Abstract
BACKGROUND
To evaluate performance of magnetic resonance (MR)-ultrasound guided fusion biopsy in diagnosing clinically significant prostate cancer (csCaP).
METHODS
1042 men underwent multi-parametric MRI (mpMRI) and fusion biopsy consecutively in a prospective trial (2009 – 2014). An expert reader graded mpMRI regions of interest (ROI) 1–5 using published protocols. The fusion biopsy device was used to obtain targeted cores from ROIs (when present) followed by a fusion-image guided 12-core systematic biopsy in all men, even if no suspicious ROI. Primary endpoint was detection of clinically significant CaP (i.e., Gleason score ≥ 7).
RESULTS
Among 825 men with ≥ 1 suspicious ROI of grade 3 or higher, 289 (35%) had csCaP. Powerful predictors of csCaP were ROI grade (grade 5 vs 3, OR 6.5, p<0.01) and prostate-specific antigen density (each increase of 0.05 ng/mL/cc, OR 1.4, p<0.01). Combining systematic and targeted biopsies detected more csCaP (n=289) than targeting (n=229) or systematic biopsy alone (n=199). Among patients with no suspicious ROI, 35 (16%) had csCaP on systematic biopsy.
CONCLUSION
In this prospective trial, MR-ultrasound fusion biopsy allowed detection of csCaP with a direct relationship with ROI grade and PSA density. The combination of targeted and systematic biopsy detected more csCaP than either modality alone; systematic biopsies revealed csCaP in 16% of men with no suspicious MRI target. Advantages of this new biopsy method are apparent, but issues of cost, training, and reliability await resolution prior to widespread adoption.
Keywords: prostate cancer, magnetic resonance imaging, biopsy, cancer staging, diagnostic imaging
INTRODUCTION
Targeted prostate biopsy utilizing multiparametric magnetic resonance imaging (mpMRI) to guide tissue sampling can improve detection of prostate cancer (CaP).1–3 This has been demonstrated in biopsy-naïve men4, men with prior negative biopsies5,6, and those considering active surveillance of CaP.7,8 However, many studies favoring the new technology are limited by small sample size or variable protocols, and the value of guided biopsy has been questioned.9–11 Furthermore, the predictive value of a ‘normal’ mpMRI and the significance of ‘normal’ regions on mpMRI, have not been adequately evaluated.
The negative predictive value (NPV) of mpMRI is critical because of claims that mpMRI may have utility—standing alone—as a cancer-screening tool for men with an elevated prostate-specific antigen (PSA) or abnormal digital rectal exam.12 In preliminary studies from our institution, 28% of Gleason score (GS) ≥ 7 prostate tumors went undetected by mpMRI, based on whole-mount prostatectomy specimens.13 The key questions are whether a ‘normal’ mpMRI should preclude immediate biopsy and, if guided biopsy performed, whether targeting alone can suffice.
To evaluate these questions, a prospective trial was designed in which men with a clinical suspicion of CaP received pre-biopsy mpMRI. All participants underwent systematic (SB) and, when indicated by the mpMRI, targeted biopsy (TB). The inclusion of both biopsy methods was uniformly applied to a large sample, partial subsets of which have been reported previously.3,5,7,14 The study design, which mandated both SB and TB in all participants regardless of MRI findings, allowed a critical appraisal of whether SB may no longer be necessary, or even desirable.1 We hypothesized that the CB would identify more cases of csCaP than either of the others alone.
METHODS
Study design
Subjects included all men who underwent MR-ultrasound fusion biopsy between September 2009 and February 2015 for either (a) an elevated PSA or abnormal digital rectal exam or (b) confirmation of low-risk CaP for patients considering active surveillance. For patients who underwent > 1 fusion biopsy, we assessed results from their first biopsy. The study was approved in advance by the University of California, Los Angeles (UCLA) Institutional Review Board.
Multiparametric magnetic resonance imaging
Subjects underwent mpMRI on a Siemens TrioTrim Somatom 3-Tesla magnet (Siemens Medical Solutions, Malvern, Pennsylvania) with a trans-abdominal external phased-array coil. Regions of interest (ROIs) were delineated and graded 1–5 using a scoring system established before the Prostate Imaging and Reporting and Data System (PI-RADS) was described.2 The UCLA scoring system incorporates T2-weighted imaging, diffusion weighted imaging (DWI), and dynamic contrast enhancement (DCE) (Supplemental Table 1).2 We defined primary ROI based on highest ROI grade, then lowest apparent diffusion coefficient (ADC) from the DWI, then largest diameter in millimeters.
