Abstract
The medial temporal lobe (MTL), encompassing the hippocampus and parahippocampal gyrus (PHG), is a heterogeneous structure which plays a critical role in memory and cognition. Here we investigate functional architecture of the human MTL along the long-axis of the hippocampus and PHG. The hippocampus showed stronger connectivity with ventral striatum, ventral tegmental area and amygdala — regions important for integrating reward and affective signals, whereas the PHG showed stronger connectivity with unimodal and polymodal association cortices. In the hippocampus, the anterior node showed stronger connectivity with the anterior medial temporal lobe and the posterior node showed stronger connectivity with widely-distributed cortical and subcortical regions including those involved in sensory and reward processing. In the PHG, differences were characterized by a gradient of increasing anterior to posterior node connectivity with core nodes of the default mode network. Left and right MTL connectivity patterns were remarkably similar, except for stronger left than right MTL connectivity within the hippocampus and PHG. Graph theoretical analysis of MTL-based networks revealed higher node centrality of the posterior, compared to anterior and middle hippocampus. The PHG showed prominent gradients in both node degree and centrality along its anterior to posterior axis. Our findings highlight several novel aspects of functional heterogeneity in connectivity along the long-axis of the human MTL and provide new insights into how its network organization supports integration and segregation of signals from distributed brain areas. The implications of our findings for a principled understanding of distributed pathways that support memory and cognition are discussed.
Keywords: medial temporal lobe, hippocampus, connectivity, network, fMRI, memory
Introduction
The medial temporal lobe (MTL) is a heterogeneous brain structure with multiple distinct roles in memory and cognition (Eichenbaum et al. 2007; Squire et al. 2004; Squire and Zola-Morgan 1991; Bird and Burgess 2008; Maguire et al. 2000). The hippocampus and parahippocampal gyrus (PHG) are two major anatomically distinct divisions of the MTL, spanning the dorsal and ventral aspects along its anterior-posterior axis (Squire and Zola-Morgan 1991; Squire et al. 2004). Studies in animals have shown that the hippocampus and PHG have different cortical and subcortical afferent and efferent projections (Aggleton et al. 2005; Goldman-Rakic et al. 1984; Kobayashi and Amaral 2003; Suzuki and Amaral 1994a), but very little is known about large-scale functional connectivity and network profiles of the human MTL at the whole brain level. Knowledge of the large-scale functional organization of the hippocampus and the PHG is critical for understanding how each of the major MTL subdivisions makes distinct contributions to memory and cognition through their functional interactions with other distributed brain regions.
Most of our current knowledge of MTL circuitry is based on anatomical tract-tracing studies in animals (Aggleton et al. 2005; Suzuki and Amaral 1994a, f). Research in monkeys and rodents has demonstrated that the MTL receives widespread projections from multiple unimodal and polymodal association cortices. Most of these cortical inputs converge, via the perirhinal cortex (PRC) and the parahippocampal cortex (PHC) regions in the PHG, onto the entorhinal cortex (ERC), which functions as the main interface between the hippocampus and neocortex (Van Hoesen et al. 1972; Krimer et al. 1997; Buzsaki 1996). The ERC, in turn, projects to the hippocampus, which is positioned at the top of a hierarchical circuitry within the MTL (Burwell 2000; van Strien et al. 2009; Powell et al. 2004; Sakai and Miyashita 1991). In addition to massive inputs from neocortical areas, the hippocampus also receives ascending projections from subcortical structures including the striatum and midbrain (Bird and Burgess 2008; Lisman and Grace 2005; Luo et al. 2011; Voermans et al. 2004). Thus, research in animal models has provided strong evidence that the hippocampus and PHG subdivisions (including PHC, PRC and ERC) have distinct patterns of anatomical connectivity.
The extent to which findings from histological tract-tracing studies in animals relate to the anatomical and functional organization of the human MTL is as yet unclear. To date, only a few studies have examined the structural and functional connectivity of the human MTL. Powell and colleagues (Powell et al. 2004) used diffusion tensor imaging (DTI) to examine structural connectivity of the human PHG. They identified white matter pathways between the PHG and multiple cortical areas including the anterior and posterior temporal lobe, orbitofrontal cortex and extrastriate occipital lobe, as well as pathways linking the PHG and the hippocampus within the MTL (Powell et al. 2004). However, this study was restricted to connectivity of the anterior PHG along the ventral visual processing pathways, thus providing a limited view of MTL circuitry. Granziera and colleagues used diffusion spectrum imaging (DSI) to identify the hippocampus-mammillary body pathway via the fornix and connections between the lateral subiculum and the cingulate cortex (Granziera et al. 2011). Recently, Zeineh and colleagues combined high-resolution DTI with a detailed segmentation of the MTL to identify tracks representing the major pathways within the MTL, including the ventral cingulum bundle, the perforant pathways, subicular projections to the fornix and the ERC, and entorhinal projections to the PRC and the PHC (Zeineh et al. 2012). Due to the limited field-of-view used in these studies, connectivity outside of the MTL was not examined. Thus, the large-scale anatomical connectivity of the human MTL remains poorly characterized.
In contrast to the limited investigations of anatomical connectivity of the human MTL, a large number of task-related fMRI studies have focused on differential activation of MTL subdivisions associated with different aspects of memory and cognitive processes, including item, associative and relational memory (Davachi et al. 2003; Henke et al. 1999; Davachi 2006; Qin et al. 2007; Qin et al. 2009; Staresina and Davachi 2010), recollection- and familiarity-based recognition memory (Diana et al. 2007; Ranganath et al. 2004; Yassa and Stark 2008), memory strength (Qin et al. 2011; Shrager et al. 2008; Song et al. 2011; Diana and Ranganath 2011), novelty detection and pattern separation (Kirwan and Stark 2004; Yassa and Stark 2008; Bakker et al. 2008). Within the anterior PHG, the PRC has been reported to be essential for item-based processing for objects and identities (Davachi et al. 2003; Henke et al. 1999; Qin et al. 2009; Staresina and Davachi 2010), while the anterior and posterior PHC (at the posterior PHG) preferentially contribute to domain-general and domain-specific (such as spatial-related) contextual information, respectively (Aminoff et al. 2007; Bar et al. 2008a; Bar et al. 2008c; Litman et al. 2009; Engelien et al. 2000; Schon et al. 2004; Aminoff et al. 2013). Collectively, these studies point to material and context-specific functional distinctions along the anterior-posterior axis of the PHG. In contrast, functional dissociations along the anterior-posterior axis of the hippocampus are less well understood. Many functional neuroimaging studies have demonstrated that the hippocampus plays an important role in reward- and motivation-based learning due to its complex interaction with striatum, basal ganglia and dopaminergic midbrain regions (Shohamy and Wagner 2008; Wimmer and Shohamy 2012). While some fMRI studies have suggested that the anterior and posterior hippocampus both play domain-general roles in declarative memory (Davachi 2006; Spaniol et al. 2009; Diana et al. 2007; Eichenbaum et al. 2007), others have proposed that the posterior hippocampus is more important for recollective memory and spatial navigation (Diana et al. 2007; Maguire et al. 2000; Ranganath et al. 2004) and that the anterior hippocampus plays a more dominant role in novelty- and emotion-related processes (Poppenk and Moscovitch 2011; Poppenk et al. 2008; Strange and Dolan 2006; Strange et al. 1999). How brain circuitry associated with various MTL subdivisions supports these distinct mnemonic and cognitive functions is poorly understood. Here we use intrinsic functional connectivity analysis in combination with novel brain network analysis of the human MTL to address critical gaps in our knowledge of human MTL circuitry.
Intrinsic functional connectivity analysis has emerged as a powerful systems neuroscience approach for uncovering key architectural features of large-scale circuits associated with individual brain areas (Bressler and Menon 2010; Fox and Raichle 2007; Greicius et al. 2003; Qin et al. 2012). Since its first use in mapping the somatomotor system (Biswal et al. 1995), intrinsic functional connectivity analysis has been used to characterize multiple brain systems, including those involved in sensory processing, language, emotion, and attention (Greicius et al. 2003; Fox et al. 2006; Hampson et al. 2002; Lowe et al. 1998). The first such studies involving the MTL arose from findings that this region is part of the default mode network (Greicius et al. 2004; Buckner et al. 2008), a brain network supporting internally-oriented processes and autobiographical memory (Raichle et al. 2001; Greicius et al. 2003; Buckner and Carroll 2007; Vincent et al. 2006). Combining resting-state fMRI with DTI, Greicius and colleagues showed functional and structural connections between the MTL and the retrosplenial cortex as well as connections between the medial prefrontal cortex and posterior cingulate cortex (Greicius et al. 2009). The next series of studies in the field examined the intrinsic functional organization of the human MTL in more detail by examining connectivity of different segments along its axis. Kahn and colleagues reported two separate pathways associated with the anterior and posterior segments of the MTL (Kahn et al. 2008). Specifically, the posterior PHC was correlated with lateral parietal cortex and posterior medial cortex, whereas the anterior hippocampus and the ERC were correlated with the lateral temporal cortex. Similar evidence has been provided by Poppenk and Moscovitch that the anterior and posterior subdivisions of the hippocampus are involved in two different pathways, with the posterior pathway supporting recollection memory and the anterior pathway being associated with social and emotional processes (Poppenk and Moscovitch 2011). Kahn and Shohamy recently identified robust functional connectivity between the hippocampus and mesolimbic pathways in humans (Kahn and Shohamy 2013). However, these studies did not directly contrast functional connectivity profiles associated with different MTL subdivisions, nor did they characterize differences in connectivity between the hippocampus and PHG with subcortical and mesolimbic pathways at the whole brain level.
More recent studies have focused on intrinsic functional connectivity within the MTL. Lacy and Stark demonstrated that within the MTL, the hippocampal subfields and PHG regions have distinct functional connectivity profiles (Lacy and Stark 2012). They found that hippocampal subfields had relatively higher correlations with each other both within and across hemispheres but did not have strong correlations with other MTL cortices. This study, however, focused on the hippocampus and PHG only. Similarly, Libby and colleagues characterized distinct functional connectivity profiles of the PRC and PHC with hippocampal subfields (Libby et al. 2012). They reported dissociations between anterior-posterior functional connectivity for hippocampal subfields CA1 and subiculum. While these studies have provided important insights into the local organization of the MTL, they leave unclear the precise dissociation of large-scale functional connectivity patterns for the human MTL subdivisions at the whole brain level and their differential connectivity profiles associated with the anterior-posterior axis of the hippocampus and the PHG. Critically, none of these previous studies have examined differential connectivity with cortical and subcortical structures that are important for emotion- and reward-related processes. Little is known about the large-scale network organization of the human MTL circuits, knowledge of which is crucial for advancing our understanding of the information processing architecture of the human MTL.
In the past decade, graph theoretical approaches have provided novel quantitative metrics for characterizing the intrinsic functional organization of complex brain networks (Bullmore and Sporns 2009; Bassett and Bullmore 2006; Supekar et al. 2009). Graph theory has been used to quantify structural and functional network properties at the macroscopic whole-brain as well as microscopic cellular levels in humans and animals (Bullmore and Sporns 2009). As with many other species, human brain networks demonstrate a small-world non-random topology (Bullmore and Sporns 2009). Human structural and functional brain networks, derived from structural and functional MRI, as well as DTI, data demonstrate short path length, high degree of clustering, and modularity with clusters linked by highly connected cortical ‘hubs’ (Bullmore and Sporns 2009; Bassett and Bullmore 2006). These properties have been widely used as quantitative metrics of global brain network organization (Bullmore and Sporns 2009; Di Martino et al. 2014; Supekar et al. 2008). Crucially, no previous studies have investigated quantitative network metrics and organization associated with the large-scale functional organization of individual MTL subregions.
