Abstract
BACKGROUND
Impaired cardiac function in doxorubicin-treated childhood cancer survivors is partly mediated by disruption of mitochondrial energy production. Doxorubicin intercalates into mitochondrial DNA (mtDNA) disrupting genes encoding for polypeptides that make ATP.
METHODS
This cross-sectional study examined mtDNA copy numbers/cell and oxidative phosphorylation (OXPHOS) in peripheral blood mononuclear cells (PBMCs) in 64 childhood survivors of high-risk acute lymphoblastic leukemia (ALL) treated on Dana-Farber Cancer Institute Childhood ALL protocols who had received doxorubicin alone (42%) or with dexrazoxane (58%), a cardioprotectant. Mitochondrial DNA copies per cell and OXPHOS enzyme activities of nicotinamide adenine dinucleotide (NADH) dehydrogenase (Complex I, CI) and cytochrome c oxidase (Complex IV, CIV) were measured by quantitative real time-polymerase chain reaction (qRT-PCR) immunoassay and thin layer chromatography, respectively.
RESULTS
At a median follow-up of 7.8 years after treatment, the median number of mtDNA copies per cell for patients treated with doxorubicin alone was significantly higher than for those who also received dexrazoxane (medians, 1106.3 and 310.5; P=0.001). No significant differences were detected between groups for CI or CIV activities.
CONCLUSIONS
Doxorubicin-treated survivors had increased PBMC mtDNA copies/cell and concomitant use of dexrazoxane was associated with lower mtDNA copies/cell. Due to a possible compensatory increase in mtDNA copies/cell to maintain mitochondrial function in the setting of mitochondrial dysfunction, overall OXPHOS activity was not different between groups. The long-term sustainability of this compensatory response in these survivors at risk for cardiac dysfunction over their lifespan is concerning.
Keywords: Childhood cancer survivors, dexrazoxane, doxorubicin, mitochondrial function, mitochondrial DNA
INTRODUCTION
Childhood cancer survival rates have improved dramatically over recent decades. The five-year event-free survival rate for childhood acute lymphoblastic leukemia (ALL) in 2011 was 82%, a significant improvement from less than 50% in the 1970s (1). Much of this success can be attributed to the widely used and highly effective anthracycline, doxorubicin. However, long-term follow-up studies of this growing population have revealed that doxorubicin treatment is associated with cardiac late-effects years later (2-10).
Doxorubicin-associated cardiotoxicity can occur both during and years after therapy, progressing from asymptomatic left ventricular (LV) dysfunction, to cardiomyopathy, heart failure, and in some cases, the need for heart transplantation or cardiac death (7,9,11,12). Identifying patient risk factors such as female sex, anthracycline cumulative dose and dose-rate, trisomy 21, younger age at diagnosis, black race, prior and concomitant cardiotoxic therapy, preexisting risk for developing cardiovascular disease or identified cardiovascular disease, and increasing time since cancer treatment has helped characterize those at higher risk for these anthracycline-associated cardiac late-effects (5,13-17), although predisposition to cardiac damage varies substantially among individuals.
Recent evidence suggests that doxorubicin-associated cardiotoxicity is at least partially due to the generation of semiquinone free radicals by doxorubicin-iron complexes in the myocardium. Furthermore, one animal study found a dose-dependent increase of doxorubicin in mitochondria, indicating that doxorubicin accumulates in the mitochondria and leads to an increase in mitochondrial iron levels (18). Reaction of these free radicals with oxygen (reactive oxygen species) leads to lipid peroxidation and ultimately DNA damage (19-22). Dexrazoxane, a cardioprotectant, chelates iron, thereby reducing iron from forming doxorubicin-iron complexes and, as a result, lowering free radical DNA damage (23).
Mitochondria contain mitochondrial DNA (mtDNA) that is separate from nuclear DNA and encodes 13 polypeptides of oxidative phosphorylation (OXPHOS). Preclinical studies have associated doxorubicin exposure with irreversible cardiac mitochondrial dysfunction (24-26); clinical histological studies have observed doxorubicin-related structural abnormalities; and one recent study of childhood ALL survivors found that gene polymorphisms of mitochondrial expression may affect mtDNA replication and possibly indicate susceptibility to doxorubicin (27). We have found that doxorubicin-treated long-term survivors of childhood ALL had significantly more mitochondrial DNA mutations or polymorphisms than healthy children (28). Our findings were similar to what has been observed in murine late doxorubicin cardiotoxicity where increased peripheral blood lymphocytes contained mitochondrial mutations that were related to respiratory chain defects and late onset cardiomyopathy (29). These studies demonstrate that doxorubicin has deleterious effects on mitochondrial structure and DNA, which could impact mtDNA copy numbers and OXPHOS.
Further, preclinical studies on anthracycline exposure in mitochondria have shown disrupted OXPHOS, particularly in OXPHOS enzyme activities of NADH dehydrogenase (complex I (CI) and cytochrome c oxidase (complex IV (CIV) activity (30,31), as well as changes in the mtDNA in the form of mutations, deletions, and reduced copy numbers per cell in heart tissue (32,33). If the mitochondria of doxorubicin-exposed cancer survivors are indeed impaired, either through DNA intercalation or oxidative stress, they may undergo clonal expansion of their mtDNA in order to compensate for mutations or deletions that could lead to normal OXPHOS and ATP production (34) in the myocardium. Studies in rats treated with dexrazoxane and doxorubicin demonstrated that concomitant dexrazoxane treatment prevented doxorubicin-induced cardiac mitochondrial dysfunction by maintaining OXPHOS activities and mtDNA integrity (32,35).