MR-US fusion biopsy
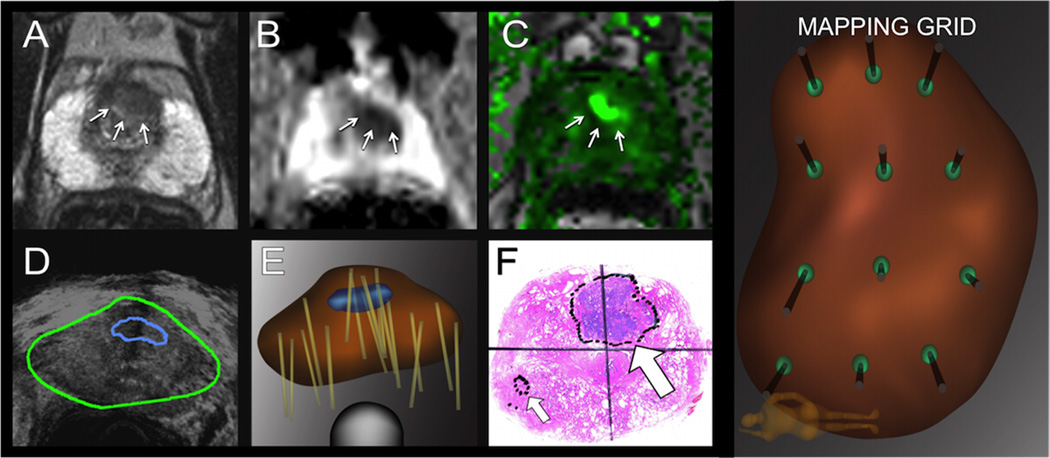
Figure 1 shows the steps in the process of fusion biopsy. MRI images were transferred electronically to an Artemis fusion device (Eigen, Grass Valley, CA) immediately before a transrectal ultrasound was performed. Fusion of MR and real-time ultrasound images was then completed.2 Men with ROIs underwent TB, with ~1 core/3 mm of the longest ROI axis. After TB was obtained, patients underwent 12-core SB via a scalable grid incorporated in the software of the Artemis device.
Figure 1.
Pathway for performance of MR-US fusion biopsy in a sample patient. From the mpMRI, a region of interest (ROI (arrows) is identified on 3 sequences: T2 (A), DWI (B), and DCE (C). MR images are co-registered with real-time transrectal ultrasound in the image-fusion device (Artemis, Eigen) (D). Biopsies (tan lines) are performed on a 3-dimensional reconstruction of the prostate made by the fusion device; the model incorporates the ROI (in blue) as an anterior target; targeted and systematic cores are obtained (E). The radical prostatectomy specimen processed with whole-mount sectioning shows the index tumor corresponding to the ROI (large arrow) (F). Small arrow points to a secondary lesion. Men with no suspicious ROIs on mpMRI had systematic biopsies taken via a 12-point scalable grid, performed with the fusion biopsy device (systematic grid, coronal view).
Primary outcome of interest was detection of clinically significant CaP (csCaP), defined here as any GS ≥ 7.15 We compared the performance of different fusion biopsy strategies (i.e., TB alone, SB alone, combination of both (CB)) in detecting csCaP among patients with ≥1 ROI of grade 3 or greater.
Statistical Analysis
Descriptive statistics were performed to summarize clinical, radiographic, and biopsy characteristics. Chi-square and Fisher’s exact tests were used to evaluate the association between clinical characteristics and presence of csCaP. McNemar’s test was used to compare the performance of different biopsy strategies and detection of (a) csCaP, (b) low-risk CaP (i.e., GS 3+3=6), and (c) high-risk CaP (i.e., GS ≥ 8). Multivariable logistic regression models were used to estimate odds ratios (OR) for the presence of csCaP based on pertinent covariates. The efficacy of the logistic model was estimated using the area under the receiver operator characteristic curve and the goodness of fit using the Hosmer-Lemeshow test. Chi-square and Fisher’s exact tests were also performed to assess the relationship between covariates and presence of CaP among patients with a negative MRI (i.e., no ROIs of grade 3 or more). Tests were 2-sided and considered statistically significant if p<0.05. Statistical analysis was performed using Stata 11 (StataCorp, College Station, TX).
RESULTS
Table 1 lists characteristics of the analytic cohort. Among 1042 patients, 324 (31%) had csCaP found on fusion biopsy: 289 with at least one suspicious ROI and 35 with a normal mpMRI. 825 (79%) had ≥1 ROI of grade 3 or greater, and 217 (21%) had no suspicious lesions noted on MRI. Median time to biopsy after mpMRI was 20 days (IQR 7–43). Men were divided nearly evenly among those with no prior biopsy (32%), prior negative biopsy (31%), and previously positive biopsy, i.e., active surveillance patients (37%). Regarding maximum ROI grade, 42% had a low-suspicion grade 3 lesion, 29% moderate-suspicion grade 4 lesion, and 8% with a high-suspicion grade 5 ROI.
Table 1.