To address key gaps in the literature and to gain a better understanding of the large-scale functional organization of the MTL, we conducted a comprehensive functional connectivity and graph-based brain network analysis of distinct divisions of the hippocampus and PHG. We used resting-state fMRI data acquired from thirty-six healthy young adults ages 19–22 using an optimized spiral in-out pulse sequence known to reduce signal dropout and increase signal-to-noise ratio (Glover 2012; Glover and Law 2001; Glover and Thomason 2004). We used two parallel arrays of nodes along the longitudinal axis of the hippocampus and the PHG in each hemisphere (Figure 1A). Three nodes were chosen in the hippocampus along its anterior, middle and posterior segments to characterize intrinsic functional organization patterns along the hippocampal anterior-posterior axis. Four nodes were chosen along the PHG axis – in the anterior PRC, in the transition area between ERC and PRC, in the anterior PHC and in the posterior PHC – based on their aforementioned distinct contributions to memory and cognition. These seven nodes, representing distinct subdivisions of the hippocampus and the PHG, allowed us to systematically characterize the large-scale functional connectivity of MTL. In addition, graph analytical metrics were used to quantify and contrast the relative importance of each of the seven subdivisions within the MTL functional connectivity network. Specifically, we used node degree and centrality, two complementary graph-theoretical measures that have been widely used to capture the relative importance of a node in a network (Bolland 1988; Bullmore and Sporns 2009). The node degree provides information about how densely each node connects with all other network nodes, and eigenvector centrality measures the influence of a node in a network (Bullmore and Sporns 2009). A node with high centrality plays a crucial role in efficient communication and information transfer (Bullmore and Sporns 2009). Crucially, nodes with high degree and centrality are thought to play a key role in facilitating information transfer across the entire network, and together these measures capture fundamental properties of network organization (Bullmore and Sporns 2009). We used these two metrics to characterize differences in the importance of each node in the MTL-based target network. Based on previous findings from anatomical and functional neuroimaging studies, we predicted that the PHG would show greater connectivity with unimodal and polymodal association areas, whereas the hippocampus would exhibit greater connectivity with subcortical and other limbic areas. We further predicted that the PHG and hippocampus would show heterogeneous patterns of functional connectivity along their anterior-posterior axes and that these patterns would be further reflected in distinct network measures of node degree and centrality.
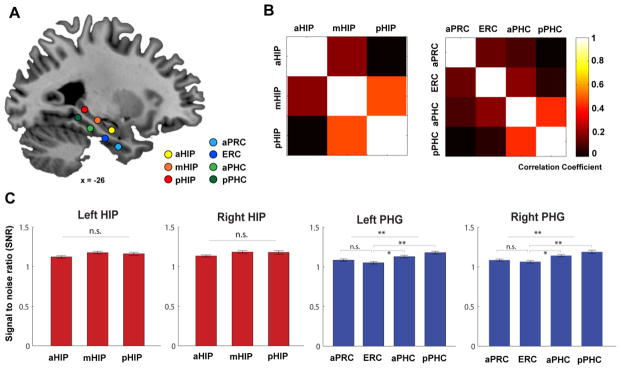
Figure 1. Nodes of interest in the MTL and their inter-node correlation.
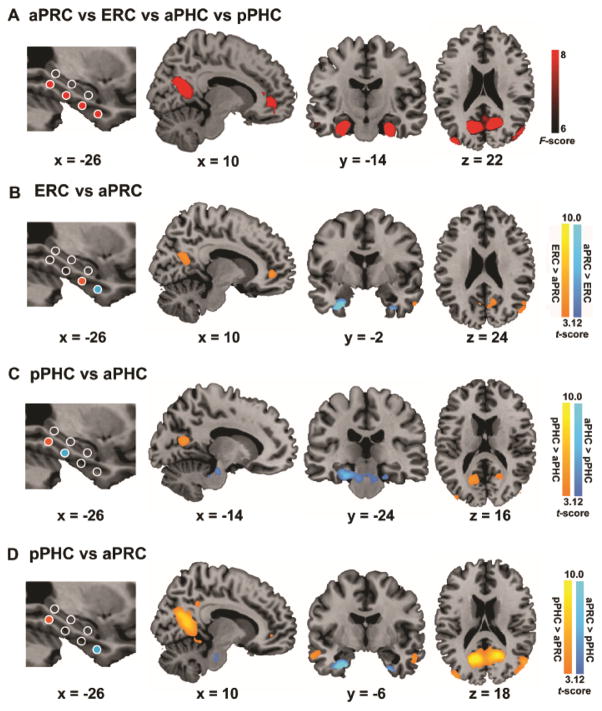
(A) Sagittal view showing node locations along the long axis of the left hippocampus and parahippocampal gyrus (PHG). Nodes are superimposed on a high-resolution T1-weighted brain template in stereotaxic MNI space. (B) Correlation matrix maps indicating mean inter-node correlation computed by partial correlation between three hippocampal nodes and four PHG nodes respectively. (C) The temporal signal-to-noise ratio for spontaneous BOLD signal fluctuations within the frequency band of interest over other frequency bands in the hippocampal and PHG nodes. Notes: aHIP, anterior hippocampus; mHIP, middle hippocampus; pHIP, posterior hippocampus; aPRC, anterior perirhinal cortex; ERC, entorhinal cortex; aPHC, anterior parahippocampal cortex; pPHC, posterior parahippocampal cortex.
Materials and Methods
Participants
Thirty-six (17 males, 19 females) young, healthy, right-handed adults (mean age 20.58 ± 1.04) participated in this study after giving written, informed consent. All participants reported no history of neurological or psychiatric disorders. The study protocol was approved by the Stanford University Institutional Review Board.
Data Acquisition
For the resting-state fMRI scan, participants were instructed to keep their eyes closed but not fall asleep and remain still for the duration of an 8-minute scan. Whole brain functional imaging data were acquired on a 3T GE Signa Scanner (General Electric, Milwaukee, WI) using a custom-built head coil with a T2*-sensitive gradient echo spiral in-out pulse sequence based on blood oxygenation level-dependent contrast (BOLD) (Glover and Law 2001). Specifically, we used an optimized spiral in-out pulse sequence with optimized parameter settings such as optimization of the B0 shim, reduced slice thickness, optimized slice orientation to the inhomogeneity gradients, and the use of shorter echo times. This has been demonstrated by a series of studies to reduce the effect of macroscopic susceptibility-induced field gradients generated near air-tissue interfaces and to increase both signal-to-noise ratio and BOLD contrast-to-noise ratio in T2*-weighted images (Glover and Law 2001; Glover and Thomason 2004; Glover 2012). A total of 29 axial slices (4.0 mm thickness, 0.5 mm skip) parallel to the anterior commissure-posterior commissure (AC-PC) line and covering the whole brain were imaged with the following parameters: 2000 ms TR, 30 ms TE, 80° flip angle, field of view 20 cm, 256 × 256 × 132, and matrix size 64 × 64, providing an in-plane spatial resolution of 3.125 mm. To reduce blurring and signal loss arising from field inhomogeneities, an automated high-order shimming method based on spiral acquisitions was used before acquiring functional images. A linear shim correction was applied separately for each slice during reconstruction using a magnetic field map acquired automatically by the pulse sequence at the beginning of the scan (Glover and Lai 1998).
Data Preprocessing
Data were preprocessed using SPM8 software (http://www.fil.ion.ucl.ac.uk/spm). The first eight volumes were discarded to allow signal equilibration effects stabilization of the MR signal. Remaining functional Images were realigned to correct for head motion, and any data affected by head motion over 2 mm or rotation more than 1° was excluded. The realigned images were corrected for errors in slice-timing, spatially transformed to standard stereotaxic space based on the Montreal Neurologic Institute (MNI) coordinate system, resampled every 2 mm using sinc interpolation, and smoothed with a 6-mm full-width half-maximum Gaussian kernel to reduce spatial noise. Voxel-wise time series were then filtered using a temporal bandpass filter (0.008 to 0.10 Hz).
Definition of MTL Nodes of Interest
We used two parallel arrays of nodes along the longitudinal axis of the hippocampus and the PHG. A total of 7 nodes were defined in the left hemisphere and a parallel set of 7 nodes were defined in the right hemisphere (Figure 1A; Table 1). In each hemisphere, 3 nodes were located in the hippocampus: head (anterior, aHIP), body (middle, mHIP) and tail (posterior, pHIP). The other 4 nodes were located along the PHG axis in the anterior PRC, the ERC, the anterior PHC (aPHC) and posterior PHC (pPHC). The precise locations of the nodes were based on convergent evidence from previous studies in humans about distinct anatomical and functional profiles of the MTL subdivisions and functional heterogeneity along the hippocampal long-axis. Specifically, the head, body and tail of the hippocampus were anatomically defined based on previous guidelines (Hackert et al. 2002; Greicius et al. 2003), suggesting the anterior 35% of the coronal slices as ‘head’, the intermediate 45% as ‘body’, and the remaining 20% as ‘tail’. The anterior and posterior nodes of the hippocampus were defined by the uncal apex appropriately anterior to y = −21 mm in MNI space as suggested by Poppenk and Moscovitch (2011). We then took the middle points of MNI y-axis of these three portions as the anterior, middle and posterior nodes of interest (Table 1).
Table 1.
Coordinates for seven seed regions of interest
| x | y | z | |
|---|---|---|---|
| aHipp | (±) 24 | −14 | −20 |
| mHipp | (±) 26 | −26 | −12 |
| pHipp | (±) 26 | −34 | −4 |
| aPRC | (±) 26 | −4 | −36 |
| ERC | (±) 26 | −16 | −28 |
| aPHC | (±) 26 | −30 | −20 |
| pPHC | (±) 26 | −40 | −12 |
Notes: aHipp, mHipp and pHipp represent the anterior, middle, posterior hippocampus respectively; aPRC, anterior perirhinal cortex; ERC, posterior perirhinal cortex proximate to entorhinal cortex; aPHC, anterior parahippocampal cortex; pPHC, posterior parahippocampal cortex. Coordinates for seeds in the right and left hemispheres were defined in the MNI stereotaxic space.
The four nodes in the PHG were based on anatomical landmarks and currently known models of its functional specialization based on human neuroimaging studies (Davachi, 2006). The anatomical landmarks of the aPRC, ENC and PHC were defined in MNI-coordinate space according to guidelines provided by Insausti and colleagues (1998) (Insausti et al. 1998) and Pruessner and colleagues (2002) (Pruessner et al. 2002). The location of the anterior PRC node was based on previous fMRI studies showing domain-specific object recognition and item memory (Kahn et al. 2008; Staresina et al. 2011; Libby et al. 2012), and the ENC node is at the transition area between the PRC and PHC (Insausti et al. 1998). The anterior and posterior nodes of the PHC were defined according to their functional dissociation in spatial and non-spatial contextual processing (Bar, 2004; Aminoff and Bar, 2007). As shown in Figures 1B and C, the correlation coefficients between pairs of nodes within the hippocampus and PHG ranged from 0.12 to 0.56, indicating that there is substantial non-shared variance between these nodes.
Signal-to-noise ratio in MTL Nodes of Interest
To quantify resting-state fMRI signals in the anterior and posterior MTL nodes and rule out gross differences arising from potential susceptibility artifacts, we computed the temporal signal-to-noise ratio (tSNR) of spontaneous BOLD signal fluctuations for the frequency band (0.008–0.1) of interest over other frequency bands for each node involved in our analysis. That is, tSNR = S(x)/S(y), where S(x) represents the power spectrum of frequency band of interest (0.008–0.1) and S(y) represents the power spectrum of other frequency bands. As shown in Figure 1C, we observed a very similar pattern of tSNR in 3 nodes in the hippocampus as well as 4 nodes within the PHG for each hemisphere, suggesting the robustness of BOLD fluctuations along the long-axis of the hippocampus and PHG.
Functional Connectivity Analysis
For each node ROI, time series were extracted by averaging across all voxels. Each of the resulting ROI time series was used as a covariate of interest in an individual subject-level general linear model. A global time series computed across whole brain voxels and 6 motion parameters were included as additional covariates of no interest to remove confounding effects of physiological noise and potential head movement-related artifacts. Separate functional connectivity analyses were first conducted for each ROI and subject.
Corresponding contrast images for each node’s functional connectivity map from the individual level analysis were submitted to a second-level group analysis. Group level functional connectivity of each node was statistically analyzed using one sample t test with a stringent threshold of p < 0.001 with family-wise error (FWE) correction on a whole-brain level. Direct comparisons between functional connectivity maps of multiple nodes in the hippocampus and the PHG were performed using one-way analysis of variance (ANOVA), with a height threshold of p < 0.001 and an extent threshold of p < 0.01, corrected for multiple spatial comparisons using Monte Carlo simulations (Hayasaka et al. 2004; Nichols et al. 2005; Nichols and Hayasaka 2003).