Therefore, we examined mtDNA and OXPHOS enzyme activities in peripheral blood mononuclear cells (PBMCs) in childhood survivors of high-risk ALL who received doxorubicin alone or dexrazoxane with doxorubicin.
MATERIALS AND METHODS
Participants
Following institutional review board approval, patients were enrolled on DFCI ALL Consortium Protocol 05-336 if they met all of the following eligibility criteria: were previously diagnosed with ALL and treated on DFCI Childhood ALL protocols, had no prior relapse (in first complete remission), and had no prior allogeneic stem cell transplant. For this cross-sectional study only high-risk ALL patients were included and additional inclusion criteria were established: patients had to have been diagnosed with high-risk ALL at least 4 years prior and did not have a history of secondary malignancies treated with chemotherapy or radiation. Informed consent was obtained from patients who were >18 years old or from parents of patients ≤18 years old. The total planned cumulative doxorubicin dosage for high-risk ALL patients was 300-360 mg/m2. All patients received doxorubicin alone or doxorubicin plus dexrazoxane.
Sample collection
Peripheral blood was collected at the time of enrollment. All samples were shipped overnight at room temperature to a central processing laboratory. Whole blood was diluted 1:1 with basic medium saline/PBS. Samples were centrifuged on lymphocyte separation solution (LSS, Organon Biosciences, Kenilworth, NJ) and the mononuclear cell layer was isolated and washed twice with two volumes of PBS. Cell samples were enumerated and adjusted with PBS to achieve a concentration of 1 × 106 cells/mL and again centrifuged and aspirated. Resulting mononuclear cells constituting primarily lymphocytes were cryopreserved by freezing at -70 °C until mtDNA isolation and analysis. All samples were processed within 30 hours of collection.
PBMC isolation
Blood samples were drawn into ethylenediaminetetraacetic acid and heparin containing vacutainers at each study site. PBMCs were isolated within 8 hours via a Ficoll gradient (GE Healthcare, Waukesha, WI) and platelets were removed by two phosphate-buffered saline washes and centrifugation at 300 relative centrifugal force. The PBMCs were then pelleted and frozen at -80°C.
Acquisition of mtDNA copy numbers and OXPHOS activity
PBMC mtDNA copies/cell were measured by quantitative real time-polymerase chain reaction. DNA was extracted from frozen PBMC using a Qiagen DNA kit (Qiagen, Valencia, CA). Standardization of real-time PCR was performed using LightCycler FastStart DNA Master SYBR Green I with the Roche LightCycler instrument (Roche, Indianapolis, IN). A dilution series of the control plasmid containing the 90 base-pair mtDNA NADH dehydrogenase, subunit 2 and the 98 base-pair Fas Ligand gene was prepared to set up the standard. Each sample and standard was run in a duplicate 20 μl reaction mixture. At the conclusion of the PCR, a melt curve analysis was started at 65°C, and the temperature was increased half a degree every 30 sec for 60 cycles. The results were analyzed with Version 4.0 LightCycler software (36).
CI and CIV were measured by immunoassay and thin layer chromatography, respectively. Each vial of viable PBMC was thawed and washed in 0.5 ml of PBS twice before addition of 0.5 ml of ice-cold extraction buffer [1.5% lauryl maltoside, 25mM Hepes (pH 7.4), 100 mM NaCl, plus protease inhibitors (Sigma, P-8340)]. Samples were mixed gently and kept on ice for 20 minutes, and then they were spun in a micro-centrifuge at 16,400 rpm at 4°C for 20 minutes to remove insoluble cell debris. The supernatant, an extract of detergent-solubilized cellular proteins, was then assayed with the OXPHOS dipstick assays. All samples were loaded on dipsticks with equal amounts of total cell protein or enzyme activity using an amount previously established with control samples to generate signals within the linear range of the assay. Therefore, the resulting dipstick signals were directly proportional to the amount of OXPHOS protein or enzyme activity in the sample. Quantitation of dipstick signals was done by densitometric scanning (36). OXPHOS CI and CIV were specifically measured, as they are the beginning and end of the electron transport chain and are partially encoded by mtDNA. Furthermore, preclinical studies on anthracycline exposure observed disrupted OXPHOS, particularly in CI and CIV, in heart tissue (30,31). We expect that any disturbances to OXPHOS would be measurable at these complexes.
Statistical analysis
A Wilcoxon rank-sum test was used to compare continuous measures between treatment groups. A multiple linear regression model for each continuous outcome measure (PBMC mtDNA copy number and CI and CIV enzyme activity measures) was constructed to test for differences between treatment groups while adjusting for patient and sample characteristics including age at study registration (<10 years versus ≥10 years), sex, T-cell immunophenotype, initial white blood cell count (WBC), and red blood cell (RBC) contamination. The cumulative doxorubicin dose was also included in the modeling on a subset of the data, excluding those patients dosed as mg/kg. Due to the skewness of the distributions, the values for mtDNA copy number and CI and CIV enzyme activities were log10 transformed prior to modeling. All analyses were performed using SAS 9.2. P-values were two-sided and considered significant at the 0.05 level.
