Abstract
Background
The effects of exposure to childhood trauma (CT) may be transmitted across generations, however the time period(s) and mechanism(s) have yet to be clarified. We address the hypothesis that intergenerational transmission may begin during intrauterine life via the effect of maternal CT exposure on placental-fetal stress physiology, specifically placental corticotrophin-releasing hormone (pCRH).
Methods
The study was conducted in a sociodemographically-diverse cohort of 295 pregnant women. CT exposure was assessed using the Childhood Trauma Questionnaire. Placental CRH concentrations were quantified in maternal blood collected serially over the course of gestation. Linear mixed effects and Bayesian piecewise linear models were employed to test hypothesized relationships.
Results
Maternal CT exposure (CT+) was significantly associated with pCRH production. Compared to non-exposed women, CT+ was associated with an almost 25% increase in pCRH towards the end of gestation, and the pCRH trajectory of CT+ women exhibited an approximately two-fold steeper increase after the pCRH inflection point at 19 wks gestation.
Conclusions
To the best of our knowledge, this finding represents the first report linking maternal CT exposure with placental-fetal stress physiology, thus identifying a potential novel biological pathway of intergenerational transmission that may operate as early as during intrauterine life.
Keywords: childhood trauma, placental CRH, developmental programming, intergenerational transmission, preconceptional stress, pregnancy
INTRODUCTION
Traumatic events that occur during a woman’s pregnancy likely impact the development of her as-yet-unborn child. But could traumatic exposures that may have occurred before the woman became pregnant, perhaps even as early as during her own childhood, also impact fetal development? As a first step towards addressing this question, we establish here an association during gestation between a woman’s exposure to trauma in her own childhood and the physiology of the developing fetal-placental unit, with a focus on the production and trajectory of the major placental-fetal stress hormone - placental corticotrophin-releasing hormone (pCRH).
Childhood trauma (CT) represents one of the most pernicious stressors in our society. Estimates from the CDC and others suggest a majority of children are exposed to one or more traumatic events in their lifetimes (1, 2), and that 30–40% of adult women have experienced at least one, and 15–25% more than one, type of childhood trauma (3, 4). The long-term sequelae of CT exposure are well established, and include adverse psychological, biological, biophysical and behavioral states, and increased likelihood of developing mental and physical disorders such as depression, posttraumatic stress disorder (PTSD), drug addiction, obesity, cardiovascular, metabolic and autoimmune disease (5–14). Emerging evidence now suggests the long shadow cast by childhood trauma may not be restricted to only the lifespan of abused women, but also may be transmitted to another yet even more vulnerable population - their children - who have been shown to exhibit alterations in stress physiology systems (15–18), behavioral disorders such as conduct problems, internalizing and externalizing behavioral problems, and autism spectrum disorder (19–22), and obesity (23). The mechanisms and pathways underlying such intergenerational mother-to-child transmission are not well understood, and their elucidation is an area of considerable interest and importance.
The prevailing paradigm posits such intergenerational transmission likely occurs after childbirth during the periods of infancy and childhood via the effects of CT exposure-related maternal dysfunctional states (e.g., depression, low maternal sensitivity, substance use) on the quality of mother-child relationships and parenting (22, 24–26). We seek to extend this existing paradigm. We advance an interdisciplinary, translational framework to postulate that the process of intergenerational transmission may start earlier during the highly sensitive period of fetal development. We propose that the developing embryo/fetus may sense and respond to biological cues in the maternal compartment that reflect the long-term biological, biophysical, psychological or behavioral consequences that CT exposed women may bring to their pregnancy. Intergenerational transmission in utero is expected to be determined by, 1) the degree to which the developing fetus can receive biological signals indicative of maternal CT-related alterations in her peripheral physiology, and 2) the extent to which such signals participate directly or indirectly in fetal development and phenotypic specification. Based on the consideration that are no direct neural or vascular connections between the maternal and fetal compartments, and all communication is mediated by the placenta, an organ of fetal origin, we suggest that feto-placental stress-responsive systems, specifically the placental corticotrophin-releasing hormone (pCRH) system, represents an attractive candidate pathway.
In non-pregnant state CRH is secreted primarily by hypothalamic PVN neurons and plays a central role in coordinating the central and peripheral stress response (27). During pregnancy, the placenta of higher primates synthesizes and releases CRH in an exponentially increasing manner into the fetal and maternal compartments (28). pCRH is known to play a major, obligatory role in the initiation, maintenance, and progression of gestation, fetal development and parturition (29–32). Moreover, pCRH production is stress-sensitive. Its in vitro production is regulated in a positive, dose-dependent manner in response to all the major biological effectors of stress (33–35), and in vivo evidence suggests it is sensitive to suboptimal maternal physiological, clinical, social and environmental exposures (36–40). pCRH likely serves as a key communication signal between the mother and her as-yet-unborn child. We and others have reported that variation in pCRH concentration in pregnancy is associated with several key fetal and infant developmental and health outcomes (41–46). Thus, the placental CRH system appears to play a tripartite role as a sensor, transducer and effector of the consequences of intrauterine perturbations on the developing fetal brain and peripheral systems (47).
The goal of this study was to establish evidence of an association between history of maternal CT exposure and pCRH production across gestation after accounting for potential confounding factors. Because pCRH production increases in an approximately exponential manner across gestation, we sought to elucidate the precise nature of the effect by performing analyses to determine the association of maternal CT exposure with the initial production and/or the rate of change of pCRH production over gestation. While the present study is not designed to address questions related to potential mechanisms underlying any observed maternal CT-related alterations in feto-placental stress physiology, we did address the issue of whether the effects of maternal CT exposure persist after accounting for the effects of salient gestational conditions that occur more frequently in CT+ mothers, such as clinical/obstetric complications in the index pregnancy, biophysical state (higher body-mass index; BMI), unhealthy maternal behaviors (smoking), and unfavorable maternal psychological state (depression), in order to estimate the potential impact of maternal childhood trauma on feto-placental stress physiology over and beyond that reflected in current gestational state or conditions.
METHODS AND MATERIALS
Participants
The study was conducted in a sociodemographically-diverse cohort of 295 pregnant women attending prenatal care at two university-based medical centers in southern California (see Table 1). All participants had singleton, intrauterine pregnancies with no known cord, placental, or uterine anomalies, fetal congenital malformations, or presence of any conditions known to be associated with dysregulated neuroendocrine function or corticosteroid medication use. All study procedures were approved by the IRBs of the respective institutions, and all participants provided written informed consent.
Table 1.
