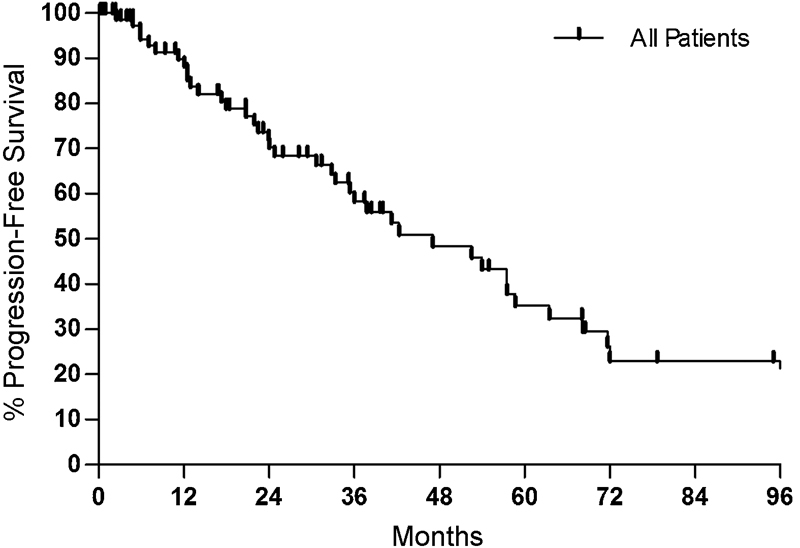
Kaplan–Meier plot for all treatments. The 1- and 5-year progression-free survival rates were 88.2% and 35.2%, respectively.
There were 19 of 41 recurrences (46.3%) within the surgery-only group. Twenty-four of the 42 patients (57.1%) receiving adjuvant RT had tumor recurrence at last clinical follow-up. When evaluating all treatments for both primary and recurrent disease, adjuvant RT was not associated with statistically significant differences in rates of tumor control. Rates of 1- and 5-year PFS were 97.4% and 37% with and 72.9% and 36.5% without adjuvant RT (p = 0.7). Differences in sex (p = 0.59), tumor diameter (p = 0.53), number of recurrent tumors (p = 0.18), cranial neuropathies (p = 0.28), diplopia (p = 0.43), or other visual deficits (p = 0.71) were not statistically significant between patients who underwent surgery only and those who also received adjuvant radiotherapy. However, patients who received radiotherapy following resection were older and more likely to have had STR. Mean ages for surgery-only and surgery with radiotherapy groups were 42.7 (standard deviation [SD]: 14.8) and 52.5 (SD: 15.1) years, respectively (p = 0.004). Rate of GTR was 48.8% and 12.5% within the surgery-only and surgery with adjuvant radiotherapy groups, respectively (p < 0.001).
Subset analysis was performed based on number of repeat treatments, and a similar relationship was found when controlling for number of resections. For first-time resections, 1- and 5-year PFS was 76.5% and 30.6% for surgery only and 100% and 51.3% for surgery with RT, respectively (p = 0.08) (Fig. 2). Following repeat resections for recurrent tumors, 1- and 5-year PFS was 72.9% and 36.5% without, and 74% and 23.5% with adjuvant RT (p = 0.45).
Fig. 2.
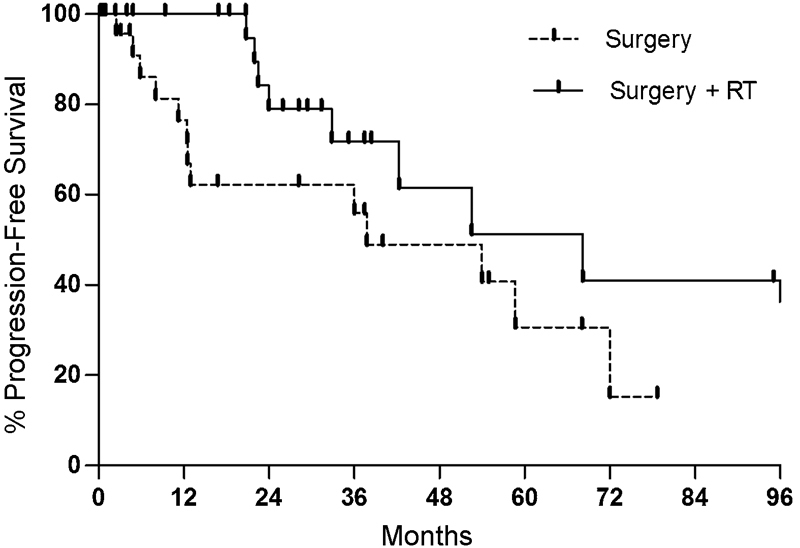
Kaplan–Meier plot comparing rates of progression-free survival between surgery with (solid line) and without (dotted line) adjuvant radiation therapy (RT) (p = 0.08) in the treatment of primary tumors. Surgery includes both gross total resection and subtotal resection, and RT includes both stereotactic radiosurgery and stereotactic radiation therapy.
