Abstract
Abnormally active glycogen synthase kinase-3 (GSK3) contributes to pathological processes in multiple psychiatric and neurological disorders. Modeled in mice, this includes increasing susceptibility to dysregulation of mood-relevant behaviors, impairing performance in several cognitive tasks, and impairing adult hippocampal neural precursor cell (NPC) proliferation. These deficits are all evident in GSK3α/β knockin mice, in which serine-to-alanine mutations block the inhibitory serine phosphorylation regulation of both GSK3 isoforms, leaving GSK3 hyperactive. It was unknown if both GSK3 isoforms perform redundant actions in these processes, or if hyperactivity of one GSK3 isoform has a predominant effect. To test this, we examined GSK3α or GSK3β knockin mice in which only one isoform was mutated to a hyperactive form. Only GSK3β, not GSK3α, knockin mice displayed heightened vulnerability to the learned helplessness model of depression-like behavior. Three cognitive measures impaired in GSK3α/β knockin mice demonstrated differential regulation by GSK3 isoforms. Novel object recognition was impaired in GSK3β, not GSK3α, knockin mice, whereas temporal order memory was not impaired in GSK3α or GSK3β knockin mice, and coordinate spatial processing was impaired in both GSK3α and GSK3β knockin mice. Adult hippocampal NPC proliferation was severely impaired in GSK3β knockin mice, but not impaired in GSK3α knockin mice. Increased activity of GSK3β, in the absence of over-expression or disease pathology, is sufficient to impair mood regulation, novel object recognition, and hippocampal NPC proliferation, whereas hyperactive GSK3α individually does not impair these processes. These results demonstrate that hyperactivity of the two GSK3 isoforms execute non-redundant effects on these processes.
Keywords: glycogen synthase kinase-3, depression, cognition, neurogenesis, novel object recognition
Introduction
Glycogen synthase kinase-3 (GSK3) is involved in many cellular signaling mechanisms that regulate multiple cellular functions, and abnormally active GSK3 has been linked to a wide variety of central nervous system (CNS) diseases (Beurel et al., 2015). GSK3 refers to two kinase isoforms, GSK3α and GSK3β, which are 85% homologous, both are ubiquitously expressed throughout the brain, and they share many functions. However, there is growing evidence that the two isoforms act on a number of distinct targets (Kaidanovich-Beilin and Woodgett, 2011; Beurel et al., 2015). Abnormally active GSK3 has been linked to pathological processes in a wide variety of diseases of the CNS, such as mood disorders (Jope, 2011), schizophrenia (Beaulieu et al., 2009), several conditions involving cognitive impairments (King et al., 2014), such as Alzheimer’s disease (Martinez and Perez, 2008) and Fragile X syndrome (Mines and Jope, 2011), and a variety of other conditions (Beurel et al., 2015). Thus, it is important to identify actions of GSK3 that may contribute to these pathologies, and a first step in this process is to determine if either isoform has a predominant role in such actions.
GSK3 is primarily regulated by phosphorylation of an N-terminal serine, serine-21 in GSK3α and serine-9 in GSK3β. Phosphorylation on these sites inhibits the activity of GSK3, and diminished phosphorylation is thought to contribute to hyperactive GSK3 associated with multiple CNS pathological conditions, such as those noted above. The functional effects of impaired inhibitory serine-phosphorylation of GSK3 can be studied with GSK3α21A/21A/β9A/9A knockin mice (McManus et al 2005; Polter et al., 2010) that have serine-to-alanine mutations that prevents the inhibition by serine-phosphorylation of GSK3. These mutations maintain GSK3 maximally active, but importantly within the physiological range since both GSK3 isoforms are expressed at normal levels. Thus, these mice provide the opportunity to interrogate detrimental actions of hyperactive GSK3 in mice in the absence of additional pathological processes that occur in mouse models of CNS diseases. Notably, the GSK3α/β knockin mice display heightened sensitivity to stress-induced mood-relevant behaviors (Polter et al., 2010), impaired performance on several cognitive tasks (Pardo et al., 2015), and impaired adult hippocampal neural precursor cell proliferation (Eom and Jope; 2009; Pardo et al., 2015). However, little is known about the relative roles of hyperactive GSK3α and GSK3β in causing these deficits.
To address this, we examined separately GSK3α knockin mice and GSK3β knockin mice to test if activation of either isoform predominantly regulates susceptibility to the learned helplessness model of depression-like behavior, performance in three cognitive tasks, novel object recognition, temporal order memory, and coordinate spatial processing, and hippocampal neural precursor cell proliferation in adult mice. The results demonstrate that active GSK3β, but not active GSK3α, is sufficient to increase susceptibility to learned helplessness, impair novel object recognition, and reduce hippocampal neural precursor cell proliferation.
Materials and methods
Mice
Male adult (7–12 weeks old) GSK3α21A/21A knockin mice, GSK3β9A/9A knockin mice, and littermate wild-type C57BL/6J mice were used, except where female mice (7–12 weeks old) are specified. These strains display no overt phenotypes and neither GSK3α knockin mice nor GSK3β knockin mice display differences from wild-type mice in locomotor activity in a novel open field (Mines et al., 2013). Mice were housed in groups of 3–5 in standard cages in light and temperature controlled rooms and were treated in accordance with NIH and the University of Miami Institutional Animal Care and Use Committee regulations.