Patient characteristics (n = 1,042)
| Prior negative (n = 324) |
Biopsy-naïve (n = 329) |
Prior positive (n = 389) |
|
|---|---|---|---|
| Covariate | N (%) | N (%) | N (%) |
|
Age at biopsy, years (median, IQR) |
65.7 (59.3–70.2) | 64.4 (58.5–69.4) | 65.1 (59.6–69.5) |
| Race | |||
| Caucasian | 248 (77) | 270 (82) | 312 (83) |
| African-American | 17 (5) | 18 (5) | 24 (6) |
| Asian | 35 (11) | 22 (7) | 24 (6) |
| Hispanic/Latino | 14 (4) | 9 (3) | 22 (6) |
| Other/Unknown | 10 (3) | 10 (3) | 7 (2) |
|
PSA, ng/mL (median, IQR) |
7.6 (5.0–11.5) | 5.8 (4.4–8.1) | 4.8 (3.0–6.9) |
|
Prostate volume, cc (median, IQR) |
57.7 (39.8–83.5) | 45.0 (33.0–61.5) | 43.0 (32.3–60.4) |
|
Time between MRI to biopsy, days (median, IQR) |
21 (7–43) | 19 (7–43) | 20 (8–49) |
|
Maximum diameter of ROI, mm (median, IQR) |
11.0 (9.0–14.5) | 11.0 (8.0 – 14.0) | 10.0 (8.0–14.0) |
|
Number of ROI ≥ Grade 3 |
|||
| 0 | 48 (15) | 45 (14) | 85 (22) |
| 1 | 162 (50) | 186 (56) | 183 (47) |
| 2 | 98 (30) | 81 (25) | 98 (25) |
| 3 | 16 (5) | 17 (5) | 23 (6) |
| Maximum ROI grade | |||
| No lesion/Grade 1–2 | 59 (18) | 56 (17) | 102 (26) |
| Grade 3 | 148 (46) | 129 (39) | 158 (49) |
| Grade 4 | 87 (27) | 109 (32) | 105 (27) |
| Grade 5 | 30 (9) | 35 (11) | 24 (6) |
|
ADC of Index ROI (median, IQR) |
982 (871–1096) | 985 (875–1104) | 999 (870–1126) |
| Anterior lesion | 100 (31) | 148 (45) | 130 (33) |
Abbreviations: MRI – magnetic resonance imaging; US – ultrasound; SD – standard deviation; PSA – prostate-specific antigen; IQR – interquartile range; ROI – region of interest; ADC – apparent diffusion coefficient
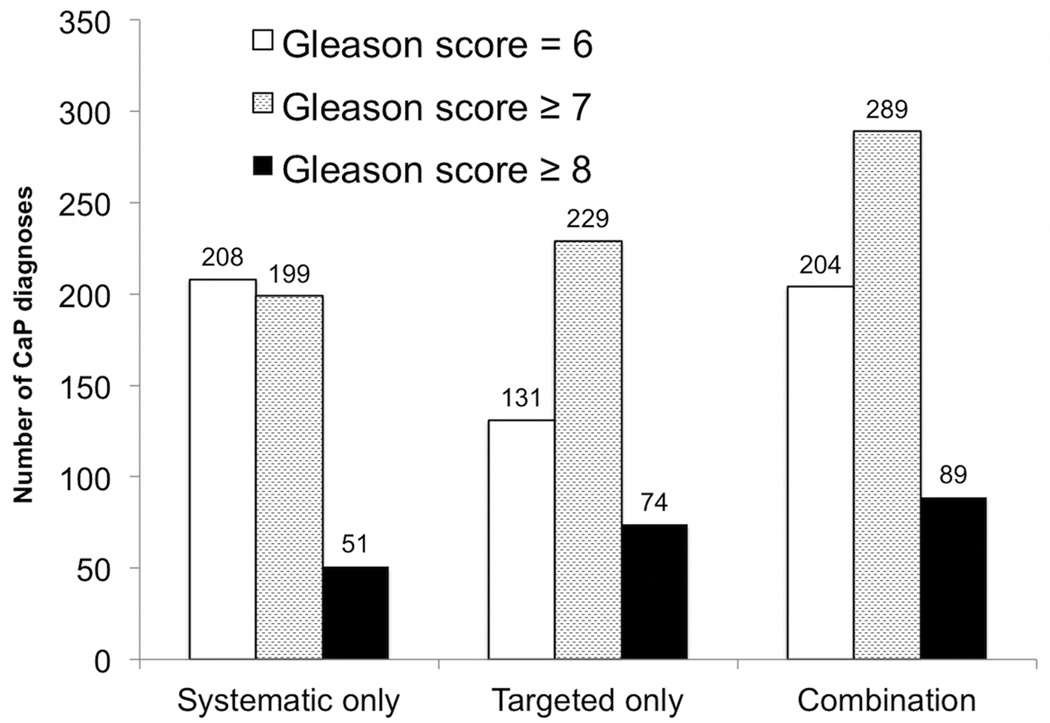
The performance of CB compared to SB-only or TB-only strategies is detailed in Figure 2. Among 825 patients with ≥1 ROI of grade 3 or higher, CB identified 289 cases of csCaP (vs 229 cases for TB-only and 199 cases for SB-only, p<0.001). The combined approach also identified more high-risk CaP cases (i.e., GS 8 or more) than either approach alone (89 cases vs 74 TB-only, p<0.001; 51 cases SB-only, p<0.001). 204 men were diagnosed with GS 6 disease with CB (vs 208 SB-only (p<0.001) and 131 TB-only (p<0.001)). Thus, adding SB to TB resulted in 60 additional csCaP diagnoses (7% of ROI cohort), 15 additional high-risk CaP cases (2% of ROI cohort), and 73 additional GS 6 cases (9% of cohort) that would have otherwise been undiagnosed by only targeting ROIs. Using the CB approach, the number needed to biopsy to identify one additional csCaP or high-risk CaP was 14 or 55, respectively. Thus, the CB approach would result in 1 additional low-risk CaP case per csCaP case, and 5 additional low-risk CaP cases per high-risk CaP. In a separate analysis, the number of targeted cores taken was related to the detection rate of csCaP (OR 1.44, p<0.001), but the number of systematic cores was not (OR 0.93, p>0.05).
