Abstract
Starting biotech or pharmaceutical companies is traditionally thought to be based around a scientist, their technology platform or a clinical candidate spun out from another company. Between us we have taken a different approach and formed two small early stage companies after initially leveraging the perspective of a parent with a child with a life-threatening rare disease. Phoenix Nest (http://www.phoenixnestbiotech.com/) was co-founded to work on treatments for Sanfilippo syndrome a devastating neurodegenerative lysosomal storage disorder. In the space of just over 3 years we have built up collaborations with leading scientists in academia and industry and been awarded multiple NIH small business grants. The second company, Collaborations Pharmaceuticals Inc. (http://www.collaborationspharma.com/) was founded to address some of the other 7000 or so rare diseases as well as neglected infectious diseases. The Rare Pediatric Disease Priority Review Voucher is likely the most important incentive for companies working on rare diseases with very small populations. This may also be partially responsible for the recent acquisitions of rare disease companies with late stage candidates. Lessons learned in the process of starting our companies are that rare disease parents or patients can readily partner with a scientist and fund research through NIH grants rather than venture capital or angel investors initially. This process may be slow so patience and perseverance is key. We would encourage other pharmaceutical scientists to meet rare disease parents, patients or advocates and work with them to further the science on their diseases and create a source of future drugs.
Keywords: Entrepreneurship, neglected diseases, rare diseases, Sanfilippo syndrome, Small companies
Introduction
Rare diseases are those that each affect 200,000 persons or fewer in the USA. They are also generally characterized by there being over 7000 of them, with only a few hundred having treatments and in some cases these can be incredibly expensive (1). The families affected by these rare diseases are also in most cases highly motivated to raise funds and reach out to researchers and pharma and biotech companies (2). The importance of research on rare diseases is also becoming increasingly visible (3–6) as the convergence of new therapeutic approaches, incentives to work on these diseases (7, 8) and the value of the companies involved reaches an all-time high. This represents an opportunity that other academic and industrial pharmaceutical researchers need to be aware of as it is likely they could contribute and this may also be an alternative source of funding for them.
Vouchers as incentives
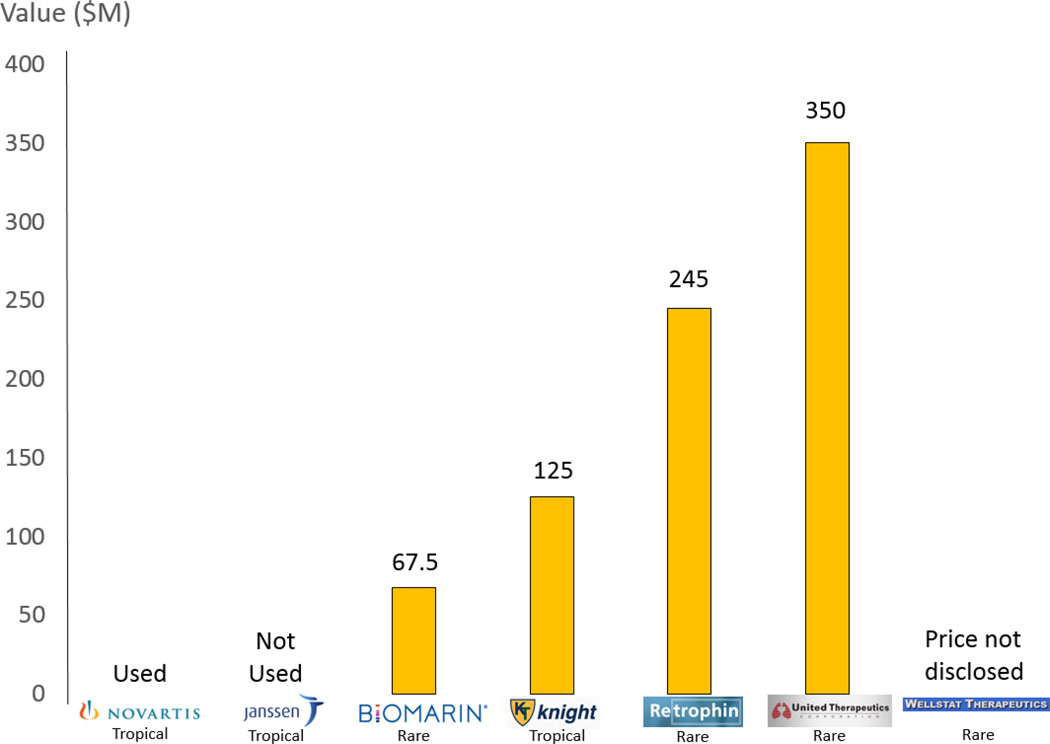