Immunoblot analysis
Mouse hippocampi were rapidly dissected in ice-cold phosphate-buffered saline. Brain regions were homogenized in ice-cold lysis buffer containing 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, 10% glycerol, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 μg/ml pepstatin, 1 mM phenylmethanesulfonyl fluoride, 50 mM NaF, 1 mM sodium orthovanadate, and 100 nM okadaic acid. The lysates were centrifuged at 20,800xg for 10 min. Protein concentrations in the supernatants were determined using the Bradford protein assay (Bradford 1976). Lysates were mixed with Laemmli sample buffer (2% SDS) and placed in a boiling water bath for 5 min. Proteins (10 μg) were resolved in SDS-polyacrylamide gels, transferred to nitrocellulose, and incubated with primary antibodies to phospho-Ser21-GSK3α (#9316L; Cell Signaling Technology), phospho-Ser9-GSK3β (#9336; Cell Signaling Technology) and total GSK3α/β (#05-412; Millipore). Immunoblots were developed using horseradish peroxidase-conjugated goat anti-mouse, or goat anti-rabbit IgG, followed by detection with enhanced chemiluminescence.
Behavioral procedures
Learned helplessness was measured using a modified learned helplessness protocol, referred to as the reduced intensity inescapable shock protocol, which allows detection of increased susceptibility to learned helplessness because most wild-type mice do not develop learned helplessness with this protocol (Polter et al., 2010; Beurel et al., 2011). Mice were placed in one side of a Gemini Avoidance system shuttle box (San Diego Instruments, San Diego, CA, USA) with the gate between chambers closed. To induce learned helplessness, 180 inescapable foot shocks were delivered at an amplitude of 0.3 mA and an average 4 sec shock duration, and a randomized inter-shock interval of 5–25 sec. Twenty-four hours after inescapable foot shocks, mice were returned to the shuttle box and the escape task was tested by giving 30 escape trials with each trial involving a 0.3 mA foot shock for a maximum duration of 24 sec. The door of the chamber opens at the beginning of the foot shock administration to allow mice to escape. Latency to escape the shock was recorded using Gemini software, and trials in which the mouse did not escape within the 24 sec time limit were counted as escape failures. Mice with greater than 15 escape failures were defined as learned helpless.
Three cognitive tasks were tested in the following order on three consecutive days: novel object recognition, temporal order memory, and coordinate spatial processing, carried out as previously described (King and Jope, 2013; Pardo et al., 2015). Mice were habituated to the testing room with a white noise generator (55 dB) for 1 hr, each mouse was tested in a different cognitive task each day, 70% ethanol was used to clean each apparatus and object used between each test session, test sessions were filmed, and films were scored by an investigator blind to the genotype. Time spent exploring an object only included the mouse sniffing or touching the object with its nose, vibrissa, mouth, or forepaws. As described previously (Pardo et al., 2015), novel object recognition was measured by allowing each mouse to explore two identical objects for 5 min, and after a 5 min period in an opaque chamber, mice were allowed to explore an unused familiar object and a novel object for 5 min. The discrimination index was calculated as the times ((exploring novel object – exploring familiar object)/total time of object exploration) × 100). To measure temporal order memory, each mouse underwent three sessions exploring three unique sets of objects (Objects 1, 2, 3). During the test session, the mouse was allowed to explore an unused copy of Object 1 and an unused copy of Object 3 for 5 min. Intact temporal order memory is evident when mice spend more time exploring the first object presented (Object 1) than the most recent object presented (Object 3). The discrimination index was calculated as the times ((exploring Object 3 – exploring Object 1)/total time of object exploration) × 100). For the coordinate spatial processing task, each mouse was allowed to explore two novel objects that were 45 cm apart for 15 min. After 5 min in an opaque chamber, each mouse was allowed to explore the same two objects that had been moved closer together (30 cm) for 5 min. Increased exploration of the objects during the test session compared with the last 5 min of the habituation phase is considered a measure of memory of the distance between objects. The exploration ratio was calculated as time (exploring during the 5 min test session)/(exploring during the 5 min test session plus the last 5 min of the habituation session).
Neural precursor cell proliferation
Adult mouse hippocampal neural precursor cell proliferation was measured as described previously (Eom and Jope, 2009; Pardo et al., 2015). 5-Bromo-2′-deoxyuridine (BrdU; 100 mg/kg; Sigma-Aldrich, St Louis, MO) was administered to mice i.p. three times at 2 hr intervals, and after 24 hr mice were perfused and brains were sliced and analyzed as previously described (Eom and Jope, 2009; Pardo et al., 2015) using anti-BrdU antibody (1:500; BU1/75; Abcam) and cell nuclei were stained with 0.2 μg/ml bisbenzimide (Hoechst 33258; Sigma). BrdU positive cells in the granule cell layer of the dentate gyrus and the subgranular zone were counted in each section and analyzed by unbiased stereology using the StereoInvestigator system (MicroBrightField, Williston, VT).
Statistical analysis
Statistical significance was assessed by one-way ANOVA with genotype as a factor followed by Bonferroni’s multiple comparison tests (for Figure 1B,C, 2B,D,E, 3B,D,E and 4B), or by Student’s t-test (for percent time spent with each object in Figure 2A,C and 3A,C).