However, in a separate analysis of STR for primary tumors, adjuvant RT demonstrated superior rates of tumor control compared with STR alone (p = 0.036) (Fig. 3). Tumor recurrence was present in eight patients (61.5%) initially receiving only STR and in nine patients (40.9%) receiving STR with adjuvant RT. Rates of 1- and 5-year PFS were 73.3% and 20.1%, respectively, for STR followed by RT, and 100% and 62.5%, respectively, for STR alone (Table 2). Additionally, differences in rates of tumor control between the GTR-only group (1- and 5-year PFS 80% and 40%, respectively) and the STR with adjuvant RT group were not statistically significant (p = 0.59).
Fig. 3.
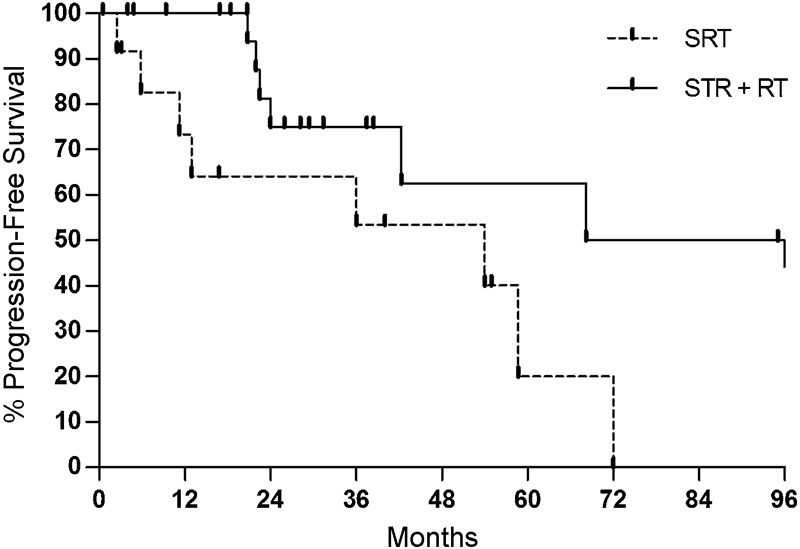
Kaplan–Meier plot comparing rates of progression-free survival between subtotal resection (STR) with (solid line) and without (dotted line) adjuvant radiation therapy (RT) (p = 0.036). SRT, stereotactic radiation therapy.
Table 2. Role of adjuvant radiation therapy following surgical resection.
| Surgery subgroup | Total | Recurrences (%) | 1-y PFS, % | 5-y PFS, % | p | |
|---|---|---|---|---|---|---|
| All surgeries | S | 41 | 19 (46.3) | 79.5 | 32.9 | 0.7 |
| S + RT | 42 | 24 (57.1) | 97.4 | 37 | ||
| Primary resections | S | 31 | 13 (41.9) | 76.5 | 30.6 | 0.08 |
| S + RT | 26 | 11 (42.3) | 100 | 51.3 | ||
| Subtotal resection | STR | 13 | 8 (61.5) | 73.3 | 20.1 | 0.036 |
| STR + RT | 22 | 9 (40.9) | 100 | 62.5 | ||
| Repeat resections | S | 10 | 6 (60) | 72.9 | 36.5 | 0.45 |
| S + RT | 16 | 13 (81.3) | 74 | 23.5 |
Abbreviations: PFS, progression-free survival; RT, radiation therapy; S, surgery only; S + RT, surgery and adjuvant radiation therapy; STR, subtotal resection.
Modality of Radiation Therapy
Mean follow-up for patients receiving surgery followed by SRS was 48.3 months. Of these patients, five had received some form of RT prior to SRS. Mean follow-up following SRT was 63.3 months. Mean time between surgery and RT was 5.1 months for SRS and 6.3 months for SRT. Differences in time to RT and length of follow-up were not statistically significant between those who received SRS and SRT. Five of the eight patients had received prior RT.
Of the eight patients receiving SRS, five (62.5%) demonstrated tumor growth at the latest clinical follow-up. The 1- and 5-year PFS rates were 100% and 20%, respectively, for patients receiving SRS. Of the 34 patients receiving SRT, 19 had tumor recurrence (55.9%), and 1- and 5-year PFS rates were 97% and 40.6%, respectively. Differences in rates of tumor control were not statistically significant between SRS and SRT (p = 0.28) (Fig. 4).
Fig. 4.
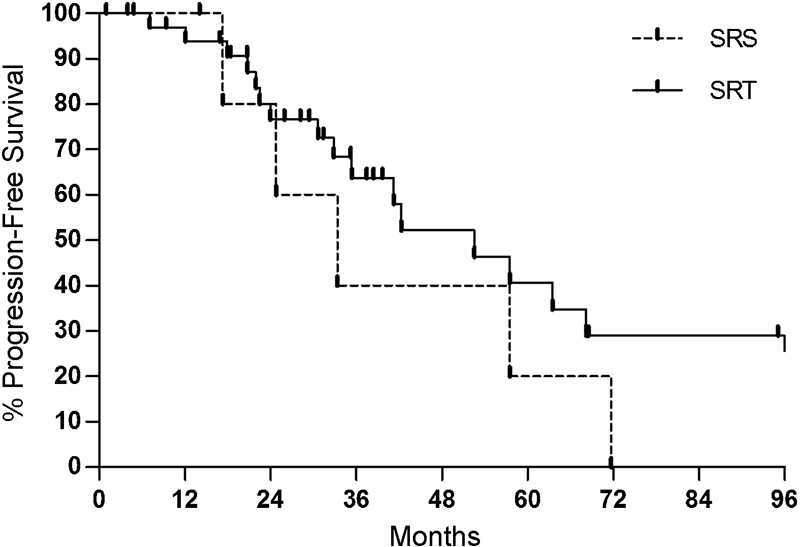
Kaplan–Meier plot comparing rates comparing progression-free survival following postoperative stereotactic radiosurgery (SRS) (dotted line) versus stereotactic radiation therapy (SRT) (solid line) (p = 0.28). Surgeries includes both gross total resection and subtotal resection.