Frequencies and means (SD) of maternal characteristics in the whole sample and in women meeting criteria for any type of childhood trauma vs. women without childhood trauma.
| Characteristic | Complete sample N = 295 |
CT-group (no childhood trauma) N=169 (57.3%) |
CT+ group (≥ 1 type of childhood trauma) N=126 (42.7%) |
|---|---|---|---|
| Race/ethnicity, n (%) | |||
| Non-Hispanic White | 96 (32.5%) | 67 (39.6%) | 29 (23.0%)** |
| Hispanic | 100 (33.9%) | 54 (32.0%) | 46 (36.5%) |
| African American | 58 (19.7%) | 31 (18.3%) | 27 (21.4%) |
| Childhood SES, mean ± SD | 10.92 ± 3.12 | 11.51 ± 2.75 | 10.08 ± 13.42*** |
| Presence of any obstetric risk condition, n (%) | 78 (26.4%) | 41 (24.3%) | 37 (29.4%) |
| Smoking in pregnancy = 1, n (%) | 26 (8.8%) | 11 (6.5%) | 15 (11.9%) |
| Drug use in pregnancy = 1, n (%) | 14 (4.7%) | 5 (3.0%) | 9 (7.1%) |
| Alcohol in pregnancy = 1, n (%) | 48 (16.3%) | 24 (14.2%) | 24 (19.0%) |
| Family income index, mean ± SD | 6.36 ± 3.28 | 6.79 ± 3.39 | 5.79 ± 3.04* |
| Age at delivery, yrs, mean ± SD | 28.92 ± 5.90 | 29.12 ± 6.08 | 28.64 ± 5.66 |
| Age at first delivery, yrs, mean ± SD | 24.72 ± 6.04 | 25.33 ± 6.28 | 23.89 ± 5.63* |
| Parity, mean ± SD | 1.07 ± 1.07 | 0.97 ± 1.00 | 1.21 ± 1.14† |
| Pre-pregnancy BMI, mean ± SD | 26.26 ± 6.24 | 25.87 ± 6.10 | 26.80 ± 6.40 |
| Depression (CES-D), mean ± SD | 0.70 ± 0.46 | 0.57 ± 0.37 | 0.87 ± 0.51*** |
Note. CT = childhood trauma; SES = socioeconomic status; BMI = Body-Mass Index; CES-D = Center for Epidemiological Studies - Depression
p < .10;
p < .05;
p < .01;
p < .001 compared to CT−
Procedures
The study employed a prospective, longitudinal design with serial assessments over the course of gestation. Participants were recruited in the first trimester of gestation. Study visits occurred up to a maximum of 5 times over the course of their pregnancy at T1 15 ± 0.7 wks (mean ± SEM) (range 13.3 – 17.5), T2 20.3 ± 0.8 wks (range 17.0 – 23.2), T3 25.6 ± 0.8 wks (range 24 – 27.3), T4 30.7 ± 0.6 wks (range 29.6 – 32.4), and T5 36.5 ± 0.8 wks (range 33.5 – 38.5) gestation. Study visit procedures included the collection of maternal venous blood, administration of structured clinical and psychosocial interviews and questionnaires, and fetal ultrasonography. Gestational age was confirmed by obstetric ultrasonographic biometry performed before 20 wks gestation using standard clinical criteria (48).
Measures
Maternal childhood trauma exposure
Maternal exposure to CT was ascertained at the T2 visit using the Childhood Trauma Questionnaire (CTQ, 49), one of the most widely-used instruments for determination of abuse and neglect experiences in childhood and adolescence (50). This 28-item measure assesses five dimensions of childhood maltreatment: emotional abuse (EA), physical abuse (PA), sexual abuse (SA), emotional neglect (EN), and physical neglect (PN). Details of scoring are described in the Supplemental Material. Cut-off values for moderate or greater exposure were used to create dichotomous variables of exposure for each CTQ subscale (emotional abuse ≥13; physical abuse ≥10; sexual abuse ≥8; emotional neglect ≥15; and physical neglect ≥10) and then summed to compute a score reflective of the total number of moderate to severe abuse and neglect categories of exposure (Total CT, with a range between 0 and 5). The Total CT score was used as the principal predictor in statistical analysis.
Placental CRH
pCRH concentration was determined in maternal venous blood collected at the study visits. It is important to note that the vast majority of the CRH that is detectable in maternal peripheral blood during pregnancy is of placental origin (51). Several extra-hypothalamic sites of CRH production do exist, including reproductive tissues and lymphocytes. However, in contrast to CRH produced by the placenta, CRH from these other tissues acts locally in an autocrine-paracrine manner (52–54). Thus, because the placenta (an organ of fetal origin) is the source of CRH measured in the plasma of pregnant women, the term pCRH is used.
A 20-ml blood sample was withdrawn by antecubital venipuncture into siliconized EDTA vacutainers and immediately chilled to 6°C. Samples were centrifuged at 2,000 g for 15 min, and the plasma was decanted into polypropylene tubes containing 500 KIU/ml aprotinin (Sigma Chemical Company, St. Louis, Mo., USA). Plasma samples were then stored at −70°C until assayed. CRH levels (pg/ml) were determined from extracted samples by radioimmunoassay (Bachem Peninsula Laboratories LLC, San Carlos, CA, USA, see Supplemental Material for assay details). Intra- and inter-assay coefficients of variance ranged from 5% to 15%, with a minimum detectable dose of 2.04 pg/ml. 9.5% of the pCRH samples were excluded due to high coefficients of variance (CV) > 15%. Specifically, of the 295 participants included in our study, 283 had a T1 visit with 82% (231) having usable CRH data based on the CV criterion; 289 had a T2 visit with 94% (272) having usable CRH data; 99 had a T3 visit with 92% (91) having usable CRH data; 89 had a T4 visit and 100% had usable CRH data; and 82 had a T5 visit with 96% (79) having usable CRH data (see Supplemental Material for further details).
Confounding
To address the possibility that any observed associations between maternal CT exposure and pCRH concentrations across gestation reflect residual confounding of the effects of “third” variables that may be causally related to both factors, we included measures of childhood socioeconomic status (SES) and race/ethnicity in the first analytic model because these factors may have preceded the event of maternal CT exposure (2, 39, 55). Both these variables were assessed at the T1 visit. Childhood SES was quantified using a 15-item measure that characterized distinct aspects of economic status during childhood (childhood SES mean ± SD = 10.8 ±3.1; range: 0–15).
Covariates
The second analytic model included additional covariates of sociodemographic, biophysical, obstetric, behavioral and psychological factors in the index (current) pregnancy that have been associated with pCRH production or childhood trauma (11, 36, 39, 40, 55–59). Sociodemographic (current SES, assessed at T1), behavioral (smoking, drug use, and alcohol consumption during pregnancy, assessed at each study visit) and psychological state (depressive symptoms, assessed at each study visit), were ascertained using standardized structured interviews and questionnaires. The 9-item short version of the Center for Epidemiological Studies Depression (CES-D, 60, 61) scale was used to quantify depressive symptomatology. To account for singular missing items the mean responses were calculated for each time point and then combined into a mean CES-D score over all assessment time points. Biological verification of self-reported smoking and illicit drug use was performed in urine using INSTANT-VIEW Multi-Test Drugs of Abuse Panel (Alfa Scientific Designs Inc., Poway, CA, USA). Biophysical characteristics (pre-pregnancy BMI), parity, and the presence of obstetric risk conditions in the index pregnancy (hypertension, diabetes, anemia, vaginal bleeding, infection, oligohydramnios, and placental abruption) were abstracted from the antepartum and delivery medical record, as previously described (46).