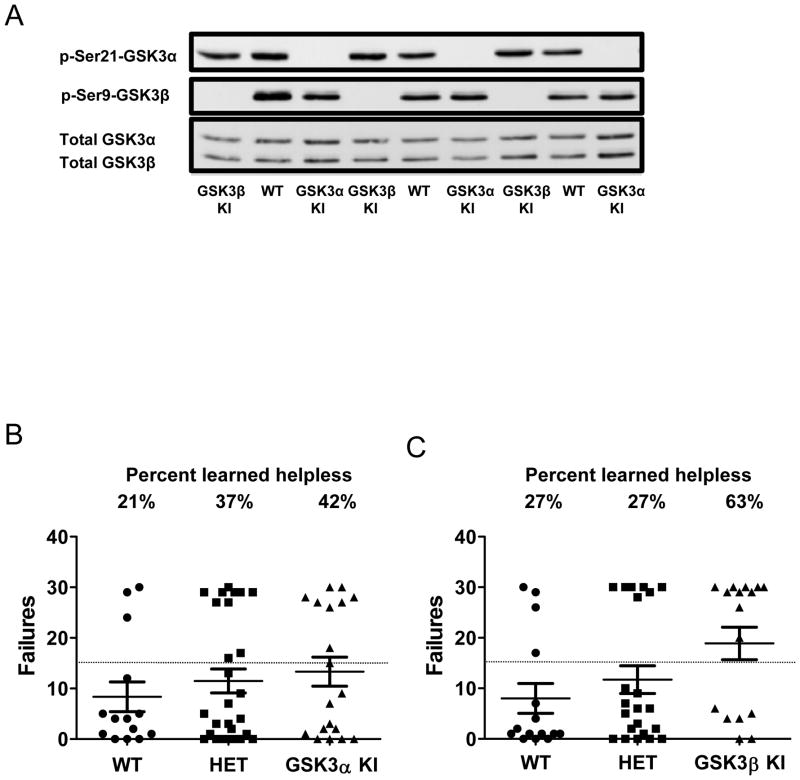
Figure 1. Susceptibility to the learned helplessness model of depression-like behavior is selectively promoted by active GSK3β, not GSK3α.
(A) Immunoblots showing the absence of serine-21 phosphorylation of GSK3α in GSK3α knockin (KI) mice and of serine-9-GSK3β in GSK3β knockin mice compared with wild-type (WT) mice in the hippocampus of three representative mice per group. (B) Wild-type (WT) mice (n=14), heterozygous (HET) GSK3α knockin (KI) mice (n=27) and homozygous GSK3α KI mice (n=19), or (C) wild-type (WT) mice (n=15), heterozygous GSK3β KI mice (n=22) and homozygous GSK3β KI mice (n=16), were subjected to the reduced intensity inescapable foot shocks described in the Methods followed in 24 hr by escapable foot shocks, and the numbers of failure to escape are shown for each individual mouse. Means ± SEM; *p<0.05 compared to WT mice.
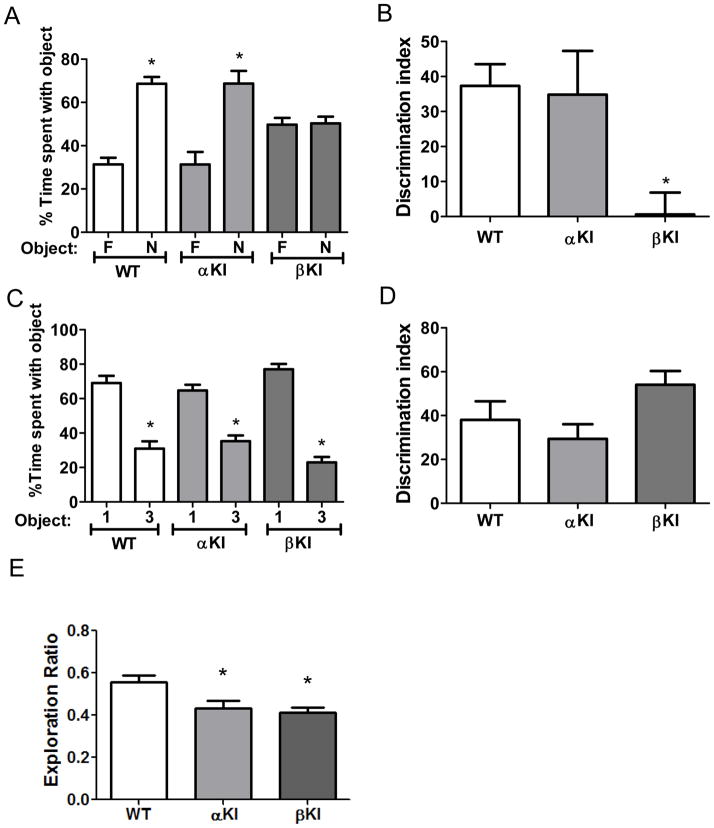
Figure 2. GSK3 isoform-dependence of performance in three cognitive tasks in male mice.
(A,B) Performance of wild-type (WT) mice (n=11), GSK3α knockin (αKI) mice (n=12), and GSK3β knockin (βKI) mice (n=10) on novel object recognition. (A) Percent time spent exploring the novel (N) and familiar (F) object. Means ± SEM; *p<0.05 compared to time spent with the familiar object. (B) Discrimination index. Means ± SEM; *p<0.05 compared to WT mice. (C,D) Performance of WT mice (n=12), GSK3α KI mice (n=12) and GSK3β KI mice (n=9) on the temporal order task. (C) Percent time spent exploring the first (1) and last (3) object presented. Means ± SEM; *p<0.05 compared to time spent with object 1. (D) Discrimination index. Means ± SEM. (E) Performance of WT mice (n=12), GSK3α KI mice (n=12) and GSK3β KI mice (n=9) mice in the coordinate spatial processing task. Means ± SEM; *p<0.05 compared to WT mice (one-way ANOVA, F(2,32)=6.14).
