Abstract
Purpose
This study tested the hypothesis that encapsulation of influenza vaccine in microneedle patches increases vaccine stability during storage at elevated temperature.
Methods
Whole inactivated influenza virus vaccine (A/Puerto Rico/8/34) was formulated into dissolving microneedle patches and vaccine stability was evaluated by in vitro and in vivo assays of antigenicity and immunogenicity after storage for up to 3 months at 4, 25, 37 and 45°C.
Results
While liquid vaccine completely lost potency as determined by hemagglutination (HA) activity within 1–2 weeks outside of refrigeration, vaccine in microneedle patches lost 40–50% HA activity during or shortly after fabrication, but then had no significant additional loss of activity over 3 months of storage, independent of temperature. This level of stability required reduced humidity by packaging with desiccant, but was not affected by presence of oxygen. This finding was consistent with additional stability assays, including antigenicity of the vaccine measured by ELISA, virus particle morphological structure captured by transmission electron microscopy and protective immune responses by immunization of mice in vivo.
Conclusions
These data show that inactivated influenza vaccine encapsulated in dissolving microneedle patches has enhanced stability during extended storage at elevated temperatures.
Keywords: influenza vaccine immunogenicity, microneedle patch, thermostability, vaccine stability
INTRODUCTION
Currently, almost all vaccines are formulated in liquid form, which must be kept at refrigerated temperature to maintain vaccine quality (1). Due to this strict temperature requirement, vaccine management typically operates within a cold chain, which is a series of temperature controls during transport, storage and distribution of vaccine from the site of manufacturing to the final destination of delivery. However, even an established cold chain does not guarantee the quality of vaccine, as any accidental exposure to heat or unintentional freezing of vaccines during transport and storage can damage the vaccine (2, 3). Motivated by the high costs associated with maintaining the cold chain and dealing with its failures, vaccine with thermostability that abrogates the need for refrigeration is highly desired. Thermally stable vaccine not only allows greater accessibility to populations in developing countries with poor cold-chain infrastructure, it also enables more efficient and widespread distribution of vaccine through pharmacies and by mail in industrialized countries to combat seasonal and pandemic outbreaks of diseases like influenza (4).
One way to increase vaccine stability is by converting liquid vaccine into a dry powder form. Biopharmaceuticals, such as vaccines, can be dried by several means, including air drying, vacuum drying, drying with desiccant, and lyophilization (5). Generally, lyophilization is the preferred method of drying biopharmaceuticals (6). However, during the freeze-drying process, the vaccine particles and vaccine proteins are subjected to several potentially damaging stresses including freezing and drying stresses that may lead to changes in secondary and tertiary structure of the vaccine proteins as well as physical changes such as aggregation (e.g., due to freeze concentration) (7, 8). Addition of sugar into the vaccine formulation, often to create a sugar glass, has been used to protect vaccines from the aforementioned causes during lyophilization (5, 9).
Although injection using hypodermic needles is the most common method of administering vaccines, this method is painful and has low patient acceptance. In many developing countries, for more than 50% of injections are believed to be carried out using unsafe injection practices (10), which is a leading cause of bloodborn pathogen transmission. An alternative to injection is the use of a microneedle patch, which addresses these issues by offering more a patient compliant and safer delivery method that administers vaccines into the epidermis and superficial dermis layer of the skin using an easy-to-apply patch (11–13).
The microneedle patch is more acceptable to patients because it does not hurt and is simple to administer (14, 15) and is safer because microneedles can be made out of safe, water-soluble excipients that dissolve in the skin and leave no sharps waste (16–18). Moreover, microneedle patches store and deliver vaccines in a dry, solid form, which is only dissolved in the interstitial fluid of the skin upon application. Additional studies have shown that vaccination in the skin is more immunogenic than vaccine delivery to the muscle, probably due to the presence of Langerhans and dermal dendritic cells in the skin (13, 19–23).
In this study, we designed patches made of dissolving microneedles that encapsulate whole inactivated influenza virus (A/Puerto Rico/8/34). The microneedles were made out of polyvinyl alcohol (PVA), which is a biocompatible synthetic polymer that was used to provide mechanical strength to the microneedles for skin insertion (24), and sucrose and trehalose, which were used mainly as vaccine stabilizers. The goal of this study was to evaluate the thermal stability of inactivated influenza vaccine encapsulated in microneedles at different storage temperatures under different packaging conditions. Hemagglutination (HA) assay, as well as ELISA and electron microscopy were used to measure vaccine stability. Finally, microneedle patches stored at different temperatures over an extended period of time were evaluated for in vivo immunogenicity in mice.
MATERIALS AND METHODS
Vaccine and Microneedle Formulation Preparation
Influenza virus A/Puerto Rico/8/34 (H1N1) was grown in the allantoic cavity of 9 days old embryonated hen's eggs (Hyline, Douglusville, GA) for 72 h and purified from allantoic fluid by centrifugation through a sucrose gradient (10 to 60%) prepared in phosphate buffered saline without Ca2+ and Mg2+ (PBS; phosphate buffer, NaCl and DI water; pH 7.4; Cellgro, Tewksbury, MA) at 28,000 RPM for 1 h at room temperature in an SW-28 rotor (Beckman Coulter, Brea, CA). After purification, the virus was re-suspended in PBS and then inactivated by incubating with formalin (0.04%) at 4°C for 72 h followed by dialysis against PBS to remove excess formalin (25). Inactivated virus was assayed for total protein concentration by a BCA assay (Pierce, Rockford, IL), and then re-suspended in PBS at 1 mg/ml (total protein concentration) and stored in a −80°C freezer in aliquots until use to serve as the whole inactivated influenza vaccine stock solution.
The vaccine casting solution was prepared by diluting the whole inactivated influenza vaccine stock solution with PBS into 0.5 mg/ml (total protein concentration determined by a BCA assay). Trehalose (D-(+)-trehalose dehydrate, Sigma-Aldrich, St. Louis, MO) was added into the vaccine solution at a concentration of 10 mg/ml.
To prepare the matrix casting solution, PVA (MW 2000, ACROS Organics, Geel, Belgium) was dissolved in DI water at 90°C at a ratio of 0.75 g PVA per 1 ml of DI water. Sucrose (≥99.5%, Sigma-Aldrich, St. Louis, MO) was added to the PVA solution at a ratio of 1 g sucrose per 1 ml of PVA solution, which was then centrifuged at 1500×g for 1 h to remove air bubbles. This matrix formulation was used in all experiments, unless otherwise noted.