The tropical disease voucher was initially developed to incentivize companies for working on treatments for selected tropical diseases in 2007 (9). The Rare Pediatric Disease Priority Review Voucher was created under the Food and Drug Administration Safety and Innovation Act (FDASIA) and was based on this tropical disease voucher (10). A "rare pediatric disease" in this case is defined specifically as one which "primarily affects individuals aged from birth to 18 years, including age groups often called neonates, infants, children and adolescents," and is a rare disease according to federal statute. These vouchers can be used by the winner or sold to others for their use or resale, which is exactly what has happened in most cases. The first rare pediatric disease voucher was awarded to BioMarin in 2014 and they sold it to Sanofi and Regeneron for $67 million (Figure 1). The second voucher was awarded to United Therapeutics in 2015 and was sold for $350M to Abbvie in the most recent sale. In 2014, Knight Therapeutics sold their tropical disease priority voucher for $125 million. The third pediatric voucher was awarded in 2015 to Asklepion Pharmaceuticals, but was passed on to Retrophin when they bought the company. Their voucher was then sold to Sanofi for $245 million. It would appear that the price of these vouchers has increased over time and this could be due to their scarcity. Price may also be dependent on what drug the purchaser uses it on at the FDA and its perceived market value (Figure 1). FDASIA contained a clause which limited the FDA to awarding as few as 3 of the pediatric disease vouchers. The FDASIA legal wording writes "[FDA] may not award any priority review vouchers…after the last day of the 1-year period that begins on the date that the Secretary awards the third rare pediatric disease priority voucher under this section". In other words, this means the Rare Pediatric Disease Priority Review Voucher program will formally end on March 17, 2016—1 year after Retrophin received the priority review voucher—unless Congress takes additional action. So what needs to happen for the voucher program to continue? There is legislation now being considered in the US House of Representatives (the 21st Century Cures Act) (11, 12), which would extend the Rare Pediatric Disease Priority Review Voucher system for 3 years. The extension would only apply for rare pediatric diseases which are serious or life-threatening. Further, the new legislation would not allow companies to double dip (obtain both a tropical disease voucher and a pediatric voucher for the same drug/ disease). It is feasible that the value of these vouchers may continue to increase as companies realize their value in potentially helping to bring a drug to market faster. A recent voucher transaction between Wellstat Therapeutics and AstraZeneca did not disclose the price (13).
Figure 1.
