Summary
Pentatricopeptide repeat (PPR) proteins are helical repeat proteins that bind RNA and influence gene expression in mitochondria and chloroplasts. Several PPR proteins in plants harbor a carboxy-terminal Small-MutS-Related (SMR) domain, but functions of the SMR appendage are unknown. To address this issue, we studied a maize PPR-SMR protein denoted PPR53 (GRMZM2G438524), which is orthologous to the Arabidopsis protein SOT1 (AT5G46580). Null ppr53 alleles condition a chlorotic, seedling lethal phenotype and a reduction in plastid ribosome content. Plastome-wide transcriptome and translatome analyses revealed strong defects in the expression of the ndhA and rrn23 genes, which were superimposed on secondary effects resulting from a decrease in plastid ribosome content. Transcripts with processed 5′-ends mapping approximately 70-nt upstream of rrn23 and ndhA are absent in ppr53 mutants, and the translational efficiency of the residual ndhA mRNAs is reduced. Recombinant PPR53 binds with high affinity and specificity to the 5′ proximal region of the PPR53-dependent 23S rRNA, suggesting that PPR53 protects this RNA via a barrier mechanism similar to that described for several PPR proteins lacking SMR motifs. However, recombinant PPR53 did not bind with high affinity to the ndhA 5′ UTR, suggesting that PPR53’s RNA stabilization and translation enhancing effects at the ndhA locus involve the participation of other factors.
Keywords: pentatricopeptide repeat, SMR domain, chloroplast, plastid, RNA processing, translational activator, Zea mays
Introduction
Gene expression systems in mitochondria and chloroplasts are derived from those in their bacterial progenitors, but they have acquired many new features during their coevolution with the eucaryotic host (reviewed in Barkan, 2011a; Hammani and Giege, 2014). For example, organellar RNAs in plants are modified by RNA editing, RNA splicing, and by exo- and endonucleolytic processing events that are either unusual or absent in bacteria. Many of these events are mediated by nucleus-encoded RNA binding proteins in the alpha solenoid superfamily, which are characterized by tracts of tandem helical repeats (Hammani et al., 2014). The pentatricopeptide repeat (PPR) protein family (Small and Peeters, 2000) is the canonical example of this phenomenon. PPR proteins are found in all eucaryotes and function almost exclusively in organellar gene expression (reviewed in Barkan and Small, 2014). They influence a wide range of molecular processes, including RNA editing, RNA stabilization, group II intron splicing, RNA cleavage and translation. Many PPR proteins bind specific RNA sequences and do so, at least in part, via a modular 1 repeat-1 nucleotide binding mode in which several amino acids in each repeat specify the bound nucleotide via a predictable “code” (Barkan et al., 2012; Takenaka et al., 2013; Yagi et al., 2013a).
PPR proteins fall into several subfamilies based on the nature of their repeats and on the presence or absence of additional functional domains (Lurin et al., 2004). Roughly half of the PPR proteins in plants consist of PPR tracts and little else; functions ascribed to these “pure” PPR proteins include enhancement of group II intron splicing, RNA stabilization, definition of processed RNA termini, translational activation and repression, and the promotion of site-specific RNA cleavages (reviewed in Barkan and Small, 2014). Several of these functions have been shown to result from passive steric effects caused by the high affinity binding of an RNA segment along the extended surface of a long PPR tract. For example, this type of activity defines and stabilizes specific processed RNA termini (Pfalz et al., 2009; Ruwe and Schmitz-Linneweber, 2012; Zhelyazkova et al., 2012; Fujii et al., 2013; Haili et al., 2013), and guides RNA folding in a manner that increases translational efficiency (Prikryl et al., 2011; Zoschke et al., 2013b). Another large subset of PPR proteins in plants harbor variant repeat tracts followed by characteristic carboxy-terminal domains that are required to direct sites of RNA editing in mitochondria and chloroplasts (reviewed in Yagi et al., 2013b).
A small PPR subfamily is characterized by a PPR tract followed by a carboxy-terminal Small MutS Related (SMR) domain (reviewed in Liu et al., 2013). These “PPR-SMR” proteins comprise approximately eight orthologous groups in angiosperms. The PPR-SMR subfamily has attracted considerable attention due to the physiological functions ascribed to several of its members. For example, GUN1 influences the transcription of nuclear genes in response to certain types of plastid dysfunction (Koussevitzky et al., 2007), albeit through unknown mechanisms. PTAC2 associates with the plastid-encoded RNA polymerase (PEP) and is required for its activity (Pfalz et al., 2006), but the basis for this effect is unknown. Orthologous proteins denoted SVR7 in Arabidopsis thaliana and ATP4 in Zea mays have overlapping but distinct effects on chloroplast gene expression. SVR7 was recovered in a genetic screen for suppressors of a leaf variegation phenotype (Liu et al., 2010). Loss of SVR7 function causes mild defects in plant growth, chloroplast rRNA processing, and the accumulation of the chloroplast ATP synthase (Liu et al., 2010; Zoschke et al., 2013a). By contrast, ATP4 is required for the translation of the atpB open reading frame (ORF) and for the accumulation of specific processed transcript isoforms from the atpF, psaJ, and rpl14 loci (Zoschke et al., 2012; Zoschke et al., 2013b). It has been shown that ATP4 associates in vivo with the 5′UTR of the atpB mRNA (Zoschke et al., 2012), but beyond that, mechanisms underlying these effects are unknown.
It seems likely that the SMR domain expands the functional repertoire of the PPR tract, but there is little information about the nature of its contribution. SMR domains in other contexts have been shown to harbor DNA or RNA endonuclease activity and to bind branched DNA structures (reviewed in Fukui and Kuramitsu, 2011). In addition, SMR domains are structurally similar to several known DNA and RNA binding domains (reviewed in Fukui and Kuramitsu, 2011). These findings provide a foundation for hypotheses concerning the functions of the SMR domain in PPR-SMR proteins, but alternative possibilities are also plausible.
To elucidate the functions of the SMR domain in PPR-SMR proteins, we studied the molecular functions of the maize PPR-SMR protein encoded by gene GRMZM2G438524. This protein, denoted here as PPR53, is orthologous to Arabidopsis SOT1 (AT5G46580). Our results indicate that PPR53 harbors several molecular functions that have been ascribed also to PPR proteins lacking an SMR domain: it can serve as a site-specific blockade to 5′→3′ RNA decay and it can stimulate the translation of a specific mRNA. We detected two sites of PPR53 action, one in a precursor to the 23S rRNA and the other in the ndhA 5′UTR. PPR53 is required for the accumulation of 23S rRNA precursors with a 5′ end mapping 73 nucleotides upstream of mature 23S rRNA. PPR53 binds directly to this site, as shown by in vitro RNA binding assays and by the fact that its sequence matches that predicted for the PPR53 binding site based on the PPR code. PPR53 also stabilizes RNAs with a processed 5′-end 66 nucleotides upstream of ndhA and increases the translational efficiency of the ndhA open reading frame (ORF). However, PPR53 did not bind with high affinity to the ndhA 5′UTR in vitro, and the sequence of the ndhA 5′UTR shows little similarity to PPR53’s predicted binding site. These features are reminiscent of an activity described for the PPR-SMR protein ATP4, which is required to support the action of the pure PPR protein PPR10 when it stabilizes the 3′-terminus of psaJ mRNA (Zoschke et al., 2012). As such, we suggest that PPR53 promotes the binding of a different, as yet unidentified protein to the ndhA 5′UTR, which acts directly to block a 5′→3′ exoribonuclease and to stimulate ndhA translation.
Results
Disruption of ppr53 causes a mild global loss of plastid-encoded proteins and a severe loss of the plastid NDH complex
PPR53 and its orthologs consist of a predicted chloroplast targeting sequence followed by a conserved segment lacking known functional motifs, eleven PPR motifs, and an SMR domain (Figure 1a and Figure S1 in Supporting Information). Two insertions in ppr53 were identified during the systematic sequencing of Mu transposon insertions in mutants in the Photosynthetic Mutant Library (Belcher et al., 2015), each of which cosegregated with a recessive mutation conditioning a range of chlorotic phenotypes (Figure 1a). Complementation crosses between plants that were heterozygous for each allele produced heteroallelic progeny with chlorotic phenotypes (Figure 1a), confirming that the mutant phenotypes result from disruption of the ppr53 locus. Both alleles are likely to be null alleles based on the positions of the insertions in the gene and the fact that mRNA derived from sequences flanking the insertions was below the limit of detection by RT-PCR (Figure 1b). The basis for the variability in phenotype is unknown, but might be due to the segregation of polymorphic modifier loci, to unusual sensitivity to local environmental conditions, or to stochastic effects early in development.