Microneedle Patch Fabrication
Microneedle patches were fabricated using established fabrication techniques with minor modifications (26). Briefly, vaccine casting solution was loaded into polydimethylsiloxane (PDMS) molds containing 10×10 arrays of pyramidal cavities. Each mold cavity (300×300×600 μm: L×W×H) had a center-to-center spacing of 640 μm, such that 1.8 μl of vaccine casting solution filled the 100 mold cavities. The molds filled with the vaccine casting solution were partially dried under centrifugation (GS-15R, Beckman, Fullerton, CA) at 3200×g at 20°C for 3 min. Next, matrix casting solution was applied to molds to fill any remaining space in the mold cavities and form a backing that connected all the microneedles into a single patch. These molds were placed under vacuum and centrifuged as above to fill the molds.
The molds were then lyophilized for approximately 24 h as follows. The molds were frozen at −20°C, ramped to −40°C for 1 h, and then vacuumed at 2.67 Pa at −40°C for an additional 10 h. While the pressure was kept constant (2.67 Pa), the temperature was gradually ramped up from −40 to 0°C for 1 h, from 0 to 20°C for 1 h and from 20°C to room temperature (25°C) for another 10 h. In some cases, microneedle patches were dried by air drying overnight at either 25 or 45°C.
After lyophilization, double-sided tape (444 Double-Sided Polyester Film Tape, 3 M, St. Paul, MN) was adhered onto the backside of the microneedle arrays and used to detach the microneedle arrays from the molds by peeling. For the in vivo immunization study, the detached microneedle array was taped onto a scanning electron microscopy mount (Structure Probe, West Chester, PA), which was used as a handle for manual insertion of microneedles into the animal skin.
Microneedle Patch Packaging and Storage
Microneedle patches containing vaccine were stored under three different packaging conditions. The first set of microneedle patches was placed in open glass vials exposed to ambient air and humidity inside the building. The second set of microneedle patches was placed in glass vials with 1 g of desiccant (calcium sulfate, Drierite, Xenia, OH). These vials were tightly capped and further sealed with parafilm. The third set of microneedle patches was also sealed in glass vials with desiccant and with the air replaced with nitrogen gas. Microneedle patches were stored at 4°C in a refrigerator, 25°C in a lab bench drawer, or 37 or 45°C in temperature-controlled incubators. Liquid solutions of inactivated influenza virus in vials were similarly stored with and without desiccant and oxygen at the four temperatures. Microneedle patches and vaccine solutions were removed and assayed for stability after 0, 1, 7, 14, 30, 60 and 90 days.
Microneedle Patch Reconstitution for Analysis
To dissolve and isolate the vaccine from microneedle patches for sample analysis, each microneedle patch was dissolved in 1 ml of DI water. The resulting solution containing vaccine and microneedle matrix excipients was transferred to a centrifuge tube and centrifuged at 29,000×g (Eppendorf centrifuge 5804) at 4°C for 30 min. The supernatant was discarded and the vaccine pellet was re-suspended with PBS to 100 μl for further analysis.
Hemagglutination Assay
One hundred microliter of re-suspended vaccine solution sample was added to a round-bottom 96-well plate (Corning Costar Corp, Cambridge MA). The positive control was prepared by diluting 1.7 μl of 0.5 mg/ml unprocessed vaccine solution with PBS into 100 μl. The vaccine solutions were serially diluted two-fold with PBS. Chicken red blood cells (RBC) (Lampire, Pipersville, PA) were diluted to 0.5% and added to each well containing the diluted vaccine solution (1:1 ratio by volume). After 1 h of incubation at 25°C, the HA potency was recorded based on the maximal dilution that exhibited hemagglutination of the red blood cells (27).
Capture ELISA
Goat anti-H1N1 influenza virus antibodies (United States Biological, Swampscott, MA) were used as the capture antibody and coated onto 96-well plates by incubating 100 μl of the antibody solution at a dilution of 1:500 in each well overnight at 4°C. The plates were then washed with PBS plus 0.5% Tween-20 (PBST) followed by blocking with PBST plus 2% BSA for 2 h at room temperature. After blocking, 100 μl of vaccine sample were added to the wells and incubated for 2 h at room temperature followed by washing with PBST. After washing, the amount of vaccine antigen bound to the plates was determined by ELISA using mouse anti-hemagglutinin antibodies as the primary antibodies and horseradish peroxidase (HRP) conjugated goat anti-mouse IgG antibodies (Southern Biotech, Birmingham, AL) as the secondary antibodies. After washing, 50 μl of tetramethylbenzidine (TMB; Sigma-Aldrich, St. Louis, MO) was added to each well for 20 min at room temperature to develop color until the reaction was stopped by addition of 50 μl of 0.2 N HCl. The optical density of each well was determined by microplate reader (BioTek Synergy 2 Multi-Mode Microplate Reader, Winooski, VT) at 450 nm.
Negative Staining and Electron Microscopy
To examine the morphology of encapsulated vaccine, 10 μl of re-suspended vaccine solution was applied onto formvar- and carbon-coated grids (EM science, Gibbstown, NJ) for 3 min. Extra vaccine suspension was absorbed by filter paper. The grids were immediately stained with 2% potassium phosphotungstate. Excess stain was removed by filter paper. The samples were examined on a Hitachi 7500 transmission electron microscope (Tokyo, Japan).
Immunization of Mice and Lethal-Dose Challenge
Mice were vaccinated using microneedle patches and subsequently challenged with a lethal dose of influenza virus, as described previously [26] and with approval from the Emory University Institutional Animal Care and Use Committee (IACUC). In brief, female BALB/c mice (6–8 weeks old; Charles River Laboratory, Charleston, SC; 6 animals per group) were vaccinated with 0.6 μg of inactivated influenza vaccine by microneedle patch or by intramuscular injection after storage of the vaccine under the various conditions described above. Serum samples were collected 2 weeks after vaccination for serological analysis. Four weeks after vaccination, mice were challenged intranasally with a lethal dose of mouse-adapted influenza virus A/PR/8/34 (50×LD50) and monitored on a daily basis for weight loss and signs of illness. Mice with severe signs of illness and >25% weight loss were euthanized according to IACUC guidelines. All mice were euthanized 6 weeks after vaccination.
Serological Analysis
Serum samples were analyzed for HAI activity following established protocols (28). Briefly, mouse serum was heat-inactivated for 1 h at 56°C and then treated overnight with receptor destroying enzyme (Kenka Seiken, Tokyo, Japan) at 37°C, according to the manufacturer's instruction. Then, 25 μl aliquots of two-fold serially diluted serum samples were mixed with 25 μl virus solution containing four hemagglutinin units of A/Puerto Rico/8/34 influenza virus at 37°C and incubated for 1 h, followed by incubation with 50 μl of 0.5% chicken red blood cells (LAMPIRE Biological Laboratories) for 45 min at 25°C. HAI titers were defined as the reciprocal of the highest serum dilution that inhibited hemagglutination.