Figure 2.
Diagnostic performance of systematic biopsy, targeted biopsy, and combined approach among patients, whose mpMRI revealed at least one ROI of grade ≥3 (n=825). Number of patients diagnosed with CaP (vertical axis) vs biopsy strategy is shown. Combining targeted and systematic biopsies resulted in detection of 60 clinically-significant cancers undetected by either alone (light gray, p<0.001 vs systematic and targeted alone), and an additional 15 high-risk cases (black, p<0.001 vs systematic and targeted approach).
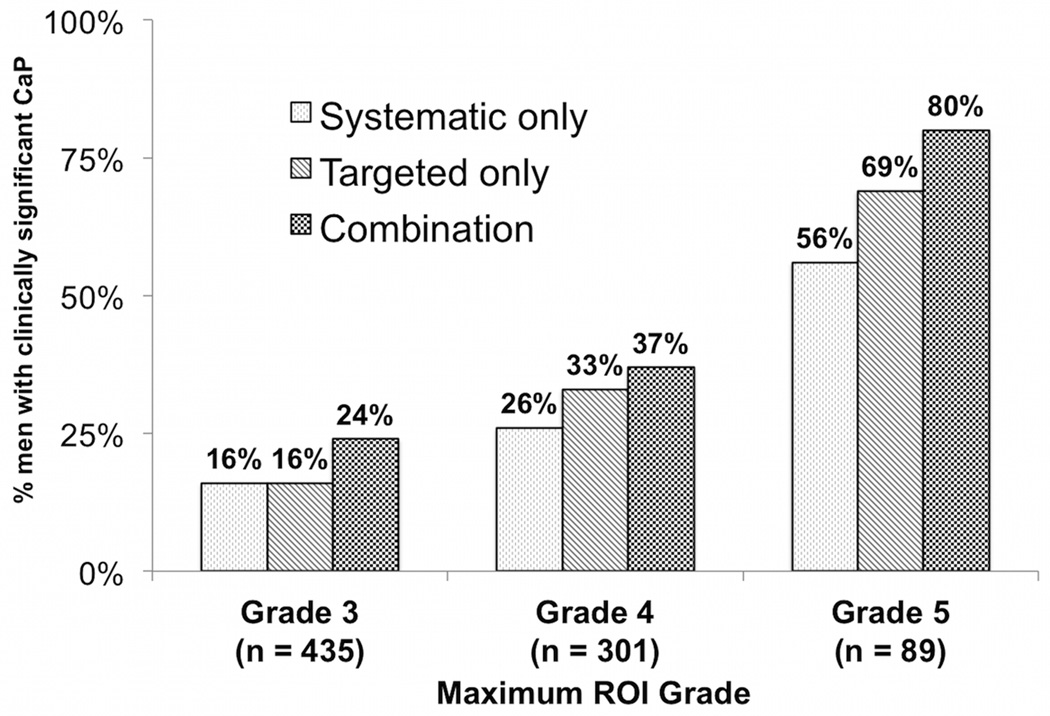
Figure 3 displays the relationship between ROI grade and the presence of csCaP, among the 825 men with an ROI of grade 3 or greater. The presence of csCaP was directly related to ROI grade. 80% of men with a grade 5 ROI had evidence of GS ≥ 7 CaP (vs 24% for grade 3 ROI, p<0.001). The CB outperformed TB or SB alone for all ROI grades (all p<0.001). There was a direct association with ROI size and presence of GS ≥ 7 CaP (24% for < 8 mm vs 41% > 14 mm, OR 1.04 per mm, p<0.001).
Figure 3.
Relationship between ROI grade and presence of cancer. This figure shows the proportion of patients with ≥ 1 ROI on MRI (n=825) with a diagnosis of csCaP (n=289, 35%) (y-axis) stratified by ROI grade (x-axis). Combination biopsy (black checked bars) outperformed systematic biopsy (dark diagonal bars) and targeted biopsy (light hatched bars) across all ROI grades (p<0.001). Overall, 80% of patients with a grade 5 ROI had csCaP (vs 24% grade 3 ROI, OR 9.05, 95% CI 4.96 – 16.50).