Tropical and rare pediatric disease priority review vouchers have increasing value over time
Rare disease company acquisitions
While there are certainly many companies with billion dollar market caps focused on rare diseases e.g. Genzyme, Shire, BioMarin etc., there are many more smaller companies. These smaller companies even without approved products are becoming important targets for acquisitions. Recent rare disease company acquisitions include the following examples: Shire PLC made an unsolicited offer to acquire rare-disease treatment maker Baxalta Inc. for roughly $30.6 billion in stock, a company which has over a dozen FDA approved products for rare diseases. Other recent acquisitions include Amicus Therapeutics acquisition of the rare disease company Scioderm. Scioderm’s phase III candidate is for Epidermolysis Bullosa and would be eligible for the priority review voucher. Amicus would pay $229 million, $361 million for clinical and regulatory milestones and $257 for sales milestones. In addition they would pay up to $100 million for the proceeds of selling the voucher. The total potentially due to the shareholders is $947 million. Alexion offered to acquire Synageva for $8.4 billion. Synageva does not have an approved product but it has one in late stage trials with a potential market of 3000. Gilead acquired EpiTherapeutics ApS for $65 million, which has produced a library of first-in-class, selective small molecule inhibitors of epigenetic regulation of gene transcription, in particular histone demethylases. Roche acquired Trophos for up to EUR 470 million. Trophos’ proprietary screening platform generated olesoxime for spinal muscular atrophy. These last four acquisitions are examples of much larger companies buying smaller rare disease companies which do not yet have an approved treatment. Clearly, the earlier the stage of the product, the lower the value. An example of a rare disease company focused on several rare diseases (with very small patient populations) yet with many therapeutics in the clinic, is Ultragenyx which has a market cap of over $3.5bn at the time of writing. If this company is ultimately successful in bringing these treatments to market it could become a target for a larger rare disease company. Perhaps one of the reasons for this current focus on rare diseases is that they could lead to either the tropical disease or rare pediatric disease priority review voucher.
Starting rare disease companies
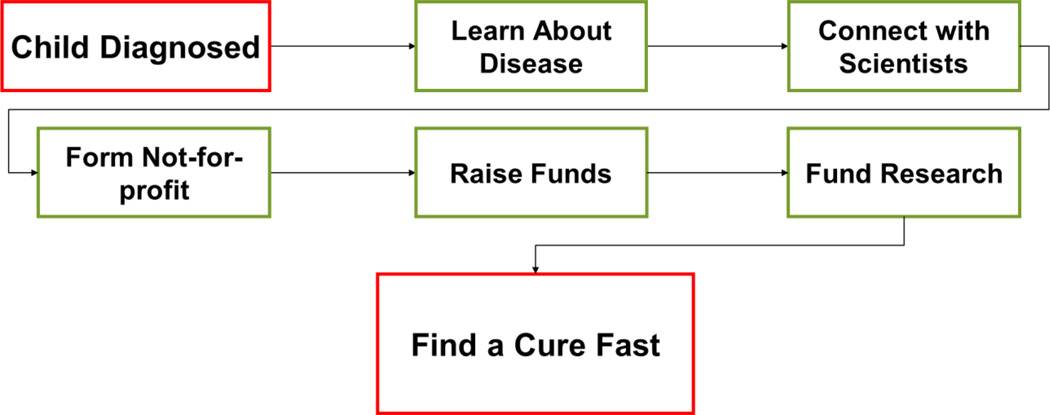
We can learn from these rare disease companies and at the same time try to attempt to do it differently. In the past three years we have formed two companies that are both focused on early stage rare disease drug discovery. One of us (JW) is the parent of a child with a rare disease called Sanfilippo Syndrome (Mucopolysaccharidosis, MPS IIIC) which is caused by genetic deficiency of heparan sulfate acetyl CoA: α-glucosaminide N-acetyltransferase, (HGSNAT). MPS III is a devastating neurodegenerative lysosomal storage disorder of childhood. The three other subtypes of the disease include: MPS IIIA (heparan N-sulfatase); MPS IIIB (α-N-acetylglucosaminidase); and MPS IIID (N-acetylglucosamine 6-sulfatase) (14). There are multiple steps a parent can take to try to find a treatment or cure for a rare disease (Figure 2). Parents of children with rare diseases have formed successful companies previously including Lysogene (15) which was initiated to develop a gene therapy for MPS IIIA, and has lead to a clinical trial (16). After meeting in 2011 we resolved to start a company in 2012 which is called Phoenix Nest, Inc. (17) so that we could pursue NIH Small Business Technology Transfer (STTR) and Small Business Innovation Research (SBIR) grant funding for MPS IIIC. This would be essential as the disease has few patients and is of little or no interest to venture capital (VC) funding (Table 1). The ability to apply for such grants needs to include considerable set up time to just be able to physically submit a proposal through the quite onerous registrations required (Table 2). Finding an academic collaborator that has a therapeutic or approach important for your disease of interest is key for an STTR. Initially we tried multiple STTR proposals with a collaborator in Canada without success due to reviewers looking upon research outside the USA unfavorably (Table 2). This grant-based start-up strategy is certainly not fast and a lot of waiting is involved. Compared to the continual fund raising in a not-for-profit as well as maintaining a 501c3 status, a for-profit is easier to manage and the potential benefits of a successful grant application are greater. We submitted 5 proposals including resubmissions (Table 3) before we were able to successfully obtain our first NIH grant with a collaborator (1R41NS089061-01) within two years of starting the company. However, this grant was on a different subtype of the disease called MPS IIID which represents a different enzyme to our initial focus. Our aim is that working on this will help to raise additional funding that will ultimately assist MPS IIIC research.