Serum samples were also analyzed for virus-specific IgG by ELISA following established protocols (28). The coating antigens were purified His-tagged hemagglutinin proteins. Each ELISA plate was coated with serial two-fold dilutions of purified mouse IgG with known concentration to generate a standard curve. The standard curves were used to calculate the concentrations of influenza hemagglutinin-specific antibodies in serum samples.
RESULTS
Microneedle Fabrication
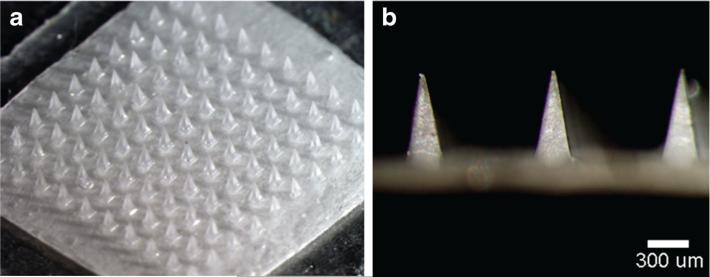
We fabricated microneedle patches containing inactivated influenza vaccine by encapsulating the vaccine within a water-soluble microneedle matrix that was molded in the shape of a microneedle patch and dried by lyophilization. The microneedles were arranged in a 10×10 array of solid microneeedles made of PVA and sucrose (Fig. 1). Each pyramidal needle had a 300×300 μm square base and tapered to a sharp tip with a 600 μm height. Although not visually apparent in the figure, the vaccine was encapsulated only within the microneedles themselves (which are inserted into the skin) and not in the patch backing due to the use of a double-casting molding process that first applied formulated vaccine into the tips of the microneedle molds and then applied the vaccine-free microneedle matrix material to form the rest of the patch.
Fig. 1.
Microneedle patch encapsulating inactivated influenza vaccine. (a) A patch containing a 10×10 array of pyramidal microneedle viewed from above. (b) A side view of individual microneedles.
Effect of Microneedle Patch Drying Method on HA Potency
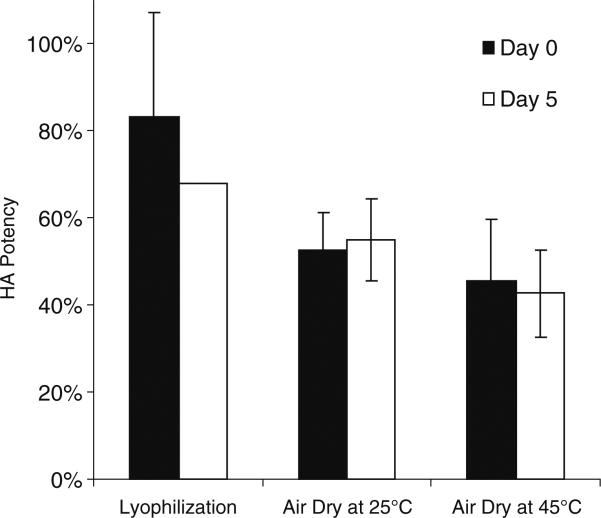
Vaccine stability was assessed by using HA activity as a measure of influenza vaccine antigenicity. Correctly drying the liquid vaccine casting solution during microneedle fabrication is important to maintaining vaccine's stability. We therefore studied the effect of three different drying methods on HA potency after microneedle fabrication: lyophilization, air drying at 25°C and air drying at 45°C.
Immediately after fabrication, microneedles dried by lyophilization maintained 82% HA potency relative to unprocessed vaccine solution stored at 4°C (Fig. 2). This was significantly greater (Student's t-test, p<0.05) than after air drying at 25 and 45°C, which maintained 52 and 45% HA potency, respectively. After 5 days of storage at 25°C in the presence of desiccant, HA potency was between 43 and 68%, which no longer exhibited a significant difference among the microneedle patches dried at the three different conditions (Student's t-test, p>0.05).
Fig. 2.
Effect of microneedle drying conditions on HA potency of vaccine: lyophilization, air drying at 25°C overnight and air drying at 45°C overnight. Day 0 indicates assay of microneedle patches immediately after drying. Day 5 indicates assay of microneedle patches after storage in a sealed container containing desiccant at 25°C for 5 days. 100% potency is defined as the potency of unprocessed liquid vaccine stored at 4°C. Data show average±standard deviation (s.d.) (n=4 replicates).
Effect of Microneedle Patch Formulation on HA Potency
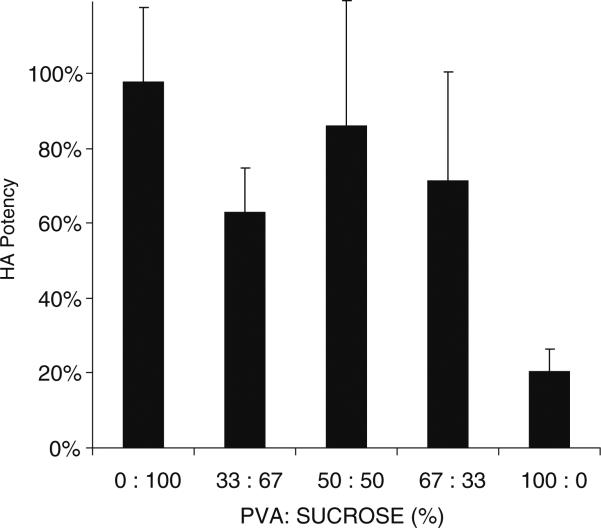
Microneedle patch formulation can also affect vaccine stability. Trehalose was previously found to help stabilize inactivated influenza vaccine (29), so we left trehalose in the formulation of the vaccine casting solution. However, we sought to vary the composition of the microneedle matrix casting solution, since the PVA in that solution is needed to provide mechanical strength to the microneedles and the sucrose is needed to provide vaccine stability (but it is mechanically weak).
We found that microneedle patches made of 100% PVA were only able to maintain 20% HA potency, presumably due to the lack of sucrose (Fig. 3). A formulation containing 33% sucrose with 67% PVA maintained 71% HA potency, which was significantly greater (Student's t-test, p<0.05). Further increasing sucrose did not significantly increase HA potency (ANOVA, p>0.05), but is expected to weaken the microneedles, because PVA provides microneedles with mechanical strength. We therefore concluded that the 33% sucrose formulation was optimal.
Fig. 3.
Effect of microneedle matrix formulation on HA potency of vaccine. Microneedle patches were fabricated using a vaccine casting solution containing 10 mg/ml trehalose and a matrix casting solution containing different ratios of PVA to sucrose, and were then dried by lyophilization. HA potency was measured immediately after drying. Data show average±s.d. (n=4).