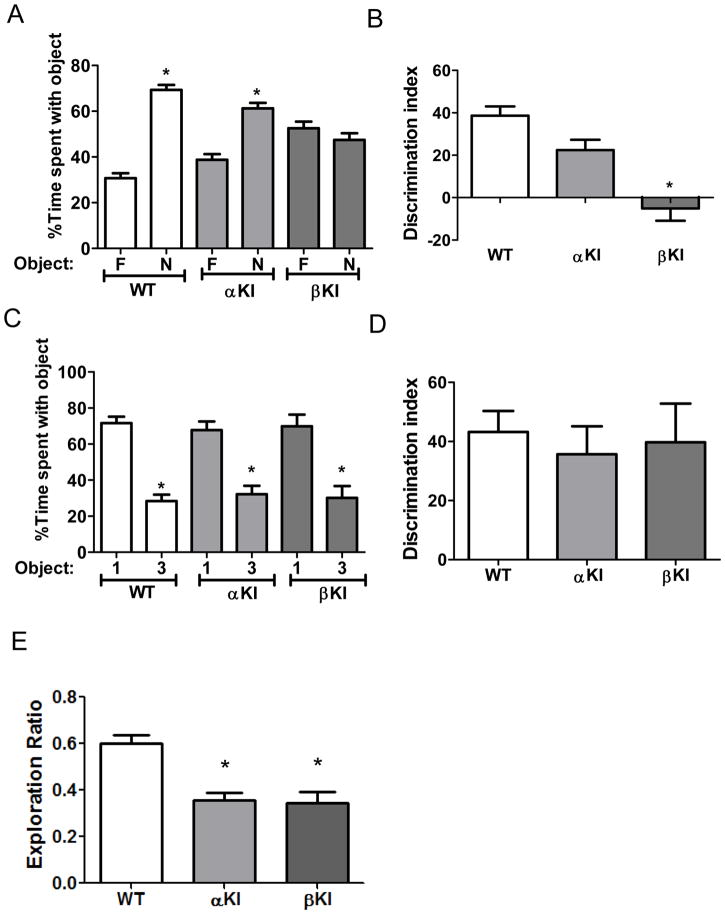
Figure 3. GSK3 isoform-dependence in performance in three cognitive tasks in female mice.
(A,B) Performance of wild-type (WT) mice (n=8), GSK3α knockin (αKI) mice (n=8), and GSK3β knockin (βKI) mice (n=8) on novel object recognition. (A) Percent time spent exploring the novel (N) and familiar (F) object. Novel object was explored more than the familiar object by wild-type mice (familiar 30.7±2.2 vs. novel 69.30±2.2 % of total time exploring objects; t(14)=12.29, p<.01) and by GSK3α knockin mice (familiar 38.8±2.4 vs. novel 61.2±2.4 % of total time exploring objects; t(14)=6.5, p<.01), but not by GSK3β knockin mice (familiar 52.5±2.9 vs. novel 47.5±2.9 % of total time exploring objects; t(14)=1.23, p=ns) Means ± SEM; *p<0.05 compared to time spent with the familiar object (Student’s t-test). (B) Discrimination index. Means ± SEM; *p<0.05 compared to WT mice (one-way ANOVA, F(2,23)=18.86). (C,D) Performance of WT mice (n=15), GSK3α KI mice (n=12) and GSK3β KI mice (n=8) on the temporal order task. (C) Percent time spent exploring the first (1) and last (3) object presented. The percent of time exploring the first object was higher than the last object by wild-type mice (Object 1: 71.6±3.6 vs Object 3: 28.4±3.6, t(28)=8.60, p<.01), by GSK3α knockin mice (Object 1:67.8±4.7 vs Object 3: 32.2±4.7, t(22)=5.34, p<.01), and by GSK3β knockin mice (Object 1: 69.9±6.5 vs Object 3: 30.1±6.5, t(14)=4.30, p<.01) Means ± SEM; *p<0.05 compared to time spent with object 1 (Student’s t-test). (D) Discrimination index. Means ± SEM; (one-way ANOVA, F(2,34)=0.19, p=ns compared to WT mice). (E) Performance of WT mice (n=15), GSK3α KI mice (n=13) and GSK3β KI mice (n=8) mice in the coordinate spatial processing task. Means ± SEM; *p<0.05 compared to WT mice (one-way ANOVA, F(2,35)=15.84; p<0.01).
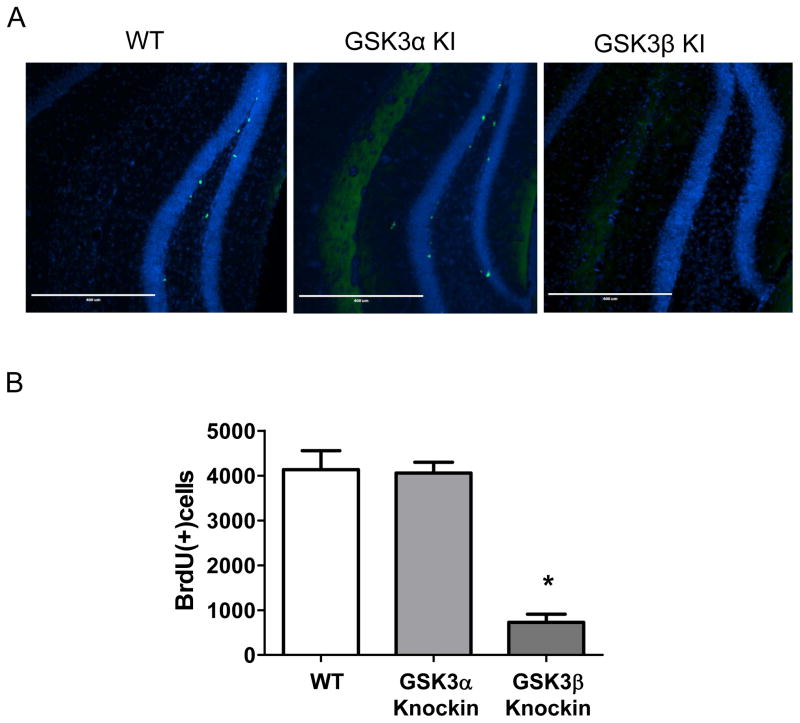
Figure 4. Adult hippocampal neural precursor cell proliferation is selectively impaired by active GSK3β, not by active GSK3α.