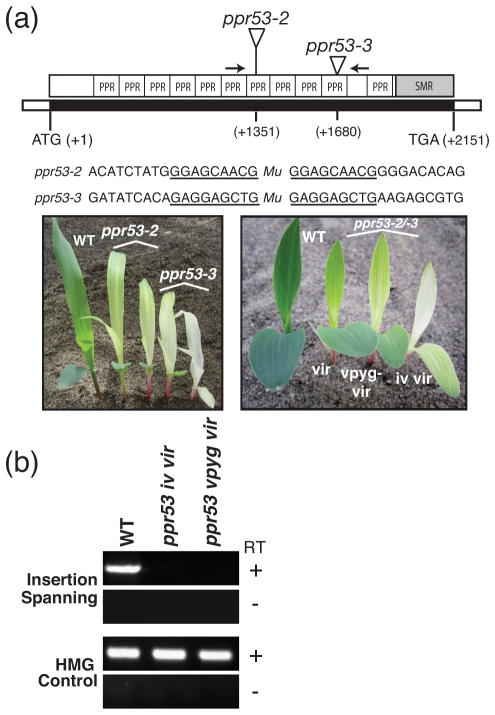
Figure 1. Overview of ppr53 mutant alleles.
(a) The ppr53 gene (locus GRMZM2G438524) lacks introns. Positions of the Mu insertions in alleles characterized here are diagrammed in the context of the domain architecture of the PPR53 protein. The sequence context of each insertion including the 9 bp target site duplication (underlined) and the phenotypes of the plants are shown below. Plants were grown for 8 days in soil. Plants that are homozygous for each single allele (left) as well as the heteroallelic progeny of complementation crosses (right) exhibit variable chlorotic phenotypes (vir-virescent; vpyg-very pale yellow green; iv-ivory). Arrows mark the positions of primers used for RT-PCR in panel (b).
(b) Reverse-transcriptase PCR demonstrating the absence of ppr53 mRNA in ppr53 mutants. Seedling leaf RNA from ppr53-2/ppr53-3 mutant individuals with the indicated pigment phenotypes (see panel a) was analyzed by RT-PCR, using primers that flank the insertions (see arrows in panel (a)). Reactions lacking reverse transcriptase (RT) and amplification of mRNA encoding High Mobility Group (HMG) protein (GRMZM2G024976) were performed as negative and positive controls, respectively.
Given the functions established for characterized PPR proteins and the fact that PPR53 is an abundant component of the chloroplast nucleoid (Majeran et al., 2012), it seemed likely that the chlorotic phenotypes of ppr53 mutants result from a defect in chloroplast gene expression. As a first step toward characterizing chloroplast gene expression in ppr53 mutants, immunoblots of seedling leaf extracts were probed to detect core subunits of each photosynthetic complex harboring plastid-encoded proteins: photosystem I (PSI), photosystem II (PSII), the cytochrome b6f complex, the ATP synthase, the NADH dehydrogenase-like complex (NDH), and Rubisco (Figure 2). The failure to synthesize or assemble any core subunit of these complexes is generally accompanied by a reduction in the levels of closely-associated subunits. Mutant ppr53 individuals exhibiting a range of chlorotic phenotypes were compared with a mutant lacking ATP4, which is the most closely related paralog of PPR53 (Liu et al., 2013) (see Figure S1). As shown previously (Zoschke et al., 2012), atp4 mutants have a strong and rather specific loss of ATP synthase subunits (AtpB and AtpF). By contrast, ppr53 mutants displayed a severe deficiency for NdhH, a subunit of the NDH complex. This strong NDH defect is superimposed on a moderate loss of components of the ATP synthase, PSII, PSI, cytochrome b6f, and Rubisco. The latter deficiencies ranged from roughly 25%–50% of normal levels, with the severity of the protein defects correlating with that of the chlorotic phenotype. Taken together, these results suggested that PPR53 plays a critical role in the expression of at least one subunit of the NDH complex, and that it also boosts overall plastid gene expression.
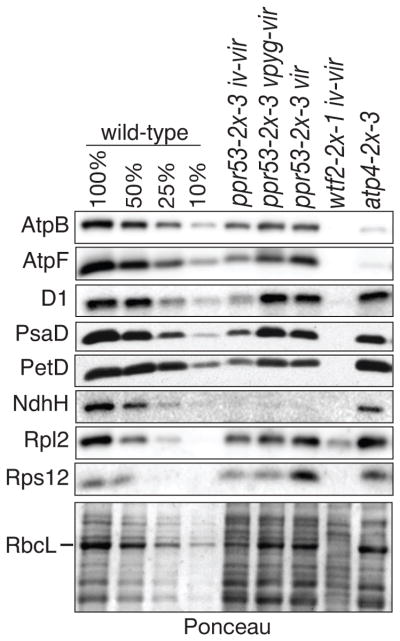
Figure 2. Immunoblot analysis of core subunits of photosynthetic complexes in ppr53 mutants.
Replicate immunoblots of seedling leaf extract (5 μg protein or the indicated dilutions) were probed with antibodies specific for subunits of the ATP synthase (AtpB, AtpF), photosystem II (D1), photosystem I (PsaD), the cytochrome b6f complex (PetD), the NADH dehydrogenase (NdhH), and the large and small ribosomal subunits (Rpl2 and Rps12, respectively). RbcL (the large subunit of Rubisco) can be seen on the Ponceau S-stained blot below. A mutant lacking the PPR53 paralog ATP4 (Zoschke et al., 2012) and a mutant with a plastid ribosome deficiency similar in magnitude to ppr53 mutants (wtf2, whose ribosome deficiency is documented below) were included for comparison. Mutant samples are annotated with their pigment phenotypes as defined in Figure 1a.
Genome-wide analyses of the plastid transcriptome and translatome in ppr53 mutants revealed defects in ndhA and rrn23 expression
To further define the effects of PPR53 on plastid gene expression, the expression of all chloroplast genes in ppr53 mutants was analyzed with a ribosome profiling method that provides a genome-wide, quantitative read-out of mRNA abundance and ribosome occupancy (Zoschke et al., 2013b). The method employs high-resolution microarrays to quantify “ribosome footprints” (mRNA segments that are protected by ribosomes from nuclease attack) derived from all chloroplast open reading frames, at a resolution of ~30 nucleotides. The ratios of ribosome footprint signals in the wild-type relative to the ppr53 mutant are plotted according to position on the chloroplast genome in Figure 3b. A prominent peak was detected that mapped to ndhA, indicating that ppr53 mutants have a strong defect in ndhA expression. A modest decrease in expression of the adjacent downstream gene ndhI is apparent in a high-resolution view of the data (Figure 3e). The abundance of ribosome footprints on other genes encoding NDH subunits was similar to that in the wild-type. The loss of NdhH protein in ppr53 mutants can be explained by the defect in ndhA expression, as NdhA and NdhH belong to NDH subcomplex A, whose members fail to accumulate when one subunit is not synthesized or assembled (reviewed in Ifuku et al., 2011).
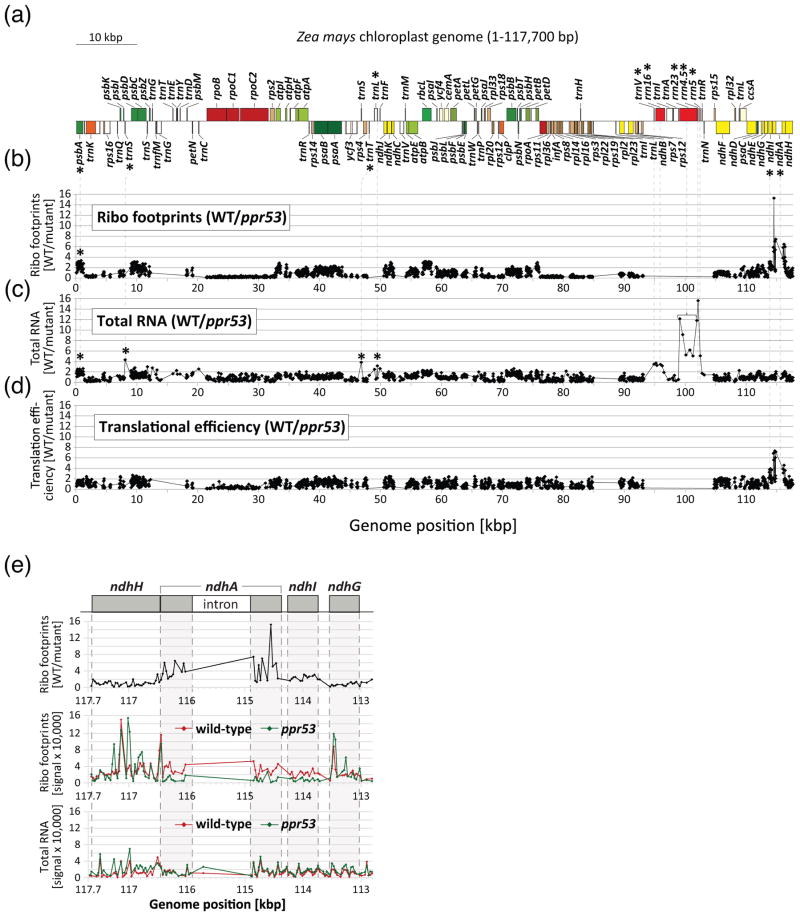
Figure 3. Genome-wide analyses of the chloroplast translatome and transcriptome in ppr53 mutants. The data used to generate these plots are provided in Data S1.