Stability of Vaccine in Microneedle Patches Stored at Elevated Temperature
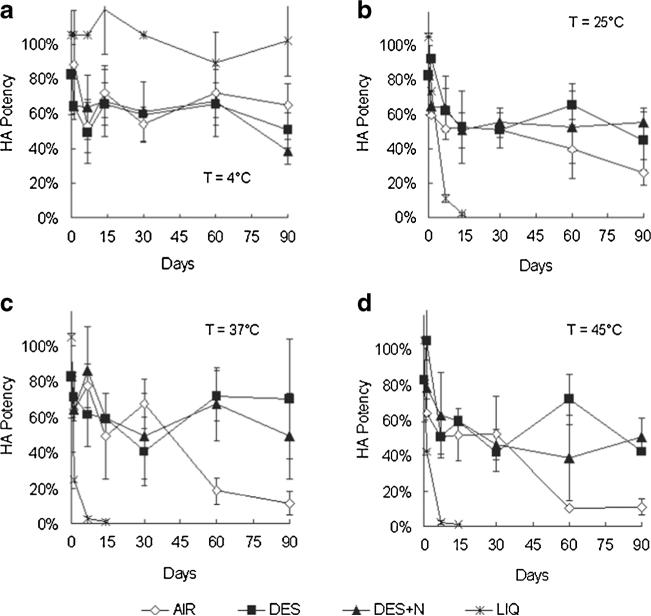
Having assessed stability during the drying process of fabricating microneedle patches, we next studied the vaccine stability during extended storage at elevated temperatures. First, unprocessed liquid vaccine was found to maintain full stability for 3 months during storage at 4°C (Fig. 4a, one-way ANOVA, p<0.05), but lost essentially all HA potency within 1–2 weeks when stored at 25, 37 or 45°C (Fig. 4b–d, one-way ANOVA, p>0.05).
Fig. 4.
Three-months stability of inactivated influenza vaccine as a function of storage temperature, desiccation and oxygen content. Unprocessed vaccine solution was stored in sealed microcentrifuge tubes (LIQ). Microneedle patches containing vaccine were packaged in: open containers subject to ambient humidity (AIR); sealed containers containing air in the head space and desiccant to maintain low humidity (DES); and sealed containers filled with nitrogen gas in the headspace and desiccant (DES+N). Samples were stored for up to 90 days at 4°C (a), 25°C (b), 37°C (c) and 45°C (d). Data show average±s.d. (n≥3).
In contrast, microneedle patches stored in desiccated packaging lost roughly 50% HA potency during the first week or storage (Fig. 4a–d, one-way ANOVA, p>0.05), but then had no significant additional loss of potency for the remainder of the 3 months study at all four temperatures tested (one-way ANOVA, p<0.05). Microneedle patches exposed to air (i.e., without desiccant) initially showed similar stability to the desiccated samples, but after 3 months at 37 and 45°C, HA potency dropped to approximately 10%, which was significantly less than in the desiccated patches (Student's t-test, p>0.05). Microneedle patches stored with both desiccant and a nitrogen gas headspace containing little or no oxygen provided no significant additional vaccine stability compared to microneedle patches stored with desiccant and with air in the headspace (one-way ANOVA, p<0.05). Altogether, these data show that microneedle patches confer much better stability than liquid vaccine, and that storage of microneedles in a reduce humidity environment provided by desiccant is beneficial, but the removal of oxygen from the environment did not have a significant effect.
Assessment of Thermostability by Antigenic Binding Properties
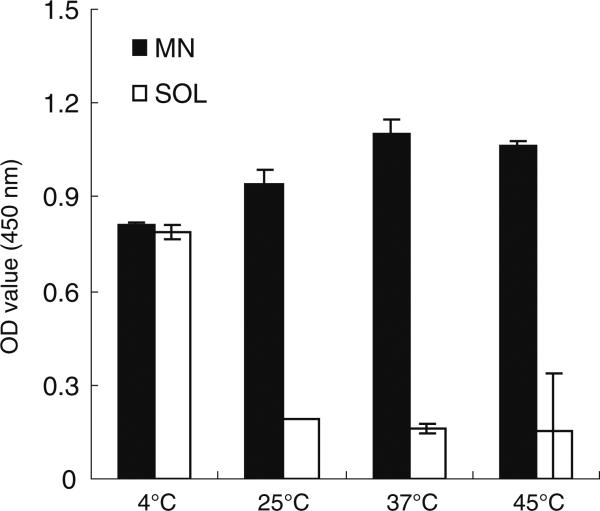
To further validate the findings of thermostability based on HA potency, we used capture ELISA as an additional stability assessment based on the antibody binding specificity of the hemagglutinin in the vaccine. As with the HA assay, vaccine in liquid formulation retained binding activity at 4°C, but lost most of its activity after 2 weeks’ storage at 25, 37 or 45°C (Fig. 5, Student's t-test, p>0.05). In contrast, microneedle patches retained binding activity at all temperatures tested.
Fig. 5.
Stability of inactivated influenza vaccine after storage for 2 weeks as a function of storage temperature. Microneedle patches (MN) encapsulating vaccine and unprocessed vaccine solution (SOL) were stored at 4, 25, 37 and 45°C with desiccant and with air in the headspace. Capture ELISA was employed to detect antibody binding specificity and is reported as optical density (OD) value. We interpret the liquid vaccine (SOL) stored at 4°C as the positive control. Data show average±s.d. (n=4).
The ELISA assay (Fig. 5) indicated greater stability compared to the HA assay (Fig. 4). This might be because antigencity as measured by ELISA only requires that individual hemagglutinin proteins retain their ability to bind to the antibody, whereas the HA assay requires hemagglutination, which involves the assembly of HA proteins into multivalent clusters enabling coordination of multiple hemagglutinin binding events on at least two red blood cells.
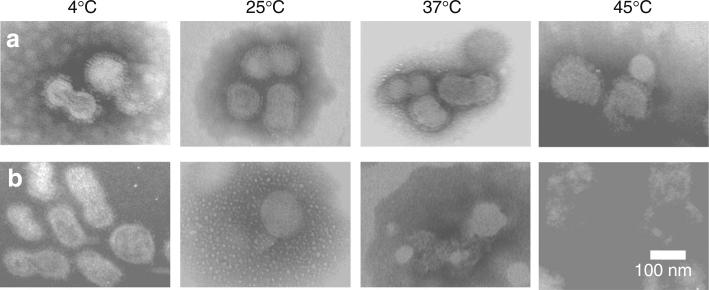
Assessment of Thermostability by Inactivated Influenza Virus Morphology
To better understand the causes of damage to vaccine antigenicity during storage, we further examined vaccine stability based on morphological integrity of the virus particles. When viewed by transmission electron microscopy (TEM) after negative staining with potassium phosphotungstate, the vaccine from microneedle patches stored for 2 weeks at all of the four temperatures exhibits virus-particle structures with intact circular contours of lipid bilayers, which prevented the negative-staining dye from entry into the virus particle as shown by the bright particle core (Fig. 6a). In contrast, while the unprocessed liquid vaccine stored at 4°C retained normal morphology, the unprocessed vaccine stored at 25, 37 and 45°C showed ruptured particle structures in which the dye leaked across the lipid bilayer and into the particles’ core (Fig. 6b).