(A) Immunohistochemical detection of BrdU-positive cells (green) in the dentate gyrus of wild-type (WT) mice, GSK3α knockin (KI) mice and GSK3β KI mice. Nuclei are labeled with bisbenzimide (blue). (B) Unbiased stereological quantitation of BrdU-positive cells in the hippocampal dentate gyrus of WT mice (n=8), GSK3α KI mice (n=6), and GSK3β KI mice (n=6). Means ± SEM; *p<0.01 compared to WT mice.
Results
GSK3α/β knockin mice display increased susceptibility to the learned helplessness model of depression-like behavior (Polter et al., 2010). To test if either GSK3α or GSK3β predominantly contributes to this effect, GSK3α knockin mice and GSK3β knockin mice expressing serine-to-alanine mutations disabling the inhibitory regulation by phosphorylation of serine-21 or serine-9, respectively (Figure 1A), were evaluated for vulnerability to learned helplessness compared with wild-type mice. Heterozygous and homozygous GSK3α knockin mice displayed susceptibility equivalent to wild-type mice in the learned helplessness paradigm, as 21–42% of mice displayed learned helplessness in these cohorts (Figure 1B). In contrast, 63% of homozygous GSK3β knockin mice displayed learned helplessness, which was significantly greater than the 27% of wild-type mice and 27% of heterozygous GSK3β knockin mice that developed learned helplessness (one-way ANOVA, F(2,52)=3.10, p<0.05; Figure 1C). Thus, abnormally active GSK3β increases the susceptibility of mice to the learned helplessness model of depression-like behavior.
Execution of novel object recognition, temporal order memory, and coordinate spatial processing was previously found to be impaired in GSK3α/β knockin mice (Pardo et al., 2015). Novel object recognition was significantly impaired in GSK3β knockin mice but was not impaired in GSK3α knockin mice compared with wild-type mice (Figure 2A and 2B). Whereas the novel object was explored more than the familiar object by wild-type mice (familiar 31.3±3.1 vs. novel 68.7±3.1 % of total time exploring objects; t(20)=8.50, p<0.01) and GSK3α knockin mice (familiar 31.3±5.9 vs. novel 68.7±5.9 % of total time exploring objects; t(22)=4.52, p<0.01), GSK3β knockin mice did not explore the novel object more than the familiar object (familiar 49.7±3.1 vs. novel 50.3±3.1 % of total time exploring objects). Therefore, the discrimination index was impaired in GSK3β knockin mice (one-way ANOVA, F(2,31)=5.043), but not GSK3α knockin mice, demonstrating that abnormally active GSK3β, but not GSK3α, is sufficient to impair novel object recognition.
In contrast, to novel object recognition, temporal order memory was not impaired in GSK3α knockin mice or GSK3β knockin mice (Figure 2C and 2D). A significantly greater percent of time exploring the first object was displayed by wild-type mice (Object 1: 69.0±4.2, Object 3: 31.0±4.2; t(22)=6.36, p<0.01,), GSK3α knockin mice (Object 1: 64.7±3.3, Object 3: 35.3±3.3; t(22)=6.22, p<0.01) and GSK3β knockin mice (Object 1: 77.0±3.1, Object 3: 23.0±3.1; t(16)=12.21, p<0.01), resulting in no significant differences in the discrimination index between wild-type mice, GSK3α knockin mice, and GSK3β knockin mice. Since temporal order memory was impaired in GSK3α/β knockin mice (Pardo et al., 2015), these results demonstrate that both isoforms of GSK3 must be abnormally active to impair temporal order memory.
Coordinate spatial processing was significantly impaired in both GSK3α knockin mice and GSK3β knockin mice, demonstrating that coordinate spatial processing is sensitive to abnormal activity of either GSK3 isoform. Thus, activation of either GSK3α or GSK3β is sufficient to impair coordinate spatial processing.
In summary, abnormally active GSK3β is sufficient to impair novel object recognition, whereas abnormally active GSK3α and GSK3β are both required for impairing temporal order memory, revealing that for these two tasks GSK3α and GSK3β have non-redundant functions. In contrast, coordinate spatial memory can be impaired by abnormal activation of either GSK3 isoform, suggesting redundant functions of GSK3α and GSK3β in this process.
We tested if the differential effects of hyperactive GSK3α and GSK3β in each of these three cognitive tasks were influenced by sex. However, female mice displayed the same outcomes of hyperactive GSK3 isoforms in each of the tests as those displayed by male mice: only female GSK3β knockin mice, not GSK3α knockin mice, exhibited impaired novel object recognition, neither female GSK3α nor GSK3β knockin mice displayed impaired temporal ordering, and both female GSK3α and GSK3β knockin mice were impaired in the coordinate spatial processing task (Fig. 3). Thus, the detrimental effects of hyperactivity of each GSK3 isoform were equivalent in male and female mice in these three cognitive tasks.
Adult hippocampal neural precursor cell proliferation is impaired by ~40% in double GSK3α/β knockin mice (Eom and Jope, 2009; Pardo et al., 2015). In contrast to GSK3α/β knockin mice, adult hippocampal neural precursor cell proliferation in GSK3α knockin mice (4058 ± 242) was not significantly different from wild-type mice (4133 ± 422) (Figure 4). However, GSK3β knockin mice displayed severely impaired hippocampal neural precursor cell proliferation (727 ± 185; one-way ANOVA F(2,19)=31.93; p<0.01) that was only 18% of the level of wild-type mice (Figure 4). Thus, abnormally active GSK3β is particularly detrimental to adult hippocampal neural precursor cell proliferation, whereas this process is impervious to abnormal activation of GSK3α.