(a) Map of the maize chloroplast genome showing just one of the two large inverted repeats. Asterisks mark genes showing reduced expression in ppr53 mutants.
(b) Ratio of ribosome footprint signals in the wild-type relative to ppr53 mutants. Heteroallelic progeny of complementation crosses with a mild pigment phenotype (vir seedlings in Figure 1a) were used for these experiments.
(c) Ratio of RNA abundance in the wild-type relative to ppr53 mutants. RNA extracted from an aliquot of the material used for ribosome profiling was hybridized to a replicate microarray.
(d) Relative translational efficiencies were calculated as the ratio of ribosome footprint ratios to the total RNA ratios.
(e) High resolution view of data from the ndhA region. The upper panel shows the ratio of ribosome footprint signal in wild-type relative to the mutant, whereas the lower panels show the individual wild-type and mutant values for both ribosome footprints and total RNA. Two regions in ndhH show much stronger ribosome footprint signal in the mutant than in the wild-type (see middle panel); this was a reproducible finding but its basis is unknown. Data points from the ndhA intron are sparse (RNA) or absent (ribosome footprints) due to sparse coverage on the array and low signal.
Hybridization of seedling leaf RNA from wild-type and mutant seedlings to the same microarray format (Figure 3c) showed that ndhA RNA accumulates to near normal levels in ppr53 mutants, indicating that the defect in ndhA expression is largely due to decreased translational efficiency (Figure 3d). In addition, the transcriptome data revealed a substantial decrease in the abundance of rRNAs of the large ribosomal subunit (23S, 4.5S and 5S). Several other minor differences between the mutant and wild-type transcriptomes were observed (e.g. a slight loss of psbA RNA, 16S rRNA and several tRNAs), but similar changes have been observed in other mutants with modest chloroplast ribosome deficiencies (Williams-Carrier et al., 2014) suggesting that these are secondary effects of the reduced ribosome content in ppr53 mutant chloroplasts (see below).
PPR53 is required for the accumulation of RNAs with processed 5′-ends upstream of ndhA and rrn23
Several PPR proteins that activate the translation of specific chloroplast RNAs also stabilize processed mRNAs from the same gene (Barkan et al., 1994; Pfalz et al., 2009; Cai et al., 2011; Zoschke et al., 2013b). To determine whether PPR53 has dual effects of this nature, transcripts from the ndhA transcription unit were analyzed by RNA gel blot hybridization (Figure 4). The ndhA gene includes a group II intron and is embedded in a polycistronic transcription unit that gives rise to a complex population of processed RNAs (see, for example, del Campo et al., 2000). The transcripts from the ndhA transcription unit have not been comprehensively mapped in any species. We inferred the positions of some transcripts based on their size, the probes to which they hybridized, and the positions of processed termini that have been mapped in barley and/or maize (Zhelyazkova et al., 2012) (Figure 4a). However, the large size and comigration of many of the transcripts precluded their firm placement on the map.
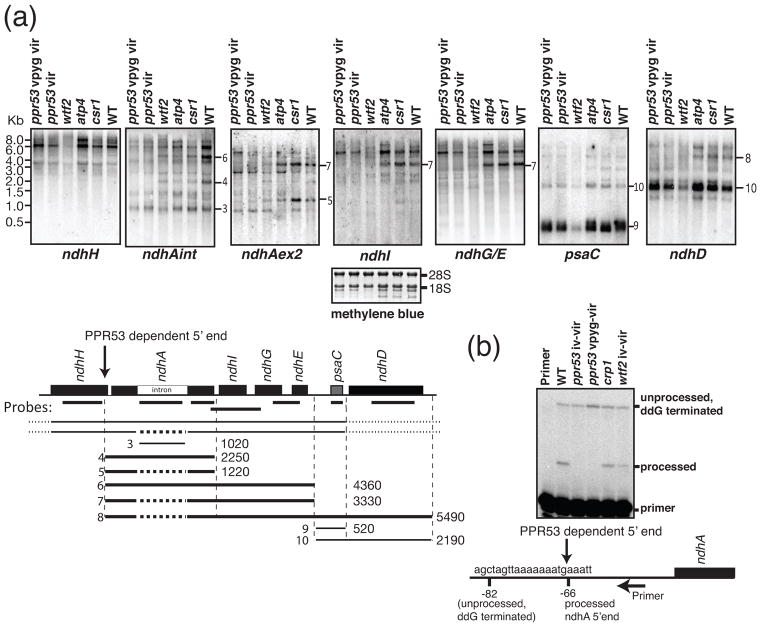
Figure 4. Analysis of ndhA transcripts in ppr53 mutants.
(a) RNA gel blot analysis of transcripts from the ndhA transcription unit. Replicate blots of seedling leaf RNA (5 μg) were hybridized to the probes indicated on the map below. Two heteroallelic ppr53 individuals with distinct pigment phenotypes (vpyg vir and vir; see Figure 1) were analyzed. RNA from atp4, wtf2, and csr1 mutants was analyzed for comparison; wtf2 mutants have a plastid rRNA deficiency that is similar in magnitude to that of ppr53 mutants (see Figure 5), whereas csr1 mutants have pleiotropic losses of thylakoid membrane complexes due to the absence of the chloroplast SRP receptor (Asakura et al., 2004). A representative methylene blue stained blot is shown below as a loading control. Transcripts whose positions could be confidently assigned based on these results are diagrammed below the map, annotated with their approximate length in nucleotides; thick lines denote transcripts that are missing in ppr53 mutants. Spliced transcripts are illustrated with a dashed line in the place of the ndhA intron.
(b) Poisoned-primer extension assay to display the ratio of processed to unprocessed transcripts with a 5′ end 66 nucleotides upstream of ndhA. A primer mapping a short distance downstream of the processed 5′ end was used in a reverse transcription reaction with seedling leaf RNA. Dideoxyguanosine (ddG) was included to terminate reverse transcription on unprocessed RNA at the first cytidine residue upstream of the processed end.
Despite this limitation, the results show that several ndhA transcript isoforms were missing in ppr53 mutants but accumulate normally in other non-photosynthetic mutants that were analyzed as controls (Figure 4a). This is not due to the failure to splice the ndhA intron because the excised intron accumulated to normal levels in ppr53 mutants (see transcript 3 in Figure 4a), and both spliced and unspliced transcript isoforms were missing (see for example transcripts 4, 5, and 6 in Figure 4a). Instead, the results suggested that PPR53 is required for the accumulation of transcripts with a 5′ end mapping a short distance upstream of the ndhA gene (see transcripts 4 through 8 in Figure 4a). A processed 5′ end mapping 66 nucleotides upstream of ndhA has been mapped in barley and maize (Zhelyazkova et al., 2012), and the RNA gel blot data suggested that transcripts with this end require PPR53 for their accumulation. To test this possibility, we used a poisoned-primer extension assay, which compiled all 5′-processed ndhA transcript isoforms into one band, and all isoforms with distal 5′ ends into a second band (Figure 4b). As predicted, transcripts with the −66 5′ terminus were undetectable in ppr53 mutants but accumulated normally in two other non-photosynthetic mutants. The loss of these processed RNAs was not accompanied by an increase in precursors, suggesting that PPR53 functions to stabilize the processed RNAs rather than to promote processing. The poisoned-primer extension data show that approximately 50% of ndhA transcripts have the PPR53-dependent 5′ end in wild-type plants. The two-fold loss of ndhA RNA in ppr53 mutants was not of sufficient magnitude to stand out in the microarray transcriptome data.