Table 2 lists results from our multivariable regression models estimating the relationship between clinical factors and the presence of csCaP on fusion biopsy. The strongest predictor of csCaP on fusion biopsy was ROI grade, where men with a grade 5 ROI had nine times the odds of csCaP, compared to men with grade 3 ROI (OR 9.05, 95% CI 4.96 – 16.50). Presence of csCaP was directly related to age, PSA, PSA density, number of targeted cores and inversely related to prostate volume. Adding ROI size to the model did not significantly alter the area under the receiver operating characteristic curve and the effect of ROI size was no longer significant (p=0.115).
Table 2.
Covariates of csCaP among patients with an ROI ≥ grade 3 (n=825).
| Unadjusted OR (95% CI) |
Adjusted OR (95% CI)1 |
Adjusted OR (95% CI)2 |
|
|---|---|---|---|
| Age at biopsy (per 10 years) | 1.46 (1.21 – 1.76) | 1.40 (1.13 – 1.73) | 1.63 (1.31 – 2.02) |
| PSA (per 5 ng/mL) | 1.24 (1.12 – 1.39) | -- | 1.29 (1.12 – 1.49) |
| Prostate volume (per 10 cc) | 0.76 (0.71 – 0.82) | -- | 0.70 (0.64 – 0.76) |
|
PSA density (per 0.05 ng/mL/cc) |
1.75 (1.58 – 1.94) | 1.33 (1.23 – 1.43) | -- |
| ROI grade | |||
| Grade 3* | -- | -- | -- |
| Grade 4 | 1.91 (1.38 – 2.63) | 1.55 (1.10 – 2.19) | 1.61 (1.14 – 2.27) |
| Grade 5 | 12.80 (7.28 – 22.41) | 7.98 (4.43 – 14.38) | 9.05 (4.96 – 16.50) |
Abbreviations: csCaP – clinically significant prostate cancer; OR – odds ratio; CI – confidence interval; PSA – prostate-specific antigen; ROI – region of interest;
reference group;
– included covariates for age, PSA density, ROI grade and number of targeted cores and has an area under receiver operating characteristic curve = 0.781;
– included covariates for age, PSA, prostate volume, ROI grade and number of targeted cores and has an area under receiver operating characteristic curve = 0.794.
Compared to men with prior negative biopsies, those undergoing their first prostate biopsy had a two-fold risk of csCaP (OR 2.0, 95% CI 1.39–2.86). Nearly three out of four patients with a prior negative conventional biopsy, who had either a negative MRI (79%) or a ROI < grade 3 (70%) had a negative fusion biopsy. On the other hand, most patients with grade 5 ROIs had csCaP: 83% of biopsy-naïve men, 72% of men with prior negative biopsies, and 76% of men with prior positive biopsies. The summary of cancer detection stratified by biopsy indication (biopsy-naïve, prior negative, and prior positive biopsy) and biopsy strategy, based on mpMRI findings, is seen in Supplmental Table 2.
Among 217 men who did not have any suspicious lesions noted on MRI, fusion biopsy showed CaP and csCaP in 93 (42%) and 35 (16%) cases, respectively. Presence of any CaP in the setting of a normal mpMRI was directly associated with age and inversely associated with prostate volume (p<0.05), and it was most common among men with prior positive biopsies (59% vs 21% prior negative, p<0.05). Age and PSA density were directly associated with csCaP in the setting of a normal mpMRI (p<0.05), and csCaP was most common among men who had previously positive biopsy (22% vs 9% prior negative, p=0.05).
DISCUSSION
Three principal findings derive from this prospective study of 1042 men undergoing MR-ultrasound fusion biopsy. First, two factors, ROI grade and PSA density, were strongly and directly related to the presence of csCaP. Men with grade 5 ROIs had nine times the odds of csCaP, compared to men with grade 3 ROIs. Second, the combination of targeted and systematic biopsies resulted in the detection of more csCaP than either method alone. This difference was clinically important; 60 men were diagnosed with csCaP on systematic biopsy that would have been missed targeted biopsy alone. Third, a considerable number of men with negative mpMRIs were found to harbor potentially important CaP: one in eight men without suspicious lesions on mpMRI were diagnosed with csCaP by systematic biopsy. The study design, which included systematic biopsy regardless of MRI findings, thus provided a critical test of the negative predictive value of MRI in detection of csCaP.
In predicting csCaP from MRI, grade of the ROI was by far the most important factor. Among patients with a grade 5 ROI, the presence of aggressive disease was the usual finding, where 8 of 10 men with these high-suspicion regions harbored high-grade CaP. These results are concordant with a number of small, retrospective studies, where increasing suspicion of MRI lesions is associated with aggressive disease appearing on fusion biopsy.2,7,16 Prior work by our group demonstrated that ROI grade is directly related to reclassification beyond Epstein criteria15 for a small cohort of men considering active surveillance7 and among a limited group of men with prior negative prostate biopsies.5 The results presented herein confirm these preliminary studies among a large cohort of men and provide helpful information for men considering fusion biopsy after mpMRI of the prostate.