Prognostic Factors
Delivery of lower total radiation dose, older age, and treatment of recurrent disease were negative prognostic factors following surgery with adjuvant RT (Table 3). The most important prognostic factor of tumor control was total radiation dose in patients receiving SRT. Eleven patients received a total ≤ 6,000 cGy, and 16 patients received > 6,000 cGy. Mean cGy was 5,141 cGy (range: 4,500–5,600 cGy) for the low-dose group and 7,162 cGy (range: 6,300–8,272 cGy) for the high-dose group. Rates of tumor progression were 63.6% and 37.5% for low- and high-dose SRT, respectively. On Kaplan–Meier log–rank analysis, a clear benefit was seen in high-dose compared with low-dose SRT (p = 0.009) (Fig. 5). Moreover, for the treatment of primary tumors, high-dose SRT following resection was associated with improved rates of tumor control (100% 5-year PFS) compared with surgery alone (30.6% 5-year PFS) (p = 0.013). However, low-dose adjuvant SRT did not produce better rates of tumor control than surgery alone (p = 0.86).
Table 3. Prognostic factors and rates of progression-free survival following postoperative radiation therapy.
| Prognostic factor | Total | Recurrences (%) | 1-y PFS, % | 5-y PFS, % | p | |
|---|---|---|---|---|---|---|
| Modality | SRS + S | 8 | 5 (62.5) | 100 | 20 | 0.28 |
| SRT + S | 34 | 19 (55.9) | 97 | 40.6 | ||
| Prescribed dose | cGy < 6,000 | 11 | 7 (63.6) | 100 | 0 | 0.009 |
| cGy > 6,000 | 16 | 6 (37.5) | 93.8 | 69.6 | ||
| Time to RT | < 6 mo | 28 | 16 (57.1) | 92 | 42.6 | 0.3 |
| > 6 mo | 9 | 5 (55.6) | 100 | 0 | ||
| Age | < 45 y | 10 | 4 (40) | 100 | 74.1 | 0.03 |
| > 45 y | 32 | 20 (62.5) | 93.2 | 24.6 | ||
| Subtype | Chondroid | 9 | 4 (44.4) | 100 | 34.3 | 0.45 |
| Classical | 33 | 20 (60.6) | 96.8 | 38.8 | ||
| Tumor diameter | < 3.5 cm | 19 | 13 (68.4) | 100 | 29.6 | 0.15 |
| > 3.5 cm | 19 | 8 (42.1) | 94.4 | 35.9 | ||
| Sex | Male | 24 | 12 (50) | 100 | 28.4 | 0.49 |
| Female | 18 | 12 (66.7) | 94.1 | 25.3 | ||
| Tumor type | Primary | 26 | 11 (42.3) | 100 | 51.27 | 0.045 |
| Recurrent | 16 | 13 (81.3) | 93.7 | 23.56 |
Abbreviations: RT, radiation therapy; SRS + S, stereotactic radiosurgery following surgery; SRT + S, stereotactic radiation therapy following surgery.
Fig. 5.
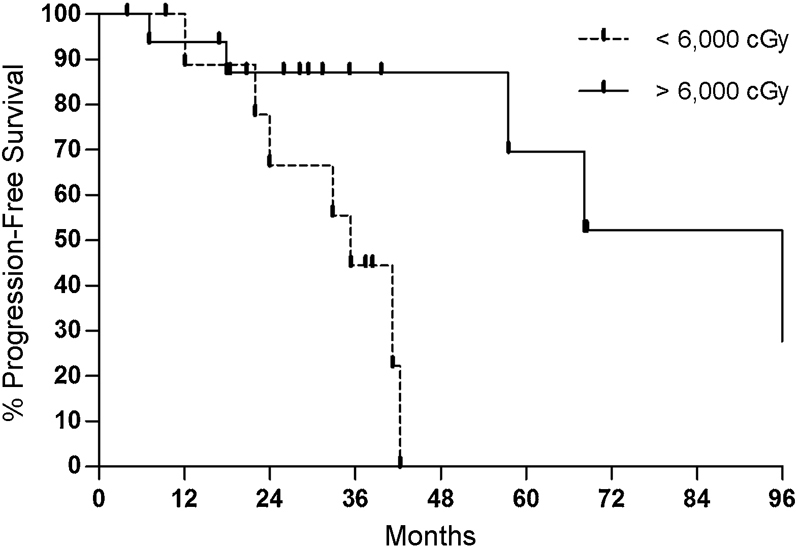
Kaplan–Meier plot comparing rates comparing progression-free survival between high-dose (solid line) and low-dose (dotted line) stereotactic radiation therapy (p = 0.009).