Analogous experiments were used to characterize the rRNA deficiencies detected in the transcriptome analysis. RNA gel blot hybridizations confirmed that all plastid rRNAs accumulate to reduced levels in ppr53 mutants (Figure 5a). The probe for rrn4.5 and the more downstream of two rrn23 probes detected a (presumed) precursor of approximately 2 kb that accumulates to increased levels in ppr53 mutants but not in a different mutant with a plastid rRNA deficiency of similar magnitude (wtf2). The Arabidopsis PPR53 ortholog SOT1 is required for the accumulation of a 23S rRNA precursor with a processed 5′ end mapping 73-nucleotides upstream of the mature 23S rRNA (Ian Small, personal communication). A primer extension assay showed that the pre-23S rRNA isoform with this 5′-end is likewise missing in ppr53 mutants (Figure 5b). This isoform accumulates to normal levels in mutants lacking the closely related protein ATP4, and it is found at elevated levels in the wtf2 mutant control (see Figure 5a). Interestingly, the PPR53-dependent pre-23S 5′ end was undetectable even in mutants with the mildest visual phenotype (ppr53-vir) (Figures 1a and 5b) whereas the severity of the deficiency for mature rRNAs correlated with the severity of the chlorophyll deficiency (Figure 5a). This suggests that the absence of this 23S rRNA precursor is not the sole cause of the loss of plastid ribosomes in ppr53 mutants.
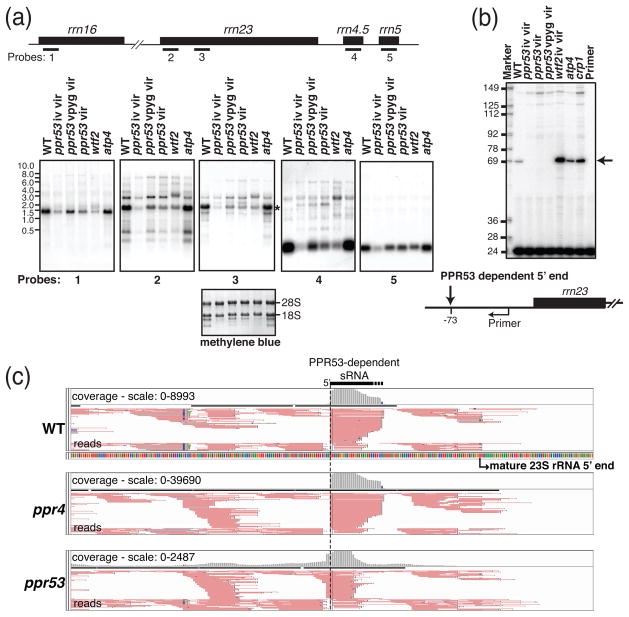
Figure 5. Analysis of chloroplast rRNAs in ppr53 mutants.
(a) RNA gel blot analysis of transcripts from the rrn transcription unit. Replicate blots of seedling leaf RNA were hybridized to the probes indicated on the map. Three heteroallelic ppr53 individuals with distinct pigment phenotypes (iv vir, vpyg vir and vir; see Figure 1) were analyzed. RNAs from the non-photosynthetic mutants atp4 and wtf2 were analyzed for comparison. The wtf2 mutant is shown to illustrate pleiotropic effects resulting from the loss of plastid ribosomes. An image of one of the blots stained with methylene blue is shown below to illustrate equal loading of cytosolic 28S and 18S rRNAs.
(b) Primer extension assay to quantify processed transcripts with a 5′-end mapping 70 nucleotides upstream of rrn23. A 5′-end labeled 24-nucleotide primer starting four nucleotides upstream of mature 23S rRNA was used to prime reverse transcription on 5 μg seedling leaf RNA. The ribosome-deficient mutant wtf2 (see panel a) as well as two other non-photosynthetic mutants (atp4 and crp1) accumulate increased levels of the −70 23S rRNA precursor (see arrow), whereas it is undetectable in all three ppr53 mutant individuals. The abundance of longer rrn23 processing intermediates is similar in all mutant samples analyzed, as shown also on the RNA gel blot in panel a (see probe 2 data).
(c) PPR53-dependent sRNA mapping to the 5′ end of the PPR53-dependent rrn23 precursor. Screen captures from the Integrated Genome Viewer show reads as pink lines and a histogram of read counts in gray (above). The 5′-end of the PPR53-dependent sRNAs corresponds with that of the PPR53-dependent pre-23S rRNA. sRNA reads from flanking regions serve as internal standards. Data from a ppr4 mutant are shown to control for effects resulting from the loss of plastid ribosomes. The loss of this PPR53-dependent sRNA is accompanied by an increase in the abundance of an sRNA with a 5′-end 2-nucleotides upstream. The basis for this effect is unknown.
Recombinant PPR53 binds with specificity to a sequence near the 5′ end of the PPR53-dependent pre-23S rRNA
The “footprints” of some PPR proteins accumulate as small RNAs (sRNAs) in vivo due to protection by the bound protein (Ruwe and Schmitz-Linneweber, 2012; Zhelyazkova et al., 2012). An abundant sRNA in wild-type seedlings has a 5′-end matching that of the PPR53-dependent 5′-end upstream from rrn23 (Figure 5c). This sRNA is missing in ppr53 mutants (Figure 5c) but accumulates to high levels in ppr4 mutants, which have a plastid ribosome deficiency of similar magnitude (Schmitz-Linneweber et al., 2006). These results suggested that this sRNA is PPR53’s in vivo footprint. Furthermore, the amino acid code for RNA recognition by PPR motifs (Barkan et al., 2012; Yagi et al., 2013a) predicts that the first nine contiguous PPR motifs in PPR53 will bind the sequence UGGAYGUAG (Figure 6a). A very similar sequence, UGGACGUUG, maps near the 5′end of the PPR53-dependent sRNA and pre-23S rRNA (Figure 6b), making this an excellent candidate for a direct PPR53 binding site.
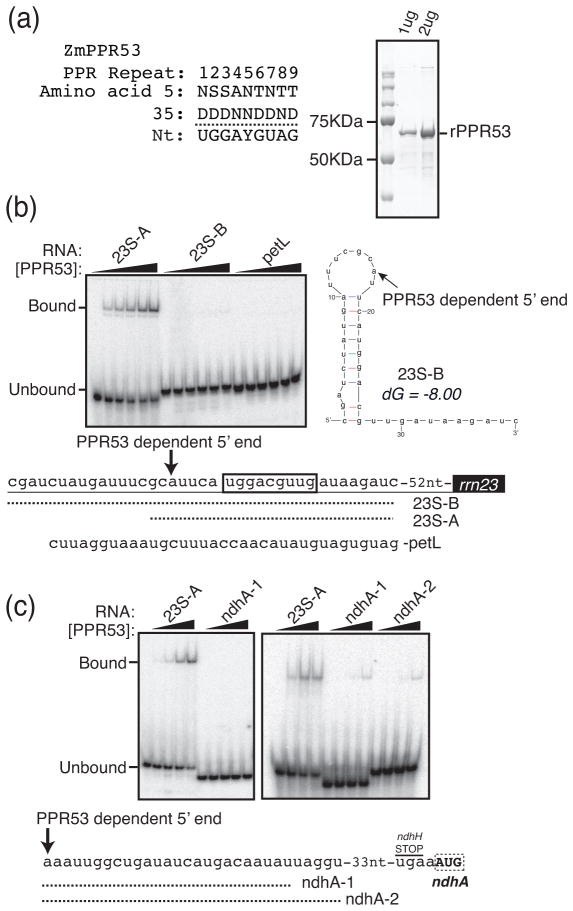
Figure 6. RNA binding activities of recombinant PPR53.
(a) Predicted PPR53 sequence specificity. The identities of the fifth and 35th amino acid in each of the first nine PPR motifs in PPR53 are shown (amino acid numbering scheme of (Yin et al., 2013)), along with the predicted nucleotide specificity of each repeat (Barkan et al., 2012). The 10th PPR motif was not included in the prediction because its highly degenerate sequence does not suggest a nucleotide preference based on current understanding of the PPR code. The final PPR motif, which sits adjacent to the SMR domain, was not considered because it is not contiguous with the first ten motifs. A Coomassie-stained SDS-polyacrylamide gel of recombinant PPR53 (rPPR53) is shown to the right.