Figure 2.
The Rare disease parent’s pathway to develop treatments for their child
Table 1.
Challenges of starting a rare disease company
|
Table 2.
Challenges of SBIR and STTR grants
|
Table 3.
A new company timeline: Phoenix Nest, Inc.
|
Grant funding and rare disease companies
It is likely that such an approach based entirely on grant funding might work in other countries although we have no personal experience of this or whether similar competitive sources of funding to the STTR/SBIR program for small businesses are available. Ownership of intellectual property (IP) by the small business grantee is required in the USA. If successful and a grant is obtained, in the there are also additional requirements that need to be fulfilled before funding is issued, which is common to academics with NIH grants, but would usually be performed by a grant administrator. In addition we have incurred extensive legal costs to set up contracts with our collaborator in order to set up laboratory space in their vicinity. Ultimately, there needs to be some education of reviewers of SBIR and STTR grants that deal with rare diseases, so that there is a greater appreciation for the limited rare disease expertise in the USA in some areas as well as the potential for return on investment (ROI) (Table 2). While our patient population globally is very small for Sanfillipo Syndrome, the likely major consideration for financial ROI should be seen as the rare pediatric disease priority review voucher (see above) which at the very least is worth ~$67.5M (8). Our work on other forms of Sanfilippo (e.g. MPS IIIB) which occur more frequently could provide more revenue and more potential opportunities for vouchers. Our aim at Phoenix Nest, Inc. is to build a rare disease company that is focused on Sanfilippo Syndrome and is self-sustaining. If we can reach the market for one of our treatments in the next 5 years and obtain at least one priority review voucher, we will be well on our way towards this goal. Subsequently, we have also in-licensed small molecules as chaperones for MPS IIIC and MPSIIID which we have leveraged in grant proposal writing. We are looking to also add a license for a gene therapy for MPS IIIC to round out our portfolio. Currently all of our resources are spent on supporting research and development, with minimal overhead, as we leverage collaborative researchers and tools. We will also need to hire more expertize or consultants as we reach regulatory or manufacturing stages, but in summary our approach is lean even for a virtual company.