Younger age was associated with improved rates of tumor control following surgery and adjuvant RT. Overall local failure rates following surgery and RT was 40% in patients < 45 years, and 62.5% in those > 45 years. The 1- and 5-year PFS was 100% and 74% for the younger group, and 93.2% and 25% for the older group (p = 0.03).
Recurrent tumors were more likely to progress locally following resection and RT. The number who failed local control for initial treatment was 11 of 26 (42.3%) and for recurrent tumors was 13 of 16 (81.25%). Rates of 1- and 5-year PFS were 100% and 51.3% for first-time treatment and 93.7 and 23.6% for recurrent disease, respectively.
Classic versus chondroid histologic subtype, tumor size prior to surgical resection, length of time between surgery and adjuvant therapy, and gender were not predictive of rates of tumor control following surgery with adjuvant RT. Mean time from surgery to RT was 6.1 months (range: 1–37 months). Mean time to SRS and SRT was 5.1 months and 6.3 months, respectively (p = 0.69). Shorter period of time to adjuvant RT (< 6 months) was not associated with improved rates of tumor control.
Discussion
Here we review the experience of a single institution treating 57 consecutive patients undergoing 83 surgeries either alone or with adjuvant SRS or SRT, to examine the role of adjuvant RT following surgical resection and identify factors that predict tumor progression following treatment. Local recurrence of chordomas is clinically important, associated with a 21-fold increased risk of tumor-associated mortality.14 The surgical goal at our institution focuses on maximal resection balanced with preservation of neurologic function and quality of life. Given these aims, GTR was achieved in 38.6% and 12.5% of primary and recurrent tumors, respectively. Given the proximity to sensitive neurovascular structures and invasion to adjacent bone, STR followed by adjuvant therapy is often performed in patients with the goal of preserving neurologic function. Several radiotherapy modalities are available including radiosurgery, SRT, and proton beam therapy. Proton beam therapy has been shown to be safe, and small retrospective studies have demonstrated promising rates of tumor control.21 30 31 However, comparisons of the efficacy of various adjuvant modalities in skull-based chordomas is difficult due to the limited published experience comprising mainly small retrospective series due to the rarity of this neoplasm.32 Studies have also utilized a combination of proton and photon therapies, and others have reported their experience with chordomas, chondrosarcomas, and other skull-based neoplasms together.26 33 Proton beam radiotherapy was not used at our institution, and all patients received either SRS or SRT following surgery.
Adjuvant radiotherapy was mainly used in the setting of STR with the aim of minimizing the risk of local tumor progression within our institution. Although greater extent of resection is a strong predictor of recurrence, GTR of chordomas is not always achievable due to involvement with sensitive neurovascular structures. Although the surgery-only and the surgery with adjuvant radiotherapy groups were not randomized, there were no differences in demographic features, presenting symptoms, tumor size, or number of recurrent tumors between these two groups. However, patients within the adjuvant radiotherapy group were at a higher risk of recurrence because rates of GTR were much lower in the adjuvant radiotherapy compared with the surgery-only groups. Although the radiotherapy group disproportionately underwent STR and consequently carried an increased risk of recurrence, the present study aims to elucidate key prognostic factors within this subset of patients undergoing adjuvant radiotherapy. RT produced only moderate response rates in chordoma. Within our cohort, RT did not uniformly benefit all patients, but rather only benefited patients with primary tumors in which total resection was not achieved. For all patients, treatment with RT compared with surgery alone demonstrated a trend toward improved rates of 1-year (100% versus 76.5%) and 5-year PFS (51.3% versus 30.6%). However, this difference did not reach statistical significance on Kaplan–Meier log–rank analysis. This may be limited by the wide heterogeneity of follow-up. Patients receiving adjuvant RT were 5.5 times more likely to have receiving a subtotal initial resection, showing a selection bias for larger residual volume in receiving RT.
Subgroup analysis was performed based on extent of resection attained. Within the subgroup of patients where complete resection was not achieved, irradiation of residual tumor was associated with improved outcomes. Rates of 1- and 5-year PFS were 100% and 62.5% following STR with RT, which were significantly improved compared with STR alone (1- and 5-year PFS of 73.3% and 20.1%, respectively). A similar benefit was not observed following GTR of primary tumors. Thus RT is particularly important in the management of residual tumor, but its benefit is not seen if complete excision is achieved. Although the size of postoperative residual tumor predicted RT efficacy, tumor size prior to resection did not predict RT outcomes following RT.