Men with increased PSA density were at considerable risk of csCaP on fusion biopsy, with an odds ratio of 1.3 per increase of 0.05 ng/mL/cc. Increased PSA density has been recognized as a risk factor for csCaP since Epstein’s criteria for clinically insignificant disease were established.15 Several active surveillance protocols use elevated PSA density as an exclusion criterion for enrollment.17,18 Other studies have shown the association with elevated PSA density and significant CaP noted on fusion biopsy.19 Along with ROI grade, our study confirms PSA density as an important risk factor for the presence of csCaP on fusion biopsy.
The present study design and findings differ somewhat from the recently published work of Siddiqui et al. at the National Cancer Institute (NCI) (Supplemental Table 3). In the NCI study, men with a negative mpMRI (n=182) were excluded from biopsy; in the present study, all men underwent systematic biopsy, even if MRI was negative. By performing systematic biopsy regardless of mpMRI findings, 42% of men with no suspicious lesions on mpMRI were found to harbor CaP; moreover, one-third of the cancers found were clinically significant, resulting in a change in management for these men. Systematic freehand biopsy added very little to detection of csCaP in the NCI study, but software-guided systematic biopsy was of considerable importance to detection of csCaP in the present work.
Several factors that could explain these observed differences in cancer detection on systematic biopsy. First, 43% of the NCI patients had undergone prior negative biopsy (vs 31% in the present report), indicating that more men in the NCI study had hard-to-detect tumors than in the present group. Furthermore, an anterior location was more common in the NCI study (44%) than in the present work (36%). Anterior tumors often go undetected by conventional systematic biopsy (in as many as 50% of cases).20 Further, the MRI grading systems used were not the same in the two studies. Neither system was the contemporary version of PI-RADS v2, which is now the ‘industry standard.’21 Another possible contributing factor to the observed differences is the technique used for systematic biopsy. Freehand biopsies using transrectal ultrasound guidance, as in the NCI report, may provide different tissue findings than systematic biopsies using a defined grid or template, as in the present report. For instance, conventional prostate biopsies are hampered by a risk of falsely negative results, reported to be as high as 47% in some series.22 When experienced urologists performed freehand ultrasound-guided biopsies on a phantom prostate, biopsy sites were widely divergent between individual operators, were frequently clustered, and left large parts of the prostate un-sampled.23 Thus, all of these factors may have played a role in the lower detection rate of the systematic biopsies in the NCI report.
The concept of using mpMRI to obviate prostate biopsy, if the imaging reveals no targets, should be regarded with caution.12,24 In a recent meta-analysis, the NPV of mpMRI was found to range from 65 – 94%, depending on how that finding was validated.25 In a retrospective study of 193 men, Itatani et al. reported a NPV of 89.6% for mpMRI identification of csCaP.12 However, in the Itatani series, conventional transrectal ultrasound-guided biopsy was performed, which may result in a lower detection rate than the systematic technique. In the current report, negative predictive values of 56% for any cancer and 85% for csCaP were observed. These data suggest that a negative mpMRI should not routinely replace biopsy as a method to rule out the presence of csCaP.
The present report focuses on diagnosis of csCaP, rather than high-risk CaP. Siddiqui et al. found that to detect one additional high-risk case, 200 combination biopsies would be required, the implication being that systematic biopsies may be unnecessary. However, high-risk cases are often of large volume and less difficult to detect. Clinically significant tumors (i.e., GS 7) may not always be apparent on mpMRI and are often smaller and more difficult to detect than large-volume, high-risk tumors. Furthermore, detection of csCaP will often change patient management, at least increasing vigilance of follow-up. Using the systematic technique as described herein, 14 combination biopsies would be needed to detect one additional csCaP and for high-risk tumor (i.e. GS > 8) detection, 55 combination biopsies. Thus, the combination of systematic and targeted biopsies, as described in the present report, is necessary for optimal characterization of whole-organ pathology and assessment of biologic potential.
Our findings should be interpreted within the context of some methodological limitations. First, we chose not to use an endorectal coil for the present investigation. Use of an endorectal coil for MRI is acknowledged to improve staging of CaP. However, comparisons of external phased array versus endorectal coil imaging show equivalent performance with each modality for CaP detection.26,27 A recent comparison of these acquisition techniques28 found more cancers with use of an endorectal coil than the body coil. However, in this report, the endorectal coil provided only a 10% improvement in sensitivity for the dominant tumor (85% vs 75%); only 20 patients were included; and MRI method was not fully multi-parametric. Thus, with the goal of defining a practical diagnostic modality for widespread adoption, the choice to employ the more patient-friendly body coil was a key consideration of the experimental design.