To characterize functional connectivity patterns of each node to specific target brain regions, mean parameter estimates (β) of these regions were extracted from individual contrast parameter images by using the MarsBar toolbox (http://marsbar.sourceforge.net). To avoid selection bias and circularity, thirty-five target ROIs were defined with a 6-mm sphere around peak voxels of significant clusters from a group-level analysis of functional connectivity for the hippocampus and PHG, collapsing across all 7 node ROIs along the longitudinal axis. Mean parameter estimates, representing connectivity strength of each node ROI with corresponding target masks, were then plotted using polar plots to summarize overall functional connectivity patterns throughout the whole brain – this included target ROIs in subcortical structures as well as motor, temporal, frontal, occipital and parietal cortices.
Network Construction
The regional BOLD-fMRI time series were extracted for each of 49 regions (or nodes), consisting of 7 MTL subdivisions (nodes) in the left hemisphere, 7 MTL nodes in the right hemisphere, and 35 target ROIs (see above) of the overall left and right MTL (collapsing across individual divisions). The BOLD time series of 49 nodes were then correlated (Pearson’s r) region-by-region for each participant, creating a 49 × 49 correlation matrix. Fisher’s z-transform was applied to the correlation values to ensure normality. These correlations, representing functional connectivity between distinct ROIs, were used for network visualization and for graph theoretical analysis.
Graph Theoretical Network Analysis
A 49-node undirected weighted graph representing MTL functional connectivity network was constructed using the correlation matrix for each participant. We computed two node-level metrics – degree and eigenvector centrality – for each graph. The degree of every node was computed by counting the number of edges incident on that node. Eigenvector centrality is a self-referential measure of centrality – that is, nodes have high eigenvector centrality if they connect to other nodes that have high eigenvector centrality. The eigenvector centrality of node i is equivalent to the ith element in the eigenvector corresponding to the largest eigenvalue of the adjacency matrix (Bullmore and Sporns 2009).
The Brain Connectivity Toolbox (http://www.indiana.edu/~cortex/connectivity_toolbox.html) was used to compute these metrics for each MTL subdivision. ANOVA was then used to quantify network properties of the hippocampus and PHG along their respective anterior-posterior axes.
Results
Distinct functional connectivity profiles of the hippocampus and the PHG
We first focused on the left hemisphere and examined functional connectivity patterns of nodes of interest in the hippocampus and the PHG separately. We found that spontaneous activity in the MTL nodes was strongly correlated with activity in a widely distributed set of cortical and subcortical regions (Figure S1, Table S1). We then examined differential connectivity between the hippocampus and the PHG. Compared to the PHG, the hippocampus showed greater connectivity with multiple subcortical structures, mainly the amygdala, caudate, putamen, thalamus, nucleus accumbens (NAcc), and VTA/substantial nigra (SN) (Figure 2A, Table S2). In contrast, the PHG showed greater connectivity with multiple cortical regions, including multiple unimodal and polymodal association areas in occipital, parietal and temporal lobes, retrosplenial cortex, posterior cingulate cortex and precuneus, middle and anterior cingulate cortex, and insular cortex (Figure 2A, Table S2). Parallel analysis of the hippocampus and the PHG in the right hemisphere revealed very similar functional connectivity patterns (Figure S2).
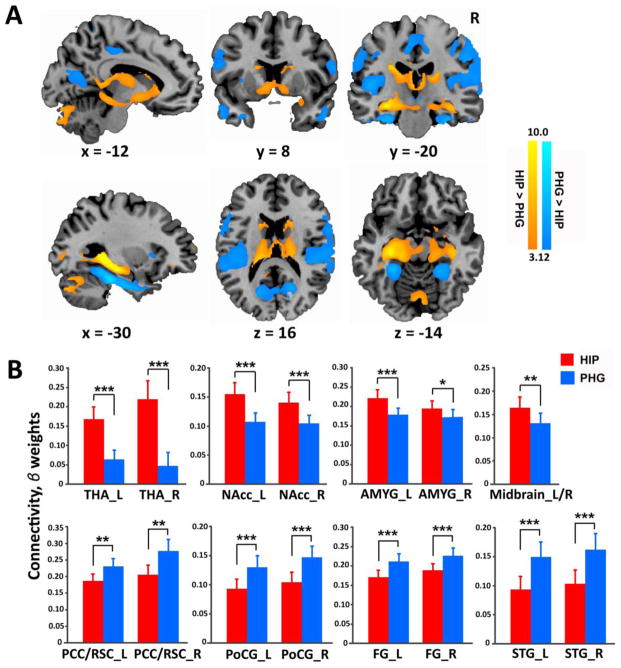
Figure 2. Functional connectivity of the hippocampus and PHG.
(A) Direct comparisons of functional connectivity associated with the entire hippocampus versus the entire PHG. (B) Bar graphs show mean functional connectivity strength of the hippocampus (coded in red) and PHG (coded in blue) with four subcortical and four cortical target regions of interest (ROI). Error bars represent standard error of mean (SEM). t values were generated by paired t-test. Notes: * p < 0.05; ** p < 0.01; *** p < 0.001. HIP, hippocampus; PHG, parahippocampal gyrus; THA, thalamus; NAcc, nucleus accumbens; AMYG, amygdala; PCC/RSC, posterior cingulate cortex/retrosplenial cortex; PoCG, postcentral gyrus; FG, fusiform gyrus; STG, superior temporal gyrus; R, right; L, left.
Additional analyses were performed using target ROIs associated with the hippocampus and PHG. To avoid selection bias and circularity, target ROIs were defined with a 6-mm sphere around peak voxels of significant clusters from a group-level analysis of functional connectivity for the overall hippocampus and the overall PHG collapsing across seven nodes along the longitudinal axis. Separate paired t-tests on the mean functional connectivity strength of each of these target ROIs revealed that, compared to the PHG, the hippocampus had significantly greater functional connectivity with the thalamus, NAcc, amygdala, and midbrain. In contrast, compared to the hippocampus, the PHG exhibited significantly greater connectivity with the posterior cingulate cortex/retrosplenial cortex (PCC/RSC), postcentral gyrus, fusiform gyrus, and superior temporal gyrus (Figure 2B; Table 2). These analyses confirmed distinct functional connectivity profiles associated with the hippocampus and PHG.
Table 2.
Statistics of T and F tests from ROI analyses
| Comparisons | Hem | T/F | P | |
|---|---|---|---|---|
|
| ||||
| HIP > PHG | Thalamus | L | t (35) = 5.56 | < 0.0001 |
| R | t (35) = 6.05 | < 0.0001 | ||
| NAcc | L | t (35) = 4.96 | < 0.0001 | |
| R | t (35) = 5.11 | < 0.0001 | ||
| Amygdala | L | t (35) = 4.81 | < 0.0001 | |
| R | t (35) = 2.67 | < 0.05 | ||
| Midbrain | L/R | t (35) = 3.48 | < 0.005 | |
| HIP < PHG | PCC/RSC | L | t (35) = 3.33 | < 0.005 |
| R | t (35) = 3.37 | < 0.005 | ||
| PCG | L | t (35) = 3.64 | < 0.001 | |
| R | t (35) = 3.97 | < 0.0005 | ||
| FG | L | t (35) = 4.42 | < 0.0001 | |
| R | t (35) = 3.87 | < 0.0005 | ||
| STG | L | t (35) = 4.08 | < 0.0005 | |
| R | t (35) = 3.98 | < 0.0005 | ||
| aHIP vs. mHIP vs. pHIP | Caudate | L | F(2, 35) = 5.58 | <0.01 |
| R | F(2,35) = 6.05 | <0.005 | ||
| Putamen | L | F(2, 35) = 22.30 | < 0.0001 | |
| R | F(2, 35) = 14.09 | < 0.0001 | ||
| NAcc | L | F(2, 35) = 21.82 | < 0.0001 | |
| R | F(2, 35) = 12.62 | < 0.0001 | ||
| Midbrain | L/R | F(2, 35) = 15.16 | < 0.0001 | |
| Amygdala | L | F(2, 35) = 9.29 | < 0.0001 | |
| R | F(2, 35) = 4.50 | < 0.01 | ||
| PCG | L | F(2, 35) = 8.13 | < 0.001 | |
| R | F(2, 35) = 8.21 | < 0.001 | ||
| Temporal pole | L | F(2, 35) = 3.91 | < 0.03 | |
| R | F(2, 35) = 4.58 | < 0.01 | ||
| MPFC | L | F(2, 35) = 7.96 | < 0.001 | |
| R | F(2, 35) = 14.01 | < 0.0001 | ||
| FG | L | F(2, 35) = 7.94 | < 0.001 | |
| R | F(2, 35) = 9.13 | < 0.0001 | ||
| PCC/RSC | L | F(2, 35) = 3.91 | < 0.03 | |
| aPRC vs. ENC vs. aPHC vs. pPHC | PCC/RSC | L | F(3, 35) =25.02 | < 0.0001 |
| R | F(3,35) = 16.07 | < 0.0001 | ||
| Angular gyrus | L | F(3, 35) = 3.86 | < 0.012 | |
| R | F(3,35) = 9.36 | < 0.0001 | ||
Notes: NAcc, nucleus accumbens; PCC, posterior cingulate cortex; RSC, retrosplenial cortex; PCG, postcentral gyrus; FG, fusiform gyrus; MPFC, medial prefrontal cortex; STG, superior
Inter-hemispheric differences in functional connectivity of the hippocampus and the PHG
To examine inter-hemispheric differences in functional connectivity of the hippocampus, we compared connectivity maps of the hippocampus between the left and right hemispheres. Compared to the right hippocampus, the left hippocampus showed significantly higher functional connectivity with the left hippocampus and left PHG as well as the bilateral NAcc (clusters colored in warm yellow; Figure 3A). The right hippocampus showed significantly higher connectivity only with the right middle hippocampus (a cluster colored in cool blue).
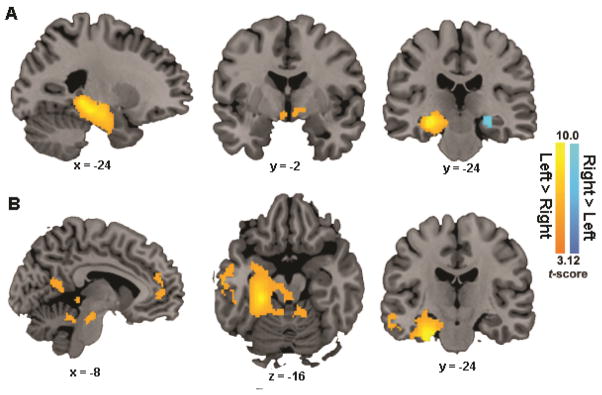
Figure 3. Inter-hemispheric differences in functional connectivity of the hippocampus and the PHG.
(A) Clusters showing significant differences in functional connectivity between the left and right hippocampal nodes; (B) Clusters showing significant differences in functional connectivity between the left and right PHG. Notes: HIP, hippocampus; PHG, parahippocampal gyrus.
For the inter-hemispheric differences in PHG functional connectivity, we found that the left compared to the right PHG showed significantly higher connectivity with several regions within the MTL along the left PHG long-axis and extending into the amygdala, the left anterior temporal lobe, the middle temporal gyrus, the left retrosplenial cortex, the left medial prefrontal cortex, the bilateral midbrain and the cerebellum (Figure 3B). In the opposite contrast (right > left), however, we found no reliable inter-hemispheric differences. These results suggest that the left hemisphere connectivity was stronger than the right in both the hippocampus and PHG.
Functional connectivity profiles along the long-axis of the hippocampus
Next, we examined differences in functional connectivity across three ROIs spanning the anterior-posterior axis of the left hippocampus. One-way ANOVA revealed a significant main effect of region (aHIP versus mHIP versus pHIP) in multiple cortical and subcortical regions. Cortical regions included anterior and posterior cingulate cortex, insular cortex, ventromedial prefrontal cortex, superior frontal gyrus, retrosplenial cortex, precuneus and widespread unimodal and polymodal association areas in occipital and temporal lobes. Subcortical regions of caudate, putamen, thalamus, NAcc and VTA/SN also showed a main effect of hippocampal subdivisions (Figure 3A, Table S3). Parallel analysis for corresponding nodes in the right hippocampus revealed an almost identical pattern of results (Figure S3A).