Fig. 6.
Morphology of inactivated influenza virus imaged by transmission electron microscopy after storage for 2 weeks. Vaccine in microneedle patches (a) and in unprocessed liquid solution (b) were stored at 4, 25, 37 and 45°C with desiccant and with air in the headspace.
Immunogenicity of Vaccine Administered to Mice Using Microneedle Patches Stored at Elevated Temperature
As a final and most important assessment of vaccine stability, we vaccinated mice with microneedle patches after storage for 1 month at 4, 25 and 45°C and compared their immunogencity and protective efficacy with intramuscular injection of liquid vaccine stored at the same temperatures. As positive controls, we also vaccinated with microneedle patches and liquid vaccine that had been stored for just 1 day at 4°C. As a negative controls, we administered vaccine-free patches and intramuscular injection of PBS.
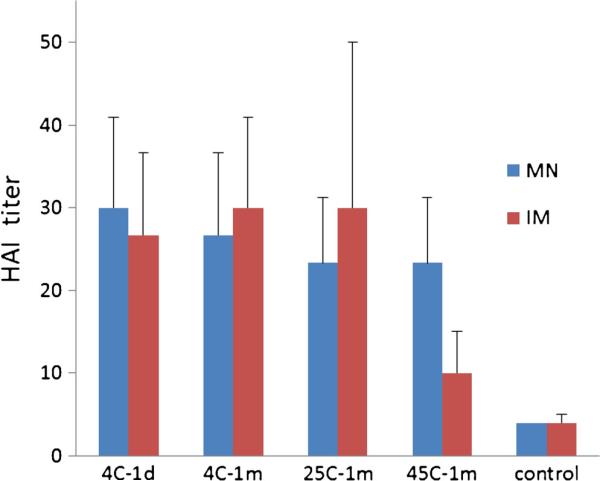
We found that vaccination with microneedle patches stored for 1 month at temperatures at all three of the temperatures tested generated HAI titers that were indistinguishable from freshly made microneedle patches and intramuscular injection of liquid vaccine stored for 1 day at 4°C (one-way ANOVA, p>0.05, Fig. 7). In contrast, while liquid vaccine stored for 1 month at 4 or 25°C and injected intramuscularly also induced antibody responses with HAI activities similar to the positive controls, the HAI titers after vaccination with liquid vaccine stored for 1 month at 45°C were significantly weaker compared to the other immunization groups (one-way ANOVA, p<0.05).
Fig. 7.
Hemagglutination inhibition (HAI) activity 3 weeks after vaccination of mice with microneedle patches (MN) and unprocessed liquid vaccine injected intramuscularly (IM). Microneedle patches and liquid vaccine were stored at 4, 25 or 45°C for 1 month (with desiccant and with air in the headspace) or were stored for 1 day at 4°C. Negative control animals received IM injection of PBS. Data show average±s.d. (n=6).
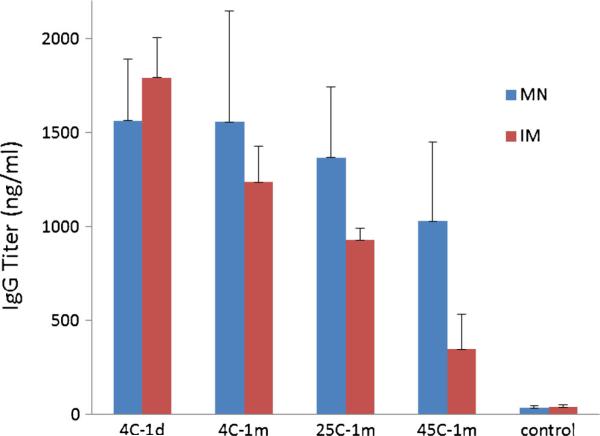
Antibody responses were further characterized by measuring hemagglutinin-specific IgG titers after vaccination. Similar to the HAI responses, IgG titers were similar among animals vaccinated with microneedle patches stored for 1 month at various temperatures compared to microneedle patches or liquid vaccine stored for 1 day at 4°C (one-way ANOVA, p<0.05, Fig. 8), but liquid vaccine stored for 1 month at 45°C induced significantly lower IgG titers compared to microneedle patches or liquid vaccine stored for 1 day at 4°C (one-way ANOVA, p<0.05).
Fig. 8.
Hemagglutinin-specific IgG titers 3 weeks after vacination of mice with microneedle patches (MN) and unprocessed liquid vaccine injected intramuscularly (IM). Microneedle patches and liquid vaccine were stored at 4, 25 or 45°C for 1 month or were stored for 1 day at 4°C. Data show average±s.d. (n=6).
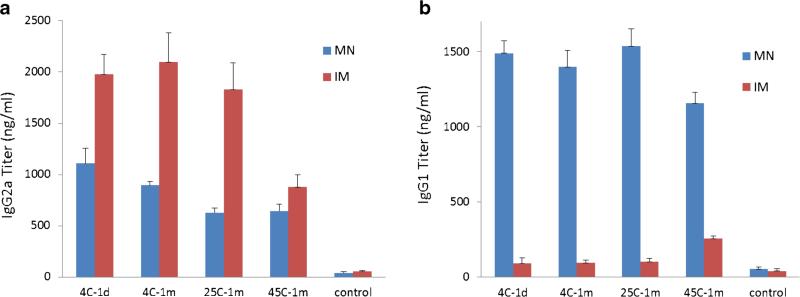
Further examination of antibody isotypes showed that intramuscular injection of liquid vaccine generated stronger IgG2a responses compared to microneedle patch vaccination (two-way ANOVA, p<0.05, Fig. 9a) among patches stored at 4 and 25°C, but intramuscular vaccination exhibited a significant decrease in IgG2a titers after storage at 45°C (one-way ANOVA, p<0.05). In contrast, IgG1 titers were much higher after microneedle patch vaccination compared to intramuscular vaccination of liquid vaccine at all storage conditions and were generally independent of storage conditions (two-way ANOVA, p>0.05, Fig. 9b).
Fig. 9.
Hemagglutinin-specific IgG isotype titers 3 weeks after vaccination of mice with microneedle patches (MN) and unprocessed liquid vaccine injected intramuscularly (IM): IgG2a (a) and IgG1 (b). Microneedle patches and liquid vaccine were stored at 4, 25 or 45°C for 1 month or were stored for 1 day at 4°C. Data show average±s.d. (n=6).