Adjuvant RT following STR demonstrated comparable rates of tumor control compared with complete resection without residual tumor. The rate of 5-year PFS was 62.5% following STR with adjuvant radiotherapy/radiosurgery and 40% following GTR. This was corroborated by Eid et al in a review of 30 skull base and spinal chordomas with a mean follow-up of 78 months.34 The report similarly demonstrated no difference in PFS between resection with wide excision (median PFS: 26%) and subtotal resection followed either by SRS or external photon beam RT (median PFS: 35%).34 The authors attributed the comparable outcomes to SRS, which has demonstrated in several case series to provide improved rates of tumor control for residual tumors following resection and recurrent chordomas.34 35 36 37 38
Treatment of recurrent remains difficult because repeat resections and RT can be limited by scar tissue, distorted native anatomy, and increased risk for adverse outcomes. Complete tumor resection was much less likely to be achieved in patients with recurrent tumors compared with primary tumors (12.5% versus 36.6%; p = 0.0043). Additionally, adjuvant RT for patients with recurrent tumors who have had previous irradiation for primary chordomas had significantly poorer rates of tumor control. Rates of recurrence following RT were 42.3% for primary and 81% for previously treated tumors. Given that the likely clinical course of recurrent tumors is further recurrence despite redo resections and repeat courses of RT, these patients likely harbor a subset of locally aggressive and particularly radioresistant tumors. The limited reports on retreatment have included reirradiation39 and repeat resection.36 40 Delivery of adequate irradiation is limited by treatment toxicities following a previously irradiated field. Although patients often elect for repeat surgeries, these repeat operations carry a higher risk of morbidity.28
There is some consensus in the literature of the role of RT for intracranial chordomas. Several studies have demonstrated an added benefit of adjuvant RT.34 35 36 37 38 However, in a large comprehensive review of all published cases of intracranial chordomas comprising 560 patients, adjuvant RT was not associated with improved clinical outcomes compared with patients receiving surgery alone.41 Five-year rates of survival were 56% versus 54%, respectively.41 As demonstrated in the present analysis, this discrepancy is associated with the selective benefit of adjuvant RT in only a subset of tumors, specifically in primary tumors with residual disease following STR.
The reported rate of recurrence associated with adjuvant SRS for intracranial chordomas is 21 to 80%.37 38 42 43 44 45 Although our findings fall within this range, the wide reported range is likely due to heterogeneous residual tumor size, treatment parameters, dosimetric factors, prescribed dose, and length of follow-up among studies. SRT and SRS were well tolerated and produced similar rates of tumor control following surgical resection, with tumor recurrence rates of 62.5% and 55.9%, respectively. Although differences in rates of recurrence following SRS and SRT were not statistically significant within the present study, statistical power is limited by the sample size. Larger studies will be needed to elaborate on the differences in rates of tumor control between these two different adjuvant treatment strategies. Treatment algorithms may depend on patient- and institution-specific factors. Benefits of SRS include high-dose irradiation in a single session and a steep dose gradient that maximizes delivered radiation dose and minimizes damage to adjacent radiosensitive neurovascular structures. However, large residual tumor size following surgery may limit the efficacy of SRS, and alternative therapies may be preferable. There is little consensus on the superiority of one radiation modality over another because comparative studies are limited and too underpowered for conclusive generalizations. Comparisons between studies is challenging due to the variability in the delivery of RT, and the efficacy of SRS compared with SRT will depend on several factors such as residual tumor size, tumor location, and irregularity of tumor shape. Patient-specific treatment plans, including the use of adjuvant RT and type of RT used, were designed by a multidisciplinary team reflecting multifactorial considerations such as patient preference and evaluation, clinical status, and surgical outcome.
The most important prognostic factor associated with SRT was total dose because chordomas respond better to a higher dose of RT. For those receiving SRT, higher dose (> 6,000 cGy) resulted in lower rates of tumor progression (63.6% versus 37.5%). Chordomas are considered relatively resistant to RT, and high doses of RT are required for progression control.30 46 47 48 Other studies have corroborated our findings. In a study of 48 patients with intracranial and spinal chordomas, Catton et al reported a 100% tumor recurrence rate following conventional fractionated RT with lower total doses of 4,000 to 6,000 cGy.49 Pearlman et al further demonstrated significantly improved overall rates of tumor control in patients receiving higher doses (> 8,000 cGy) compared with those receiving 4,000 to 6,000 cGy (80% versus 20%, respectively).50
Although chordomas respond only to high-dose radiation, there is not a clear consensus on the optimal dose. While some authors have shown minimal doses between 6,000 and 6,500 cGy to be required for successful tumor control, other have demonstrated improved outcomes with higher doses.21 26 51 52 Consistent with the findings in the present study, high-dose adjuvant SRT following resection was associated with significant improvements in rates of tumor control than surgery alone. However, this benefit was lost in low-dose adjuvant SRT, which led to comparable outcomes with surgery alone. Whether there is a dose-dependent relationship is unclear, and larger studies will be needed to identify the optimal dose that balances control of local recurrence and minimization of adverse effects of therapy.
Limitations
One limitation is lack of statistical power in analyzing the role of RT following GTR. The small number of patients within this subgroup is both due to the rarity of the tumor and the frequency in which GTR was achieved. Similarly, larger prospective studies will be needed to further elucidate the role of SRS in comparison with SRT and identify prognostic factors specific to SRS because the small number of patients undergoing SRS in the present study precluded a thorough analysis. The present study carries biases inherent to all retrospective and nonrandomized studies. Confounding factors that may influence tumor recurrence were not controlled for between the groups that did and did not receive adjuvant radiotherapy. Although heterogeneous lengths of follow-up limited a meaningful analysis of survival, tumor recurrence and rates of PFS served as surrogate measures for predicting long-term outcomes in this review.