The next steps for Phoenix Nest and our collaborators are to obtain phase II STTR funding (pending) to further develop the technology from the phase I grants. Between the maximum combined Phase I and Phase II STTR funding allowable (~$1.75M) this should be enough to produce the key proof-of-concept studies for a treatment, whether small molecule or biologic. Beyond this we will need to perform toxicology testing. We can leverage several NIH programs in order to ensure this happens as quickly as possible. Following the STTR program we could apply for a CREATE award. For example the NINDS CREATE Bio Discovery Track: Optimization in preparation for development of Biotechnology products and biologics (U44)’ (18) would potentially fund pre-IND studies. To be eligible there needs to be a clear and convincing proof of concept (dose response relationship) and in vivo efficacy using clinically relevant outcome measures at the site of action. The minimal requirement is demonstration of in vivo efficacy in the animal model. Preliminary findings need to be at the stage where IND enabling studies are feasible at the end of 4 years of funding. In addition there are other funding opportunities such as the SBIR/STTR Commercialization Readiness Pilot Program (19) as well as other resources available through NIH NCATS including TRND that might be more relevant to small molecule projects. There is also access to clinical trials through NeuroNEXT for molecule or device projects relevant to NINDS. Beyond this there is the Orphan drug Act (20) and ultimately the rare pediatric disease priority review voucher (12) which we can take advantage of. Therefore, in the absence of a sizeable patient population our hope of an ROI rests almost entirely on the rare pediatric disease priority review voucher which we hope will ultimately attract investment from VC and Angel investors.
The challenges described above have not deterred one of us (SE) from starting a second company, Collaborations Pharmaceuticals Inc. (21) (Table 4), focused on collaborating with researchers working on other rare (excluding Sanfilippo Syndrome) and neglected diseases. This company has also worked with several collaborators to submit STTR and SBIR grants. From interaction with many different rare disease parent advocates it is clear they are also funding early stage exploratory research in academia for their diseases of interest and few consider starting a company to potentially commercialize the research. They therefore represent a valuable partner resource for finding and connecting with early stage technologies in academia. We would encourage scientists with little experience of rare diseases to reach out and offer whatever assistance you can to such parent lead foundations. In fact we would gladly offer advice to anyone wishing to start a rare disease company (22). It is likely those with an intimate knowledge of the disease, even without entrepreneurial experience, could be of value to the global economy by starting such companies. If we are to stand a chance of treating more rare diseases we also need to foster more collaboration and recruit scientists from outside. Starting companies focused on rare diseases may be an approach to catalyze this.
Table 4.
A new company timeline: Collaborations Pharmaceuticals, Inc.
|
Summary
Clearly the intent and motivation of our efforts is to collaborate closely with academic researchers doing drug or therapeutic discovery to fund their work so that it reaches the patient in a timely manner. The gap-to-approval for new molecular entities (NME) has recently been recognized to take longer for academic versus industry (23), so our efforts could help identify missing data ultimately required by the FDA in the Investigational New Drug Application (IND) and New Drug Application (NDA) process and perhaps shorten this process. (23).This is equally relevant to pharmaceutical researchers developing new approaches or technologies for drug delivery or targeting. A major goal of these companies has to be identifying as many patients as possible and understanding the disease process before clinical trials. To find patients we need to have a global presence which can be assisted by the rare disease foundations and global patient advocates (Figure 3). The development of a registry (24) is an important approach to connect with patients and a natural history study (25) is also essential to understand how a rare disease develops and to help identify biomarkers for future clinical trials.
Figure 3.
An example of an infographic (highlighting a registry, natural history study and treatments) shared on social media online as part of an effort to raise awareness for the disease to help identify patients.
We have briefly described our strategy of patient-driven rare disease companies that may be a useful vehicle to push for more translational research in collaboration with scientists in academia. We would encourage other rare disease parents and researchers to start companies and learn from our and others’ experiences. Due to the limited pool of funding for these diseases, enhanced collaboration between foundations, academics and companies facilitated by groups like ours and funded by governments may prevent unnecessary redundancies and broaden the impact of the ongoing research efforts. Ultimately the goal has to be to successfully deliver approved treatments to the patient, that are in turn affordable. With the help of incentives like vouchers and periods of extended marketing exclusivity, other companies, academics and rare disease parents will see this as a viable approach and a useful model for other rare diseases. We hope that additional incentives could also be provided to focus on diseases with very small numbers of patients in order to translate more treatments from academia.