Statistical Analysis
We used the likelihood-ratio test to compare a model with the main and interaction effects of CT with a simpler model that excluded these two terms. The likelihood ratio test statistic −2log(LR) indicates improvement of the model fit upon including CT as a predictor. Since the likelihood ratio test does not differentiate between the main and interaction effect of CT, and because there are multiple observations per subject, we employed linear mixed effects models to test our primary hypothesis that pCRH production over gestation differs as a function of maternal CT exposure (see Supplementary Material). We performed a log-transformation of pCRH values to linearize the approximately exponential increase in the rate of pCRH production over gestation (28). To estimate the rate of pCRH change over gestation we included an interaction effect between the total CT score and gestational age at assessment, with gestational age centered at the first study assessment time point (15 weeks gestation). Maternal race/ethnicity and childhood SES were included in this model. Secondary analyses were performed to estimate whether maternal CT exposure exerts effects over and above those of gestational conditions, including maternal socio-demographic, biophysical, obstetric, behavioral, and psychological factors. Model diagnostics were performed using the HLMdiag package (62).
Next, to test our hypothesis that there is no initial difference in pCRH production between CT+ and CT− women but that the difference would emerge later and become progressively larger with advancing gestation, Bayesian piecewise linear modeling was employed to determine whether there is a constant multiplicative shift in the non-linear growth rate of pCRH concentrations across gestation, or whether one or more points of time in gestation exist when significant changes in the rate of pCRH increase across gestation occur (i.e. knot points). Our model was trained on 70% of the data, and its predictive performance (measured in terms of mean squared error, MSE) was evaluated on the remaining 30% of the data (63). The procedure was stratified by CT status. All models were adjusted for the covariates described above.
RESULTS
Approximately one half (57.3%) of the study participants reported no or low exposure childhood trauma; 19.3% reported exposure to a single category of abuse or neglect; and 23.4% reported exposure to multiple types of maltreatment (see Table S1 in Supplemental Material). The mean number of traumas (total CT) in the exposed population was 2.06 (± 1.22 SD). The percentage of participants scoring above the cut-off for depression ranged between 7.8% and 11.1% at the different time points during pregnancy.
Compared to CT− women, CT+ women had a lower childhood SES, lower current income, were younger at their first delivery, had marginally higher parity, higher depressive symptomatology, and were less likely to be of non-Hispanic White race/ethnicity (Table 1).
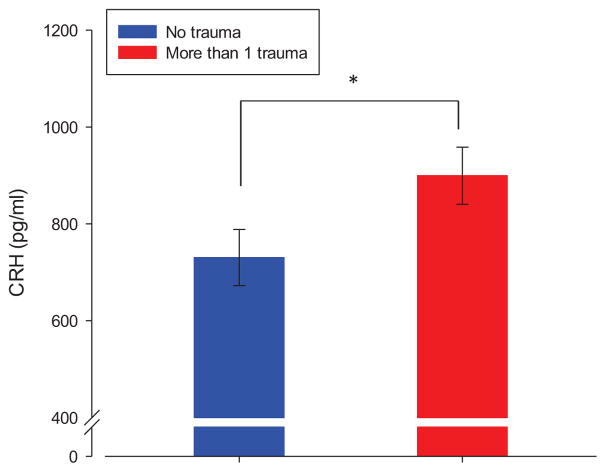
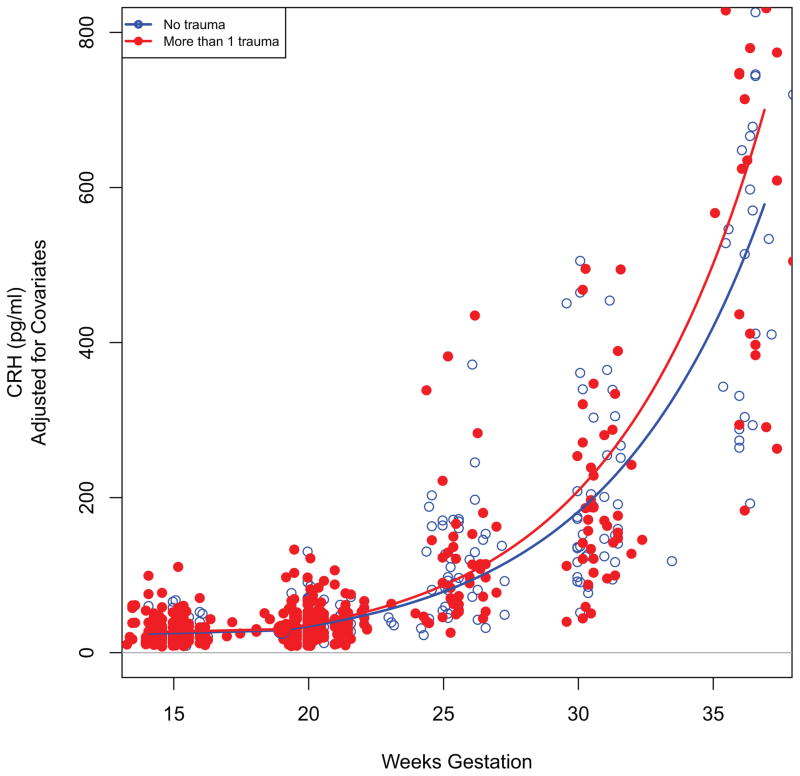
The likelihood-ratio test comparing the models with and without total CT revealed a significant effect of CT exposure on pCRH production across gestation (−2log(LR) = 7.56, p = 0.02). Thus, the total CT score significantly contributed to the model’s explanation of the pCRH variation. The results of the mixed effects model revealed that the strength of the association between CT exposure and pCRH production increased over the course of gestation, as indicated by the significant interaction between total CT and gestational age at assessment (Table 2). To interpret the results, the logarithmized pCRH values were retransformed by exponentiation, which provides an estimate of the change in median CRH as a function of CT and GA. Specifically, at 15 weeks gestation (the first assessment time point), exposure to one additional trauma category corresponded to less than 1% increase in median CRH value, but at 36 weeks gestation (the last assessment time point) each additional trauma category corresponded to a 12.1% increase in median pCRH (see Figure 1). Given that women in the CT+ group were exposed to a mean of 2.06 categories of trauma, their average CT exposure corresponded to a 24.9% higher concentration in median pCRH towards the end of gestation compared to CT− women (Figures 1 and 2).
Table 2.
Model 1: Effect estimates of the linear mixed effects model with log-pCRH as the outcome and Total CT and potentially confounding covariates as predictors.
| Likelihood ratio test (comparing null with full model) = 7.56, p=0.02 | |||||
|---|---|---|---|---|---|
| Estimate | SE | df | t-value | p-value | |
| GA | 0.136 | 0.004 | 415 | 38.351 | <0.001* |
| Total CT | 0.005 | 0.029 | 264 | 0.185 | 0.85 |
| Total CT* GA | 0.005 | 0.002 | 415 | 2.356 | 0.02* |
| White | 0.091 | 0.098 | 264 | 0.237 | 0.36 |
| Hispanic | 0.024 | 0.103 | 264 | 0.237 | 0.81 |
| African-American | −0.083 | 0.114 | 264 | −0.726 | 0.47 |
| Childhood SES | 0.010 | 0.012 | 264 | 0.790 | 0.43 |
Note. GA = gestational age at assessment; CT = childhood trauma; SES = socioeconomic status
p < .05
Figure 1.