(b) Gel mobility shift assay showing interaction between rPPR53 and an RNA mapping to the 5′ proximal region of the PPR53-dependent pre-23S rRNA isoform. The sequences of the three radiolabeled RNAs are shown below. The predicted binding site of PPR53 is boxed and the position of the PPR53-dependent 5′ end is marked with an arrow. Reactions contained 100 pmol RNA and protein at 0 nM, 12 nM, 37 nM, 110 nM, 330 nM, or 1 μM. The predicted secondary structure (Mfold, with default parameters) of the 23S-B RNA is shown to the right. (c) Gel mobility shift assays to detect an interaction between PPR53 and the ndhA 5′UTR. Reactions contained 100 pmol RNA and protein as in (b) except that the 12 nM dilution was omitted. The sequences of the two ndhA RNAs are shown below. These RNAs correspond to short and long versions of an sRNA detected in barley (Zhelyazkova et al., 2012) and maize (Figure S2). All binding assays were performed under identical conditions with one exception: binding assays shown in the right panel of Figure 6c included 3 mM MgCl2 whereas the other assays lacked MgCl2.
To test whether PPR53 can bind with specificity to this RNA sequence, purified recombinant PPR53 (rPPR53) was used in gel mobility shift assays with various radiolabeled synthetic RNAs (Fig. 6b). PPR53 bound a 24-mer that included its predicted binding site (RNA 23S-A) but did not bind an RNA of similar length from the petL 5′UTR that is bound by the PPR protein PGR3 (Cai et al., 2011). This biochemical data, in conjunction with the facts that this RNA includes a strong match to PPR53’s predicted binding site and coincides with a PPR53-dependent sRNA and processed 5′ end, provide strong evidence that PPR53 binds this site in vivo. Interestingly, PPR53 failed to bind an RNA that extends 17 nucleotides upstream of the PPR53-dependent 5′ end (RNA 23S-B in Figure 6b). This longer RNA is predicted to fold into a stem-loop structure that includes the first six nucleotides of the predicted PPR53 binding site in the stem (Figure 6b). Given that sequence-specific interactions between PPR motifs and RNA involve the Watson-Crick face of RNA bases (Yin et al., 2013), it is not surprising that this hairpin impedes protein binding. That being said, the existence of this structure raises the question of how PPR53 gains access to its binding site in vivo.
PPR53 also acts at the ndhA locus, where it is required both for the accumulation of mRNAs with a processed end 66 nt upstream of the start codon and for optimal translational efficiency. An sRNA with hallmarks of a PPR footprint maps to the PPR53-dependent ndhA 5′ end (Ruwe and Schmitz-Linneweber, 2012; Zhelyazkova et al., 2012) and is missing in ppr53 mutants (Figure S2), suggesting that this may be a direct PPR53 binding site. However, the sequence of this sRNA lacks strong similarity to PPR53’s binding site as predicted by the PPR code (Fig. 6a,c). We tested the ability of rPPR53 to bind two synthetic RNAs corresponding to the shorter and longer of the sRNAs mapping to this region (Figure 6c), but neither RNA bound significantly to rPPR53 in vitro. These RNAs are not predicted to form stable secondary structures, so RNA-based masking of a binding site is unlikely to account for this negative result. In light of the positive binding results at rrn23, these results suggest that PPR53’s effects on ndhA expression and sRNA accumulation require the participation of another protein.
Discussion
Results presented here show that the PPR-SMR protein PPR53 plays an essential role in the biogenesis of the photosynthetic apparatus by promoting the expression of the chloroplast ndhA and rrn23 genes. PPR53 binds directly to a sequence ~70-nt upstream from the mature 5′ end of 23S rRNA and is required for the accumulation of a pre-23S rRNA isoform with a 5′ end several nucleotides upstream of this binding site. The failure of PPR53 to bind this site is likely to underlie the plastid rRNA deficiencies in ppr53 mutants, which, in turn, can account for their modest global losses of photosynthetic complexes harboring plastid-encoded proteins. PPR53 is also required for the accumulation of a processed mRNA with a 5′ end mapping 66 nucleotides upstream of ndhA and increases ndhA translational efficiency. This activity can account for the severe loss of the NDH-like complex in ppr53 mutants. Genome-wide ribosome profiling and transcriptome assays did not detect any additional chloroplast gene expression defects in ppr53 mutants, other than minor changes that are likely to be secondary effects. Thus, it seems likely that all aspects of the ppr53 mutant phenotype arise from the ndhA and rrn23 expression defects.
PPR53’s most closely related paralog, ATP4, has also been functionally characterized (Zoschke et al., 2012; Zoschke et al., 2013b). The PPR code predicts that ATP4 and PPR53 bind similar sequences (ATP4-UGGACXUAG; PPR53-UGGAYGUAG- see Figure S1). Nonetheless, the documented functions for these proteins are entirely different. ATP4 is required for the translation of the atpB mRNA and interacts in vivo with the atpB 5′UTR (Zoschke et al., 2012). ATP4 is also required for the accumulation of processed RNAs with termini near atpF, psaJ, and rpl14, but other chloroplast genes are expressed at near normal levels in atp4 mutants (Zoschke et al., 2012; Zoschke et al., 2013b). It is possible that both PPR53 and ATP4 have additional functions that are redundant and therefore not apparent in each single mutant. In any case, the distinct mutant phenotypes of atp4 and ppr53 mutants highlight the challenges of connecting PPR proteins to their in vivo binding sites and functions based solely on current understanding of the amino acid code for RNA recognition by PPR tracts.
Mechanism of PPR53 action at rrn23
Our results strongly suggest that PPR53 promotes the biogenesis of the chloroplast 50S ribosomal subunit by binding an RNA sequence spanning approximately −69 to −53 with respect to the 5′ end of mature 23S rRNA. However, it remains unclear how this interaction increases the output of mature ribosomes. PPR53 is a plant-specific protein, but it acts in the context of a ribosome maturation pathway that retains considerable similarity to that in the chloroplast’s bacterial ancestor. As in bacteria, chloroplast rRNAs are processed from a single large precursor (reviewed in Deutscher, 2009; Germain et al., 2013). The order of rRNA genes in the chloroplast genome is the same as that in its bacterial ancestor except that the chloroplast 23S and 4.5S rRNAs are derived from the 5′ and 3′ regions, respectively, of the ancestral 23S rRNA (Edwards and Kossel, 1981). In bacteria, rRNA processing is guided by sequences flanking each mature rRNA, which form long-range duplexes that are cleaved by enzymes in the ribonuclease III family (reviewed in Redko et al., 2008; Deutscher, 2009). Additional processing events are carried out by various endo- and exo-ribonucleases, and the final maturation steps are coupled to ribosome assembly and to translational activity itself.
In chloroplasts, a ribonuclease III-family enzyme called Mini-III is believed to cleave a 23S-4.5S rRNA precursor to simultaneously produce the mature 5′ end of 23S rRNA and the 3′ end of 4.5S rRNA (Hotto et al., 2015), in a process that is similar to the final steps in 23S rRNA processing in B. subtilis (Redko et al., 2008). The substrate for this cleavage event in chloroplasts is believed to be an RNA duplex of ~20 base pairs formed by complementary sequences in the 5′-proximal region of 23S rRNA and 3′ proximal region of 4.5S rRNA (Takaiwa and Sugiura, 1982; Hotto et al., 2015). As PPR53 binds approximately 30 nucleotides upstream from the sequences involved in this proposed duplex, it is well placed to influence this step in rRNA maturation. This arrangement is reminiscent of that reported for the octotricopeptide repeat protein RAP, which binds upstream of the mature 5′ end of chloroplast 16S rRNA (Kleinknecht et al., 2014) and promotes the accumulation of 30S ribosomal subunits.
How might PPR53 promote the maturation and/or accumulation of 23S rRNA? One consequence of PPR53’s binding upstream of rrn23 is clear: this interaction provides a steric block to a 5′→3′ exonuclease, thereby stabilizing a pre-23S rRNA isoform with a 5′ end at position −69. This view is supported by: (i) the position of the PPR53 binding site immediately downstream of the PPR53-dependent processed 5′ end; (ii) the presence of a PPR53-dependent sRNA spanning the binding site; and (iii) the fact that longer precursors do not accumulate to increased levels in ppr53 mutants. It is well established that some pure PPR proteins promote the accumulation of processed mRNA isoforms via an analogous barrier mechanism (reviewed in Barkan and Small, 2014). That said, the relationship between PPR53’s RNA stabilization effect and its effect on the accumulation of 50S ribosomal subunits remains uncertain. One possibility is that the pre-23S rRNA isoform that is stabilized by PPR53 is an important intermediate along the rRNA processing pathway. Arguing against this possibility is the fact that this precursor appears to be completely absent in homozygous mutants that exhibit a range of ribosome and pigment deficiencies (see Figures 1 and 5). An alternative possibility is that PPR53 influences the formation of an RNA duplex that guides the concerted 5′ processing of 23S rRNA and 3′ processing of 4.5S rRNA. The 5′ and 3′ portions of this duplex are separated by more than 3000 nucleotides. As such, PPR53 might protect the 5′ proximal region from degradation until the 3′ portion is available for pairing, or it might maintain the 5′ proximal sequences in an unpaired state prior to the availability of its 3′ partner. The RNA stabilization and RNA remodeling scenarios for PPR53 are not mutually exclusive.