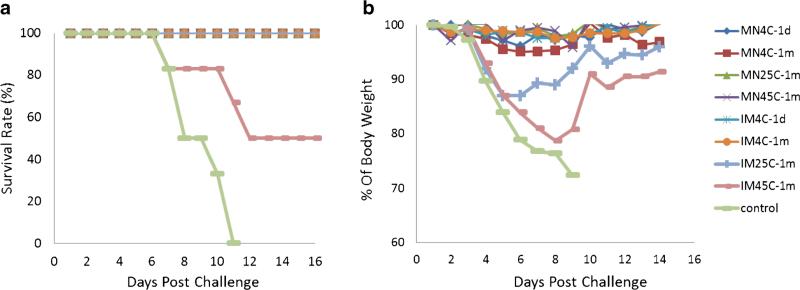
Four weeks after immunization, the mice were challenged with lethal dose of live, mouse-adapted A/Puerto Rico/8/34 virus. All mice vaccinated with microneedle patches stored at any temperature were completely protected against the virus challenge (Fig. 10a) with no significant weight loss (Fig. 10b). In contrast, the mice immunized with intramuscular injection of liquid vaccine stored for 1 month at 45°C only conferred 50% protection and was associated with significant weight loss and illness. Also, mice immunized with injection of liquid vaccine stored for 1 month at 25°C exhibited weight loss after challenge, although they all survived the lethal challenge.
Fig. 10.
Survival (a) and weight loss (b) of mice after challenge with a lethal dose (50×LD50) of live, mouse-adapted A/Puerto Rico/8/34 virus. Mice were challenged 4 weeks after vaccination with microneedle patches (MN) or unprocessed liquid vaccine injected intramuscularly (IM). Microneedle patches and liquid vaccine were stored at 4, 25 or 45°C for 1 month. Control animals received IM injection of PBS. Data show average survival rate or percentage of body weight at each time point in comparison with mouse body weight on Day 0 of challenge for each group (n=6).
DISCUSSION
Biopharmaceuticals, such as vaccines, are known to be more stable in dried powder form than in liquid solution (5, 30), but dried vaccines require reconstitution in liquid for injection. Reconstitution involves additional time, cost and risk (31). Microneedle patches store and deliver vaccine in a dried state without the need of reconstitution (11–13). However, drying the vaccine during microneedle patch fabrication must be done correctly in order to preserve vaccine activity.
In this study, we encapsulated inactivated influenza virus vaccine in dissolving microneedles, which dissolve in the skin, thereby avoiding reconstitution as well as generating no sharps waste. The fabrication process involved casting a liquid solution containing the vaccine onto a mold and drying it into the mold cavities to form microneedles. This drying process can cause stresses on the vaccine, such as the change of pH, ionic strength, osmolarity and dehydration (32).
We adopted two strategies to minimize vaccine stability loss during microneedle fabrication and storage. We first focused on identifying a drying method that best maintained vaccine stability. Out of the three methods we evaluated, lyophilization caused the least damage to the vaccine's HA potency. The second strategy focused on the microneedle formulation that encapsulates the vaccine. Formulation plays a critical role in vaccine stability. For example, sugar glass technology has been described in the literature for protecting proteins, liposomes and viruses from drying stresses (5, 33–35), and specifically the sugar trehalose has been shown to protect inactivated influenza vaccine activity during fabrication of coated microneedles (29).
Guided by this literature, we incorporated trehalose in the vaccine casting solution. For the microneedle matrix solution that needed to contain PVA to provide mechanical strength to the microneedles, we found that inclusion of at least 33% sucrose (a less-costly sugar) was helpful to protect the vaccine from stresses during lyophilization when included with PVA in the matrix solution.
Using this microneedle patch design, we found that the inactivated influenza vaccine retained a significant amount of its activity independent of storage temperature (4, 25, 37 and 45°C) for 3 months. Vaccine stability was determined by HA potency in addition to hemagglutinin-specific antibody binding, morphological analysis by TEM and in vivo tests of protective immunity after vaccination of mice. We further found that vaccine stability during storage was enhanced by the inclusion of desiccant to control humidity, but was not significantly affected by removal of oxygen during storage. This contrasts with the stability of liquid vaccine, which lost essentially all activity within 1–2 weeks without refrigeration. These findings complement prior studies of influenza vaccine stabilization in microneedles (36–40) and bioneedles (41).
At all storage conditions, HA potency of the vaccine dropped during fabrication and then dropped further during the first 2 weeks of storage by roughly 50%, but then remained stable for the rest of the 3 month storage period as long as desiccant was present. The initial drop in HA potency loss at day 0 is likely associated with the drying process during fabrication, as discussed above. The additional drop in HA potency over the following 2 weeks may be due to additional drying of the microneedle patch, which was mostly dry after fabrication, but continued to dry further during storage. Once fully dried, HA potency remained stable for the rest of the 3 months. The samples stored without desiccant were not able to fully dry and therefore continued to lose HA potency throughout the experiment. This mechanistic hypothesis requires additional testing.
While in vitro studies of vaccine stability provide greater throughput and therefore more data, in vivo immunogenicity studies are critical to show that a vaccine is active. HAI titers, which are believe to correlate most closely with protective efficacy of influenza vaccines, were shown to be indistinguishable after vaccination with microneedle patches stored at temperatures as high as 45°C for 1 month compared to vaccination with freshly made microneedle patches. Vaccine stored as a liquid at 45°C for 1 month generated much lower HAI titers, just above baseline values. These findings were supported by lethal-virus challenge studies, in which all mice vaccination with microneedle patches stored at any temperature were protected and had no significant weight loss, whereas mice vaccinated IM with liquid vaccine storage at 25 or 45°C experienced significant weight loss and 60% of mice vaccinated IM with liquid vaccine stored at 45°C died.
Hemagglutinin-specific IgG titers were generally consistent with the findings from HAI titer analysis. Examination of the IgG isotypes revealed that vaccination with microneedle patches, independent of storage conditions, generated relatively lower IgG2a titers than liquid vaccination IM, but generated much stronger IgG1 titer. This indicates that microneedle patch vaccination generates a more balanced immune response between Th1- and Th2-mediated pathways (1).
A thermostable microneedle patch for influenza vaccination could benefit public health in many ways. In developing countries, distributing vaccine without losing vaccine quality is still a challenge, because the cold-chain is often not affordable or may not be available, especially the “last mile” of distribution to villages (1, 2). A vaccine that can withstand elevated temperature during storage can be partially or fully removed from the cold chain, thereby simplifying vaccine distribution, lowering cost and reduced vaccine wastage due to cold chain failures. The ease of administration by minimally trained personnel, generation of no sharps waste, elimination of the need for reconstitution, reduced size and weight of the vaccine and other benefits of the microneedle patch dosage form make this delivery method even more attractive to address challenges in developing countries.