RESULTS
Sixty-four patients provided samples at a median of 7.8 years (range, 2.9-30.2 years) after doxorubicin treatment (Table 1). The median cumulative dose of doxorubicin was 300 mg/m2 (120-382 mg/m2) and 37 patients (58%) received concomitant dexrazoxane.
Table 1.
Patient characteristics of all high-risk ALL patients (n=64).
| n (%) | |
|---|---|
| Time to sample from DOX or DEX+DOX treatment years, median (range) | 7.8 (2.9, 30.2) |
| Protocol | |
| 77-01 | 1 (1) |
| 81-01 | 1 (1) |
| 87-01 | 4 (6) |
| 91-01 | 13 (20) |
| 95-01 | 14 (22) |
| 00-01 | 31 (48) |
| Sex | |
| Male | 33 (52) |
| Female | 31 (48) |
| Agea, median years (range) | 5.1 (1.4, 17.1) |
| <10 years | 41 (64) |
| ≥10 years | 23 (36) |
| T-cell immunophenotype | |
| Yes | 7 (11) |
| No | 57 (89) |
| Initial WBC (×103/μL), median (range) | 32.2 (1.3, 354.0) |
| Treatment | |
| DOX | 27 (42) |
| DEX+DOX | 37 (58) |
| Cumulative dose of DOX (mg/m2)b, median (range) | 300 (120, 382) |
Age at the time of protocol registration.
n=50 reported.
DOX, doxorubicin; DEX, dexrazoxane; DEX+DOX, administration of dexrazoxane prior to every doxorubicin treatment; WBC, white blood cell count.
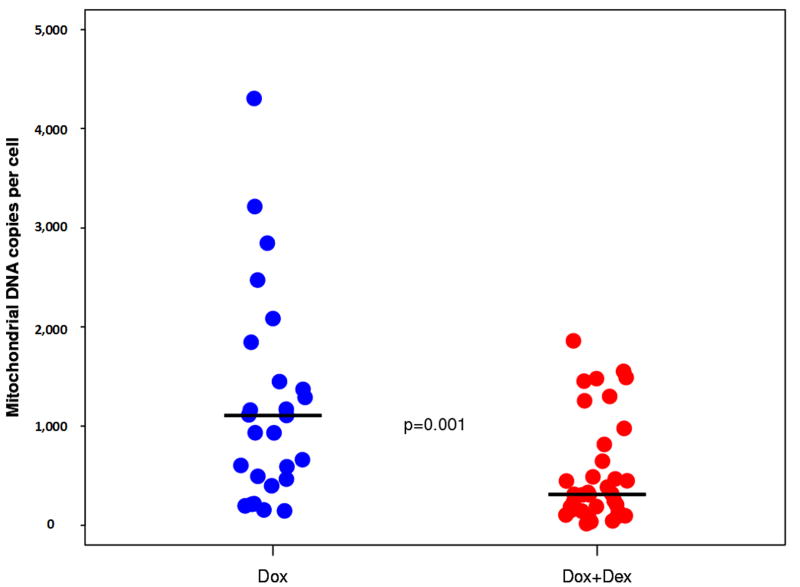
In a univariate analysis, a significant difference was detected for mtDNA copy number between patients who received doxorubicin only (median, 1106.3 copies/cell; range, 144.2-6746.8 copies/cell) and those who also received dexrazoxane (310.5 copies/cell; 15.3-1859.2 copies/cell) (P=0.001; Figure 1). No significant differences were found between treatment groups in CI or CIV enzyme activities (Table 2). However, the median CI activity was significantly higher for males (12.6 OD/μg × 103; n=31; range, 5-41.4) compared to females (6.7 OD/μg × 103; n=27; range, 5.0-27.7) (P=0.037), but not for CIV. Similarly, there was no significant difference in the mtDNA copies/cell when comparing girls to boys.
Figure 1.
MtDNA levels for doxorubicin alone without dexrazoxane versus dexrazoxane administered before each dose of doxorubicin. DEX, dexrazoxane; DOX, doxorubicin; DOX+DEX, administration of dexrazoxane prior to every doxorubicin treatment.
*Horizontal black bars indicate median.
Table 2.
Mitochondrial Parameters and Treatment
| n | DOX Median (range) | n | DEX+DOX Median (range) | P-value | |
|---|---|---|---|---|---|
| MtDNA copies/cell | 27 | 1106.3 (144.2-6746.8) | 35 | 310.5 (15.3-1859.2) | 0.001 |
| CI activity (OD/μg × 103) | 25 | 10.5 (5.0-31.3) | 33 | 11.7 (5.0-41.4) | 0.97 |
| CIV activity (OD/μg × 103) | 25 | 9.8 (5.0-20.1) | 34 | 8.1 (4.7-23.4) | 0.40 |
CI, Complex I; CIV, Complex IV; DOX, doxorubicin; DEX, dexrazoxane; DEX+DOX, administration of dexrazoxane prior to every doxorubicin treatment; OD, optical density.