Scatterplot depicting the relationship between maternal childhood trauma and placental CRH over the course of gestation. The black square represents the point of inflection at 19 weeks of gestation. Analyses were performed using the continuous total CT variable as predictor and logCRH as outcome and including all covariates. For illustration purposes the difference in non-logarithmized CRH values between the two groups representing no trauma exposure and exposure to more than one trauma are presented in the figure.
Figure 2.
Association between maternal childhood trauma and placental CRH concentrations at 36 weeks gestation. Error bars represent standard error of the mean (SEM). Analyses were performed using the continuous total CT variable as predictor and logCRH as outcome and including all covariates. For illustration purposes the difference in non-logarithmized CRH values between the two groups representing no trauma exposure and exposure to more than one trauma are presented in the figure.
The results of the second linear mixed effects model (to ascertain whether the effects of maternal CT exposure on pCRH persist after accounting for the effects of salient gestational conditions that may be associated with both childhood trauma and placental physiology) remained altogether unchanged, indicating that childhood trauma appears to exert an effect on pCRH over and above these potential covariates ((−2log(LR) = 7.21, p = 0.02; see Table 3)). The analyses were repeated including only subjects with at least two pCRH measures, which did not appreciably change the results. The significance of the likelihood ratio test comparing the model with and without CT as a predictor improved slightly (−2log(LR) = 8.85, p = 0.01). The parameter estimates of the mixed effects model are shown in Table S2.
Table 3.
Model 2: Effect estimates of the linear mixed effects model with logCRH as the outcome and CT Total and full list of covariates as predictors.
| Likelihood ratio test (comparing null with full model) = 7.21, p=0.02 | |||||
|---|---|---|---|---|---|
| Estimate | SE | df | t-value | p-value | |
| GA | 0.135 | 0.004 | 384 | 35.556 | <0.001* |
| Total CT | 0.004 | 0.032 | 236 | 0.132 | 0.90 |
| Total CT* GA | 0.005 | 0.002 | 384 | 2.322 | 0.02* |
| White | 0.082 | 0.103 | 236 | 0.794 | 0.43 |
| Hispanic | 0.031 | 0.108 | 236 | 0.282 | 0.78 |
| African-American | −0.019 | 0.125 | 236 | −0.150 | 0.88 |
| Childhood SES | 0.006 | 0.013 | 236 | 0.460 | 0.65 |
| Parity | −0.062 | 0.033 | 236 | −1.863 | 0.06 |
| Family Income | 0.008 | 0.012 | 236 | 0.686 | 0.49 |
| Pre-preg BMI | −0.008 | 0.006 | 236 | −1.362 | 0.18 |
| Obstetric risk | 0.091 | 0.077 | 236 | 1.176 | 0.24 |
| Smoking | −0.147 | 0.121 | 236 | −1.219 | 0.22 |
| Drugs | 0.062 | 0.172 | 236 | 0.364 | 0.72 |
| Alcohol | −0.091 | 0.091 | 236 | −1.008 | 0.32 |
| Depression | 0.042 | 0.080 | 236 | 0.530 | 0.60 |
Note. GA = gestational age at assessment; CT = childhood trauma; SES = socioeconomic status; Pre-preg BMI = pre-pregnancy body mass index
p < .05
The Bayesian piecewise linear models of CRH trajectory analysis trained three models - a simple linear regression (with 0 knots), and two piece-wise linear models with 1 and 2 knots, respectively. Based on the resulting MSEs (0.375, 0.328, 0.329 respectively), the model with 1 knot was selected as the best-fitting model (lowest MSE term). The estimate for the location of the knot point was at 19 weeks gestation. There were no substantial differences between the CT+ and CT− groups in terms of the knot’s location, however, their pCRH trajectories were different in terms of the intercept and slopes before and after the knot (see Table 4). The change in the second slope (i.e. rate of change after 19 weeks gestation) differed significantly between subjects with and without CT. In the CT− group, the median of pCRH increased multiplicatively by 1.09 (i.e., 9%) per week from 19 wks gestation onwards, whereas in the CT+ group the median of pCRH increased multiplicatively by 1.20 (i.e., 20%) per week from 19 wks gestation onwards. Thus, the exponential increase in pCRH production over the latter part of gestation among CT+ women is approximately double that of CT− women.
Table 4.
Point estimates and 95% probability intervals for the regression parameters of the piecewise linear models for subjects with complete trajectories.
| Point Estimate | 95% Probability Interval | |
|---|---|---|
| Intercept for CT− | 3.36 | 3.18–3.54 |
| Intercept for CT+ | 3.27 | 3.06–3.47 |
| Difference in the intercept between CT+ and CT− | −0.09 | −0.36–0.17 |
| First slopea for CT− | 0.06 | 0.00–0.12* |
| First slopea for CT+ | 0.00 | −0.07–0.06 |
| Difference in the first slopea between CT+ and CT− | −0.06 | −0.15–0.02 |
| Second slopeb for CT− | 0.09 | 0.02–0.15* |
| Second slopeb for CT+ | 0.18 | 0.11–0.26* |
| Difference in the second slopeb between CT+ and CT− | 0.09 | 0.00–0.20* |
Note. Included are all subjects with at least one observation in each trimester; CT = childhood trauma.
The term ‘first slope’ refers to the slope of the log CRH trajectory before the inflection point of 19 wks gestation.
The term ‘second slope’ refers to the slope of the log CRH trajectory after the inflection point of 19 wks gestation.
p < .05
DISCUSSION
To the best of our knowledge, this is the first study to establish an association between a woman’s exposure to trauma in her own childhood and placental-fetal stress physiology during pregnancy. Consistent with our hypothesis, pCRH production over gestation was significantly greater in women exposed to childhood trauma, with a graded effect such that exposure to two types of trauma (the mean number in the exposed group) corresponded to an almost 25% increase in pCRH concentrations towards the end of gestation. Maternal CT exposure was not associated with the initial production or a shift in the gestational time point of a change in the non-linear rate of pCRH increase (which occurred at 19 weeks gestation), but with an approximately two-fold greater increase in the rate of pCRH production over the second half of gestation. These effects remained statistically significant after accounting for the effects of potential confounding variables and other gestational conditions related to either pCRH production or exposure to childhood trauma.
Previous studies have established an association between a woman’s history of CT exposure and the subsequent course and outcome of her pregnancy (56, 64, 65), maternal psychological states and conditions in pregnancy (depression, anxiety, PTSD (57, 66)), and maternal gestational physiology (endocrine, immune/inflammatory state) (56, 67–69). The current findings extend this research to the physiological function of the placental-fetal unit, which is a critical link in making the case for the biological plausibility of intergenerational transmission during the intrauterine period of life.