How does PPR53 stimulate ndhA expression?
PPR53 is required to stabilize transcripts with a 5′-end mapping 66 nucleotides upstream from the ndhA start codon, and also increases ndhA translational efficiency. Dual RNA stabilization/ translation enhancing effects have been reported for several other helical repeat RNA binding proteins in chloroplasts (Barkan et al., 1994; Vaistij et al., 2000; Felder et al., 2001; Pfalz et al., 2009; Boulouis et al., 2011; Cai et al., 2011; Zoschke et al., 2013b; Lefebvre-Legendre et al., 2015). Several of these proteins have been shown to bind to an RNA segment mapping immediately downstream of the 5′ end they stabilize (Pfalz et al., 2009; Cai et al., 2011; Prikryl et al., 2011; Hammani et al., 2012; Loizeau et al., 2013) and to protect the bound RNA from ribonucleases in vivo, as reflected by the accumulation of their “footprints” as sRNAs (Hammani et al., 2012; Ruwe and Schmitz-Linneweber, 2012; Zhelyazkova et al., 2012; Loizeau et al., 2013). The RNA stabilization activity of such proteins results from a steric blockade to 5′→3′ exonucleolytic degradation. An sRNA maps to the 5′ end of PPR53-dependent ndhA transcripts (Ruwe and Schmitz-Linneweber, 2012; Zhelyazkova et al., 2012) and fails to accumulate in ppr53 mutants (Figure S2). The simplest interpretation of these results is that PPR53 binds to the region corresponding to this sRNA and that this interaction simultaneously blocks 5′→3′ degradation and enhances translation. However, there is minimal sequence similarity between the ndhA sRNA and the PPR53 binding site as predicted by the PPR code (Figure 6). Furthermore, recombinant PPR53 did not bind this sequence in vitro under conditions in which its interaction with the site upstream of rrn23 was unambiguous. Given this body of data, we favor the possibility that a different, as yet unknown PPR-like protein binds to and protects the 5′proximal region of processed ndhA RNA, and that PPR53 promotes this interaction. There is evidence that the PPR-SMR protein ATP4 cooperates with a pure PPR protein in an analogous manner: PPR10 binds the psaJ 3′UTR where it blocks 3′→5′ RNA degradation (Pfalz et al., 2009; Prikryl et al., 2011), and ATP4 is required for PPR10’s activity at this site in vivo (Zoschke et al., 2012). This cooperation might involve direct protein-protein interactions. Alternatively, the PPR-SMR partner might aid RNA binding by the second protein via transient RNA interactions that reduce occluding secondary structure. In any case, these findings imply that the loss of a particular sRNA in a mutant lacking a PPR-like protein does not always reflect the existence of a direct binding site for that protein within the sRNA sequence.
PPR53 enhances ndhA translational efficiency while also stabilizing the −66 processed ndhA 5′ end. Several other helical repeat RNA binding proteins acting at distinct chloroplast loci have analogous dual effects on the accumulation of specific processed RNAs and translational efficiency (Barkan et al., 1994; Felder et al., 2001; Pfalz et al., 2009; Cai et al., 2011; Zoschke et al., 2013b; Wang et al., 2015). These correlations can be explained in two ways: either the RNA isoforms that are stabilized by these proteins are intrinsically more translatable than are their precursors, or the presence of the protein a short distance upstream of the start codon stimulates translation. Although we cannot distinguish between these possibilities for PPR53, biochemical evidence supports the latter view for PPR10 and HCF107, whose binding upstream of atpH and psbH, respectively, precludes the formation of secondary structures that would otherwise occlude the ribosome binding sites (Prikryl et al., 2011; Hammani et al., 2012).
Function of the SMR domain?
A prime motivation for this study was to gain insight into the function of the SMR motif in PPR-SMR proteins. PPR53’s stabilization of pre-23S rRNA can be accounted for by a passive barrier effect analogous to that of pure PPR proteins. However, PPR53’s effects at ndhA and the effects of its paralog ATP4 at psaJ cannot be explained by activities attributed thus far to pure PPR proteins. Some SMR domains have endonuclease activity (reviewed in Fukui and Kuramitsu, 2011; Liu et al., 2013) but the molecular defects in ppr53 mutants do not provide evidence for an endonuclease activity in PPR53. Furthermore, we did not detect endoribonuclease activity when recombinant PPR53 was incubated with radiolabeled RNAs in vitro (see unbound RNAs in Figure 6, for example). One possible theme to emerge from the limited body of molecular data for PPR-SMR proteins is suggested by the ability of both ATP4 and PPR53 to support the action of a different protein as an RNA stabilizer (see above). Biochemical analysis of these and other examples will likely be required to elucidate the functions of the enigmatic SMR moiety of proteins in the PPR-SMR subfamily.
Experimental Procedures
Plant material
PPR53 corresponds to maize locus GRMZM2G438524 and is orthologous to Arabidopsis AT5G46580. The ppr53 mutant alleles were identified during the systematic sequencing of Mu transposon insertions in non-photosynthetic mutants in the maize Photosynthetic Mutant Library (Belcher et al., 2015). Molecular analyses used the heteroallelic progeny of complementation crosses between ppr53-2/+ and ppr53-3/+ plants. Phenotypically wild-type siblings segregating in the same plantings served as controls. Mutants lacking the closely related protein ATP4 (GRMZM2G128665) (Zoschke et al., 2012; Zoschke et al., 2013b) were included in some assays to illustrate the distinct functions of PPR53 and ATP4. A different ribosome deficient mutant, wtf2, was used to control for secondary effects that result from the loss of plastid ribosomes. csr1 mutants, which lack the thylakoid SRP receptor (Asakura et al., 2004), and crp1 mutants, which lack a PPR protein required for the expression of several chloroplast ORFs (Zoschke et al., 2013b), were used as additional controls. Plants were grown in soil in cycles of 16 h light (~300 μmol photons × m−2 × s−1)/28 °C and 8 h dark/26°C. Leaf tissue was harvested one hour after the start of the light cycle on the eighth day after sowing, frozen in liquid nitrogen and stored at −80 °C until use.
RNA and protein analyses
RNA was extracted from the second leaf and used for RNA gel blot, primer extension and poisoned primer extension analyses as described (Barkan, 1998; Barkan, 2011b). Probes used for these experiments are shown in Table S1. Proteins were extracted from the apical half of the second leaf. Antibody to Rps12 was purchased from Agrisera (AS12-2114). Antibodies to NdhH and Rpl2 were generously provided by Tsuyoshi Endo (Kyoto University) and Alap Subramanian (University of Arizona), respectively. Other antibodies were generated by us and have been described previously (Zoschke et al., 2012).
Genome wide analyses of the chloroplast transcriptome, translatome, and sRNAs
The ribosome profiling and transcriptome assays were performed as described previously (Zoschke et al., 2013b). The total RNA and ribosome footprint plots shown in Figure 3 are based on two and three biological replicates, respectively. Each biological replicate was analyzed on microarrays with three replicate spots for each probe. Only probes with at least five (of nine) spots (ribosome profiles) or three (of six) spots (total RNA) that passed the background filter (signal>background) are presented in the Figure. Data were normalized as described previously (Zoschke et al., 2013b). The normalized data are available in Dataset S1. The sRNA data were derived from RNAs between ~15 and 40 nucleotides that were purified from seedling leaf RNA. Sequencing libraries were prepared with the NEBNext Multiplex Small RNA Library Prep Set.