Conclusion
Although aggressive resection with wide margins remains the most significant predictor for improved outcomes, GTR may not always be feasible for skull base chordomas. Adjuvant RT plays an important role in the management of these tumors. However, chordomas are relatively resistant to RT, and adjuvant RT following surgery does not uniformly improve the outcomes of all patients. Adjuvant RT improved rates of tumor control only in primary tumors in which total resection was not achieved. Recurrent or previously irradiated tumors did not benefit from adjuvant radiotherapy. If adjuvant RT is required, choice of modality should depend on patient- and institution-specific factors. Larger studies will be needed to further elaborate on differences in tumor- and patient-specific prognostic factors and outcomes between SRT and SRS. High-dose radiation is required for tumor response because lower doses were not associated with improved outcomes compared with surgery alone.
Acknowledgments
Isaac Yang (senior author) was partially supported by a Visionary Fund Grant, an Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research UCLA Scholars in Translational Medicine Program Award, the Jason Dessel Memorial Seed Grant, the UCLA Honberger Endowment Brain Tumor Research Seed Grant, and the STOP CANCER Research Career Development Award.
References
- 1.Rich T A, Schiller A, Suit H D, Mankin H J. Clinical and pathologic review of 48 cases of chordoma. Cancer. 1985;56(1):182–187. doi: 10.1002/1097-0142(19850701)56:1<182::aid-cncr2820560131>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 2.Cummings B J, Esses S, Harwood A R. The treatment of chordomas. Cancer Treat Rev. 1982;9(4):299–311. doi: 10.1016/s0305-7372(82)80042-x. [DOI] [PubMed] [Google Scholar]
- 3.Forsyth P A, Cascino T L, Shaw E G. et al. Intracranial chordomas: a clinicopathological and prognostic study of 51 cases. J Neurosurg. 1993;78(5):741–747. doi: 10.3171/jns.1993.78.5.0741. [DOI] [PubMed] [Google Scholar]
- 4.Almefty K, Pravdenkova S, Colli B O, Al-Mefty O, Gokden M. Chordoma and chondrosarcoma: similar, but quite different, skull base tumors. Cancer. 2007;110(11):2457–2467. doi: 10.1002/cncr.23073. [DOI] [PubMed] [Google Scholar]
- 5.Arnold H, Herrmann H D. Skull base chordoma with cavernous sinus involvement. Partial or radical tumour-removal? Acta Neurochir (Wien) 1986;83(1–2):31–37. doi: 10.1007/BF01420505. [DOI] [PubMed] [Google Scholar]
- 6.Colli B O, Al-Mefty O. Chordomas of the skull base: follow-up review and prognostic factors. Neurosurg Focus. 2001;10(3):E1. doi: 10.3171/foc.2001.10.3.2. [DOI] [PubMed] [Google Scholar]
- 7.Colli B, Al-Mefty O. Chordomas of the craniocervical junction: follow-up review and prognostic factors. J Neurosurg. 2001;95(6):933–943. doi: 10.3171/jns.2001.95.6.0933. [DOI] [PubMed] [Google Scholar]
- 8.Thodou E, Kontogeorgos G, Scheithauer B W. et al. Intrasellar chordomas mimicking pituitary adenoma. J Neurosurg. 2000;92(6):976–982. doi: 10.3171/jns.2000.92.6.0976. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs B, Dickey I D, Yaszemski M J, Inwards C Y, Sim F H. Operative management of sacral chordoma. J Bone Joint Surg Am. 2005;87(10):2211–2216. doi: 10.2106/JBJS.D.02693. [DOI] [PubMed] [Google Scholar]
- 10.York J E Kaczaraj A Abi-Said D et al. Sacral chordoma: 40-year experience at a major cancer center Neurosurgery 199944174–79.; discussion 79–80 [DOI] [PubMed] [Google Scholar]
- 11.Yonemoto T, Tatezaki S, Takenouchi T, Ishii T, Satoh T, Moriya H. The surgical management of sacrococcygeal chordoma. Cancer. 1999;85(4):878–883. [PubMed] [Google Scholar]
- 12.Tzortzidis F Elahi F Wright D Natarajan S K Sekhar L N Patient outcome at long-term follow-up after aggressive microsurgical resection of cranial base chordomas Neurosurgery 2006592230–237.; discussion 230–237 [DOI] [PubMed] [Google Scholar]
- 13.Ozaki T, Hillmann A, Winkelmann W. Surgical treatment of sacrococcygeal chordoma. J Surg Oncol. 1997;64(4):274–279. doi: 10.1002/(sici)1096-9098(199704)64:4<274::aid-jso5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 14.Bergh P, Kindblom L G, Gunterberg B, Remotti F, Ryd W, Meis-Kindblom J M. Prognostic factors in chordoma of the sacrum and mobile spine: a study of 39 patients. Cancer. 2000;88(9):2122–2134. doi: 10.1002/(sici)1097-0142(20000501)88:9<2122::aid-cncr19>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 15.Baratti D, Gronchi A, Pennacchioli E. et al. Chordoma: natural history and results in 28 patients treated at a single institution. Ann Surg Oncol. 2003;10(3):291–296. doi: 10.1245/aso.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Cheng E Y, Ozerdemoglu R A, Transfeldt E E, Thompson R C Jr. Lumbosacral chordoma. Prognostic factors and treatment. Spine. 1999;24(16):1639–1645. doi: 10.1097/00007632-199908150-00004. [DOI] [PubMed] [Google Scholar]