The clinical significance in terms of intergenerational transmission of the observed maternal CT exposure-related differences in pCRH production across gestation is presently unknown. However, we note that the pattern of differences in the pCRH trajectory by CT status in our study is of similar nature and magnitude to what has previously been reported in pregnancies complicated by preeclampsia and preterm birth (70, 71). Additional support for the biological plausibility of our hypothesis that pCRH physiology may act as a mediator of intergenerational transmission of the effects of maternal CT exposure derives from the observation that many of the adverse pregnancy, fetal, birth and postnatal outcomes associated with pCRH dysregulation in pregnancy (such as intra-amniotic infection (72), growth and size at birth (46), preterm delivery (46, 71), difficult infant temperament (42), and obesity (44),) are antecedents of the same alterations in stress physiology systems and neurodevelopmental disorders that are manifest in children of CT+ mothers (15–21).
In terms of generalizability, we note the prevalence of CT in our study population was similar to that reported in previous large, population-based samples (4, 73). When stratified by CT exposure, our study population differed with respect to their childhood SES and race/ethnicity, which is consistent with previous reports in non-pregnant populations of a higher CT prevalence among individuals with a lower childhood SES and among African Americans and Hispanics compared to non-Hispanic Whites (1, 2). Moreover, our finding of an association of CT exposure with higher parity and younger age at first pregnancy also is consistent with previous reports (64, 74) and with the tenets of life history theory (75).
The two major biological pathways by which maternal CT exposure could influence pCRH physiology are via maternal endocrine and immune/inflammatory stress biology. Exposure to CT is associated in non-pregnant individuals with changes in HPA axis baseline activity as well as reactivity in response to stressors or pharmacological challenges (76), and, in pregnant women, with alterations in baseline cortisol and in the cortisol awakening response (68, 69). In contrast to their inhibitory effect on hypothalamic CRH, glucocorticoids stimulate placental CRH production (77). In turn, pCRH can stimulate the production of cortisol from the maternal and fetal adrenals (78). Maternal cortisol may, therefore, partially mediate the effect of CT exposure on pCRH. In this context, it is interesting to note that the observed inflection point in the non-linear trajectory of pCRH at 19 weeks gestation in our study coincides with the time period when the fetal adrenals start producing appreciable amounts of cortisol (79), suggesting the feto-placental unit of CT+ pregnant women may be more sensitive to the positive feedback of cortisol. Maternal stress, anxiety or depression (i.e. conditions that are among the most prevalent psychological sequelae of CT) have been associated during gestation with several adverse neurodevelopmental outcomes in the children of affected mothers (80). Alterations in the functioning or regulation of the maternal and fetal hypothalamic pituitary adrenal (HPA) axes are commonly discussed as mechanisms that may mediate these associations (81–83). It is conceivable that the psychological consequences of CT are associated with an increased secretion of cortisol from the maternal adrenal, which may stimulate pCRH production, and which, in turn, could stimulate the release of cortisol from the fetal adrenals. The second biological pathway relates to the interaction of the maternal immune/inflammatory system with pCRH. Secretion of pCRH is directly stimulated by pro-inflammatory cytokines such as IL-1 and microbial antigens (84). CRH, in turn, has been shown to have a pro-inflammatory effect in the periphery (85, 86), and increased levels of pCRH have been associated with intra-amniotic infection (72, 87). Moreover, previous studies of non-pregnant CT+ women have reported they exhibit an increased inflammatory status (88–90). Thus, the maternal environment also may modulate pCRH production via changes in the inflammatory milieu.
It has previously been suggested that the intergenerational transmission of the effects of maternal CT exposure may occur in postnatal life via the detrimental effects of maternal CT-related psychological states on maternal-child relationships and suboptimal parenting behaviors (91, 92). Prenatal and postnatal periods of intergenerational transmission are not mutually exclusive. However, it is important to ascertain whether this effect starts during intrauterine life for at least two reasons. First, the elucidation of the time windows and mechanisms underlying intergenerational transmission is necessary to develop efficacious strategies for primary prevention. Second, the characteristics of the offspring at birth (e.g., newborn temperament) may, in part, influence the nature of postnatal mother-child dyadic interactions to moderate the effects of postnatal maternal CT-related characteristics. If such offspring characteristics at birth have already been adversely impacted by maternal CT-related gestational effects, they would be expected to further accentuate the consequences of dysregulated mother-child relationships. Indeed, pCRH has been previously demonstrated to be a predictor of child temperament (42).
In summary, childhood abuse and neglect represent one of the most pervasive, persistent and pernicious stressors in our society. Emerging evidence now suggests its adverse consequences may not be restricted to the exposed women alone, but may also be transmitted to their children. It is critical to arrive at a better understanding of this process for elucidating biological pathways and developing interventions in order to ultimately break the vicious cycle of the enduring consequences of early life stress passed down from a vulnerable population of abused women to the even more vulnerable population of their unborn children. The present study represents the first step towards addressing the hypothesis that the inter-generational transmission of these adverse effects may start as early as during the child’s intrauterine period of life by establishing an association between a woman’s exposure to abuse or neglect in her own childhood and the physiology of the placental-fetal unit of her as-yet-unborn child.
Supplementary Material
Acknowledgments
Supported, in part, by US PHS (NIH) grants RO1 HD-060628, PO1 HD-047609, R01 HD-041696, R29 HD-33506 (all to PDW), RO1 MH-105538 (to CB, PDW and Damien Fair), and R01 NS-41298 (to Curt Sandman).