In vitro RNA binding assays
The protein coding region of ppr53 (GRMZM2G438524) minus that encoding the predicted chloroplast targeting sequence was cloned into the pMAL-TEV vector to produce a fusion protein consisting of an N-terminal maltose binding protein, a TEV protease site, and PPR53 starting at amino acid 53. The amino terminus of the protein after TEV cleavage begins “GSPSLSQ...”, with the G derived from the vector and the subsequent amino acids from PPR53. Protein was expressed in Arctic Express cells (Agilent). Protein was induced in log phase cultures with 0.5 mM IPTG at 12 °C for 24 h. Purification on amylose beads, TEV cleavage, and gel filtration chromatography on a Superdex 200 column were as described for PPR10 (Pfalz et al., 2009) with the exception that the lysis and column buffer consisted of 40 mM Tris-HCl pH 7.5, 600 mM NaCl and 5 mM β-mercaptoethanol. Lysis buffer additionally contained a protease inhibitor cocktail (Roche complete, EDTA free). Monodisperse protein from the gel filtration column was dialyzed against 25 mM Tris-HCl pH 7.5, 0.5 mM EDTA, 400 mM NaCl, 5 mM β-mercaptoethanol and 50% glycerol and stored at −20°C. Gel mobility shift assays employed synthetic RNAs (IDT) that were radiolabeled at their 5′-end as described previously (Williams-Carrier et al., 2008). The binding reactions contained 50 mM Tris-HCl pH 7.5, 180 mM NaCl, 0.1 mM EDTA, 3 mM DTT, 100 μg/ml Heparin, 20 μg/ml BSA, 10% Glycerol and a maximal protein concentration of 1 μM with 3-fold serial dilutions. Because some SMR domains bind divalent cations, we examined RNA binding and RNA cleavage activities of rPPR53 in the presence and absence of Mg++, but no differences in activities were detected. Mg++ was omitted in the experiments shown here, except in the right panel of Figure 6c in which rPPR53 was preincubated on ice with 15 mM MgCl2 and used in the RNA binding reactions at a final concentration of 3 mM MgCl2.
Supplementary Material
Loss of sRNAs mapping to the PPR53-dependent RNA terminus upstream of ndhA.
Comparison of PPR53 and ATP4 orthologs in maize and Arabidopsis
Table S1. Primers used to generate probes for RNA gel blots and for RT-PCR.
Data S1. Normalized values for ribosome profiling and transcriptome data plotted in Figure 3.
Significance Statement.
PPR-SMR proteins are nucleic acid-binding proteins found in plant chloroplasts and mitochondria. Members of this protein family have diverse effects on plant physiology, but little is known about their direct molecular activities. Here we show that one PPR-SMR protein is a sequence-specific RNA binding protein that promotes the expression of two chloroplast genes by influencing the stability, processing, and translation of their RNAs.
Acknowledgments
We are grateful to Tiffany Kroeger for technical assistance, Roz Williams-Carrier for identifying insertions in ppr53, Susan Belcher for mutant propagation, Margarita Rojas for preparing figures, and Ian Small and Kate Howell (University of Western Australia) for helpful discussions and sharing of data. This work was supported by grants from the National Science Foundation to A.B. (MCB-1243641 and IOS-1339130), by a postdoctoral fellowship from the Deutsche Forschungsgemeinschaft to R.Z. (Grants Zo 302/1-1 and Zo 302/2-1), and by NIH training grant 7T32GM007759 (R.G.M.).
Contributor Information
Reimo Zoschke, Email: reimo.zoschke@gmx.de.
Kenneth P. Watkins, Email: watkins@uoregon.edu.
Rafael G. Miranda, Email: rafaelm@uoregon.edu.
References
- Asakura Y, Hirohashi T, Kikuchi S, Belcher S, Osborne E, Yano S, Terashima I, Barkan A, Nakai M. Maize mutants lacking chloroplast FtsY exhibit pleiotropic defects in the biogenesis of thylakoid membranes. Plant Cell. 2004;16:201–214. doi: 10.1105/tpc.014787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan A. Approaches to investigating nuclear genes that function in chloroplast biogenesis in land plants. Methods Enzymol. 1998;297:38–57. [Google Scholar]
- Barkan A. Expression of plastid genes: organelle-specific elaborations on a prokaryotic scaffold. Plant Physiol. 2011a;155:1520–1532. doi: 10.1104/pp.110.171231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan A. Studying the structure and processing of chloroplast transcripts. In: Jarvis P, editor. Chloroplast research in Arabidopsis: Methods and protocols. New York: Humana Press; 2011b. pp. 183–197. [DOI] [PubMed] [Google Scholar]
- Barkan A, Rojas M, Fujii S, Yap A, Chong YS, Bond CS, Small I. A combinatorial amino acid code for RNA recognition by pentatricopeptide repeat proteins. PLoS Genetics. 2012;8:e1002910. doi: 10.1371/journal.pgen.1002910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan A, Small I. Pentatricopeptide Repeat Proteins in Plants. Annu Rev Plant Biol. 2014;65:415–442. doi: 10.1146/annurev-arplant-050213-040159. [DOI] [PubMed] [Google Scholar]
- Barkan A, Walker M, Nolasco M, Johnson D. A nuclear mutation in maize blocks the processing and translation of several chloroplast mRNAs and provides evidence for the differential translation of alternative mRNA forms. EMBO J. 1994;13:3170–3181. doi: 10.1002/j.1460-2075.1994.tb06616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher S, Williams-Carrier R, Stiffler N, Barkan A. Large-scale genetic analysis of chloroplast biogenesis in maize. Biochim Biophys Acta. 2015;1847:1004–1016. doi: 10.1016/j.bbabio.2015.02.014. [DOI] [PubMed] [Google Scholar]
- Boulouis A, Raynaud C, Bujaldon S, Aznar A, Wollman FA, Choquet Y. The nucleus-encoded trans-acting factor MCA1 plays a critical role in the regulation of cytochrome f synthesis in Chlamydomonas chloroplasts. Plant Cell. 2011;23:333–349. doi: 10.1105/tpc.110.078170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Okuda K, Peng L, Shikanai T. PROTON GRADIENT REGULATION 3 recognizes multiple targets with limited similarity and mediates translation and RNA stabilization in plastids. Plant J. 2011;67:318–327. doi: 10.1111/j.1365-313X.2011.04593.x. [DOI] [PubMed] [Google Scholar]
- del Campo EM, Sabater B, Martin M. Transcripts of the ndhH-D operon of barley plastids: possible role of unedited site III in splicing of the ndhA intron. Nucleic Acids Res. 2000;28:1092–1098. doi: 10.1093/nar/28.5.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher MP. Maturation and degradation of ribosomal RNA in bacteria. Prog Molecular Biology and Translational Sci. 2009;85:369–391. doi: 10.1016/S0079-6603(08)00809-X. [DOI] [PubMed] [Google Scholar]
- Edwards K, Kossel H. The rRNA operon from Zea mays chloroplasts: nucleotide sequence of 23S rDNA and its homology with E.coli 23S rDNA. Nucleic Acids Res. 1981;9:2853–2869. doi: 10.1093/nar/9.12.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder S, Meurer J, Meierhoff K, Klaff P, Bechtold N, Westhoff P. The nucleus-encoded HCF107 gene of Arabidopsis provides a link between intercistronic RNA processing and the accumulation of translation-competent psbH transcripts in chloroplasts. Plant Cell. 2001;13:2127–2141. doi: 10.1105/TPC.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S, Sato N, Shikanai T. Mutagenesis of individual pentatricopeptide repeat motifs affects RNA binding activity and reveals functional partitioning of Arabidopsis PROTON gradient regulation3. Plant Cell. 2013;25:3079–3088. doi: 10.1105/tpc.113.112193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui K, Kuramitsu S. Structure and Function of the Small MutS-Related Domain. Molecular Biology International. 2011;2011:691735. doi: 10.4061/2011/691735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain A, Hotto AM, Barkan A, Stern DB. RNA processing and decay in plastids. Wiley Interdisciplinary Reviews RNA. 2013;4:295–316. doi: 10.1002/wrna.1161. [DOI] [PubMed] [Google Scholar]
- Haili N, Arnal N, Quadrado M, Amiar S, Tcherkez G, Dahan J, Briozzo P, Colas des Francs-Small C, Vrielynck N, Mireau H. The pentatricopeptide repeat MTSF1 protein stabilizes the nad4 mRNA in Arabidopsis mitochondria. Nucleic Acids Res. 2013;41:6650–6663. doi: 10.1093/nar/gkt337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammani K, Bonnard G, Bouchoucha A, Gobert A, Pinker F, Salinas T, Giege P. Helical repeats modular proteins are major players for organelle gene expression. Biochimie. 2014;100C:141–150. doi: 10.1016/j.biochi.2013.08.031. [DOI] [PubMed] [Google Scholar]