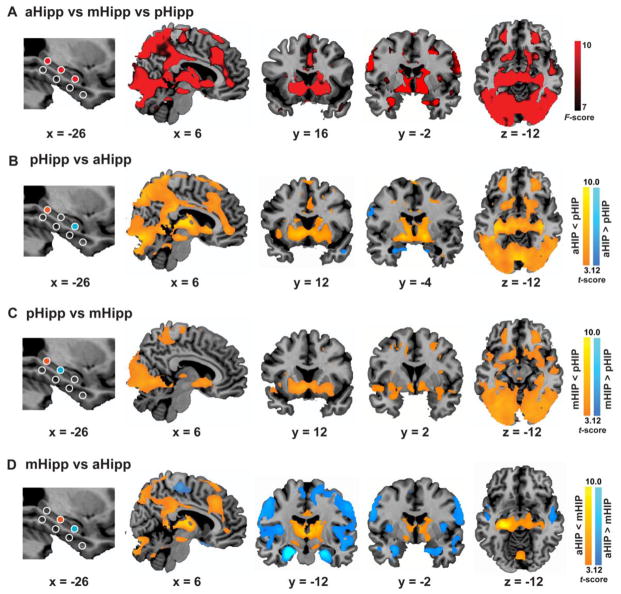
Direct pair-wise comparisons between the posterior, middle and anterior hippocampal nodes revealed that the pHIP, compared to the aHIP, had stronger connectivity with multiple cortical and subcortical regions (Figure 4B, 3C, Table S3). Cortical regions included posterior cingulate cortex, retrosplenial cortex, precuneus, anterior and middle cingulate cortex, ventromedial prefrontal cortex, middle frontal gyrus, and visual association cortex in occipital and temporal lobes. Subcortical regions included NAcc, striatum, thalamus, VTA/SN and mammillary body and declive in cerebellum. A similar but relatively weaker pattern of results was observed when contrasting the mHIP with the aHIP (Figure 4D, Table S3). Compared to the mHIP, however, the aHIP showed significantly greater connectivity with the amygdala, insula, temporal pole, fusiform gyrus, lingual gyrus, superior temporal gyrus and motor cortex (Figure 4D, Table S3). These results suggest that the pHIP shows the strongest connectivity with multiple cortical regions, the aHIP shows stronger connectivity with the bilateral anterior medial temporal lobe (including the anterior hippocampus, the entorhinal and perirhinal cortices extending into the amygdala) and the left temporal pole, and the mHIP shows intermediate patterns of connectivity (Figure 4A–D). Parallel analysis for the above comparisons in the right hippocampus revealed an almost identical pattern of results (Figure S3).
Figure 4. Heterogeneous functional connectivity along the longitudinal axis of the hippocampus.
(A) Main effect of hippocampal seed positions, generated by an omnibus F contrast from one-way ANOVA (i.e., aHIP vs mHIP vs pHIP). (B), (C) and (D) Direct pairwise comparisons for functional connectivity maps between distinct hippocampal seeds. Representative sagittal, coronal and axial slices of significant clusters (with a height threshold of p < 0.001 uncorrected and an extent threshold of p < 0.01 corrected) were overlaid on high-resolution anatomical sections in the MNI stereotaxic space.
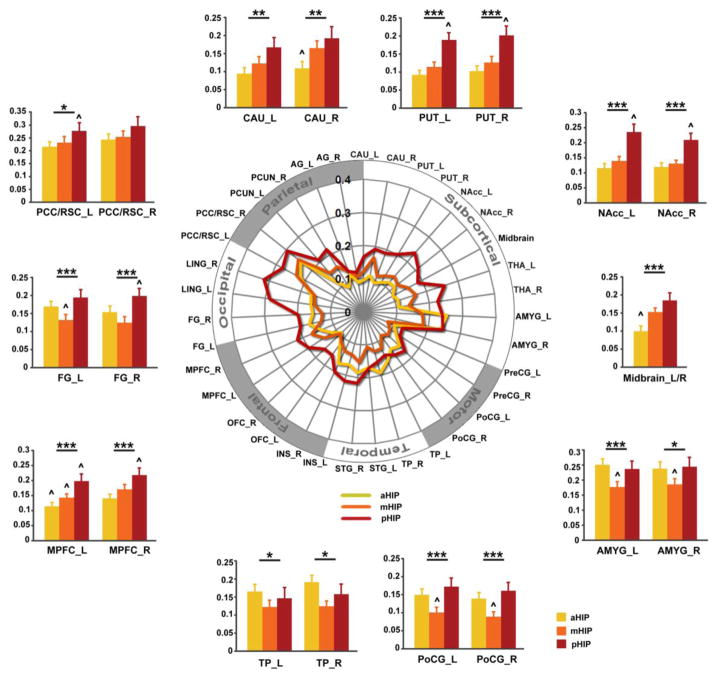
Additional ROI analyses were then performed for major target ROIs associated with the three hippocampal regions. Repeated measures ANOVA confirmed significant differences in connectivity between anterior, middle and posterior hippocampal ROIs and the caudate, putamen, NAcc, midbrain, amygdala, postcentral gyrus, temporal pole, medial prefrontal cortex, fusiform gyrus and PCC/RSC (Table 2). These results confirm that the functional connectivity of the hippocampus is characterized by decreasing connectivity strength along the posterior-to-anterior gradient with multiple posterior midline and subcortical structures. Compared to posterior and middle subdivisions, the anterior hippocampus showed stronger connectivity only with the bilateral anterior medial temporal lobe and the left temporal pole.
To better visualize the differential connectivity patterns associated with the three hippocampal subdivisions, we created schematic polar plots of each node with target regions that demonstrated stronger connectivity with the posterior hippocampus (Figure 4B & 3C). As shown in Figure 5, several subcortical structures (including striatum, thalamus and midbrain) and cortical regions (including midline prefrontal and posterior structures) showed increasing functional connectivity strength along the anterior to posterior axis of the hippocampus. Interestingly, there were also several areas including the amygdala, post central gyrus and temporal pole that showed a U-shape pattern, with the mHIP showing the lowest connectivity compared to the pHIP and the aHIP.
Figure 5. Functional connectivity gradients of the anterior, middle and posterior hippocampus nodes.
(A) Schematic polar plot illustrating connectivity patterns of three hippocampal nodes with target ROIs distributed across the whole brain. The concentric circles depict parameter estimates (β) representing the connectivity strength. (B) Bar graphs show mean connectivity strength of aHIP, mHIP and pHIP with representative target ROIs. Error bars represent SEM. Notes: * p < 0.05; ** p < 0.01; *** p < 0.001; ^, significantly from every others; PCC/RSC, posterior cingulate cortex/retrosplenial cortex; FG, fusiform gyrus; MPFC, medial prefrontal cortex; TP, temporal pole; PoCG, postcentral gyrus; AMYG, amygdala; NAcc, nucleus accumbens; PUT, putamen; CAU, caudate; R, right; L, left.
Functional connectivity profiles along the long-axis of the PHG
We examined differences in connectivity across four nodes spanning the anterior-posterior axis of the PHG. One-way ANOVA for functional connectivity of the four PHG nodes yielded a significant main effect in the default mode network (DMN) regions, including precuneus, retrosplenial cortex, ventromedial prefrontal cortex and bilateral angular gyrus (Figure 6A, Table S4). Direct comparisons between functional connectivity of the four PHG nodes revealed a gradient of decreased connectivity along the posterior to anterior axis in these DMN regions (Figures 6B–D, Table S4). Thus, compared to the PRC, the PHC showed the strongest connectivity with these regions, and the ERC and aPHC showed intermediate levels of connectivity with these target DMN regions. Parallel analysis for corresponding nodes in the right PHG revealed an almost identical pattern of results (Figure S4A–D).
Figure 6. Heterogeneous functional connectivity of the PHG nodes.
(A) Main effect of PHG nodes, generated from one-way ANOVA with an omnibus F-contrast. (B), (C) and (D) Direct pairwise comparisons for functional connectivity maps between distinct parahippocampal nodes: Representative sagittal, coronal and axial slices of significant clusters (with a height threshold of p < 0.001 and an extent threshold of p < 0.01 corrected) were overlaid on high-resolution anatomical sections in the MNI stereotaxic space.
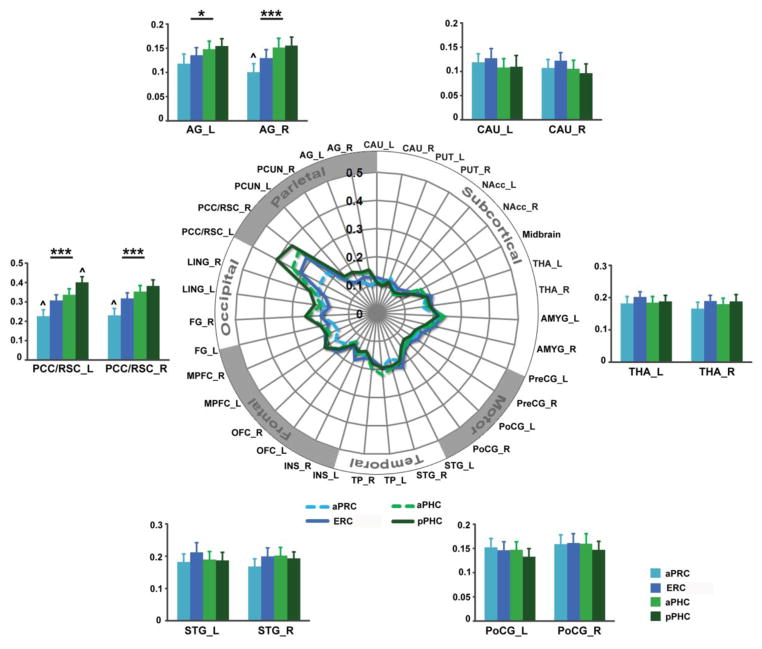
Visualization of these results using polar plots of PHG connectivity confirmed that the four PHG nodes had a similar pattern of connectivity with most subcortical and cortical target regions except core nodes of the DMN (Figure 7). Statistical significance assessed with repeated measures ANOVAs revealed significant connectivity differences of the four PHG nodes from the posterior cingulate cortex, retrosplenial cortex and angular gyrus but not from any other subcortical/cortical target regions (Figure 7, Table 2). Together, these results point toward a functional connectivity gradient, characterized by posterior-dominant connectivity with the DMN.
Figure 7. Functional connectivity gradients of four distinct PHG nodes.
(A) Schematic polar plot illustrating connectivity patterns of the parahippocampal seeds with target ROIs. The concentric circles depict the parameter estimates (β), which represent the strength of seed connectivity with target ROIs. (B) Bar graphs show mean parameter estimates (β) of aPRC, ERC, aPHC and pPHC connectivity with representative target ROIs. Error bars denote SEM. Notes: All abbreviations are same as Figure 4. Notes p < 0.05; ** p < 0.01; *** p < 0.001. ^, significantly from every other seed. Notes: PCC/RSC, posterior cingulate cortex/retrosplenial cortex; STG, superior temporal gyrus; PoCG, postcentral gyrus; THA, thalamus; CAU, caudate; AG, angular gyrus; R, right; L, left.