In a regression analysis, treatment remained associated with mtDNA copy numbers after adjusting for age, sex, T-cell immunophenotype, RBC contamination, and initial WBC cell count (P=0.001, Table 3). No other covariates were significant in the modeling. When considering a model including the cumulative dose of doxorubicin for the subset of patients dosed as mg/m2 (n=48) adjusting for the same covariates, the effect of treatment remained significant (P=0.007; model not shown). Drug treatment was not associated with CI or CIV enzyme activities. However, sex remained associated with CI enzyme activity (P=0.03; model not shown).
Table 3.
Multiple regression model for mtDNA copy number for DOX alone versus DEX+DOX
| Variable | Coefficient (β) | SE | P-value |
|---|---|---|---|
| Intercept | 2.60 | 0.28 | <0.001 |
| Treatment (DOX alone versus DEX+DOX) | 0.46 | 0.13 | 0.001 |
| Sex (male versus female) | 0.11 | 0.14 | 0.45 |
| Agea (<10 versus ≥10 years) | 0.02 | 0.14 | 0.89 |
| Immunophenotype (B-cell versus T-cell) | -0.18 | 0.21 | 0.41 |
| RBC contamination (no versus yes) | 0.02 | 0.14 | 0.89 |
| Initial WBC | -0.0006 | 0.001 | 0.60 |
Age at the time of protocol registration.
DOX, doxorubicin; DEX, dexrazoxane; DEX+DOX, administration of dexrazoxane prior to every doxorubicin treatment; RBC, red blood cell; SE, standard error; WBC, white blood cell count.
In subset analysis of each treatment group the sex difference was only present in the doxorubicin plus dexrazoxane group (male versus female median 16.4 OD/μg × 103 versus 5.3 OD/μg × 103; P=0.007), not in the doxorubicin alone group (male versus female median 9.8 versus 12.3; P=0.85).
DISCUSSION
In this study, after a median of seven years since completing doxorubicin treatment, survivors of childhood high-risk ALL treated with doxorubicin alone had three times the number of mtDNA copies/cell in PBMCs than did children treated with doxorubicin plus dexrazoxane, but OXPHOS enzyme activities did not differ between groups. In addition, mtDNA copies/cell in those who received dexrazoxane were within the normal ranges observed in our previous studies (100-350 copies/cell) (36,37).
As described earlier, in the myocardium, doxorubicin accumulates in the mitochondria, forms complexes with iron, inducing oxidative stress, which then leads to lipid peroxidation and DNA damage (18-22). Additionally, elevated oxidative stress is associated with higher mtDNA copies per cell in aging tissue (34). Dexrazoxane protects the mitochondria by chelating the iron and preventing the formation of the doxorubicin-iron complex (38). Also, in vitro dexrazoxane antagonizes doxorubicin-induced DNA damage in H9C2 cardiomyocytes through its interference with DNA topoisomerase II, which could implicate this topoisomerase in doxorubicin cardiotoxicity (39). In vivo mouse studies with a cardiomyocyte-specific deletion of DNA topoisomerase II prevented mice from developing doxorubicin progressive heart failure whereas in control mice treated with doxorubicin, the genes involved in the regulation of mitochondrial biogenesis and function were decreased (40). Together, this might explain why those who were treated with doxorubicin alone had higher mtDNA copies/cell than those who received dexrazoxane before each doxorubicin dose. This may reflect doxorubicin-associated accelerated mitochondrial aging.
Although a decrease in OXPHOS enzyme activity might be expected, due to impaired mitochondrial function, which was not the case in our study. As with aging tissue, we suspect that doxorubicin-exposed mitochondria may undergo clonal expansion to compensate for doxorubicin-induced mtDNA deletions or mutations to maintain OXPHOS and ATP production (34). A similar compensatory mechanism was seen in a study of adults with Leber’s hereditary optic neuropathy, an inherited blinding disease caused by homoplasmic point mutations in the CI subunit genes of mitochondrial DNA, which are part of the same NADH dehydrogenase subunit examined in our study. This study showed that unaffected carriers of the mutation had a higher mtDNA copy number compared with their affected relatives and controls (41). This would result in normal transcription levels of the NADH dehydrogenase subunit, resulting in normal OXPHOS enzyme activity for this enzyme. The authors postulated that a higher mtDNA copy number per cell in carriers might override some of the pathogenic effects of mitochondrial DNA mutations (41).
Lebrecht et al. found that doxorubicin-exposed rats had higher mtDNA mutations and deletions, and lower mtDNA copies/cell in cardiac tissue than controls after only a few weeks after the last doxorubicin exposure (29). It is possible that mtDNA copies/cell may have been lower in our patients initially after doxorubicin treatment and then later increased through clonal expansion to maintain steady OXPHOS levels. However, this hypothesis cannot be confirmed, as we did not measure mtDNA mutations, deletions, or copies per cell immediately after treatment or serially after completing doxorubicin therapy.