Discussion
There is increasing evidence that GSK3α and GSK3β perform some unique actions, although they often have redundant effects (Beurel et al., 2015). There is also substantial evidence that GSK3 contributes to pathological processes in a number of psychiatric and neurological disorders (Beurel et al., 2015). Therefore, we used GSK3α knockin mice and GSK3β knockin mice to determine the contribution of each abnormally activated GSK3 isoform in regulating several characteristics that had previously been shown to be altered in double GSK3α/β knockin mice that have both GSK3 isoforms mutated to constitutively active forms. We found that abnormally active GSK3β, but not GSK3α, increased susceptibility to the learned helplessness model of depression-like behavior, impaired novel object recognition, and impaired hippocampal neural precursor proliferation, whereas activation of neither isoform individually recapitulates impaired temporal order memory exhibited by GSK3α/β knockin mice, and activation of either isoform was sufficient to impair coordinate spatial processing.
GSK3α/β knockin mice display increased vulnerability to the development of learned helplessness compared with wild-type mice (Polter et al., 2010). Although definitively demonstrating that abnormally active GSK3 reduces resistance to this depression-like behavior, not addressed was the question of whether either of the GSK3 isoforms was selectively responsible for the increased vulnerability, or if activation of either isoform was sufficient to cause the same outcome. Comparisons of GSK3α and GSK3β knockin mice revealed that active GSK3β plays a predominant role in increasing susceptibility to learned helplessness. The lack of effect of active GSK3α may be attributable to this isoform not modulating resistance to learned helplessness, or it may be due to the smaller amount of GSK3α relative to GSK3β in the brain, resulting in less constitutively activated GSK3 in GSK3α knockin mice than in GSK3β knockin mice (Kaidanovich-Beilin and Woodgett, 2011). Nevertheless, it is evident that activation of GSK3β is sufficient to reduce resistance to the development of learned helplessness. Several previous findings also implicated GSK3β in mood dysregulation, but these were often studied without comparisons to the actions of GSK3α. For example, reduced GSK3β levels in heterozygous GSK3β+/− knockout mice reduced depression-like immobility in the forced swim test (O’Brien et al., 2004) and the tail suspension test (Beaulieu et al., 2008a), and reduced immobility in the tail suspension test in tryptophan hydroxylase 2 mutant mice (Beaulieu et al., 2008b). Decreased hippocampal GSK3β levels reduced depression-like immobility times in both the forced swim and tail suspension tests (Omata et al., 2011). Oppositely, transgenic expression of GSK3β in mouse brain rescued lithium-sensitive immobility in the forced swim test (O’Brien et. al., 2011), and overexpression of GSK3β in the nucleus accumbens induced a depression-like phenotype (Wilkinson et al., 2011). In postmortem ventral prefrontal cortex from subjects with depression, compared with non-depressed subjects, the activity of GSK3β was elevated, and the equivalent total activity of GSK3α plus GSK3β suggests that GSK3α activity was not different between the groups (Karege et al., 2007). Thus, there is now substantial evidence that abnormally active GSK3β is particularly important in increasing susceptibility to mood dysregulation in multiple models, but there is no clear evidence that GSK3α also participates in this outcome, although this is largely due to the previous lack of investigation.
GSK3α/β knockin mice were recently found to display impaired novel object recognition, temporal order memory, and coordinate spatial processing (Pardo et al., 2015). Examination of the GSK3 isoform-specificity of these deficits revealed distinct isoform effects among each of the cognitive tasks. GSK3β knockin mice displayed impaired novel object recognition, as previously reported (Dewachter et al., 2009), but this was not impaired in GSK3α knockin mice. Impaired novel object recognition was reported in mice overexpressing GSK3β, but overexpression is associated with neurodegenerative effects that do not occur in GSK3β knockin mice (Engel et al., 2006). Thus, novel object recognition can be impaired by abnormally active GSK3β, rather than GSK3α. However, this does not suggest that GSK3α has no role in novel object recognition, as GSK3α deficient mice exhibited impaired novel object recognition (Maurin et al., 2013). In contrast to novel object recognition, individual activation of either GSK3 isoform was not sufficient to impair performance on the temporal order task, which was impaired in GSK3α/β knockin mice (Pardo et al., 2015). Thus, intact inhibitory serine-phosphorylation of either GSK3 isoform is sufficient to maintain temporal order memory. Coordinate spatial processing was the most sensitive to increased GSK3 activity of the cognitive tasks that were examined, as constitutive activation of either GSK3α or GSK3β was sufficient to impair performance. Previously, mice overexpressing GSK3β were reported to exhibit impaired spatial learning in the Morris water maze (Hernandez et al., 2002), further indicating that excessive GSK3β impairs spatial learning and memory. Oppositely, mice deficient in GSK3α did not exhibit impaired performance in a variety of tasks with a spatial component (Kaidanovich-Beilin et al., 2009; Hurtado et al., 2012; Maurin et al., 2013). In summary, all three cognitive tasks that were examined revealed sensitivities to abnormal activation of GSK3, and novel object recognition was specifically sensitive to GSK3β activation, temporal ordering was the least sensitive as it was only impaired by activation of both GSK3 isoforms, and coordinate spatial processing was the most sensitive to impairment by active GSK3 as it was impaired by activation of either GSK3 isoform.