Footnotes
FINANCIAL DISCLOSURES
The authors declare no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.(CDC) CfDCaP . Adverse childhood experiences reported by adults --- five states, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:1609–1613. [PubMed] [Google Scholar]
- 2.Hussey JM, Chang JJ, Kotch JB. Child maltreatment in the United States: prevalence, risk factors, and adolescent health consequences. Pediatrics. 2006;118:933–942. doi: 10.1542/peds.2005-2452. [DOI] [PubMed] [Google Scholar]
- 3.Scher CD, Forde DR, McQuaid JR, Stein MB. Prevalence and demographic correlates of childhood maltreatment in an adult community sample. Child Abuse Negl. 2004;28:167–180. doi: 10.1016/j.chiabu.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Walker EA, Unutzer J, Rutter C, Gelfand A, Saunders K, VonKorff M, et al. Costs of health care use by women HMO members with a history of childhood abuse and neglect. Arch Gen Psychiatry. 1999;56:609–613. doi: 10.1001/archpsyc.56.7.609. [DOI] [PubMed] [Google Scholar]
- 5.Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, et al. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256:174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacMillan HL, Fleming JE, Streiner DL, Lin E, Boyle MH, Jamieson E, et al. Childhood abuse and lifetime psychopathology in a community sample. Am J Psychiatry. 2001;158:1878–1883. doi: 10.1176/appi.ajp.158.11.1878. [DOI] [PubMed] [Google Scholar]
- 7.Springer KW, Sheridan J, Kuo D, Carnes M. Long-term physical and mental health consequences of childhood physical abuse: results from a large population-based sample of men and women. Child Abuse Negl. 2007;31:517–530. doi: 10.1016/j.chiabu.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Widom CS. Posttraumatic stress disorder in abused and neglected children grown up. Am J Psychiatry. 1999;156:1223–1229. doi: 10.1176/ajp.156.8.1223. [DOI] [PubMed] [Google Scholar]
- 9.Thomas C, Hypponen E, Power C. Obesity and type 2 diabetes risk in midadult life: the role of childhood adversity. Pediatrics. 2008;121:e1240–1249. doi: 10.1542/peds.2007-2403. [DOI] [PubMed] [Google Scholar]
- 10.Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, et al. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation. 2004;110:1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- 11.Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, et al. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med. 2009;163:1135–1143. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 13.Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol. 2010;52:671–690. doi: 10.1002/dev.20494. [DOI] [PubMed] [Google Scholar]
- 14.Heim C, Binder EB. Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene–environment interactions, and epigenetics. Experimental Neurology. 2012;233:102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 15.Jovanovic T, Smith A, Kamkwalala A, Poole J, Samples T, Norrholm SD, et al. Physiological markers of anxiety are increased in children of abused mothers. J Child Psychol Psychiatry. 2011;52:844–852. doi: 10.1111/j.1469-7610.2011.02410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brand SR, Brennan PA, Newport DJ, Smith AK, Weiss T, Stowe ZN. The impact of maternal childhood abuse on maternal and infant HPA axis function in the postpartum period. Psychoneuroendocrinology. 2010;35:686–693. doi: 10.1016/j.psyneuen.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bierer LM, Bader HN, Daskalakis NP, Lehrner AL, Makotkine I, Seckl JR, et al. Elevation of 11β-hydroxysteroid dehydrogenase type 2 activity in Holocaust survivor offspring: Evidence for an intergenerational effect of maternal trauma exposure. Psychoneuroendocrinology. 2014;48:1–10. doi: 10.1016/j.psyneuen.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehrner A, Bierer LM, Passarelli V, Pratchett LC, Flory JD, Bader HN, et al. Maternal PTSD associates with greater glucocorticoid sensitivity in offspring of Holocaust survivors. Psychoneuroendocrinology. 2014;40:213–220. doi: 10.1016/j.psyneuen.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collishaw S, Dunn J, O’Connor TG, Golding J. Maternal childhood abuse and offspring adjustment over time. Development and psychopathology. 2007;19:367–383. doi: 10.1017/S0954579407070186. [DOI] [PubMed] [Google Scholar]
- 20.Thompson R. Mothers’ violence victimization and child behavior problems: examining the link. Am J Orthopsychiatry. 2007;77:306–315. doi: 10.1037/0002-9432.77.2.306. [DOI] [PubMed] [Google Scholar]
- 21.Roberts AL, Lyall K, Rich-Edwards JW, Ascherio A, Weisskopf MG. Association of maternal exposure to childhood abuse with elevated risk for autism in offspring. JAMA Psychiatry. 2013;70:508–515. doi: 10.1001/jamapsychiatry.2013.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plant DT, Barker ED, Waters CS, Pawlby S, Pariante CM. Intergenerational transmission of maltreatment and psychopathology: the role of antenatal depression. Psychol Med. 2013;43:519–528. doi: 10.1017/S0033291712001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts AL, Galea S, Austin SB, Corliss HL, Williams MA, Koenen KC. Women’s experience of abuse in childhood and their children’s smoking and overweight. American journal of preventive medicine. 2014;46:249–258. doi: 10.1016/j.amepre.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berlin LJ, Appleyard K, Dodge KA. Intergenerational continuity in child maltreatment: mediating mechanisms and implications for prevention. Child development. 2011;82:162–176. doi: 10.1111/j.1467-8624.2010.01547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dixon L, Hamilton-Giachritsis C, Browne K. Attributions and behaviours of parents abused as children: a mediational analysis of the intergenerational continuity of child maltreatment (Part II) J Child Psychol Psychiatry. 2005;46:58–68. doi: 10.1111/j.1469-7610.2004.00340.x. [DOI] [PubMed] [Google Scholar]
- 26.Appleyard K, Berlin LJ, Rosanbalm KD, Dodge KA. Preventing early child maltreatment: implications from a longitudinal study of maternal abuse history, substance use problems, and offspring victimization. Prev Sci. 2011;12:139–149. doi: 10.1007/s11121-010-0193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- 28.Sorem KA, Smikle CB, Spencer DK, Yoder BA, Graveson MA, Siler-Khodr TM. Circulating maternal corticotropin-releasing hormone and gonadotropin-releasing hormone in normal and abnormal pregnancies. Am J Obstet Gynecol. 1996;175:912–916. doi: 10.1016/s0002-9378(96)80024-x. [DOI] [PubMed] [Google Scholar]
- 29.Smith R. Parturition. N Engl J Med. 2007;356:271–283. doi: 10.1056/NEJMra061360. [DOI] [PubMed] [Google Scholar]
- 30.Makrigiannakis A, Zoumakis E, Kalantaridou S, Coutifaris C, Margioris AN, Coukos G, et al. Corticotropin-releasing hormone promotes blastocyst implantation and early maternal tolerance. Nat Immunol. 2001;2:1018–1024. doi: 10.1038/ni719. [DOI] [PubMed] [Google Scholar]
- 31.Giovannelli A, Greenwood SL, Desforges M, Sibley CP, Petraglia F. Corticotrophin-releasing factor and urocortin inhibit system A activity in term human placental villous explants. Placenta. 2011;32:99–101. doi: 10.1016/j.placenta.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 32.Smith R, Mesiano S, Chan EC, Brown S, Jaffe RB. Corticotropin-releasing hormone directly and preferentially stimulates dehydroepiandrosterone sulfate secretion by human fetal adrenal cortical cells. J Clin Endocrinol Metab. 1998;83:2916–2920. doi: 10.1210/jcem.83.8.5020. [DOI] [PubMed] [Google Scholar]