This study has limitations. First, studies examining doxorubicin-induced cardiotoxicity typically use cardiac tissue to measure mitochondrial function, however, we examined systemic changes in PBMC mitochondria, as acquiring cardiac mitochondria is invasive and unethical. Yet, systemic mitochondrial pathologies have been shown to correlate in PBMCs and in the tissues of interest (37,42), therefore it is possible that what we observed in PBMC mitochondria may reflect what is occurring in cardiomyocyte mitochondria. Furthermore, the transcriptome in doxorubicin-exposed cardiac cells have been shown to have a high similarity in the gene expression profiles to those in PBMCs, suggesting that the PBMC transcriptome might serve as a surrogate marker of doxorubicin-induced cardiotoxicity (43). However, further study is warranted in this area. Second, it is possible that the high number of mtDNA copies in the doxorubicin group was due to greater numbers of mitochondria present in the PBMCs. Lebrecht et al. (29) found higher citrate synthase activity, a marker for mitochondrial numbers, in cardiac cells of doxorubicin treated rats as compared to controls. An assay measuring the mitochondrial proteins citrate synthase or porin would have indicated if there were increased numbers of mitochondria in the PBMCs of our patients, but further samples were not available for testing. Future research should examine the number of mitochondria as well as the number of mtDNA copies. Lastly, we did not have sufficient echocardiograms taken at the same time as the samples to determine LV function. However, the patients enrolled in this study were sampled from a larger cohort where LV function was examined after doxorubicin alone or dexrazoxane with doxorubicin treatment [44]. While effects of dexrazoxane on LV function were mild and more significant in the females, generally there was an improvement in cardiac outcomes [44].
A recent study supports the importance of the mitochondrial respiratory chain as a target for doxorubicin cardiotoxicity. This study described a previously unidentified cardiomyocyte-signaling pathway that couples doxorubicin-induced mitochondrial respiratory chain defects and necrotic cell death in a mutually dependent and obligatory link to the BH3-only protein Bcl-2-like 19KDa-interacting protein 3 (Bnip3). Mitochondrial localization of Bnip3, increased reactive oxygen species production, loss of the mitochondrial membrane potential, and mitochondrial permeability transition pore opening resulting in doxorubicin-induced contractile failure and necrotic cell death were associated with cardiomyocyte vacuolization and disrupted mitochondria in an acute injury model that did not examine late-effects. Interventions that antagonize Bnip3 appear beneficial in preventing doxorubicin-induced mitochondrial injury and heart failure (45).
Overall, this is the first long-term study in pediatric high-risk ALL survivors to show that doxorubicin therapy affects mtDNA numbers and that concomitant dexrazoxane therapy improved mtDNA copies/cell; this was at a median of 7 years of completing treatment of doxorubicin using a median cumulative dose of 300 mg/m2. This supports the evidence that dexrazoxane adjuvant therapy in pediatric high-risk ALL patients offer systemic mitochondrial protection as observed in PBMCs, in addition to the already documented cardioprotection seen in this same patient population by measuring cardiac biomarkers of myocardial injury during doxorubicin therapy (serum cardiac troponin T) and cardiomyocyte stress (N-terminal pro brain natriuretic peptide) during therapy (23,46) and by assessment of LV structure and function by echocardiography in long-term survivors (44). The increase in mtDNA copies/cell to maintain mitochondrial function is likely compensatory in the setting of mitochondrial dysfunction. Although, overall OXPHOS activity was not different between groups. The sex differences in CI OXPHOS activity observed in our study may help explain how dexrazoxane affects girls and boys differently. Girls have greater doxorubicin cardiotoxicity than boys as long-term survivors of childhood cancer (15). Girls also derive greater doxorubicin cardioprotection than boys when treated with dexrazoxane for ALL (44). These sex differences suggest that there are differences in both mitochondrial and free radical injury protective mechanisms, similar to what is found in preclinical doxorubicin studies where these pathways have been found to be important targets for cardiotoxicity (21,25,26). The importance of ATP-binding cassette transporter and endothelial nitric oxide synthase gene mutations on the development of late cardiotoxicity in long-term survivors of doxorubicin-treated ALL was recently noted (47) and further supports the importance of mitochondrial and oxidative stress as targets. In that study, abnormal mitochondrial function after doxorubicin therapy might relate to late depressed LV contractility and in disturbed cardiac growth (47). This suggests the importance of both mitochondrial function and free radical pathways in the development of doxorubicin cardiotoxicity. Similarly, the doxorubicin cardiotoxicity free radical damage hypothesis was supported by the presence of hemochromatosis gene mutations, resulting in more iron and free radical formation by the coupling of iron with doxorubicin, are associated with more dead and dying cardiomyocytes during doxorubicin therapy for childhood ALL and in more persistent echocardiographic abnormalities years later (48). These findings support the possible role of dexrazoxane prior to anthracycline therapy to protect mitochondrial function in other healthy tissues such as the ovaries (49). We have further reported that the use of dexrazoxane before every doxorubicin dose resulted in less late cardiotoxicity for children treated for T-cell ALL (50), that its use in pediatric patients with osteosarcoma allowed dose escalation of doxorubicin to 600 mg/m2 without evidence of cardiotoxicity (51), and that its use in osteosarcoma prevented the additive cardiotoxicity associated with the concurrent use of doxorubicin and trastuzumab (52).
Additional follow-up is needed to determine if this compensatory mechanism could eventually become insufficient for maintaining OXPHOS activity in the longer term.