Acknowledgments
Much of what we have described would not have been possible without the generosity, collaboration and support of many colleagues globally who have played a major role from the beginning including: Dr. Alexey Pshezhetsky, Dr. Brian Bigger, Dr. Patti Dickson, Dr. Xiaoyi Zhang, Dr. Tsui-Fen Chou, Mr. Derek Moen, Dr. Steven Le, Dr. Shih-hsin Kan, Dr. Matthew Ellinwood, Dr. Alex Clark, Dr. Joel Freundlich, Dr. Ruben Flores, Susan Rubin, Allison Weber, Calvin Chen, Mary F. Ognibene, Allison Moore, Lori Sames, Rare Genomics Inst., Assay Depot, Taconic, Jonah’s Just Begun, JLK-Sanfilippo Research Foundation, Sanfilippo Sud, Sanfilippo Barcelona, Sanfilippo Portugal, National MPS Society, The Lukondi family, our scientific advisory board members, boards of directors and all the families with Sanfilippo Syndrome. Michele Rhee is kindly acknowledged for creating Figure 2 and her support.
Funding
NIH NINDS 1R41NS089061-01
Abbreviations
- eRA
electronic Research Administration
- FDASIA
Food and Drug Administration Safety and Innovation Act
- IND
Investigational New Drug Application
- MPS
Mucopolysaccharidosis
- NDA
New Drug Application
- NIH
National Institutes of Health
- NME
New Molecular Entities
- SBIR
Small Business Innovation Research
- STTR
Small Business Technology Transfer
- VC
Venture Capital
Footnotes
Conflict of interest
SE and JW are co-founders of Phoenix Nest, Inc. SE is a founder of Collaborations Pharmaceuticals, Inc.
References
- 1.Swinney DC, Xia S. The discovery of medicines for rare diseases. Future medicinal chemistry. 2014;6(9):987–1002. doi: 10.4155/fmc.14.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood J, Sames L, Moore A, Ekins S. Multifaceted roles of ultra-rare and rare disease patients/parents in drug discovery. Drug discovery today. 2013;18:1043–1051. doi: 10.1016/j.drudis.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Beaulieu CL, Samuels ME, Ekins S, McMaster CR, Edwards AM, Krainer AR, Hicks GG, Frey BJ, Boycott KM, Mackenzie AE. A generalizable pre-clinical research approach for orphan disease therapy. Orphanet journal of rare diseases. 2012;7:39. doi: 10.1186/1750-1172-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Litterman NK, Rhee M, Swinney DC, Ekins S. Collaboration for rare disease drug discovery research. F1000Research. 2014;3:261. doi: 10.12688/f1000research.5564.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ekins S, Litterman NK, Arnold RJ, Burgess RW, Freundlich JS, Gray SJ, Higgins JJ, Langley B, Willis DE, Notterpek L, Pleasure D, Sereda MW, Moore A. A brief review of recent Charcot-Marie-Tooth research and priorities. F1000Research. 2015;4:53. doi: 10.12688/f1000research.6160.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sames L, Moore A, Arnold R, Ekins S. Recommendations to enable drug development for inherited neuropathies: Charcot-Marie-Tooth and Giant Axonal Neuropathy. F1000Research. 2014;3:83. doi: 10.12688/f1000research.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyamoto BE, Kakkis ED. The potential investment impact of improved access to accelerated approval on the development of treatments for low prevalence rare diseases. Orphanet journal of rare diseases. 2011;6:49. doi: 10.1186/1750-1172-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kesselheim AS, Maggs LR, Sarpatwari A. Experience With the Priority Review Voucher Program for Drug Development. JAMA : the journal of the American Medical Association. 2015;314(16):1687–1688. doi: 10.1001/jama.2015.11845. [DOI] [PubMed] [Google Scholar]
- 9.Sachs-Barrable K, Conway J, Gershkovich P, Ibrahim F, Wasan KM. The use of the United States FDA programs as a strategy to advance the development of drug products for neglected tropical diseases. Drug Dev Ind Pharm. 2014;40(11):1429–1434. doi: 10.3109/03639045.2014.884132. [DOI] [PubMed] [Google Scholar]
- 10.Anon. Rare pediatric disease priority review voucher program. Available from: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/ucm375479.htm.