- 17.Walcott B P, Nahed B V, Mohyeldin A, Coumans J V, Kahle K T, Ferreira M J. Chordoma: current concepts, management, and future directions. Lancet Oncol. 2012;13(2):e69–e76. doi: 10.1016/S1470-2045(11)70337-0. [DOI] [PubMed] [Google Scholar]
- 18.Bjornsson J, Wold L E, Ebersold M J, Laws E R. Chordoma of the mobile spine. A clinicopathologic analysis of 40 patients. Cancer. 1993;71(3):735–740. doi: 10.1002/1097-0142(19930201)71:3<735::aid-cncr2820710314>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Osaka S, Kodoh O, Sugita H, Osaka E, Yoshida Y, Ryu J. Clinical significance of a wide excision policy for sacrococcygeal chordoma. J Cancer Res Clin Oncol. 2006;132(4):213–218. doi: 10.1007/s00432-005-0067-3. [DOI] [PubMed] [Google Scholar]
- 20.Samii A, Gerganov V M, Herold C. et al. Chordomas of the skull base: surgical management and outcome. J Neurosurg. 2007;107(2):319–324. doi: 10.3171/JNS-07/08/0319. [DOI] [PubMed] [Google Scholar]
- 21.Igaki H, Tokuuye K, Okumura T. et al. Clinical results of proton beam therapy for skull base chordoma. Int J Radiat Oncol Biol Phys. 2004;60(4):1120–1126. doi: 10.1016/j.ijrobp.2004.05.064. [DOI] [PubMed] [Google Scholar]
- 22.Hug E B, Slater J D. Proton radiation therapy for chordomas and chondrosarcomas of the skull base. Neurosurg Clin N Am. 2000;11(4):627–638. [PubMed] [Google Scholar]
- 23.Wold L E, Laws E R Jr. Cranial chordomas in children and young adults. J Neurosurg. 1983;59(6):1043–1047. doi: 10.3171/jns.1983.59.6.1043. [DOI] [PubMed] [Google Scholar]
- 24.Hauptman J S, Barkhoudarian G, Safaee M. et al. Challenges in linear accelerator radiotherapy for chordomas and chondrosarcomas of the skull base: focus on complications. Int J Radiat Oncol Biol Phys. 2012;83(2):542–551. doi: 10.1016/j.ijrobp.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Erdem E, Angtuaco E C, Van Hemert R, Park J S, Al-Mefty O. Comprehensive review of intracranial chordoma. Radiographics. 2003;23(4):995–1009. doi: 10.1148/rg.234025176. [DOI] [PubMed] [Google Scholar]
- 26.Noël G, Habrand J L, Jauffret E. et al. Radiation therapy for chordoma and chondrosarcoma of the skull base and the cervical spine. Prognostic factors and patterns of failure. Strahlenther Onkol. 2003;179(4):241–248. doi: 10.1007/s00066-003-1065-5. [DOI] [PubMed] [Google Scholar]
- 27.Dahlin D C, MacCarty C S. Chordoma. Cancer. 1952;5(6):1170–1178. doi: 10.1002/1097-0142(195211)5:6<1170::aid-cncr2820050613>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 28.Tamaki M, Aoyagi M, Kuroiwa T, Yamamoto M, Kishimoto S, Ohno K. Clinical course and autopsy findings of a patient with clival chordoma who underwent multiple surgeries and radiation during a 10-year period. Skull Base. 2007;17(5):331–340. doi: 10.1055/s-2007-986438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raffel C, Wright D C, Gutin P H, Wilson C B. Cranial chordomas: clinical presentation and results of operative and radiation therapy in twenty-six patients. Neurosurgery. 1985;17(5):703–710. doi: 10.1227/00006123-198511000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Munzenrider J E, Liebsch N J. Proton therapy for tumors of the skull base. Strahlenther Onkol. 1999;175 02:57–63. doi: 10.1007/BF03038890. [DOI] [PubMed] [Google Scholar]
- 31.Hug E B, Loredo L N, Slater J D. et al. Proton radiation therapy for chordomas and chondrosarcomas of the skull base. J Neurosurg. 1999;91(3):432–439. doi: 10.3171/jns.1999.91.3.0432. [DOI] [PubMed] [Google Scholar]
- 32.Amichetti M, Cianchetti M, Amelio D, Enrici R M, Minniti G. Proton therapy in chordoma of the base of the skull: a systematic review. Neurosurg Rev. 2009;32(4):403–416. doi: 10.1007/s10143-009-0194-4. [DOI] [PubMed] [Google Scholar]
- 33.Noël G, Habrand J L, Mammar H. et al. Combination of photon and proton radiation therapy for chordomas and chondrosarcomas of the skull base: the Centre de Protonthérapie D'Orsay experience. Int J Radiat Oncol Biol Phys. 2001;51(2):392–398. doi: 10.1016/s0360-3016(01)01634-0. [DOI] [PubMed] [Google Scholar]