Acknowledgments
Financial support: This study was supported in part by grants from the National Institutes of Health (HL072705, HL078522, HL053392, CA127642, CA068484, HD052104, AI50274, CA068484, HD052102, HL087708, HL079233, HL004537, HL087000, HL007188, HL094100, HL095127, HD80002); Children’s Cardiomyopathy Foundation; Women’s Cancer Association; Lance Armstrong Foundation; STOP Children’s Cancer Foundation; Parker Family Foundation, Scott Howard Fund; and the Michael Garil Fund.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to disclose.
References
- 1.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64:83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 2.Lipshultz SE. Exposure to anthracyclines during childhood causes cardiac injury. Semin Oncol. 2006;33:S8–14. doi: 10.1053/j.seminoncol.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 3.Lipshultz SE. Heart failure in childhood cancer survivors. Nat Clin Pract Oncol. 2007;4:334–5. doi: 10.1038/ncponc0818. [DOI] [PubMed] [Google Scholar]
- 4.Lipshultz SE, Adams MJ. Cardiotoxicity after childhood cancer: beginning with the end in mind. J Clin Oncol. 2010;28:1276–81. doi: 10.1200/JCO.2009.26.5751. [DOI] [PubMed] [Google Scholar]
- 5.Lipshultz SE, Alvarez JA, Scully RE. Anthracycline associated cardiotoxicity in survivors of childhood cancer. Heart. 2008;94:525–33. doi: 10.1136/hrt.2007.136093. [DOI] [PubMed] [Google Scholar]
- 6.Lipshultz SE, Cochran TR, Wilkinson JD. Screening for long-term cardiac status during cancer treatment. Circ Cardiovasc Imaging. 2012;5:555–8. doi: 10.1161/CIRCIMAGING.112.977751. [DOI] [PubMed] [Google Scholar]
- 7.Lipshultz SE, Colan SD, Gelber RD, Perez-Atayde AR, Sallan SE, Sanders SP. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;324:808–15. doi: 10.1056/NEJM199103213241205. [DOI] [PubMed] [Google Scholar]
- 8.Lipshultz SE, Landy DC, Lopez-Mitnik G, Lipsitz SR, Hinkle AS, Constine LS, et al. Cardiovascular status of childhood cancer survivors exposed and unexposed to cardiotoxic therapy. J Clin Oncol. 2012;30:1050–7. doi: 10.1200/JCO.2010.33.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipshultz SE, Lipsitz SR, Sallan SE, Dalton VM, Mone SM, Gelber RD, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629–36. doi: 10.1200/JCO.2005.12.121. [DOI] [PubMed] [Google Scholar]
- 10.Lipshultz SE, Sallan SE. Cardiovascular abnormalities in long-term survivors of childhood malignancy. J Clin Oncol. 1993;11:1199–203. doi: 10.1200/JCO.1993.11.7.1199. [DOI] [PubMed] [Google Scholar]
- 11.Kremer LC, van Dalen EC, Offringa M, Ottenkamp J, Voute PA. Anthracycline-induced clinical heart failure in a cohort of 607 children: long-term follow-up study. J Clin Oncol. 2001;19:191–6. doi: 10.1200/JCO.2001.19.1.191. [DOI] [PubMed] [Google Scholar]
- 12.Green DM, Grigoriev YA, Nan B, Takashima JR, Norkool PA, D’Angio GJ, et al. Congestive heart failure after treatment for Wilms’ tumor: a report from the National Wilms’ Tumor Study group. J Clin Oncol. 2001;19:1926–34. doi: 10.1200/JCO.2001.19.7.1926. [DOI] [PubMed] [Google Scholar]
- 13.Barry E, Alvarez JA, Scully RE, Miller TL, Lipshultz SE. Anthracycline-induced cardiotoxicity: course, pathophysiology, prevention and management. Expert Opin Pharmacother. 2007;8:1039–58. doi: 10.1517/14656566.8.8.1039. [DOI] [PubMed] [Google Scholar]
- 14.Krischer JP, Cuthbertson DD, Epstein S, Goorin AM, Epstein ML, Lipshultz SE. Risk factors for early anthracycline clinical cardiotoxicity in children: the pediatric oncology group experience. Prog Pediatr Cardiol. 1997;8:83–90. doi: 10.1200/JCO.1997.15.4.1544. [DOI] [PubMed] [Google Scholar]
- 15.Lipshultz SE, Lipsitz SR, Mone SM, Goorin AM, Sallan SE, Sanders SP, et al. Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995;332:1738–43. doi: 10.1056/NEJM199506293322602. [DOI] [PubMed] [Google Scholar]
- 16.Nysom K, Holm K, Lipsitz SR, Mone SM, Colan SD, Orav EJ, et al. Relationship between cumulative anthracycline dose and late cardiotoxicity in childhood acute lymphoblastic leukemia. J Clin Oncol. 1998;16:545–50. doi: 10.1200/JCO.1998.16.2.545. [DOI] [PubMed] [Google Scholar]
- 17.Lipshultz SE, Franco VI, Miller TL, Colan SD, Sallan SE. Cardiovascular disease in adult survivors of childhood cancer. Annu Rev Med. 2015;66:161–76. doi: 10.1146/annurev-med-070213-054849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ichikawa Y, Ghanefar M, Bayeva M, Wu R, Khechaduri A, Naga Prasad SV, et al. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest. 2014;124:617–30. doi: 10.1172/JCI72931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myers C. The role of iron in doxorubicin-induced cardiomyopathy. Semin Oncol. 1998;25(suppl 10):10–4. [PubMed] [Google Scholar]