Adult mouse hippocampal neural precursor cell proliferation is impaired in GSK3α/β knockin mice by ~40% (Eom and Jope; 2009; Pardo et al., 2015). Our examination of the specific effects of hyperactive GSK3α and GSK3β demonstrate that this is entirely due to hyperactive GSK3β. Furthermore, the deficit caused by constitutively active GSK3β was an 83% impairment, signifying a major sensitivity to active GSK3β. Previous studies have shown that reduced GSK3 activity increases multiple steps of neurogenesis. For example, deletion of both GSK3α and GSK3β, but not either isoform individually, caused hyperproliferation of neural precursor cells, in part through activation of the Wnt signaling cascade, which is essential for neurogenesis (Kim et al., 2009; Valvezan and Klein, 2011). However, since the participation of GSK3 in Wnt signaling is not influenced by the serine-phosphorylation mechanism that is mutated in GSK3 knockin mice (Ding et al., 2000; McManus et al., 2005; Ng et al., 2009), and both GSK3 isoforms participate in Wnt signaling (Kim et al., 2009), changes in Wnt signaling cannot underlie the selective inhibition of neural precursor cell proliferation in GSK3β knockin mice, but not GSK3α knockin mice. Several neurogenesis-regulating pathways, including mammalian target of rapamycin (mTOR), hypoxia inducible factor-1 (HIF-1), neurotrophins and apoptosis, have been speculated to be GSK3 targets that may contribute to its regulation of neurogenesis (Valvezan and Klein, 2011). Abnormally active GSK3 has been identified in a number of disorders that appear to be associated with impaired neurogenesis, and the present results emphasize the sensitivity of neural precursor cell proliferation to hyperactive GSK3β.
Altogether, these results demonstrate that activation of each GSK3 isoform has different outcomes in regulating susceptibility to the learned helplessness model of depression-like behavior, performance in three cognitive tasks, and hippocampal neural precursor cell proliferation. Specifically, increased activity of GSK3β, in the absence of additional disease pathology, is sufficient to impede mood regulation, novel object recognition, and hippocampal neural precursor cell proliferation, whereas hyperactive GSK3α does not impair these processes. This adds to accumulating evidence that the two GSK3 isoforms have divergent functions in a number of signaling pathways, as recently reviewed (Beurel et al., 2015).
Acknowledgments
We thank the NIMH for funding this research (MH038752, MH090236, MH095380, MH104656).
Footnotes
Conflict of interest
The authors declare no financial interests or conflicts of interest.
References
- Beaulieu JM, Gainetdinov RR, Caron MG. Akt/GSK3 signaling in the action of psychotropic drugs. Annu Rev Pharmacol Toxicol. 2009;49:327–347. doi: 10.1146/annurev.pharmtox.011008.145634. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Marion S, Rodriguiz RM, Medvedev IO, Sotnikova TD, Ghisi V, Wetsel WC, Lefkowitz RJ, Gainetdinov RR, Caron MG. A β-arrestin 2 signaling complex mediates lithium action on behavior. Cell. 2008a;132:125–136. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Zhang X, Rodriguiz RM, Sotnikova TD, Cools MJ, Wetsel WC, Gainetdinov RR, Caron MG. Role of GSK3β in behavioral abnormalities induced by serotonin deficiency. Proc Natl Acad Sci USA. 2008b;105:1333–1338. doi: 10.1073/pnas.0711496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmaco Ther. 2015;148:114–131. doi: 10.1016/j.pharmthera.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Song L, Jope RS. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry. 2011;16:1068–1070. doi: 10.1038/mp.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dewachter I, Ris L, Jaworski T, Seymour CM, Kremer A, Borghgraef P, De Vijver H, Godaux E, Van Leuven F. GSK3β, a centre-staged kinase in neuropsychiatric disorders, modulates long term memory by inhibitory phosphorylation at serine-9. Neurobiol Dis. 2009;35:193–200. doi: 10.1016/j.nbd.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Ding VW, Chen RH, McCormick F. Differential regulation of glycogen synthase kinase 3β by insulin and Wnt signaling. J Biol Chem. 2000;275:32475–32481. doi: 10.1074/jbc.M005342200. [DOI] [PubMed] [Google Scholar]
- Engel T, Hernández F, Avila J, Lucas JJ. Full reversal of Alzheimer’s disease-like phenotype in a mouse model with conditional overexpression of glycogen synthase kinase-3. J Neurosci. 2006;26:5083–5090. doi: 10.1523/JNEUROSCI.0604-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom TY, Jope RS. Blocked inhibitory serine-phosphorylation of glycogen synthase kinase-3α/β impairs in vivo neural precursor cell proliferation. Biological Psychiatry. 2009;66:494–502. doi: 10.1016/j.biopsych.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández F, Borrell J, Guaza C, Avila J, Lucas JJ. Spatial learning deficit in transgenic mice that conditionally over-express GSK-3beta in the brain but do not form tau filaments. J Neurochem. 2002;83:1529–1533. doi: 10.1046/j.1471-4159.2002.01269.x. [DOI] [PubMed] [Google Scholar]