- 11.21st Century Cures Act wins in US House. Nature biotechnology. 2015;33(9):891. doi: 10.1038/nbt0915-891. [DOI] [PubMed] [Google Scholar]
- 12.Kwok AK, Koenigbauer FM. Incentives to Repurpose Existing Drugs for Orphan Indications. ACS medicinal chemistry letters. 2015;6(8):828–830. doi: 10.1021/acsmedchemlett.5b00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anon. Wellstat Therapeutics Announces FDA Approval of XURIDEN™ to Treat Rare Pediatric Disorder Hereditary Orotic Aciduria (HOA) Available from: http://www.wellstattherapeutics.com/therapeutics/XUT.pdf. [Google Scholar]
- 14.Valstar MJ, Ruijter GJ, van Diggelen OP, Poorthuis BJ, Wijburg FA. Sanfilippo syndrome: A mini-review. Journal of inherited metabolic disease. 2008;31:240–252. doi: 10.1007/s10545-008-0838-5. [DOI] [PubMed] [Google Scholar]
- 15.Anon. Lysogene. Available from: http://www.lysogene.com/. [Google Scholar]
- 16.Tardieu M, Zerah M, Husson B, de Bournonville S, Deiva K, Adamsbaum C, Vincent F, Hocquemiller M, Broissand C, Furlan V, Ballabio A, Fraldi A, Crystal RG, Baugnon T, Roujeau T, Heard JM, Danos O. Intracerebral administration of adeno-associated viral vector serotype rh.10 carrying human SGSH and SUMF1 cDNAs in children with mucopolysaccharidosis type IIIA disease: results of a phase I/II trial. Human gene therapy. 2014;25(6):506–516. doi: 10.1089/hum.2013.238. [DOI] [PubMed] [Google Scholar]
- 17.Anon. Phoenix Nest, Inc; Available from: http://www.phoenixnestbiotech.com/. [Google Scholar]
- 18.Anon. NINDS CREATE Bio Discovery Track: Optimization in preparation for development of Biotechnology products and biologics (U44) Available from: http://grants.nih.gov/grants/guide/pa-files/PAR-14-287.html.
- 19.Anon. SBIR/STTR Commercialization Readiness Pilot (CRP) Program: Technical Assistance and Late Stage Development (SB1) Available from: http://grants.nih.gov/grants/guide/pa-files/PAR-16-027.html.
- 20.Talele SS, Xu K, Pariser AR, Braun MM, Farag-El-Massah S, Phillips MI, Thompson BH, Cote TR. Therapies for inborn errors of metabolism: what has the orphan drug act delivered? Pediatrics. 2010;126(1):101–106. doi: 10.1542/peds.2009-3246. [DOI] [PubMed] [Google Scholar]
- 21.Anon. Collaborations Pharmaceuticals, Inc; Available from: http://www.collaborationspharma.com/. [Google Scholar]
- 22.Ekins S. Advice for starting a rare disease company. Available from: http://www.raredr.com/contributor/sean-ekins/2014/11/advice-for-starting-a-rare-disease-company. [Google Scholar]
- 23.Patridge EV, Gareiss PC, Kinch MS, Hoyer DW. An analysis of original research contributions toward FDA-approved drugs. Drug discovery today. 2015;20(10):1182–1187. doi: 10.1016/j.drudis.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Anon. Sanfilippo Registry. Available from: https://connect.patientcrossroads.org/?org=SanfilippoRegistry. [Google Scholar]
- 25.Anon. Jonah's Just Begun; Available from: http://jonahsjustbegun.org/natural-history-study-patient-registry/. [Google Scholar]