- 33.Korebrits C, Yu DH, Ramirez MM, Marinoni E, Bocking AD, Challis JR. Antenatal glucocorticoid administration increases corticotrophin-releasing hormone in maternal plasma. Br J Obstet Gynaecol. 1998;105:556–561. doi: 10.1111/j.1471-0528.1998.tb10158.x. [DOI] [PubMed] [Google Scholar]
- 34.Petraglia F, Sutton S, Vale W. Neurotransmitters and peptides modulate the release of immunoreactive corticotropin-releasing factor from cultured human placental cells. Am J Obstet Gynecol. 1989;160:247–251. doi: 10.1016/0002-9378(89)90130-0. [DOI] [PubMed] [Google Scholar]
- 35.Donoghue JF, Leitch IM, Boura AL, Walters WA, Giles WB, Smith R, et al. Fetal placental vascular responses to corticotropin-releasing hormone in vitro. Effects of variation in oxygen tension. Placenta. 2000;21:711–717. doi: 10.1053/plac.2000.0548. [DOI] [PubMed] [Google Scholar]
- 36.Herrmann TS, Siega-Riz AM, Hobel CJ, Aurora C, Dunkel-Schetter C. Prolonged periods without food intake during pregnancy increase risk for elevated maternal corticotropin-releasing hormone concentrations. Am J Obstet Gynecol. 2001;185:403–412. doi: 10.1067/mob.2001.115863. [DOI] [PubMed] [Google Scholar]
- 37.Hobel CJ, Dunkel-Schetter C, Roesch SC, Castro LC, Arora CP. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks’ gestation in pregnancies ending in preterm delivery. Am J Obstet Gynecol. 1999;180:S257–263. doi: 10.1016/s0002-9378(99)70712-x. [DOI] [PubMed] [Google Scholar]
- 38.Tse AC, Rich-Edwards JW, Koenen K, Wright RJ. Cumulative stress and maternal prenatal corticotropin-releasing hormone in an urban U.S. cohort. Psychoneuroendocrinology. 2012;37:970–979. doi: 10.1016/j.psyneuen.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kramer MS, Lydon J, Seguin L, Goulet L, Kahn SR, McNamara H, et al. Non-stress-related factors associated with maternal corticotrophin-releasing hormone (CRH) concentration. Paediatr Perinat Epidemiol. 2010;24:390–397. doi: 10.1111/j.1365-3016.2010.01127.x. [DOI] [PubMed] [Google Scholar]
- 40.O’Keane V, Lightman S, Marsh M, Pawlby S, Papadopoulos AS, Taylor A, et al. Increased pituitary-adrenal activation and shortened gestation in a sample of depressed pregnant women: a pilot study. J Affect Disord. 2011;130:300–305. doi: 10.1016/j.jad.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Ellman LM, Schetter CD, Hobel CJ, Chicz-Demet A, Glynn LM, Sandman CA. Timing of fetal exposure to stress hormones: effects on newborn physical and neuromuscular maturation. Dev Psychobiol. 2008;50:232–241. doi: 10.1002/dev.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis EP, Glynn LM, Dunkel Schetter C, Hobel C, Chicz-Demet A, Sandman CA. Corticotropin-releasing hormone during pregnancy is associated with infant temperament. Developmental neuroscience. 2005;27:299–305. doi: 10.1159/000086709. [DOI] [PubMed] [Google Scholar]
- 43.Sandman CA, Wadhwa PD, Chicz-DeMet A, Porto M, Garite TJ. Maternal corticotropin-releasing hormone and habituation in the human fetus. Dev Psychobiol. 1999;34:163–173. doi: 10.1002/(sici)1098-2302(199904)34:3<163::aid-dev1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 44.Gillman MW, Rich-Edwards JW, Huh S, Majzoub JA, Oken E, Taveras EM, et al. Maternal corticotropin-releasing hormone levels during pregnancy and offspring adiposity. Obesity (Silver Spring) 2006;14:1647–1653. doi: 10.1038/oby.2006.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fasting MH, Oken E, Mantzoros CS, Rich-Edwards JW, Majzoub JA, Kleinman K, et al. Maternal levels of corticotropin-releasing hormone during pregnancy in relation to adiponectin and leptin in early childhood. J Clin Endocrinol Metab. 2009;94:1409–1415. doi: 10.1210/jc.2008-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wadhwa PD, Garite TJ, Porto M, Glynn L, Chicz-DeMet A, Dunkel-Schetter C, et al. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: a prospective investigation. Am J Obstet Gynecol. 2004;191:1063–1069. doi: 10.1016/j.ajog.2004.06.070. [DOI] [PubMed] [Google Scholar]
- 47.Buss C, Entringer S, Wadhwa PD. Fetal programming of brain development: intrauterine stress and susceptibility to psychopathology. Science signaling. 2012;5:pt7. doi: 10.1126/scisignal.2003406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Brien GD, Queenan JT, Campbell S. Assessment of gestational age in the second trimester by real-time ultrasound measurement of the femur length. Am J Obstet Gynecol. 1981;139:540–545. doi: 10.1016/0002-9378(81)90514-7. [DOI] [PubMed] [Google Scholar]
- 49.Bernstein DP, Fink L. Childhood Trauma Questionnaire: a retrospective self-report manual. San Antonio, TX: The Psychological Corp; 1998. [Google Scholar]
- 50.Baker AJL. Adult recall of childhood psychological maltreatment: Definitional strategies and challenges. Children and Youth Services Review. 2009;31:703–714. [Google Scholar]
- 51.Goland RS, Wardlaw SL, Stark RI, Brown LS, Jr, Frantz AG. High levels of corticotropin-releasing hormone immunoactivity in maternal and fetal plasma during pregnancy. J Clin Endocrinol Metab. 1986;63:1199–1203. doi: 10.1210/jcem-63-5-1199. [DOI] [PubMed] [Google Scholar]
- 52.Di Blasio AM, Pecori Giraldi F, Vigano P, Petraglia F, Vignali M, Cavagnini F. Expression of corticotropin-releasing hormone and its R1 receptor in human endometrial stromal cells. J Clin Endocrinol Metab. 1997;82:1594–1597. doi: 10.1210/jcem.82.5.3923. [DOI] [PubMed] [Google Scholar]
- 53.Karalis K, Sano H, Redwine J, Listwak S, Wilder RL, Chrousos GP. Autocrine or paracrine inflammatory actions of corticotropin-releasing hormone in vivo. Science (New York, NY) 1991;254:421–423. doi: 10.1126/science.1925600. [DOI] [PubMed] [Google Scholar]
- 54.Kalantaridou SN, Zoumakis E, Makrigiannakis A, Lavasidis LG, Vrekoussis T, Chrousos GP. Corticotropin-releasing hormone, stress and human reproduction: an update. Journal of reproductive immunology. 2010;85:33–39. doi: 10.1016/j.jri.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 55.Chen Y, Holzman C, Chung H, Senagore P, Talge NM, Siler-Khodr T. Levels of maternal serum corticotropin-releasing hormone (CRH) at midpregnancy in relation to maternal characteristics. Psychoneuroendocrinology. 2010;35:820–832. doi: 10.1016/j.psyneuen.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leeners B, Stiller R, Block E, Gorres G, Rath W. Pregnancy complications in women with childhood sexual abuse experiences. Journal of psychosomatic research. 2010;69:503–510. doi: 10.1016/j.jpsychores.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 57.Seng JS, Sperlich M, Low LK. Mental health, demographic, and risk behavior profiles of pregnant survivors of childhood and adult abuse. J Midwifery Womens Health. 2008;53:511–521. doi: 10.1016/j.jmwh.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alvarez J, Pavao J, Baumrind N, Kimerling R. The relationship between child abuse and adult obesity among california women. American journal of preventive medicine. 2007;33:28–33. doi: 10.1016/j.amepre.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 59.Currie J, Widom CS. Long-term consequences of child abuse and neglect on adult economic well-being. Child maltreatment. 2010;15:111–120. doi: 10.1177/1077559509355316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 61.Santor DA, Coyne JC. Shortening the CES–D to improve its ability to detect cases of depression. Psychological Assessment. 1997;9:233. [Google Scholar]