- 20.Myers CE, McGuire WP, Liss RH, Ifrim I, Grotzinger K, Young RC. Adriamycin: the role of lipid peroxidation in cardiac toxicity and tumor response. Science. 1977;197:165–7. doi: 10.1126/science.877547. [DOI] [PubMed] [Google Scholar]
- 21.Wallace KB. Doxorubicin-induced cardiac mitochondrionopathy. Pharmacol Toxicol. 2003;93:105–15. doi: 10.1034/j.1600-0773.2003.930301.x. [DOI] [PubMed] [Google Scholar]
- 22.Vile GF, Winterbourn CC. dl-N,N’-dicarboxamidomethyl-N,N’-dicarboxymethyl-1,2-diaminopropane (ICRF-198) and d-1,2-bis(3,5-dioxopiperazine-1-yl)propane ICRF-187) inhibition of Fe3+ reduction, lipid peroxidation, and CaATPase inactivation in heart microsomes exposed to adriamycin. Cancer Res. 1990;50:2307–10. [PubMed] [Google Scholar]
- 23.Lipshultz SE, Rifai N, Dalton VM, Levy DE, Silverman LB, Lipsitz SR, et al. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med. 2004;351:145–53. doi: 10.1056/NEJMoa035153. [DOI] [PubMed] [Google Scholar]
- 24.Lipshultz SE, Miller TL, Gerschenson M, Neuberg DS, Stevenson K, Franco VI, et al. Impaired mitochondrial structure and function which is abrogated by dexrazoxane in doxorubicin-treated childhood ALL survivors. J Clin Oncol. 2012;9530 doi: 10.1002/cncr.29872. abstr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou S, Starkov A, Froberg MK, Leino RL, Wallace KB. Cumulative and irreversible cardiac mitochondrial dysfunction induced by doxorubicin. Cancer Res. 2001;61(2):771–7. [PubMed] [Google Scholar]
- 26.Zhou S, Palmeira CM, Wallace KB. Doxorubicin-induced persistent oxidative stress to cardiac myocytes. Toxicol Lett. 2001;121:151–7. doi: 10.1016/s0378-4274(01)00329-0. [DOI] [PubMed] [Google Scholar]
- 27.Kwok CS, Quah TC, Ariffin H, Tay SK, Yeoh AE. Mitochondrial D-loop polymorphisms and mitochondrial DNA content in childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2011;33:e239–44. doi: 10.1097/MPH.0b013e31820a5ece. [DOI] [PubMed] [Google Scholar]
- 28.Lipshultz SE, Walker VE, Torres SM, Walker DM, Barry E, Miller TL, et al. Frequent mitochondrial DNA mutations and polymorphisms in long-term survivors of childhood acute lymphoblastic leukemia. Blood (American Society of Hematology Annual Meeting Abstracts) 2007;110:2800. [Google Scholar]
- 29.Lebrecht D, Setzer B, Ketelsen UP, Haberstroh J, Walker UA. Time-dependent and tissue-specific accumulation of mtDNA and respiratory chain defects in chronic doxorubicin cardiomyopathy. Circulation. 2003;108:2423–9. doi: 10.1161/01.CIR.0000093196.59829.DF. [DOI] [PubMed] [Google Scholar]
- 30.Lebrecht D, Kirschner J, Geist A, Haberstroh J, Walker UA. Respiratory chain deficiency precedes the disrupted calcium homeostasis in chronic doxorubicin cardiomyopathy. Cardiovasc Pathol. 2010;19:e167–74. doi: 10.1016/j.carpath.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Chandran K, Aggarwal D, Migrino RQ, Joseph J, McAllister D, Konorev EA, et al. Doxorubicin inactivates myocardial cytochrome c oxidase in rats: cardioprotection by Mito-Q. Biophys J. 2009;96:1388–98. doi: 10.1016/j.bpj.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lebrecht D, Geist A, Ketelsen UP, Haberstroh J, Setzer B, Walker UA. Dexrazoxane prevents doxorubicin-induced long-term cardiotoxicity and protects myocardial mitochondria from genetic and functional lesions in rats. Br J Pharmacol. 2007;151:771–8. doi: 10.1038/sj.bjp.0707294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lebrecht D, Walker UA. Role of mtDNA lesions in anthracycline cardiotoxicity. Cardiovasc Toxicol. 2007;7:108–13. doi: 10.1007/s12012-007-0009-1. [DOI] [PubMed] [Google Scholar]
- 34.Lee HC, Wei YH. Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. Int J Biochem Cell Biol. 2005;37:822–34. doi: 10.1016/j.biocel.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Carvalho RA, Sousa RP, Cadete VJ, Lopaschuk GD, Palmeira CM, Bjork JA, et al. Metabolic remodeling associated with subchronic doxorubicin cardiomyopathy. Toxicology. 2010;270:92–8. doi: 10.1016/j.tox.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 36.Brogly SB, DiMauro S, Van Dyke RB, Williams PL, Naini A, Libutti DE, et al. Short communication: transplacental nucleoside analogue exposure and mitochondrial parameters in HIV-uninfected children. AIDS Res Hum Retroviruses. 2011;27:777–83. doi: 10.1089/aid.2010.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shikuma CM, Gerschenson M, Chow D, Libutti DE, Willis JH, Murray J, et al. Mitochondrial oxidative phosphorylation protein levels in peripheral blood mononuclear cells correlate with levels in subcutaneous adipose tissue within samples differing by HIV and lipoatrophy status. AIDS Res Hum Retroviruses. 2008;24:1255–62. doi: 10.1089/aid.2007.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasinoff BB, Schnabl KL, Marusak RA, Patel D, Huebner E. Dexrazoxane (ICRF-187) protects cardiac myocytes against doxorubicin by preventing damage to mitochondria. Cardiovasc Toxicol. 2003;3:89–100. doi: 10.1385/ct:3:2:89. [DOI] [PubMed] [Google Scholar]