- Hurtado DE, Molina-Porcel L, Carroll JC, Macdonald C, Aboagye AK, Trojanowski JQ, Lee VM. Selectively silencing GSK-3 isoforms reduces plaques and tangles in mouse models of Alzheimer’s disease. J Neurosci. 2012;32:7392–7402. doi: 10.1523/JNEUROSCI.0889-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope RS. Glycogen synthase kinase-3 in the etiology and treatment of mood disorders. Front Mol Neurosci. 2011;4:16–26. doi: 10.3389/fnmol.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O, Lipina TV, Takao K, van Eede M, Hattori S, Laliberté C, Khan M, Okamoto K, Chambers JW, Fletcher PJ, MacAulay K, Doble BW, Henkelman M, Miyakawa T, Roder J, Woodgett JR. Abnormalities in brain structure and behavior in GSK-3α mutant mice. Mol Brain. 2009;19(2):35. doi: 10.1186/1756-6606-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O, Woodgett JR. GSK-3: Functional Insights from Cell Biology and Animal Models. Front Mol Neurosci. 2011;16(4):40. doi: 10.3389/fnmol.2011.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karege F, Perroud N, Burkhardt S, Schwald M, Ballmann E, La Harpe R, Malafosse A. Alteration in kinase activity but not in protein levels of protein kinase B and glycogen synthase kinase-3β in ventral prefrontal cortex of depressed suicide victims. Biol Psychiatry. 2007;61:240–245. doi: 10.1016/j.biopsych.2006.04.036. [DOI] [PubMed] [Google Scholar]
- Kim WY, Wang X, Wu Y, Doble BW, Patel S, Woodgett JR, Snider WD. GSK-3 is a master regulator of neural progenitor homeostasis. Nat Neurosci. 2009;12:1390–1397. doi: 10.1038/nn.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MK, Jope RS. Lithium treatment alleviates impaired cognition in a mouse model of fragile X syndrome. Genes, Brain and Behavior. 2013;12:723–731. doi: 10.1111/gbb.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MK, Pardo M, Cheng Y, Downey K, Jope RS, Beurel E. Glycogen synthase kinase-3 inhibitors: Rescuers of cognitive impairments. Pharmacol Ther. 2014;141:1–12. doi: 10.1016/j.pharmthera.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A, Perez DI. GSK-3 inhibitors: a ray of hope for the treatment of Alzheimer’s disease? J Alzheimers Dis. 2008;15:181–191. doi: 10.3233/jad-2008-15204. [DOI] [PubMed] [Google Scholar]
- Maurin H, Lechat B, Dewachter I, Ris L, Louis JV, Borghgraef P, Devijver H, Jaworski T, Van Leuven F. Neurological characterization of mice deficient in GSK3α highlight pleiotropic physiological functions in cognition and pathological activity as Tau kinase. Mol Brain. 2013;25(6):27. doi: 10.1186/1756-6606-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus EJ, Sakamoto K, Armit LJ, Ronaldson, Shpiro N, Marquez R, Alessi DR. Role that phosphorylation of GSK3 plays in insulin and Wnt signaling defined by knockin analysis. EMBO J. 2005;24:1571–1583. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mines MA, Jope RS. Glycogen synthase kinase-3: a promising therapeutic target for fragile x syndrome. Front Mol Neurosci. 2011;1(4):35. doi: 10.3389/fnmol.2011.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mines MA, Beurel E, Jope RS. Examination of methylphenidate-mediated behavior regulation by glycogen synthase kinase-3 in mice. Eur J Pharmacol. 2013;5:252–258. doi: 10.1016/j.ejphar.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SS, Mahmoudi T, Danenberg E, Bejaoui I, de Lau W, Korswagen HC, Schutte M, Clevers H. Phosphatidylinositol 3-kinase signaling does not activate the wnt cascade. J Biol Chem. 2009;284:35308–35313. doi: 10.1074/jbc.M109.078261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien WT, Huang J, Buccafusca R, Garskof J, Valvezan JA, Berry GT, Klein PS. Essential role of glycogen synthase kinase-3 in β-arrestin-2 complex formation and lithium-sensitive behaviors. J Clin Invest. 2011;121:3756–3762. doi: 10.1172/JCI45194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien WT, Harper AD, Jove F, Woodgett JR, Maretto S, Piccolo S, Klein PS. Glycogen synthase kinase-3β haploinsufficiency mimics the behavioral and molecular effects of lithium. J Neurosci. 2004;24:6791–6798. doi: 10.1523/JNEUROSCI.4753-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata N, Chiu CT, Moya PR, Leng Y, Wang Z, Hunsberger JG, Leeds P, Chuang DM. Lentivirally mediated GSK-3β silencing in the hippocampal dentate gyrus induces antidepressant-like effects in stressed mice. Int J Neuropsychopharmacol. 2011;14:711–717. doi: 10.1017/S1461145710000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo M, King MK, Perez-Costas E, Melendez-Ferro M, Martinez A, Beurel E, Jope RS. Impairments in cognition and neural precursor cell proliferation in mice expressing constitutively active glycogen synthase kinase-3. Front Behav Neurosci. 2015;4(9):55. doi: 10.3389/fnbeh.2015.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polter A, Beurel E, Garner R, Song L, Miller C, Sweatt JD, McMahon L, Bartolucci AA, Li X, Jope RS. Deficiency in the inhibitory serine-phosphorylation of glycogen synthase kinase-3 increases sensitivity to mood disturbances. Neuropsychopharmacology. 2010;35:1761–1774. doi: 10.1038/npp.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvezan AJ, Klein PS. GSK-3 and Wnt Signaling in Neurogenesis and Bipolar Disorder. Front Mol Neurosci. 2012;30(5):1. doi: 10.3389/fnmol.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson MB, Dias C, Magida J, Mazei-Robison M, Lobo M, Kennedy P, Dietz D, Covington H, 3rd, Russo S, Neve R, Ghose S, Tamminga C, Nestler EJ. A Novel Role of the WNT-Dishevelled-GSK3β Signaling Cascade in the Mouse Nucleus Accumbens in a Social Defeat Model of Depression. J Neurosci. 2011;31:9084–9092. doi: 10.1523/JNEUROSCI.0039-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]