Functional gradients in network organization along the anterior-posterior axes of the hippocampus and the PHG
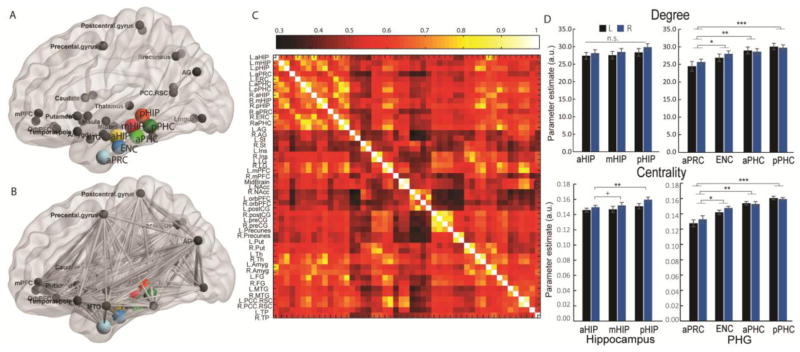
To further examine functional gradients and network organization properties along the MTL long-axis, we conducted graph theoretical analysis of MTL-based target networks collapsing across two hemispheres (Figures 8A–C). As shown in Fig. 7D–E, there is a clear pattern of gradient increases in node degree and centrality along the anterior-to-posterior axes of the hippocampus and the PHG in both the left and right hemispheres. Further analysis revealed a marginally significant gradient increase in node centrality in the right hemisphere (F(2, 105) = 2.70, p = 0.07) along the anterior-to-posterior axis in the right hippocampus, with significantly higher node centrality in the posterior relative to the anterior hippocampus (t(35) = 2.99, p = 0.001) and marginally significant higher centrality than the middle hippocampus (t(35) = 1.75, p = 0.08) (Figure 8D). There is no significant effect for node degree (F(2, 105) = 0.25, p > 0.50) in either the left or right hippocampus nor is there significant effect for node centrality in the left hippocampus (F(2, 105) < 1). These results provide novel quantitative evidence for gradients in functional network organization along the anterior-to-posterior axis of the hippocampus.
Figure 8. Graph theoretical network construction and network organization profiles for nodes along the long axis of the hippocampus and the PHG.
(A) Forty-two nodes within MTL-based intrinsic functional networks which consist of 7 nodes (colored spheres) and 37 unbiasedly defined target ROIs (darken gray spheres) across the whole brain; (B) Edges (gray lines) among 49 nodes within MTL-based intrinsic functional networks; (C) Network construction consisting of a 49-by-49 correlation matrix between nodes for each participant; (D) Network organization properties including levels of the node degree to and centrality of connections separately for each seed. Notes: Abbreviations in (C) are listed in Supplemental Materials. Notes: m.s., marginally significant with p = 0.07 or 0.08; *, p < 0.05; ** p < 0.01; ***, p < 0.001.
A parallel analysis conducted across the four nodes of the left and the right PHG separately revealed significant linear increases in node degree (left: F(3, 140) = 5.04, p < 0.003; right: F(3, 140) = 2.61, p < 0.05) and node centrality along the anterior-to-posterior axis (left: F(3, 140) = 21.33, right: F(3, 140) = 12.30, both p < 0.001), with the highest and lowest node degree and centrality in the most posterior and anterior nodes of the PHG, respectively (Figure 8E). These results provide novel quantitative evidence for prominent gradient increase in functional network organization along the anterior-to-posterior axis of the bilateral PHG.
Discussion
This study investigated the large-scale intrinsic functional organization of anatomically distinct human MTL subdivisions using optimized spiral in-out functional imaging in combination with network and graph theoretical analyses. Several features of MTL connectivity emerged from our analysis of distinct hippocampus and PHG nodes (Figure 9). First, we found a clear dissociation in connectivity between the hippocampus and the PHG. Compared to the PHG, the hippocampus showed stronger connectivity with subcortical regions including basal ganglia, midbrain, thalamus and amygdala, whereas the PHG showed significantly greater connectivity with multiple unimodal and polymodal association areas. Second, the hippocampus showed a connectivity gradient along the anterior-to-posterior axis, with stronger connectivity of the posterior hippocampus with multiple cortical regions and stronger connectivity of the anterior hippocampus with the bilateral anterior medial temporal lobe extending into the amygdala and the left temporal pole. Third, along the anterior-posterior axis of the PHG, the PRC, ERC, aPHC and pPHC showed a similar topographical pattern of connectivity with most cortical and subcortical structures, except for one key feature: posterior PHG nodes showed much stronger connectivity with the DMN. Fourth, both the hippocampus and PHG showed increased network centrality along their anterior-to-posterior axes, suggesting a key role for their posterior subdivisions in integration of signals from large-scale brain networks. Fifth, the left and right hippocampus and PHG showed a remarkably consistent pattern of functional organization, except that left hemisphere connectivity was stronger in magnitude. These findings highlight several key aspects of functional network organization within the human MTL. Below we discuss our findings in the context of previous fMRI studies of the MTL as well as histological tract-tracing studies in animals and current models of the MTL.
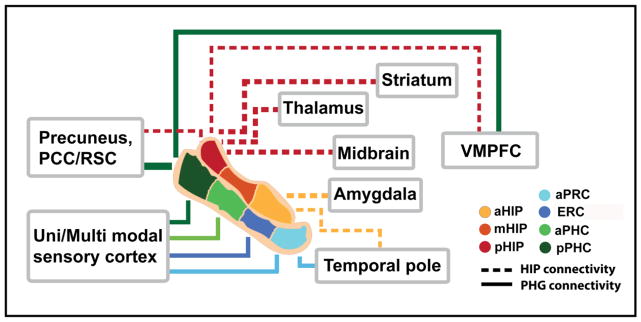
Figure 9. A schematic view of distinct functional connectivity profiles of the MTL subdivisions.
The PHG connectivity patterns are indicated by solid lines and hippocampal connectivity patterns are indicated by dash lines. The thickness of lines approximates to the strength of functional connectivity. The hippocampus shows greater connectivity with subcortical regions; in contrast, the PHG shows greater connectivity with widespread unimodal and polymodal association areas. The hippocampus shows a strongly heterogeneous pattern of connectivity along its anterior-posterior axis: the posterior hippocampus shows stronger connectivity with widespread cortical and subcortical regions including the ventral medial prefrontal cortex (VMPFC), striatum, thalamus, precuneus, and the midbrain while the anterior hippocampus shows stronger connectivity with the temporal pole and the amygdala. Unlike the hippocampus, the PHG shows more modest differences in connectivity along its anterior-posterior axis, except for the posterior PHC which shows stronger connectivity with the DMN regions including precuneus and VMPFC, and the PRC which shows stronger connectivity with the temporal pole. Notes: aHIP, anterior hippocampus; mHIP, middle hippocampus; pHIP, posterior hippocampus; aPRC, anterior perirhinal cortex; ERC, entorhinal cortex; aPHC, anterior parahippocampal cortex; pPHC, posterior parahippocampal cortex.
Distinct functional circuits associated with the hippocampus and the PHG
The hippocampus, compared to the PHG, showed preferential connectivity patterns with multiple subcortical regions, including dorsal and ventral striatum, VTA/SN and amygdala. The differential connectivity of the hippocampus with dorsal striatum provides novel evidence that the hippocampus and basal ganglia form interacting memory systems (Poldrack RA and P Rodriguez 2004; Voermans NC et al. 2004; Delgado MR and KC Dickerson 2012). Functional interactions between these pathways may underlie skill and habit formation especially during the early phases of learning (Poldrack RA et al. 2001; Poldrack RA and P Rodriguez 2004; Voermans NC et al. 2004; Dickerson KC et al. 2011; Delgado MR and KC Dickerson 2012). Notably, the hippocampus showed greater connectivity with the VTA/SN, consistent with the hippocampus-midbrain dopaminergic loop identified using tract-tracing in rodents (Lisman JE and AA Grace 2005; Luo AH et al. 2011). This circuit is thought to play an important role in reward-based learning and memory in both animals and humans (Schott BH et al. 2004; Lisman JE and AA Grace 2005; Schott BH et al. 2006; Shohamy D and AD Wagner 2008; Luo AH et al. 2011; Qin S, EJ Hermans, et al. 2012; Wolosin SM et al. 2012). Furthermore, the hippocampus also showed stronger connectivity with the amygdala, again consistent with known reciprocal anatomical connections between the amygdala complex and the hippocampus reported in both animal studies (Pitkanen A et al. 2000; Richter-Levin G and I Akirav 2000; Kishi T et al. 2006) and human fMRI studies of emotional memory (Kensinger EA and S Corkin 2004; Phelps EA 2004; Richardson MP et al. 2004; Dolcos F et al. 2005; LaBar KS and R Cabeza 2006).
In contrast, the PHG showed significantly preferential connectivity with multiple widely distributed cortical areas. These areas span several brain systems, including superior and middle temporal gyrus involved in auditory perception, inferior temporal cortex involved in visual object perception (Bancaud et al. 1994; Penfield and Perot 1963; Wheeler et al. 2000), lateral parietal regions involved in attention, episodic retrieval and spatial navigation (Vincent et al. 2006; Wagner et al. 2005; Cabeza et al. 2008; Rosenberg-Lee et al. 2011) as well as motor regions important for retrieval of action-associated events (Nyberg et al. 2001). The PHG also showed greater connectivity with several core posteromedial cortical nodes of the DMN, including posterior cingulate cortex and retrosplenial cortex. These regions have been implicated in various aspects of self-related processing and autobiographical memory (Maddock et al. 2001; Cooper et al. 2001; Maguire 2001; Cabeza and St Jacques 2007; Buckner and Carroll 2007). In line with previous findings from histological tract-tracing studies in rodents and nonhuman primates (Seltzer and Pandya 1976; Van Hoesen and Pandya 1975; Suzuki and Amaral 1994d, a), our findings suggest that the human PHG is a key convergence zone of cortical signals interacting with multiple unimodal and polymodal associations areas to bind sensory and motor representations separated in space and time (Davachi 2006; Squire and Zola-Morgan 1991; Bar 2004; Eichenbaum et al. 2007; Simons and Spiers 2003; Litman et al. 2009; Fernandez and Tendolkar 2006; Qin et al. 2007; Qin et al. 2009).
Although the left and right hippocampus showed very similar connectivity patterns with the rest of the brain, the left hippocampus showed stronger connectivity within the left MTL and with bilateral NAcc. Similarly, we also observed stronger left lateralized PHG connectivity within the left MTL, left amygdala and left anterior temporal lobe as well as the bilateral VTA and cerebellum. To our knowledge, such a pattern of stronger left hippocampus and left PHG intrinsic functional connectivity has not been reported before in human MTL studies. Task-based functional neuroimaging studies have reported lateralized hippocampal involvement in memory, with greater left hippocampus activity for verbal memory processing (Golby et al. 2001; Toga and Thompson 2003) and greater right hippocampus activity for visuo-spatial information (Iglói et al., 2010). Interestingly, hemispheric asymmetry at the CA3–CA1 pyramidal neuron synapse has recently been demonstrated in mice, with different spine morphology, glutamate receptor content, and synaptic plasticity (Kawakami et al. 2003; Shinohara et al. 2008). Critically, optogenetic silencing of CA3 pyramidal neurons in the left rather than right dorsal hippocampus impairs the formation of long-term memories in mice, suggesting a more crucial role for the left MTL in memory processing (Shipton et al. 2014). This pattern is consistent with our finding of stronger left MTL connectivity in our study. Further research is required to investigate how asymmetries in intrinsic functional connectivity contribute to lateralized hippocampal and PHG activation patterns observed in verbal and spatial tasks. Taken together, our findings provide important evidence to suggest that differential functional circuits linking the human hippocampus and the PHG might be critical for integration of disparate aspects of information representations distributed across cortical and subcortical networks involving domain-general and domain-specific perceptual information, reward, novelty, and emotional saliency (Di Martino et al. 2008; Gallagher and Chiba 1996; Hua et al. 1998; Wise 2004).