- Hammani K, Cook W, Barkan A. RNA binding and RNA remodeling activities of the Half-a-Tetratricopeptide (HAT) protein HCF107 underlie its effects on gene expression. PNAS. 2012;109:5651–5656. doi: 10.1073/pnas.1200318109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammani K, Giege P. RNA metabolism in plant mitochondria. Trends in Plant Sci. 2014;19:380–389. doi: 10.1016/j.tplants.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Hotto AM, Castandet B, Gilet L, Higdon A, Condon C, Stern DB. Arabidopsis Chloroplast Mini-Ribonuclease III Participates in rRNA Maturation and Intron Recycling. Plant Cell. 2015;27:724–740. doi: 10.1105/tpc.114.134452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifuku K, Endo T, Shikanai T, Aro EM. Structure of the chloroplast NADH dehydrogenase-like complex: nomenclature for nuclear-encoded subunits. Plant & Cell Physiol. 2011;52:1560–1568. doi: 10.1093/pcp/pcr098. [DOI] [PubMed] [Google Scholar]
- Kleinknecht L, Wang F, Stube R, Philippar K, Nickelsen J, Bohne AV. RAP, the sole octotricopeptide repeat protein in Arabidopsis, is required for chloroplast 16S rRNA maturation. Plant Cell. 2014;26:777–787. doi: 10.1105/tpc.114.122853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koussevitzky S, Nott A, Mockler TC, Hong F, Sachetto-Martins G, Surpin M, Lim J, Mittler R, Chory J. Signals from chloroplasts converge to regulate nuclear gene expression. Science. 2007;316:715–719. [PubMed] [Google Scholar]
- Lefebvre-Legendre L, Choquet Y, Kuras R, Loubery S, Douchi D, Goldschmidt-Clermont M. A nucleus-encoded chloroplast protein regulated by iron availability governs expression of the photosystem I subunit PsaA in Chlamydomonas reinhardtii. Plant Physiol. 2015;167:1527–1540. doi: 10.1104/pp.114.253906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Melonek J, Boykin LM, Small I, Howell KA. PPR-SMRs: Ancient proteins with enigmatic functions. RNA Biol. 2013;10:1501–1510. doi: 10.4161/rna.26172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yu F, Rodermel S. An Arabidopsis pentatricopeptide repeat protein, SUPPRESSOR OF VARIEGATION7, is required for FtsH-mediated chloroplast biogenesis. Plant Physiol. 2010;154:1588–1601. doi: 10.1104/pp.110.164111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loizeau K, Qu Y, Depp S, Fiechter V, Ruwe H, Lefebvre-Legendre L, Schmitz-Linneweber C, Goldschmidt-Clermont M. Small RNAs reveal two target sites of the RNA-maturation factor Mbb1 in the chloroplast of Chlamydomonas. Nucleic Acids Res. 2014;42:3286–3297. doi: 10.1093/nar/gkt1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurin C, et al. Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell. 2004;16:2089–2103. doi: 10.1105/tpc.104.022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeran W, Friso G, Asakura Y, Qu X, Huang M, Ponnala L, Watkins KP, Barkan A, van Wijk KJ. Nucleoid-enriched proteomes in developing plastids and chloroplasts from maize leaves: a new conceptual framework for nucleoid functions. Plant Physiol. 2012;158:156–189. doi: 10.1104/pp.111.188474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfalz J, Bayraktar O, Prikryl J, Barkan A. Site-specific binding of a PPR protein defines and stabilizes 5′ and 3′ mRNA termini in chloroplasts. EMBO J. 2009;28:2042–2052. doi: 10.1038/emboj.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfalz J, Liere K, Kandlbinder A, Dietz KJ, Oelmuller R. pTAC2, -6, and -12 are components of the transcriptionally active plastid chromosome that are required for plastid gene expression. Plant Cell. 2006;18:176–197. doi: 10.1105/tpc.105.036392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prikryl J, Rojas M, Schuster G, Barkan A. Mechanism of RNA stabilization and translational activation by a pentatricopeptide repeat protein. Proc Natl Acad Sci USA. 2011;108:415–420. doi: 10.1073/pnas.1012076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redko Y, Bechhofer DH, Condon C. Mini-III, an unusual member of the RNase III family of enzymes, catalyses 23S ribosomal RNA maturation in B. subtilis. Molecular Microbiology. 2008;68:1096–1106. doi: 10.1111/j.1365-2958.2008.06207.x. [DOI] [PubMed] [Google Scholar]
- Ruwe H, Schmitz-Linneweber C. Short non-coding RNA fragments accumulating in chloroplasts: footprints of RNA binding proteins? Nucleic Acids Res. 2012;40:3106–3116. doi: 10.1093/nar/gkr1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz-Linneweber C, Williams-Carrier RE, Williams-Voelker PM, Kroeger TS, Vichas A, Barkan A. A Pentatricopeptide Repeat Protein Facilitates the trans-Splicing of the Maize Chloroplast rps12 Pre-mRNA. Plant Cell. 2006;18:2650–2663. doi: 10.1105/tpc.106.046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small I, Peeters N. The PPR motif - a TPR-related motif prevalent in plant organellar proteins. Trends Biochem Sci. 2000;25:46–47. doi: 10.1016/s0968-0004(99)01520-0. [DOI] [PubMed] [Google Scholar]
- Takaiwa F, Sugiura M. The complete nucleotide sequence of a 23-S rRNA gene from tobacco chloroplasts. Eur J Biochem. 1982;124:13–19. doi: 10.1111/j.1432-1033.1982.tb05901.x. [DOI] [PubMed] [Google Scholar]
- Takenaka M, Zehrmann A, Brennicke A, Graichen K. Improved computational target site prediction for pentatricopeptide repeat RNA editing factors. PloS One. 2013;8:e65343. doi: 10.1371/journal.pone.0065343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaistij FE, Boudreau E, Lemaire SD, Goldschmidt-Clermont M, Rochaix JD. Characterization of Mbb1, a nucleus-encoded tetratricopeptide-like repeat protein required for expression of the chloroplast psbB/psbT/psbH gene cluster in Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 2000;97:14813–14818. doi: 10.1073/pnas.97.26.14813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Johnson X, Cavaiuolo M, Bohne AV, Nickelsen J, Vallon O. Two Chlamydomonas OPR proteins stabilize chloroplast mRNAs encoding small subunits of photosystem II and cytochrome b6f. Plant J. 2015;82:861–873. doi: 10.1111/tpj.12858. [DOI] [PubMed] [Google Scholar]
- Williams-Carrier R, Kroeger T, Barkan A. Sequence-specific binding of a chloroplast pentatricopeptide repeat protein to its native group II intron ligand. RNA. 2008;14:1930–1941. doi: 10.1261/rna.1077708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Carrier R, Zoschke R, Belcher S, Pfalz J, Barkan A. A major role for the plastid-encoded RNA polymerase complex in the expression of plastid transfer RNAs. Plant Physiol. 2014;164:239–248. doi: 10.1104/pp.113.228726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi Y, Hayashi S, Kobayashi K, Hirayama T, Nakamura T. Elucidation of the RNA recognition code for pentatricopeptide repeat proteins involved in organelle RNA editing in plants. PloS One. 2013a;8:e57286. doi: 10.1371/journal.pone.0057286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi Y, Tachikawa M, Noguchi H, Satoh S, Obokata J, Nakamura T. Pentatricopeptide repeat proteins involved in plant organellar RNA editing. RNA Biol. 2013b;10:1419–1425. doi: 10.4161/rna.24908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin P, et al. Structural basis for the modular recognition of single-stranded RNA by PPR proteins. Nature. 2013;504:168–171. doi: 10.1038/nature12651. [DOI] [PubMed] [Google Scholar]
- Zhelyazkova P, Hammani K, Rojas M, Voelker R, Vargas-Suarez M, Borner T, Barkan A. Protein-mediated protection as the predominant mechanism for defining processed mRNA termini in land plant chloroplasts. Nucleic Acids Res. 2012;40:3092–3105. doi: 10.1093/nar/gkr1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoschke R, Kroeger T, Belcher S, Schottler MA, Barkan A, Schmitz-Linneweber C. The Pentatricopeptide Repeat-SMR Protein ATP4 promotes translation of the chloroplast atpB/E mRNA. Plant J. 2012;72:547–558. doi: 10.1111/j.1365-313X.2012.05081.x. [DOI] [PubMed] [Google Scholar]
- Zoschke R, Qu Y, Zubo YO, Borner T, Schmitz-Linneweber C. Mutation of the pentatricopeptide repeat-SMR protein SVR7 impairs accumulation and translation of chloroplast ATP synthase subunits in Arabidopsis thaliana. J Plant Res. 2013a;126:403–414. doi: 10.1007/s10265-012-0527-1. [DOI] [PubMed] [Google Scholar]
- Zoschke R, Watkins K, Barkan A. A rapid microarray-based ribosome profiling method elucidates chloroplast ribosome behavior in vivo. Plant Cell. 2013b;25:2265–2275. doi: 10.1105/tpc.113.111567. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Loss of sRNAs mapping to the PPR53-dependent RNA terminus upstream of ndhA.
Comparison of PPR53 and ATP4 orthologs in maize and Arabidopsis
Table S1. Primers used to generate probes for RNA gel blots and for RT-PCR.
Data S1. Normalized values for ribosome profiling and transcriptome data plotted in Figure 3.