- 34.Eid A S, Chang U K, Lee S Y, Jeon D G. The treatment outcome depending on the extent of resection in skull base and spinal chordomas. Acta Neurochir (Wien) 2011;153(3):509–516. doi: 10.1007/s00701-010-0928-7. [DOI] [PubMed] [Google Scholar]
- 35.Hasegawa T, Ishii D, Kida Y, Yoshimoto M, Koike J, Iizuka H. Gamma Knife surgery for skull base chordomas and chondrosarcomas. J Neurosurg. 2007;107(4):752–757. doi: 10.3171/JNS-07/10/0752. [DOI] [PubMed] [Google Scholar]
- 36.Ito E, Saito K, Okada T, Nagatani T, Nagasaka T. Long-term control of clival chordoma with initial aggressive surgical resection and gamma knife radiosurgery for recurrence. Acta Neurochir (Wien) 2010;152(1):57–67; discussion 67. doi: 10.1007/s00701-009-0535-7. [DOI] [PubMed] [Google Scholar]
- 37.Krishnan S, Foote R L, Brown P D, Pollock B E, Link M J, Garces Y I. Radiosurgery for cranial base chordomas and chondrosarcomas. Neurosurgery. 2005;56(4):777–784; discussion 777–784. doi: 10.1227/01.neu.0000156789.10394.f5. [DOI] [PubMed] [Google Scholar]
- 38.Martin J J, Niranjan A, Kondziolka D, Flickinger J C, Lozanne K A, Lunsford L D. Radiosurgery for chordomas and chondrosarcomas of the skull base. J Neurosurg. 2007;107(4):758–764. doi: 10.3171/JNS-07/10/0758. [DOI] [PubMed] [Google Scholar]
- 39.Jensen A D, Nikoghosyan A, Ellerbrock M, Ecker S, Debus J, Münter M W. Re-irradiation with scanned charged particle beams in recurrent tumours of the head and neck: acute toxicity and feasibility. Radiother Oncol. 2011;101(3):383–387. doi: 10.1016/j.radonc.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 40.Heylen S, Michielsen J, Mahieu G, Somville J. Recurrent chordoma of the L2 vertebra: a case report. Acta Orthop Belg. 2010;76(3):416–419. [PubMed] [Google Scholar]
- 41.Jian B J, Bloch O G, Yang I, Han S J, Aranda D, Parsa A T. A comprehensive analysis of intracranial chordoma and survival: a systematic review. Br J Neurosurg. 2011;25(4):446–453. doi: 10.3109/02688697.2010.546896. [DOI] [PubMed] [Google Scholar]
- 42.Jiang B, Veeravagu A, Lee M. et al. Management of intracranial and extracranial chordomas with CyberKnife stereotactic radiosurgery. J Clin Neurosci. 2012;19(8):1101–1106. doi: 10.1016/j.jocn.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 43.Chang S D, Martin D P, Lee E, Adler J R Jr. Stereotactic radiosurgery and hypofractionated stereotactic radiotherapy for residual or recurrent cranial base and cervical chordomas. Neurosurg Focus. 2001;10(3):E5. doi: 10.3171/foc.2001.10.3.6. [DOI] [PubMed] [Google Scholar]
- 44.Dassoulas K, Schlesinger D, Yen C P, Sheehan J. The role of Gamma Knife surgery in the treatment of skull base chordomas. J Neurooncol. 2009;94(2):243–248. doi: 10.1007/s11060-009-9846-z. [DOI] [PubMed] [Google Scholar]
- 45.Kano H, Iqbal F O, Sheehan J. et al. Stereotactic radiosurgery for chordoma: a report from the North American Gamma Knife Consortium. Neurosurgery. 2011;68(2):379–389. doi: 10.1227/NEU.0b013e3181ffa12c. [DOI] [PubMed] [Google Scholar]
- 46.Foweraker K L, Burton K E, Maynard S E. et al. High-dose radiotherapy in the management of chordoma and chondrosarcoma of the skull base and cervical spine: Part 1—Clinical outcomes. Clin Oncol (R Coll Radiol) 2007;19(7):509–516. doi: 10.1016/j.clon.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Tai P T, Craighead P, Liem S K, Jo B H, Stitt L, Tonita J. Management issues in chordoma: a case series. Clin Oncol (R Coll Radiol) 2000;12(2):80–86. doi: 10.1007/s001740050116. [DOI] [PubMed] [Google Scholar]
- 48.Crockard H A, Steel T, Plowman N. et al. A multidisciplinary team approach to skull base chordomas. J Neurosurg. 2001;95(2):175–183. doi: 10.3171/jns.2001.95.2.0175. [DOI] [PubMed] [Google Scholar]
- 49.Catton C, O'Sullivan B, Bell R. et al. Chordoma: long-term follow-up after radical photon irradiation. Radiother Oncol. 1996;41(1):67–72. doi: 10.1016/s0167-8140(96)91805-8. [DOI] [PubMed] [Google Scholar]
- 50.Pearlman A W, Friedman M. Radical radiation therapy of chordoma. Am J Roentgenol Radium Ther Nucl Med. 1970;108(2):332–341. [PubMed] [Google Scholar]
- 51.Schulz-Ertner D, Nikoghosyan A, Thilmann C. et al. Results of carbon ion radiotherapy in 152 patients. Int J Radiat Oncol Biol Phys. 2004;58(2):631–640. doi: 10.1016/j.ijrobp.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 52.Weber D C, Rutz H P, Pedroni E S. et al. Results of spot-scanning proton radiation therapy for chordoma and chondrosarcoma of the skull base: the Paul Scherrer Institut experience. Int J Radiat Oncol Biol Phys. 2005;63(2):401–409. doi: 10.1016/j.ijrobp.2005.02.023. [DOI] [PubMed] [Google Scholar]