Differential functional connectivity along the long axis of the hippocampus
The differential pattern of connectivity between anterior and posterior hippocampus observed here is consistent with animal models, which have pointed toward distinct neuroanatomical profiles for dorsal and ventral hippocampus subdivisions (Moser and Moser 1998; Fanselow and Dong 2010). These studies have delineated distinct afferent and efferent pathways of the hippocampus, and have linked dorsal/posterior hippocampus pathways with visuo-spatial processing systems and the ventral/anterior hippocampus pathways with the emotion-related circuitry (Fanselow and Dong 2010). For example, dorsal CA1 and the subiculum within the posterior hippocampus contain the greatest density of place cells and head direction cells with massive projections to retrosplenial cortex and cingulate cortex that together form functional circuits important for spatial navigation and episodic memory (Frankland et al. 2004; Fanselow and Dong 2010; Teixeira et al. 2006; Harker and Whishaw 2004; Spiers and Maguire 2006). Unlike the study by Kahl and Shohamy, which reported strongest connectivity of the body (middle portion) of hippocampus with NAcc and VTA (Kahn and Shohamy 2013), we observed stronger connectivity of these core reward circuits with a more posterior hippocampus region. Our findings are more consistent with animal anatomical studies, which have reported that more dense projections from the posterior hippocampus to mammillary and anterior thalamic nuclei via the fornix (Kishi et al. 2000) are critical for habit formation and that the projections of the posterior hippocampus to NAcc and VTA are involved in linking reward and motivated behaviors (Luo et al. 2011; Lisman and Grace 2005; Poppenk et al. 2013).
Neuropsychological and functional neuroimaging studies in humans have provided evidence for a dissociation between functions of the posterior and anterior hippocampus. Several studies have demonstrated the specific role of posterior hippocampal regions in successful encoding and retrieval of information related to spatial scenes, navigation and spatial context (Ryan et al. 2010; Doeller et al. 2008; Maguire et al. 1997; Fernandez et al. 1998; Moser et al. 1993). The posterior hippocampus has also been associated with expertise in complex spatial knowledge, as in the case of London taxi drivers (Maguire et al. 2000), and with accurate recollection of spatial memory (Poppenk and Moscovitch 2011). In contrast, the differential connectivity of the anterior hippocampus with the anterior medial temporal lobe including the amygdala, may reflect strong reciprocal connections between these two regions as identified using histological tract-tracing studies in animals (Pitkanen et al. 2000; Richter-Levin and Akirav 2000; Kishi et al. 2006). Our findings are also consistent with co-activations of the anterior hippocampus and amygdala during encoding and retrieval of emotional memories (Dolcos et al. 2005; Kensinger and Corkin 2004; Richardson et al. 2004; Phelps 2004). The temporal pole is considered part of an extended limbic system given its abundant connections with limbic and paralimbic regions (Olson et al. 2007; Chabardes et al. 2002), suggesting that this region contributes to emotional and social processes via its interaction with the anterior hippocampus and amygdala (Olson et al. 2007; Glosser et al. 2003; Gorno-Tempini et al. 1998; Gorno-Tempini and Price 2001). Extending these observations, our findings suggest that functional dissociations of the hippocampus across multiple cognitive domains mirrors the organization of its intrinsic large-scale functional circuits.
Functional connectivity gradients along the long axis of the PHG
The four PHG subdivisions examined in our study – PRC, ERC, aPHC and pPHC – largely showed a similar pattern of connectivity along the anterior-posterior axis of the MTL, with unimodal and polymodal association areas in the temporal, parietal and occipital lobes. The only exception to this was the posterior PHG, which showed significantly greater connectivity with posteromedial cortex, ventromedial prefrontal cortex and bilateral angular gyri, regions that form core nodes of the DMN (Greicius et al. 2003; Raichle et al. 2001).
Our findings of differential connectivity of the posterior PHC with the retrosplenial cortex and adjacent posterior cingulate cortex are in line with anatomical models derived from monkey studies, demonstrating that the majority of projections to the posterior PHC arise from the posteromedial cortex (Goldman-Rakic et al. 1984; Suzuki and Amaral 1994a). Consistent with our findings, task-based fMRI studies have shown that the retrosplenial cortex and posterior PHC are important for spatial navigation and episodic memory for locations and scenes (Maguire et al. 2000; Epstein 2008; Engelien et al. 2000; Schon et al. 2004), while the anterior PHC is more important for recollective and associative memory for non-spatial contextual information (Aminoff et al. 2007; Bar et al. 2008a; Ranganath et al. 2004). Specifically, the recruitment of posterior cingulate cortex and medial prefrontal cortex in retrieval of autobiographical and prospective memories has been widely reported in human fMRI studies (Andrews-Hanna et al. ; Buckner and Carroll, 2007; Cabeza and St Jacques 2007). The coactivation of these regions likely arises from the dense anatomical connections of the PHC with the retrosplenial cortex as well as the cingulate cortex via the cingulum bundle (Suzuki and Amaral 1994a; Supekar et al. 2010; Greicius et al. 2009).
In addition to these differences associated with the posterior PHG, the PRC, within the anterior portion of the PHG, showed the strongest connectivity with the anterior temporal lobe (Heil et al. 1996; Odagaki et al. ; Kahn et al. 2008; Libby et al. 2012). This finding is consistent with the view that the anterior temporal lobe contributes to modality independent semantic categorization and memory by integrating multiple perceptual inputs from dorsal auditory, ventral visual and medial olfactory streams, which converge on the PRC (Courtney et al. 1998; Henson et al. 2000; Ranganath et al. 2000; Young et al.). These findings point further to differences in intrinsic functional connectivity along the anterior-posterior axis of the PHG and suggest that their distinct roles in memory and cognition may arise from differential connectivity with fronto-temporal association posteromedial cortices.
Functional gradients of network organization along the long axis of the hippocampus and the PHG
Graph theoretical analysis revealed functional gradients in network organization along the anterior-posterior long axis of the hippocampus and PHG. The most posterior nodes in the bilateral PHG as well as in the right hippocampus showed high node degree and centrality, reflecting more highly connected hubs (Bullmore and Sporns 2009, 2012) and a posterior-dominant pattern of connections with other nodes in MTL-based target networks. The posterior right hippocampus showed higher node centrality than the middle and anterior hippocampus, but no significant differences in node degree. This profile suggests that the large-scale connectivity of the hippocampus is characterized by continuous gradients (Strange et al. 2014) rather than dichotomous patterns along its anterior-posterior axis (Fanselow and Dong 2010; Moser and Moser 1998; Poppenk and Moscovitch 2011; Poppenk et al. 2008; Poppenk et al. 2013). Crucially, the observed pattern of significantly greater node centrality in the posterior hippocampus provides new quantitative data reflecting the relatively dense projections to and from the posterior hippocampus (Fanselow and Dong 2010; Moser and Moser 1998; Strange et al. 2014). Notably, greater node centrality in the posterior hippocampus, compared to the anterior and middle hippocampus, suggests that, relative to the other hippocampal subdivisions, a larger proportion of posterior hippocampus projections participate in integration of information within the MTL network.
Furthermore, we observed an even more robust increase in both node centrality as well as node degree along the anterior to posterior long axis of the bilateral PHG, with the posterior PHC and the anterior PRC demonstrating the highest and lowest values, respectively. This pattern of network organization complements our findings from whole-brain connectivity analysis and further suggests that the posterior PHC is a highly integrative hub. This finding is consistent with previous qualitative reports of converging information streams from sensory and perceptual systems into the MTL through the PHC (Davachi 2006; Squire and Zola-Morgan 1991; Bar 2004; Eichenbaum et al. 2007; Simons and Spiers 2003; Litman et al. 2009; Fernandez and Tendolkar 2006; Qin et al. 2007; Qin et al. 2009). Conversely, the PRC showed the lowest node degree and centrality – a pattern consistent with findings of more domain- and stimulus-specific mnemonic processing in both animals and humans (Daunizeau et al. 2009; Staresina et al. 2011; Mayes et al. 2007).
Taken together, these findings provide novel quantitative evidence from a large-scale brain network and graph-theoretic perspective, adding to our current understanding of gradients in intrinsic functional organization and network architecture of individual human MTL subdivisions.
Conclusions
Our study demonstrates that the intrinsic large-scale organization of the human MTL is characterized by distinct patterns of hippocampus and PHG connectivity. Compared to the PHG, the hippocampus showed stronger connectivity with multiple subcortical regions, whereas the PHG showed significantly greater connectivity with multiple unimodal and polymodal association areas. The left and right hippocampal and PHG connectivity patterns were remarkably similar, except that left hemisphere connectivity was relatively stronger than right hemisphere connectivity in both the hippocampus and PHG. Whole-brain connectivity and graph-theoretical analyses revealed functional gradients in hippocampus and PHG connectivity, node degree and centrality along the anterior-posterior axes, highlighting previously unknown aspects of their functional heterogeneity in the context of large-scale brain networks. Our findings clarify and extend current models of MTL circuitry and network organization and have important implications for a more principled understanding of MTL pathways that support memory and cognition.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (HD047520, HD059205 & K99MH105601), the Netherlands Organization for Scientific Research (NWO 446.10.010), the Child Health Research Institute (CHRI) at Stanford University and Lucile Packard Foundation for Children’s Health and the Stanford CTAS (UL1RR025744), and the Natural Science Foundation of China (61035006 & 61125304).
Footnotes
We declare that there are no conflicts of interest.