- 62.Loy A, Hofmann H. HLMdiag: A Suite of Diagnostics for Hierarchical Linear Models in R. Journal of Statistical Software. 2014;56:1–28. [Google Scholar]
- 63.Hastie T, Tibshirani R, Friedman J. Data Mining, Inference, and Prediction. 2. New York: Springer; 2009. The Elements of Statistical Learning; p. 763. [Google Scholar]
- 64.Lukasse M, Schei B, Vangen S, Oian P. Childhood abuse and common complaints in pregnancy. Birth. 2009;36:190–199. doi: 10.1111/j.1523-536X.2009.00323.x. [DOI] [PubMed] [Google Scholar]
- 65.Stevens-Simon C, McAnarney ER. Childhood victimization: relationship to adolescent pregnancy outcome. Child Abuse Negl. 1994;18:569–575. doi: 10.1016/0145-2134(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 66.Lang AJ, Rodgers CS, Lebeck MM. Associations between maternal childhood maltreatment and psychopathology and aggression during pregnancy and postpartum. Child Abuse Negl. 2006;30:17–25. doi: 10.1016/j.chiabu.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 67.Cammack AL, Buss C, Entringer S, Hogue CJ, Hobel CJ, Wadhwa PD. The association between early life adversity and bacterial vaginosis during pregnancy. Am J Obstet Gynecol. 2011;204:431 e431–438. doi: 10.1016/j.ajog.2011.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bublitz MH, Stroud LR. Childhood sexual abuse is associated with cortisol awakening response over pregnancy: Preliminary findings. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shea AK, Streiner DL, Fleming A, Kamath MV, Broad K, Steiner M. The effect of depression, anxiety and early life trauma on the cortisol awakening response during pregnancy: preliminary results. Psychoneuroendocrinology. 2007;32:1013–1020. doi: 10.1016/j.psyneuen.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 70.Ellis MJ, Livesey JH, Inder WJ, Prickett TC, Reid R. Plasma corticotropin-releasing hormone and unconjugated estriol in human pregnancy: gestational patterns and ability to predict preterm delivery. Am J Obstet Gynecol. 2002;186:94–99. doi: 10.1067/mob.2002.119188. [DOI] [PubMed] [Google Scholar]
- 71.Warren WB, Gurewitsch ED, Goland RS. Corticotropin-releasing hormone and pituitary-adrenal hormones in pregnancies complicated by chronic hypertension. Am J Obstet Gynecol. 1995;172:661–666. doi: 10.1016/0002-9378(95)90589-8. [DOI] [PubMed] [Google Scholar]
- 72.Florio P, Romero R, Chaiworapongsa T, Kusanovic JP, Torricelli M, Lowry PJ, et al. Amniotic fluid and umbilical cord plasma corticotropin-releasing factor (CRF), CRF-binding protein, adrenocorticotropin, and cortisol concentrations in intraamniotic infection and inflammation at term. J Clin Endocrinol Metab. 2008;93:3604–3609. doi: 10.1210/jc.2007-2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gerdner A, Allgulander C. Psychometric properties of the Swedish version of the Childhood Trauma Questionnaire-Short Form (CTQ-SF) Nord J Psychiatry. 2009;63:160–170. doi: 10.1080/08039480802514366. [DOI] [PubMed] [Google Scholar]
- 74.Chisholm JS, Quinlivan JA, Petersen RW, Coall DA. Early Stress Predicts Age at Menarche and First Birth, Adult Attachment, and Expected Lifespan. Human Nature. 2005;16:233–265. doi: 10.1007/s12110-005-1009-0. [DOI] [PubMed] [Google Scholar]
- 75.Belsky J. Childhood experience and the development of reproductive strategies. Psicothema. 2010;22:28–34. [PubMed] [Google Scholar]
- 76.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 77.Cheng YH, Nicholson RC, King B, Chan EC, Fitter JT, Smith R. Glucocorticoid stimulation of corticotropin-releasing hormone gene expression requires a cyclic adenosine 3′,5′-monophosphate regulatory element in human primary placental cytotrophoblast cells. J Clin Endocrinol Metab. 2000;85:1937–1945. doi: 10.1210/jcem.85.5.6552. [DOI] [PubMed] [Google Scholar]
- 78.McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R. A placental clock controlling the length of human pregnancy. Nat Med. 1995;1:460–463. doi: 10.1038/nm0595-460. [DOI] [PubMed] [Google Scholar]
- 79.Ishimoto H, Jaffe RB. Development and Function of the Human Fetal Adrenal Cortex: A Key Component in the Feto-Placental Unit. Endocrine Reviews. 2011;32:317–355. doi: 10.1210/er.2010-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O’Donnell K, O’Connor TG, Glover V. Prenatal stress and neurodevelopment of the child: focus on the HPA axis and role of the placenta. Developmental neuroscience. 2009;31:285–292. doi: 10.1159/000216539. [DOI] [PubMed] [Google Scholar]
- 81.Coe CL, Lubach GR. Fetal Programming: Prenatal Origins of Health and Illness. Current Directions in Psychological Science. 2008;17:36–41. [Google Scholar]
- 82.Huizink AC, Robles de Medina PG, Mulder EJH, Visser GHA, Buitelaar JK. Stress during pregnancy is associated with developmental outcome in infancy. Journal of Child Psychology and Psychiatry. 2003;44:810–818. doi: 10.1111/1469-7610.00166. [DOI] [PubMed] [Google Scholar]
- 83.O’Connor TG, Ben-Shlomo Y, Heron J, Golding J, Adams D, Glover V. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biological psychiatry. 2005;58:211–217. doi: 10.1016/j.biopsych.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 84.Uh A, Nicholson RC, Gonzalez GV, Simmons CF, Gombart A, Smith R, et al. Lipopolysaccharide stimulation of trophoblasts induces corticotropin-releasing hormone expression through MyD88. Am J Obstet Gynecol. 2008;199:317 e311–316. doi: 10.1016/j.ajog.2008.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang W, Nan X, Ji P, Dow KE. Corticotropin releasing hormone modulates endotoxin-induced inflammatory cytokine expression in human trophoblast cells. Placenta. 2007;28:1032–1038. doi: 10.1016/j.placenta.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 86.Theoharides TC, Singh LK, Boucher W, Pang X, Letoumeau R, Webster E, et al. Corticotropin-releasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its proinflammatory effects. Endocrinology. 1998;139:403–413. doi: 10.1210/endo.139.1.5660. [DOI] [PubMed] [Google Scholar]
- 87.Torricelli M, Novembri R, Bloise E, De Bonis M, Challis JR, Petraglia F. Changes in placental CRH, urocortins, and CRH-receptor mRNA expression associated with preterm delivery and chorioamnionitis. J Clin Endocrinol Metab. 2011;96:534–540. doi: 10.1210/jc.2010-1740. [DOI] [PubMed] [Google Scholar]
- 88.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci U S A. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, Croft JB. Cumulative childhood stress and autoimmune diseases in adults. Psychosom Med. 2009;71:243–250. doi: 10.1097/PSY.0b013e3181907888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rooks C, Veledar E, Goldberg J, Bremner JD, Vaccarino V. Early trauma and inflammation: role of familial factors in a study of twins. Psychosom Med. 2012;74:146–152. doi: 10.1097/PSY.0b013e318240a7d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pereira J, Vickers K, Atkinson L, Gonzalez A, Wekerle C, Levitan R. Parenting stress mediates between maternal maltreatment history and maternal sensitivity in a community sample. Child Abuse Negl. 2012;36:433–437. doi: 10.1016/j.chiabu.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 92.Roberts R, O’Connor T, Dunn J, Golding J, Team AS. The effects of child sexual abuse in later family life; mental health, parenting and adjustment of offspring. Child Abuse Negl. 2004;28:525–545. doi: 10.1016/j.chiabu.2003.07.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.