Second, we utilized specific institutional protocols for grading of ROIs, which may limit generalizability to other practice settings. However, our protocol varies only slightly from the validated PI-RADS grading scheme, and in-house data show that the UCLA grading system2 is highly concordant with PI-RADS. Second, our results relied on input from individual experts well versed in execution of mpMRI and MR-US fusion biopsy, respectively. These results may not be reproducible in settings among practitioners with less experience. There is also a risk of mis-registration with fusion biopsy based on a number of factors (e.g., distortion from transrectal US probe) that could explain differences in cancer detection rate between targeted and systematic biopsy. Third, our definition of csCaP as cancer with GS ≥ 7 may not capture truly significant disease, as there may be clinical implications of having high-volume GS 6 disease, and less significance with low-volume GS 7 disease. Finally, this analysis did not consider whole-mount prostatectomy specimens, precluding knowledge of the true CaP detection rate of MR-US fusion biopsy and mpMRI.13
These limitations aside, MR-US fusion biopsy appears to be most accurate when targeting of specific lesions is combined with systematic biopsy guided by software in the fusion device. The combined approach identifies more csCaP than targeted biopsy alone and provides accurate characterization of low-risk—and likely indolent— GS 6 tumors. Of note, men with high-suspicion ROI and elevated PSA density are at greatly increased risk of aggressive CaP. Finally, at this point, when biopsy is clinically indicated, a negative mpMRI should not preclude it. Template-based systematic sampling can detect cases of csCaP, even when MRI shows no suspicious targets.
Supplementary Material
Acknowledgments
Funding support:
The work was supported by the NCI at the NIH (grant R01CA158627), UCLA CTSI (grant UL1TR000124), Beckman Coulter Foundation; Jean Perkins Foundation; Steven C. Gordon Family Foundation to LM; and the American Cancer Society Postdoctoral Fellowship (grant PF-14-161-01-CPHPS) to CF.
Footnotes
There are no conflicts of interest to report.
REFERENCES
- 1.Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/Ultrasound Fusion–Guided Biopsy With Ultrasound-Guided Biopsy for the Diagnosis of Prostate Cancer. JAMA. 2015;313(4):390–397. doi: 10.1001/jama.2014.17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Natarajan S, Marks LS, Margolis DJA, et al. Clinical application of a 3D ultrasound-guided prostate biopsy system. Urol Oncol. 2011;29(3):334–342. doi: 10.1016/j.urolonc.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sonn GA, Natarajan S, Margolis DJA, et al. Targeted Biopsy in the Detection of Prostate Cancer Using an Office Based Magnetic Resonance Ultrasound Fusion Device. J Urol. 2013;189(1):86–92. doi: 10.1016/j.juro.2012.08.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abd-Alazeez M, Kirkham A, Ahmed HU. Performance of multiparametric MRI in men at risk of prostate cancer before the first biopsy: a paired validating cohort study using template prostate mapping biopsies. Pros Cancer Prostatic Dis. 2014;17(1):40–46. doi: 10.1038/pcan.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sonn GA, Chang E, Natarajan S, et al. Value of Targeted Prostate Biopsy Using Magnetic Resonance–Ultrasound Fusion in Men with Prior Negative Biopsy and Elevated Prostate-specific Antigen. Eur Urol. 2014;65(4):809–815. doi: 10.1016/j.eururo.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abd-Alazeez M, Ahmed HU, Arya M, et al. The accuracy of multiparametric MRI in men with negative biopsy and elevated PSA level—Can it rule out clinically significant prostate cancer? Urol Oncol. 2014;32(1):45.e17–45.e22. doi: 10.1016/j.urolonc.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu JC, Chang E, Natarajan S, et al. Targeted Prostate Biopsy to Select Men for Active Surveillance: Do the Epstein Criteria Still Apply? J Urol. 2014;192(2):385–390. doi: 10.1016/j.juro.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stamatakis L, Siddiqui MM, Nix JW, et al. Accuracy of multiparametric magnetic resonance imaging in confirming eligibility for active surveillance for men with prostate cancer. Cancer. 2013;119(18):3359–3366. doi: 10.1002/cncr.28216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Acar Ö, Esen T, Çolakoğlu B, Vural M, Onay A. Multiparametric MRI guidance in first-time prostate biopsies: what is the real benefit? Diagn Interv Radiol. 2015 doi: 10.5152/dir.2015.46014. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tonttila PP, Lantto J, Pääkkö E, et al. Prebiopsy Multiparametric Magnetic Resonance Imaging for Prostate Cancer Diagnosis in Biopsy-naive Men with Suspected Prostate Cancer Based on Elevated Prostate-specific Antigen Values: Results from a Randomized Prospective Blinded Controlled Trial. Eur Urol. 2015 doi: 10.1016/j.eururo.2015.05.024. (in press) [DOI] [PubMed] [Google Scholar]