References
- Aminoff E, Gronau N, Bar M. The parahippocampal cortex mediates spatial and nonspatial associations. Cereb Cortex. 2007;17 (7):1493–1503. doi: 10.1093/cercor/bhl078. [DOI] [PubMed] [Google Scholar]
- Aminoff EM, Kveraga K, Bar M. The role of the parahippocampal cortex in cognition. Trends Cogn Sci. 2013;17 (8):379–390. doi: 10.1016/j.tics.2013.06.009. S1364-6613(13)00142-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 65(4):550–562. doi: 10.1016/j.neuron.2010.02.005. S0896-6273(10)00096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science (New York, NY. 2008;319 (5870):1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancaud J, Brunet-Bourgin F, Chauvel P, Halgren E. Anatomical origin of deja vu and vivid ‘memories’ in human temporal lobe epilepsy. Brain. 1994;117 (Pt 1):71–90. doi: 10.1093/brain/117.1.71. [DOI] [PubMed] [Google Scholar]
- Bar M. Visual objects in context. Nature reviews. 2004;5 (8):617–629. doi: 10.1038/nrn1476. [DOI] [PubMed] [Google Scholar]
- Bar M, Aminoff E, Ishai A. Famous faces activate contextual associations in the parahippocampal cortex. Cereb Cortex. 2008a;18 (6):1233–1238. doi: 10.1093/cercor/bhm170. [DOI] [PubMed] [Google Scholar]
- Bar M, Aminoff E, Schacter DL. Scenes unseen: the parahippocampal cortex intrinsically subserves contextual associations, not scenes or places per se. J Neurosci. 2008c;28 (34):8539–8544. doi: 10.1523/JNEUROSCI.0987-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E. Small-world brain networks. Neuroscientist. 2006;12 (6):512–523. doi: 10.1177/1073858406293182. [DOI] [PubMed] [Google Scholar]
- Bird CM, Burgess N. The hippocampus and memory: insights from spatial processing. Nat Rev Neurosci. 2008;9 (3):182–194. doi: 10.1038/nrn2335. nrn2355. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34 (4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bolland JM. Sorting out Centrality - an Analysis of the Performance of 4 Centrality Models in Real and Simulated Networks. Soc Networks. 1988;10 (3):233–253. doi: 10.1016/0378-8733(88)90014-7. [DOI] [Google Scholar]
- Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends in cognitive sciences. 2010;14 (6):277–290. doi: 10.1016/j.tics.2010.04.004. S1364-6613(10)00089-6. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. 1124/1/1. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11 (2):49–57. doi: 10.1016/j.tics.2006.11.004. S1364-6613(06)00327-5. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nature reviews. 2009;10 (3):186–198. doi: 10.1038/nrn2575. nrn2575. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. The economy of brain network organization. Nature reviews. 2012;13 (5):336–349. doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- Burwell RD. The parahippocampal region: corticocortical connectivity. Ann N Y Acad Sci. 2000;911:25–42. doi: 10.1111/j.1749-6632.2000.tb06717.x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. The hippocampo-neocortical dialogue. Cereb Cortex. 1996;6 (2):81–92. doi: 10.1093/cercor/6.2.81. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nature reviews. 2008;9 (8):613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, St Jacques P. Functional neuroimaging of autobiographical memory. Trends Cogn Sci. 2007;11 (5):219–227. doi: 10.1016/j.tics.2007.02.005. S1364-6613(07)00078-2. [DOI] [PubMed] [Google Scholar]
- Chabardes S, Kahane P, Minotti L, Hoffmann D, Benabid AL. Anatomy of the temporal pole region. Epileptic Disord. 2002;4(Suppl 1):S9–15. [PubMed] [Google Scholar]
- Cooper BG, Manka TF, Mizumori SJ. Finding your way in the dark: the retrosplenial cortex contributes to spatial memory and navigation without visual cues. Behav Neurosci. 2001;115 (5):1012–1028. doi: 10.1037//0735-7044.115.5.1012. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Petit L, Haxby JV, Ungerleider LG. The role of prefrontal cortex in working memory: examining the contents of consciousness. Philos Trans R Soc Lond B Biol Sci. 1998;353 (1377):1819–1828. doi: 10.1098/rstb.1998.0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daunizeau J, Kiebel SJ, Friston KJ. Dynamic causal modelling of distributed electromagnetic responses. Neuroimage. 2009;47 (2):590–601. doi: 10.1016/j.neuroimage.2009.04.062. S1053-8119(09)00428-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Current opinion in neurobiology. 2006;16 (6):693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proceedings of the National Academy of Sciences of the United States of America. 2003;100 (4):2157–2162. doi: 10.1073/pnas.03371951000337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Fair DA, Kelly C, Satterthwaite TD, Castellanos FX, Thomason ME, Craddock RC, Luna B, Leventhal BL, Zuo XN, Milham MP. Unraveling the Miswired Connectome: A Developmental Perspective. Neuron. 2014;83 (6):1335–1353. doi: 10.1016/j.neuron.2014.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18 (12):2735–2747. doi: 10.1093/cercor/bhn041. bhn041. [DOI] [PubMed] [Google Scholar]
- Diana RA, Ranganath C. Recollection, familiarity and memory strength: confusion about confounds. Trends in cognitive sciences. 2011;15 (8):337–338. doi: 10.1016/j.tics.2011.06.001. S1364-6613(11)00106-9. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends in cognitive sciences. 2007;11 (9):379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Doeller CF, King JA, Burgess N. Parallel striatal and hippocampal systems for landmarks and boundaries in spatial memory. Proc Natl Acad Sci U S A. 2008;105 (15):5915–5920. doi: 10.1073/pnas.0801489105. 0801489105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc Natl Acad Sci U S A. 2005;102 (7):2626–2631. doi: 10.1073/pnas.0409848102. 0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual review of neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelien A, Stern E, Isenberg N, Engelien W, Frith C, Silbersweig D. The parahippocampal region and auditory-mnemonic processing. Annals of the New York Academy of Sciences. 2000;911:477–485. doi: 10.1111/j.1749-6632.2000.tb06750.x. [DOI] [PubMed] [Google Scholar]
- Epstein RA. Parahippocampal and retrosplenial contributions to human spatial navigation. Trends in cognitive sciences. 2008;12 (10):388–396. doi: 10.1016/j.tics.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65 (1):7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez G, Tendolkar I. The rhinal cortex: ‘gatekeeper’ of the declarative memory system. Trends in cognitive sciences. 2006;10 (8):358–362. doi: 10.1016/j.tics.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Fernandez G, Weyerts H, Schrader-Bolsche M, Tendolkar I, Smid HG, Tempelmann C, Hinrichs H, Scheich H, Elger CE, Mangun GR, Heinze HJ. Successful verbal encoding into episodic memory engages the posterior hippocampus: a parametrically analyzed functional magnetic resonance imaging study. J Neurosci. 1998;18 (5):1841–1847. doi: 10.1523/JNEUROSCI.18-05-01841.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103 (26):10046–10051. doi: 10.1073/pnas.0604187103. 0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature reviews. 2007;8 (9):700–711. doi: 10.1038/nrn2201. nrn2201. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science (New York, NY. 2004;304 (5672):881–883. doi: 10.1126/science.1094804304/5672/881. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Chiba AA. The amygdala and emotion. Curr Opin Neurobiol. 1996;6 (2):221–227. doi: 10.1016/s0959-4388(96)80076-6. S0959-4388(96)80076-6. [DOI] [PubMed] [Google Scholar]
- Glosser G, Salvucci AE, Chiaravalloti ND. Naming and recognizing famous faces in temporal lobe epilepsy. Neurology. 2003;61 (1):81–86. doi: 10.1212/01.wnl.0000073621.18013.e1. [DOI] [PubMed] [Google Scholar]
- Glover GH. Spiral imaging in fMRI. Neuroimage. 2012;62 (2):706–712. doi: 10.1016/j.neuroimage.2011.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Lai S. Self-navigated spiral fMRI: interleaved versus single-shot. Magn Reson Med. 1998;39 (3):361–368. doi: 10.1002/mrm.1910390305. [DOI] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46(3):515–522. doi: 10.1002/mrm.1222. [pii] [DOI] [PubMed] [Google Scholar]
- Glover GH, Thomason ME. Improved combination of spiral-in/out images for BOLD fMRI. Magn Reson Med. 2004;51 (4):863–868. doi: 10.1002/mrm.20016. [DOI] [PubMed] [Google Scholar]
- Golby AJ, Poldrack RA, Brewer JB, Spencer D, Desmond JE, Aron AP, Gabrieli JD. Material-specific lateralization in the medial temporal lobe and prefrontal cortex during memory encoding. Brain. 2001;124 (Pt 9):1841–1854. doi: 10.1093/brain/124.9.1841. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12 (3):719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Price CJ. Identification of famous faces and buildings: a functional neuroimaging study of semantically unique items. Brain. 2001;124 (Pt 10):2087–2097. doi: 10.1093/brain/124.10.2087. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Price CJ, Josephs O, Vandenberghe R, Cappa SF, Kapur N, Frackowiak RS. The neural systems sustaining face and proper-name processing. Brain. 1998;121 (Pt 11):2103–2118. doi: 10.1093/brain/121.11.2103. [DOI] [PubMed] [Google Scholar]
- Granziera C, Hadjikhani N, Arzy S, Seeck M, Meuli R, Krueger G. In-vivo magnetic resonance imaging of the structural core of the Papez circuit in humans. Neuroreport. 2011;22 (5):227–231. doi: 10.1097/WNR.0b013e328344f75f. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100 (1):253–258. doi: 10.1073/pnas.01350581000135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101 (13):4637–4642. doi: 10.1073/pnas.03086271010308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19 (1):72–78. doi: 10.1093/cercor/bhn059bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackert VH, den Heijer T, Oudkerk M, Koudstaal PJ, Hofman A, Breteler MM. Hippocampal head size associated with verbal memory performance in nondemented elderly. Neuroimage. 2002;7(3):1365–72. doi: 10.1006/nimg.2002.1248. [DOI] [PubMed] [Google Scholar]
- Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC. Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp. 2002;15(4):247–262. doi: 10.1002/hbm.10022. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker KT, Whishaw IQ. Impaired place navigation in place and matching-to-place swimming pool tasks follows both retrosplenial cortex lesions and cingulum bundle lesions in rats. Hippocampus. 2004;14 (2):224–231. doi: 10.1002/hipo.10159. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 2004;22 (2):676–687. doi: 10.1016/j.neuroimage.2004.01.041. S1053811904000862. [DOI] [PubMed] [Google Scholar]
- Heil M, Rosler F, Hennighausen E. Topographically distinct cortical activation in episodic long-term memory: the retrieval of spatial versus verbal information. Mem Cognit. 1996;24 (6):777–795. doi: 10.3758/bf03201102. [DOI] [PubMed] [Google Scholar]
- Henke K, Weber B, Kneifel S, Wieser HG, Buck A. Human hippocampus associates information in memory. Proceedings of the National Academy of Sciences of the United States of America. 1999;96 (10):5884–5889. doi: 10.1073/pnas.96.10.5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Dolan RJ. Confidence in recognition memory for words: dissociating right prefrontal roles in episodic retrieval. Journal of cognitive neuroscience. 2000;12 (6):913–923. doi: 10.1162/08989290051137468. [DOI] [PubMed] [Google Scholar]
- Hua S, Reich SG, Zirh AT, Perry V, Dougherty PM, Lenz FA. The role of the thalamus and basal ganglia in parkinsonian tremor. Mov Disord. 1998;13(Suppl 3):40–42. doi: 10.1002/mds.870131307. [DOI] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laakso MP, Pitkanen A. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. AJNR Am J Neuroradiol. 1998;19 (4):659–671. [PMC free article] [PubMed] [Google Scholar]
- Kahn I, Andrews-Hanna JR, Vincent JL, Snyder AZ, Buckner RL. Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. Journal of neurophysiology. 2008;100 (1):129–139. doi: 10.1152/jn.00077.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn I, Shohamy D. Intrinsic connectivity between the hippocampus, nucleus accumbens, and ventral tegmental area in humans. Hippocampus. 2013;23 (3):187–192. doi: 10.1002/hipo.22077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami R, Shinohara Y, Kato Y, Sugiyama H, Shigemoto R, Ito I. Asymmetrical allocation of NMDA receptor epsilon2 subunits in hippocampal circuitry. Science (New York, NY. 2003;300 (5621):990–994. doi: 10.1126/science.1082609. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Two routes to emotional memory: distinct neural processes for valence and arousal. Proc Natl Acad Sci U S A. 2004;101 (9):3310–3315. doi: 10.1073/pnas.03064081010306408101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Stark CE. Medial temporal lobe activation during encoding and retrieval of novel face-name pairs. Hippocampus. 2004;14 (7):919–930. doi: 10.1002/hipo.20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Tsumori T, Ono K, Yokota S, Ishino H, Yasui Y. Topographical organization of projections from the subiculum to the hypothalamus in the rat. J Comp Neurol. 2000;419 (2):205–222. doi: 10.1002/(SICI)1096-9861(20000403)419:2<205::AID-CNE5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Kishi T, Tsumori T, Yokota S, Yasui Y. Topographical projection from the hippocampal formation to the amygdala: a combined anterograde and retrograde tracing study in the rat. J Comp Neurol. 2006;496 (3):349–368. doi: 10.1002/cne.20919. [DOI] [PubMed] [Google Scholar]
- Krimer LS, Hyde TM, Herman MM, Saunders RC. The entorhinal cortex: an examination of cyto- and myeloarchitectonic organization in humans. Cereb Cortex. 1997;7 (8):722–731. doi: 10.1093/cercor/7.8.722. [DOI] [PubMed] [Google Scholar]
- Lacy JW, Stark CE. Intrinsic functional connectivity of the human medial temporal lobe suggests a distinction between adjacent mtl cortices and hippocampus. Hippocampus. 2012 doi: 10.1002/hipo.22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby LA, Ekstrom AD, Ragland JD, Ranganath C. Differential connectivity of perirhinal and parahippocampal cortices within human hippocampal subregions revealed by high-resolution functional imaging. J Neurosci. 2012;32 (19):6550–6560. doi: 10.1523/JNEUROSCI.3711-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46 (5):703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Litman L, Awipi T, Davachi L. Category-specificity in the human medial temporal lobe cortex. Hippocampus. 2009;19 (3):308–319. doi: 10.1002/hipo.20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7 (2):119–132. doi: 10.1006/nimg.1997.0315. S1053-8119(97)90315-3. [DOI] [PubMed] [Google Scholar]
- Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333 (6040):353–357. doi: 10.1126/science.1204622. 333/6040/353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104(3):667–676. doi: 10.1016/s0306-4522(01)00108-7. S0306-4522(01)00108-7[pii] [DOI] [PubMed] [Google Scholar]
- Maguire EA. The retrosplenial contribution to human navigation: a review of lesion and neuroimaging findings. Scand J Psychol. 2001;42 (3):225–238. doi: 10.1111/1467-9450.00233. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Frackowiak RS, Frith CD. Recalling routes around london: activation of the right hippocampus in taxi drivers. J Neurosci. 1997;17 (18):7103–7110. doi: 10.1523/JNEUROSCI.17-18-07103.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD. Navigation-related structural change in the hippocampi of taxi drivers. Proceedings of the National Academy of Sciences of the United States of America. 2000;97 (8):4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes A, Montaldi D, Migo E. Associative memory and the medial temporal lobes. Trends in cognitive sciences. 2007;11 (3):126–135. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci. 1993;13 (9):3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8 (6):608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25 (3):653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Nichols T, Hayasaka S. Controlling the familywise error rate in functional neuroimaging: a comparative review. Stat Methods Med Res. 2003;12 (5):419–446. doi: 10.1191/0962280203sm341ra. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Petersson KM, Nilsson LG, Sandblom J, Aberg C, Ingvar M. Reactivation of motor brain areas during explicit memory for actions. Neuroimage. 2001;14 (2):521–528. doi: 10.1006/nimg.2001.0801. S1053-8119(01)90801-8. [DOI] [PubMed] [Google Scholar]
- Odagaki Y, Nishi N, Nakagawa S, Koyama T. Activation of G proteins by neuropeptide Y and gamma-aminobutyric acid(B) receptor agonists in rat cerebral cortical membranes through distinct modes of action. J Pharmacol Exp Ther. 1999;291 (3):1250–1256. [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130 (Pt 7):1718–1731. doi: 10.1093/brain/awm052. awm052. [DOI] [PubMed] [Google Scholar]
- Penfield W, Perot P. The Brain’s Record of Auditory and Visual Experience. A Final Summary and Discussion. Brain. 1963;86:595–696. doi: 10.1093/brain/86.4.595. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol. 2004;14(2):198–202. doi: 10.1016/j.conb.2004.03.015. S0959438804000479 [pii] [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis specialization of the human hippocampus. Trends in cognitive sciences. 2013;17 (5):230–240. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Poppenk J, Moscovitch M. A hippocampal marker of recollection memory ability among healthy young adults: contributions of posterior and anterior segments. Neuron. 2011;72 (6):931–937. doi: 10.1016/j.neuron.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Poppenk J, Walia G, McIntosh AR, Joanisse MF, Klein D, Kohler S. Why is the meaning of a sentence better remembered than its form? An fMRI study on the role of novelty-encoding processes. Hippocampus. 2008;18 (9):909–918. doi: 10.1002/hipo.20453. [DOI] [PubMed] [Google Scholar]
- Powell HW, Guye M, Parker GJ, Symms MR, Boulby P, Koepp MJ, Barker GJ, Duncan JS. Noninvasive in vivo demonstration of the connections of the human parahippocampal gyrus. Neuroimage. 2004;22(2):740–747. doi: 10.1016/j.neuroimage.2004.01.011. S1053811904000448 [pii] [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kohler S, Crane J, Pruessner M, Lord C, Byrne A, Kabani N, Collins DL, Evans AC. Volumetry of temporopolar, perirhinal, entorhinal and parahippocampal cortex from high-resolution MR images: considering the variability of the collateral sulcus. Cereb Cortex. 2002;12 (12):1342–1353. doi: 10.1093/cercor/12.12.1342. [DOI] [PubMed] [Google Scholar]
- Qin S, Piekema C, Petersson KM, Han B, Luo J, Fernandez G. Probing the transformation of discontinuous associations into episodic memory: an event-related fMRI study. Neuroimage. 2007;38 (1):212–222. doi: 10.1016/j.neuroimage.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Qin S, Rijpkema M, Tendolkar I, Piekema C, Hermans EJ, Binder M, Petersson KM, Luo J, Fernandez G. Dissecting medial temporal lobe contributions to item and associative memory formation. Neuroimage. 2009;46 (3):874–881. doi: 10.1016/j.neuroimage.2009.02.039. [DOI] [PubMed] [Google Scholar]
- Qin S, van Marle HJ, Hermans EJ, Fernandez G. Subjective sense of memory strength and the objective amount of information accurately remembered are related to distinct neural correlates at encoding. J Neurosci. 2011;31 (24):8920–8927. doi: 10.1523/JNEUROSCI.2587-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Young CB, Supekar K, Uddin LQ, Menon V. Immature integration and segregation of emotion-related brain circuitry in young children. Proceedings of the National Academy of Sciences of the United States of America. 2012;109 (20):7941–7946. doi: 10.1073/pnas.1120408109. 1120408109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98 (2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Johnson MK, D’Esposito M. Left anterior prefrontal activation increases with demands to recall specific perceptual information. J Neurosci. 2000;20(22):RC108. doi: 10.1523/JNEUROSCI.20-22-j0005.2000. 20004722 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D’Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42 (1):2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Richardson MP, Strange BA, Dolan RJ. Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nat Neurosci. 2004;7 (3):278–285. doi: 10.1038/nn1190nn1190. [DOI] [PubMed] [Google Scholar]
- Richter-Levin G, Akirav I. Amygdala-hippocampus dynamic interaction in relation to memory. Mol Neurobiol. 2000;22 (1–3):11–20. doi: 10.1385/MN:22:1-3:011. MN:22:1-3:011. [DOI] [PubMed] [Google Scholar]
- Rosenberg-Lee M, Chang TT, Young CB, Wu S, Menon V. Functional dissociations between four basic arithmetic operations in the human posterior parietal cortex: a cytoarchitectonic mapping study. Neuropsychologia. 2011;49 (9):2592–2608. doi: 10.1016/j.neuropsychologia.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan L, Lin CY, Ketcham K, Nadel L. The role of medial temporal lobe in retrieving spatial and nonspatial relations from episodic and semantic memory. Hippocampus. 2010;20 (1):11–18. doi: 10.1002/hipo.20607. [DOI] [PubMed] [Google Scholar]
- Sakai K, Miyashita Y. Neural organization for the long-term memory of paired associates. Nature. 1991;354 (6349):152–155. doi: 10.1038/354152a0. [DOI] [PubMed] [Google Scholar]
- Schon K, Hasselmo ME, Lopresti ML, Tricarico MD, Stern CE. Persistence of parahippocampal representation in the absence of stimulus input enhances long-term encoding: a functional magnetic resonance imaging study of subsequent memory after a delayed match-to-sample task. J Neurosci. 2004;24 (49):11088–11097. doi: 10.1523/JNEUROSCI.3807-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. Some cortical projections to the parahippocampal area in the rhesus monkey. Exp Neurol. 1976;50(1):146–160. doi: 10.1016/0014-4886(76)90242-9. 0014-4886(76)90242-9 [pii] [DOI] [PubMed] [Google Scholar]
- Shinohara Y, Hirase H, Watanabe M, Itakura M, Takahashi M, Shigemoto R. Left-right asymmetry of the hippocampal synapses with differential subunit allocation of glutamate receptors. Proceedings of the National Academy of Sciences of the United States of America. 2008;105 (49):19498–19503. doi: 10.1073/pnas.0807461105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipton OA, El-Gaby M, Apergis-Schoute J, Deisseroth K, Bannerman DM, Paulsen O, Kohl MM. Left-right dissociation of hippocampal memory processes in mice. Proceedings of the National Academy of Sciences of the United States of America. 2014;111 (42):15238–15243. doi: 10.1073/pnas.1405648111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Wagner AD. Integrating memories in the human brain: hippocampal-midbrain encoding of overlapping events. Neuron. 2008;60 (2):378–389. doi: 10.1016/j.neuron.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrager Y, Kirwan CB, Squire LR. Activity in both hippocampus and perirhinal cortex predicts the memory strength of subsequently remembered information. Neuron. 2008;59 (4):547–553. doi: 10.1016/j.neuron.2008.07.022. S0896-6273(08)00595-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nature reviews. 2003;4 (8):637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Song Z, Wixted JT, Smith CN, Squire LR. Different nonlinear functions in hippocampus and perirhinal cortex relating functional MRI activity to memory strength. Proceedings of the National Academy of Sciences of the United States of America. 2011;108 (14):5783–5788. doi: 10.1073/pnas.1103225108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47 (8–9):1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. S0028-3932(09)00106-7. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Maguire EA. Thoughts, behaviour, and brain dynamics during navigation in the real world. Neuroimage. 2006;31 (4):1826–1840. doi: 10.1016/j.neuroimage.2006.01.037. S1053-8119(06)00101-7. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science (New York, NY. 1991;253 (5026):1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Object unitization and associative memory formation are supported by distinct brain regions. J Neurosci. 2010;30 (29):9890–9897. doi: 10.1523/JNEUROSCI.0826-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Duncan KD, Davachi L. Perirhinal and parahippocampal cortices differentially contribute to later recollection of object- and scene-related event details. J Neurosci. 2011;31 (24):8739–8747. doi: 10.1523/JNEUROSCI.4978-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Dolan RJ. Anterior medial temporal lobe in human cognition: memory for fear and the unexpected. Cognitive neuropsychiatry. 2006;11 (3):198–218. doi: 10.1080/13546800500305096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Fletcher PC, Henson RN, Friston KJ, Dolan RJ. Segregating the functions of human hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 1999;96 (7):4034–4039. doi: 10.1073/pnas.96.7.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nature reviews. 2014;15 (10):655–669. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- Supekar K, Menon V, Rubin D, Musen M, Greicius MD. Network analysis of intrinsic functional brain connectivity in Alzheimer’s disease. PLoS Comput Biol. 2008;4 (6):e1000100. doi: 10.1371/journal.pcbi.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS biology. 2009;7 (7):e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Uddin LQ, Prater K, Amin H, Greicius MD, Menon V. Development of functional and structural connectivity within the default mode network in young children. Neuroimage. 2010;52 (1):290–301. doi: 10.1016/j.neuroimage.2010.04.009. S1053-8119(10)00403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J Comp Neurol. 1994a;350 (4):497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Topographic organization of the reciprocal connections between the monkey entorhinal cortex and the perirhinal and parahippocampal cortices. J Neurosci. 1994d;14 (3 Pt 2):1856–1877. doi: 10.1523/JNEUROSCI.14-03-01856.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira CM, Pomedli SR, Maei HR, Kee N, Frankland PW. Involvement of the anterior cingulate cortex in the expression of remote spatial memory. J Neurosci. 2006;26 (29):7555–7564. doi: 10.1523/JNEUROSCI.1068-06.2006. 26/29/7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Mapping brain asymmetry. Nature reviews. 2003;4 (1):37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- Van Hoesen G, Pandya DN. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. I. Temporal lobe afferents. Brain Res. 1975;95 (1):1–24. doi: 10.1016/0006-8993(75)90204-8. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Pandya DN, Butters N. Cortical afferents to the entorhinal cortex of the Rhesus monkey. Science. 1972;175 (4029):1471–1473. doi: 10.1126/science.175.4029.1471. [DOI] [PubMed] [Google Scholar]
- van Strien NM, Cappaert NL, Witter MP. The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci. 2009;10 (4):272–282. doi: 10.1038/nrn2614. nrn2614. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96 (6):3517–3531. doi: 10.1152/jn.00048.2006. 00048.2006. [DOI] [PubMed] [Google Scholar]
- Voermans NC, Petersson KM, Daudey L, Weber B, Van Spaendonck KP, Kremer HP, Fernandez G. Interaction between the human hippocampus and the caudate nucleus during route recognition. Neuron. 2004;43 (3):427–435. doi: 10.1016/j.neuron.2004.07.009. S0896627304004349. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends in cognitive sciences. 2005;9 (9):445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Petersen SE, Buckner RL. Memory’s echo: vivid remembering reactivates sensory-specific cortex. Proc Natl Acad Sci U S A. 2000;97(20):11125–11129. doi: 10.1073/pnas.97.20.11125. 97/20/11125 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer GE, Shohamy D. Preference by association: how memory mechanisms in the hippocampus bias decisions. Science (New York, NY. 2012;338 (6104):270–273. doi: 10.1126/science.1223252. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5 (6):483–494. doi: 10.1038/nrn1406nrn1406. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Stark CE. Multiple signals of recognition memory in the medial temporal lobe. Hippocampus. 2008;18 (9):945–954. doi: 10.1002/hipo.20452. [DOI] [PubMed] [Google Scholar]
- Young KD, Bellgowan PS, Bodurka J, Drevets WC. Functional neuroimaging of sex differences in autobiographical memory recall. Hum Brain Mapp. doi: 10.1002/hbm.22144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeineh MM, Holdsworth S, Skare S, Atlas SW, Bammer R. Ultra-high resolution diffusion tensor imaging of the microscopic pathways of the medial temporal lobe. Neuroimage. 2012;62 (3):2065–2082. doi: 10.1016/j.neuroimage.2012.05.065. S1053-8119(12)00553-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.