These attributes of a thermostable microneedles patch can also benefit influenza vaccination in industrialized countries. For both seasonal and pandemic vaccination needs, it is expected to increase influenza vaccination coverage if microneedle patches could be made available, especially for self-administration at home (15). A thermostable patch would simplify stockpiling, as well as distribution in pharmacies and, potentially, via the mail without the need for refrigeration or ice packs.
CONCLUSION
This study evaluated the stability of inactivated influenza vaccine encapsulated in dissolving microneedle patches during microneedle fabrication and during extended storage. We evaluated vaccine stability using the HA assay for hemoagglutination activity, capture ELISA for antibody-binding specificity, TEM for morphological analysis and in vivo vaccination to assess antibody responses and protection against lethal-virus challenge.
During fabrication, we found that drying the vaccine using lyophilization with the inclusion of trehalose and sucrose as stabilizers enabled greater vaccine stability than air drying. During storage, vaccine encapsulated in microneedle patches stored with desiccant was thermostable, showing temperature-independent stability at 4, 25, 37 and 45°C for 3 months. In contrast, liquid vaccine lost essentially all activity after storage without refrigeration within 1–2 weeks.
These findings were supported by in vivo immunogenicity studies in mice, where microneedle patches stored for 1 month at all temperatures tested (i.e., 4, 25 and 45°C) generated HAI and IgG titers indistinguishable from freshly made patches and fully protected mice from lethal-virus challenge (i.e., 100% survival). In contrast, liquid vaccine stored for 1 month at 45°C had much weaker antibody response and led to only 50% survival of mice after challenge.
Based on these stability studies, we believe the thermostability of influenza vaccine in dissolving microneedle patches can improved influenza vaccination by reducing dependency on the cold chain, eliminating the need for reconstitution, and simplifying vaccine distribution.
Acknowledgments
We would like to thank Seong-O Choi for providing the pyramidal microneedle master structure and Donna Bondy for administrative support. This work was supported in part by the National Institutes of Health. The work was carried out at the Center for Drug Design, Development and Delivery and the Institute for Bioengineering and Bioscience at the Georgia Institute of Technology, and at the Emory Vaccine Center. Mark Prausnitz is an inventor on patents and has a significant financial interest in a company that is developing microneedle-based products (Micron Biomedical).
ABBREVIATIONS
- HA
Hemagglutination
- HRP
Horseradish peroxidase
- IACUC
Institutional animal care and use committee
- OD
Optical density
- PBS
Phosphate-buffered saline
- PBST
Phosphate-buffered saline plus 0.5% Tween-20
- PDMS
Polydimethylsiloxane
- PVA
Polyvinyl alcohol
- RBC
Red blood cell
- s.d.
Standard deviation
- TMB
Tetramethylbenzidine
- TEM
Transmission electron microscopy
Footnotes
DISCLOSURES
This potential conflict of interest has been disclosed and is being managed by Georgia Tech and Emory University.
REFERENCES
- 1.Plotkin SA, Orenstein W, Offit PA, editors. Vaccines. Saunders; Philadelphia, PA: 2012. [Google Scholar]
- 2.Chen D, Kristensen D. Opportunities and challenges of developing thermostable vaccines. Expert Rev Vaccines. 2009;8(5):547–57. doi: 10.1586/erv.09.20. [DOI] [PubMed] [Google Scholar]
- 3.Matthias DM, Robertson J, Garrison MM, Newland S, Nelson C. Freezing temperatures in the vaccine cold chain: a systematic literature review. Vaccine. 2007;25(20):3980–6. doi: 10.1016/j.vaccine.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 4.Services USDoHaH . HHS pandemic influenza plan. U.S. Department of Health and Human Services; Washington, DC: 2005. [Google Scholar]
- 5.Amorij JP, Huckriede A, Wilschut J, Frijlink HW, Hinrichs WL. Development of stable influenza vaccine powder formulations: challenges and possibilities. Pharm Res. 2008;25(6):1256–73. doi: 10.1007/s11095-008-9559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Remmele RL, Krishnan S, Callahan WJ. Development of stable lyophilized protein drug products. Curr Pharm Biotechnol. 2012;13(3):471–96. doi: 10.2174/138920112799361990. [DOI] [PubMed] [Google Scholar]
- 7.Arakawa T, Prestrelski SJ, Kenney WC, Carpenter JF. Factors affecting short-term and long-term stabilities of proteins. Adv Drug Deliv Rev. 2001;46(1–3):307–26. doi: 10.1016/s0169-409x(00)00144-7. [DOI] [PubMed] [Google Scholar]
- 8.Chang LL, Pikal MJ. Mechanisms of protein stabilization in the solid state. J Pharm Sci. 2009;98(9):2886–908. doi: 10.1002/jps.21825. [DOI] [PubMed] [Google Scholar]
- 9.Geeraedts F, Saluja V, ter Veer W, Amorij JP, Frijlink HW, Wilschut J, et al. Preservation of the immunogenicity of dry-powder influenza H5N1 whole inactivated virus vaccine at elevated storage temperatures. AAPS J. 2010;12(2):215–22. doi: 10.1208/s12248-010-9179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simonsen L, Kane A, Lloyd J, Zaffran M, Kane M. Unsafe injections in the developing world and transmission of blood-borne pathogens. Bull World Health Organ. 1999;77(10):789–800. [PMC free article] [PubMed] [Google Scholar]
- 11.Donnelly R, Douroumis D. Microneedles for drug and vaccine delivery and patient monitoring. Drug Deliv Transl Res. 2015;5(4):311–2. doi: 10.1007/s13346-015-0250-2. [DOI] [PubMed] [Google Scholar]
- 12.Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64(14):1547–68. doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koutsonanos DG, Compans RW, Skountzou I. Targeting the skin for microneedle delivery of influenza vaccine. Adv Exp Med Biol. 2013;785:121–32. doi: 10.1007/978-1-4614-6217-0_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birchall JC, Clemo R, Anstey A, John DN. Microneedles in clinical practice–an exploratory study into the opinions of healthcare professionals and the public. Pharm Res. 2011;28(1):95–106. doi: 10.1007/s11095-010-0101-2. [DOI] [PubMed] [Google Scholar]
- 15.Norman JJ, Arya JM, McClain MA, Frew PM, Meltzer MI, Prausnitz MR. Microneedle patches: usability and acceptability for self-vaccination against influenza. Vaccine. 2014;32(16):1856–62. doi: 10.1016/j.vaccine.2014.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kommareddy S, Baudner BC, Oh S, Kwon SY, Singh M, O'Hagan DT. Dissolvable microneedle patches for the delivery of cell-culture-derived influenza vaccine antigens. J Pharm Sci. 2012;101(3):1021–7. doi: 10.1002/jps.23019. [DOI] [PubMed] [Google Scholar]