- 39.Lyu YL, Kerrigan JE, Lin CP, Azarova AM, Tsai YC, Ban Y, et al. Topoisomerase IIbeta mediated DNA double-strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res. 2007;67(18):8839–46. doi: 10.1158/0008-5472.CAN-07-1649. [DOI] [PubMed] [Google Scholar]
- 40.Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18(11):1639–42. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 41.Giordano C, Iommarini L, Giordano L, Maresca A, Pisano A, Valentino ML, et al. Efficient mitochondrial biogenesis drives incomplete penetrance in Leber’s hereditary optic neuropathy. Brain. 2014;137(Pt.2):335–53. doi: 10.1093/brain/awt343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Todorova VK, Beggs ML, Delongchamp RR, Dhakal I, Makhoul I, Wei JY, Klimberg VS. Transcriptome profiling of peripheral blood cells identifies potential biomarkers for doxorubicin cardiotoxicity in a rat model. PLoS One. 2012;7(11):e48398. doi: 10.1371/journal.pone.0048398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mallon PUP, Sedwell R, Morey A, Rafferty M, Williams K, Chisholm D, et al. In vivo nucleoside reverse transcriptase inhibitors alter expression of both mitochondrial and lipid metabolism genes in the absence of depletion of mitochondrial DNA. J Infect Dis. 2005;191:1686–96. doi: 10.1086/429697. [DOI] [PubMed] [Google Scholar]
- 44.Lipshultz S, Scully R, Lipsitz S, Sallan S, Silverman L, Miller T, et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncol. 2010;11:950–61. doi: 10.1016/S1470-2045(10)70204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dhingra R, Margulets V, Chowdhury SR, Thliveris J, Jassal D, Fernyhough P, et al. Bnip3 mediates doxorubicin-induced cardiac myocyte necrosis and mortality through changes in mitochondrial signaling. Proc Natl Acad Sci USA. 2014 Dec 23;111(51):E5537–44. doi: 10.1073/pnas.1414665111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lipshultz SE, Miller TL, Scully RE, Lipsitz SR, Rifai N, Silverman LB, et al. Changes in cardiac biomarkers during doxorubicin treatment of pediatric patients with high-risk acute lymphoblastic leukemia: Associations with long-term echocardiographic outcomes. J Clin Oncol. 2012;30:1042–9. doi: 10.1200/JCO.2010.30.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krajinovic M, Elbared J, Drouin S, Bertout L, Rezgui A, Ansari M, et al. Polymorphisms of ABCC5 and NOS3 genes influence doxorubicin cardiotoxicity in survivors of childhood acute lymphoblastic leukemia. Pharmacogenomics J. 2015 Sep 8; doi: 10.1038/tpj.2015.63. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 48.Lipshultz SE, Lipsitz SR, Kutok JL, Miller TL, Colan SD, Neuberg DS, et al. Impact of hemochromatosis gene mutations on cardiac status in doxorubicin-treated survivors of childhood high-risk leukemia. Cancer. 2013;119(19):3555–62. doi: 10.1002/cncr.28256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kropp J, Roti Roti EC, Ringelstetter A, Khatib H, Abbott DH, Salih SM. Dexrazoxane diminishes doxorubicin-induced acute ovarian damage and preserves ovarian function and fecundity in mice. PLoS One. 2015 Nov 6;10(11):e0142588. doi: 10.1371/journal.pone.0142588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Asselin BL, Devidas M, Chen L, et al. Cardioprotection and safety of dexrazoxane in patients treated for newly diagnosed T-cell acute lymphoblastic leukemia or advanced stage lymphoblastic non-Hodgkin’s lymphoma: a report of the Children’s Oncology Group randomized trial POG 9404. J Clin Oncol. 2015 doi: 10.1200/JCO.2015.60.8851. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwartz CL, Wexler LH, Krailo MD, et al. Intensified chemotherapy with dexrazoxane cardioprotection in newly diagnosed nonmetastatic osteosarcoma: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2015 doi: 10.1002/pbc.25753. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ebb D, Meyers P, Grier H, et al. Phase II trial of trastuzumab in combination with cytotoxic chemotherapy for treatment of metastatic osteosarcoma with human epidermal growth factor receptor 2 overexpression: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30(20):2545–2551. doi: 10.1200/JCO.2011.37.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]