- 11.Giannarini G, Briganti A, Crestani A, Rossanese M, Montorsi F, Ficarra V. Dismiss Systematic Transrectal Ultrasound-guided and Embrace Targeted Magnetic Resonance Imaging–informed Prostate Biopsy: Is the Paradigm Ready to Shift? Eur Urol. 2015 doi: 10.1016/j.eururo.2015.05.049. (in press) [DOI] [PubMed] [Google Scholar]
- 12.Itatani R, Namimoto T, Atsuji S, et al. Negative predictive value of multiparametric MRI for prostate cancer detection: Outcome of 5-year follow-up in men with negative findings on initial MRI studies. Eur J Radiol. 2014;83(10):1740–1745. doi: 10.1016/j.ejrad.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 13.Le JD, Tan N, Shkolyar E, et al. Multifocality and Prostate Cancer Detection by Multiparametric Magnetic Resonance Imaging: Correlation with Whole-mount Histopathology. Eur Urol. 2015;67(3):569–576. doi: 10.1016/j.eururo.2014.08.079. [DOI] [PubMed] [Google Scholar]
- 14.Le JD, Stephenson S, Brugger M, et al. Magnetic Resonance Imaging-Ultrasound Fusion Biopsy for Prediction of Final Prostate Pathology. J Urol. 2014;192(5):1367–1373. doi: 10.1016/j.juro.2014.04.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and Clinical Findings to Predict Tumor Extent of Nonpalpable (Stage T1 c) Prostate Cancer. JAMA. 1994;271(5):368–374. [PubMed] [Google Scholar]
- 16.Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic Resonance Imaging/Ultrasound Fusion Guided Prostate Biopsy Improves Cancer Detection Following Transrectal Ultrasound Biopsy and Correlates With Multiparametric Magnetic Resonance Imaging. J Urol. 2011;186(4):1281–1285. doi: 10.1016/j.juro.2011.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bul M, Zhu X, Valdagni R, et al. Active Surveillance for Low-Risk Prostate Cancer Worldwide: The PRIAS Study. Eur Urol. 2013;63(4):597–603. doi: 10.1016/j.eururo.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Carter HB, Kettermann A, Warlick C, et al. Expectant Management of Prostate Cancer With Curative Intent: An Update of The Johns Hopkins Experience. J Urol. 2007;178(6):2359–2365. doi: 10.1016/j.juro.2007.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdi H, Zargar H, Goldenberg SL, et al. Multiparametric magnetic resonance imaging–targeted biopsy for the detection of prostate cancer in patients with prior negative biopsy results. Urol Oncol. 2015;33(4):165.e1–165.e7. doi: 10.1016/j.urolonc.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Ouzzane A, Puech P, Lemaitre L, et al. Combined Multiparametric MRI and Targeted Biopsies Improve Anterior Prostate Cancer Detection, Staging, and Grading. Urology. 2011;78(6):1356–1362. doi: 10.1016/j.urology.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 21.American College of Radiology. [cited 2015 Jun 10];PI-RADS v 2: Prostate Imaging and Reporting and Data System, Version 2 [Internet] Available from: http://www.acr.org/~/media/ACR/Documents/PDF/QualitySafety/Resources/PIRADS/PIRADS%20V2.pdf.
- 22.Taira AV, Merrick GS, Galbreath RW, et al. Performance of transperineal template-guided mapping biopsy in detecting prostate cancer in the initial and repeat biopsy setting. Prostate Cancer Prostatic Dis. 2010;13(1):71–77. doi: 10.1038/pcan.2009.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han M, Chang D, Kim C, et al. Geometric Evaluation of Systematic Transrectal Ultrasound Guided Prostate Biopsy. J Urol. 2012;188(6):2404–2409. doi: 10.1016/j.juro.2012.07.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore CM, Petrides N, Emberton M. Can MRI replace serial biopsies in men on active surveillance for prostate cancer? Curr Opin Urol. 2014;24(3):280–287. doi: 10.1097/MOU.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 25.de Rooij M, Hamoen E, Fütterer JJ. Accuracy of multiparametric MRI for prostate cancer detection: a meta-analysis. Am J Radiol. 2014;202(2):343–351. doi: 10.2214/AJR.13.11046. [DOI] [PubMed] [Google Scholar]
- 26.Sosna J, Pedrosa I, DeWolf WC, Mahallati H, Lenkinski RE, Rofsky NM. MR imaging of the prostate at 3 tesla. Acad Radiol. 2004;11(8):857–862. doi: 10.1016/j.acra.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 27.Park BK, Kim B, Kim CK, Lee HM, Kwon GY. Comparison of Phased-Array 3.0-T and Endorectal 1.5-T Magnetic Resonance Imaging in the Evaluation of Local Staging Accuracy for Prostate Cancer. J Comp Assist Tech. 2007;31(4):534–538. doi: 10.1097/01.rct.0000250108.85799.e1. [DOI] [PubMed] [Google Scholar]
- 28.Turkbey B, Merino MJ, Gallardo EC, et al. Comparison of endorectal coil and nonendorectal coil T2W and diffusion-weighted MRI at 3 Tesla for localizing prostate cancer: Correlation with whole-mount histopathology. J Magnet Res Imag. 2014;39(6):1443–1448. doi: 10.1002/jmri.24317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.