- 17.Lee JW, Park JH, Prausnitz MR. Dissolving microneedles for transdermal drug delivery. Biomaterials. 2008;29(13):2113–24. doi: 10.1016/j.biomaterials.2007.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sullivan SP, Koutsonanos DG, Del Pilar MM, Lee JW, Zarnitsyn V, Choi SO, et al. Dissolving polymer microneedle patches for influenza vaccination. Nat Med. 2010;16(8):915–20. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koutsonanos DG, del Pilar MM, Zarnitsyn VG, Jacob J, Prausnitz MR, Compans RW, et al. Serological memory and long-term protection to novel H1N1 influenza virus after skin vaccination. J Infect Dis. 2011;204(4):582–91. doi: 10.1093/infdis/jir094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearton M, Pirri D, Kang SM, Compans RW, Birchall JC. Host responses in human skin after conventional intradermal injection or microneedle administration of virus-like-particle influenza vaccine. Adv Healthc Mater. 2013;2(10):1401–10. doi: 10.1002/adhm.201300006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pulit-Penaloza JA, Esser ES, Vassilieva EV, Lee JW, Taherbhai MT, Pollack BP, et al. A protective role of murine langerin(+) cells in immune responses to cutaneous vaccination with microneedle patches. Sci Rep. 2014;4:6094. doi: 10.1038/srep06094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song JM, Kim YC, Lipatov AS, Pearton M, Davis CT, Yoo DG, et al. Microneedle delivery of H5N1 influenza virus-like particles to the skin induces long-lasting B- and T-cell responses in mice. Clin Vaccine Immunol. 2010;17(9):1381–9. doi: 10.1128/CVI.00100-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaric M, Lyubomska O, Poux C, Hanna ML, McCrudden MT, Malissen B, et al. Dissolving microneedle delivery of nanoparticle-encapsulated antigen elicits efficient cross-priming and Th1 immune responses by murine Langerhans cells. J Investig Dermatol. 2015;135(2):425–34. doi: 10.1038/jid.2014.415. [DOI] [PubMed] [Google Scholar]
- 24.DeMerlis CC, Schoneker DR. Review of the oral toxicity of polyvinyl alcohol (PVA) Food Chem Toxicol. 2003;41(3):319–26. doi: 10.1016/s0278-6915(02)00258-2. [DOI] [PubMed] [Google Scholar]
- 25.Sha Z, Compans RW. Induction of CD4(+) T-cell-independent immunoglobulin responses by inactivated influenza virus. J Virol. 2000;74(11):4999–5005. doi: 10.1128/jvi.74.11.4999-5005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu LY, Choi SO, Prausnitz MR. Fabrication of dissolving polymer microneedles for controlled drug encapsulation and delivery: bubbles and pedestal designs. J Pharm Sci. 2010;99(10):4228–38. doi: 10.1002/jps.22140. [DOI] [PubMed] [Google Scholar]
- 27.Wen Z, Ye L, Gao Y, Pan L, Dong K, Bu Z, et al. Immunization by influenza virus-like particles protects aged mice against lethal influenza virus challenge. Antivir Res. 2009;84(3):215–24. doi: 10.1016/j.antiviral.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu Q, Zarnitsyn VG, Ye L, Wen Z, Gao Y, Pan L, et al. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc Natl Acad Sci U S A. 2009;106(19):7968–73. doi: 10.1073/pnas.0812652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J Control Release. 2010;142(2):187–95. doi: 10.1016/j.jconrel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cicerone MT, Pikal MJ, Qian KK. Stabilization of proteins in solid form. Adv Drug Deliv Rev. 2015;93:14–24. doi: 10.1016/j.addr.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clements CJ, Larsen G, Jodar L. Technologies that make administration of vaccines safer. Vaccine. 2004;22(15–16):2054–8. doi: 10.1016/j.vaccine.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Brandau DT, Jones LS, Wiethoff CM, Rexroad J, Middaugh CR. Thermal stability of vaccines. J Pharm Sci. 2003;92(2):218–31. doi: 10.1002/jps.10296. [DOI] [PubMed] [Google Scholar]
- 33.Bieganski RM, Fowler A, Morgan JR, Toner M. Stabilization of active recombinant retroviruses in an amorphous dry state with trehalose. Biotechnol Prog. 1998;14(4):615–20. doi: 10.1021/bp980057d. [DOI] [PubMed] [Google Scholar]
- 34.Schebor C, Burin L, Buera MP, Aguilera JM, Chirife J. Glassy state and thermal inactivation of invertase and lactase in dried amorphous matrices. Biotechnol Prog. 1997;13(6):857–63. doi: 10.1021/bp970093x. [DOI] [PubMed] [Google Scholar]
- 35.Hinrichs WL, Prinsen MG, Frijlink HW. Inulin glasses for the stabilization of therapeutic proteins. Int J Pharm. 2001;215(1–2):163–74. doi: 10.1016/s0378-5173(00)00677-3. [DOI] [PubMed] [Google Scholar]
- 36.Chen X, Fernando GJ, Crichton ML, Flaim C, Yukiko SR, Fairmaid EJ, et al. Improving the reach of vaccines to low-resource regions, with a needle-free vaccine delivery device and long-term thermostabilization. J Control Release. 2011;152(3):349–55. doi: 10.1016/j.jconrel.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 37.Choi HJ, Yoo DG, Bondy BJ, Quan FS, Compans RW, Kang SM, et al. Stability of influenza vaccine coated onto microneedles. Biomaterials. 2012;33(14):3756–69. doi: 10.1016/j.biomaterials.2012.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirobe S, Azukizawa H, Hanafusa T, Matsuo K, Quan YS, Kamiyama F, et al. Clinical study and stability assessment of a novel transcutaneous influenza vaccination using a dissolving microneedle patch. Biomaterials. 2015;57:50–8. doi: 10.1016/j.biomaterials.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Mistilis MJ, Bommarius AS, Prausnitz MR. Development of a thermostable microneedle patch for influenza vaccination. J Pharm Sci. 2015;104(2):740–9. doi: 10.1002/jps.24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vassilieva EV, Kalluri H, McAllister D, Taherbhai MT, Esser ES, Pewin WP, et al. Improved immunogenicity of individual influenza vaccine components delivered with a novel dissolving microneedle patch stable at room temperature. Drug Deliv Transl Res. 2015;5(4):360–71. doi: 10.1007/s13346-015-0228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soema PC, Willems GJ, van Twillert K, van de Wijdeven G, Boog CJ, Kersten GF, et al. Solid bioneedle-delivered influenza vaccines are highly thermostable and induce both humoral and cellular immune responses. PLoS One. 2014;9(3):e92806. doi: 10.1371/journal.pone.0092806. [DOI] [PMC free article] [PubMed] [Google Scholar]