Abstract
Background
In rat models of spinal cord injury at least three different strategies can be used to promote long-term cortical reorganization: (1) active exercise above the level of the lesion; (2) passive exercise below the level of the lesion; and (3) serotonergic pharmacotherapy. Whether and how these potential therapeutic strategies – and their underlying mechanisms of action – interact remains unknown.
Methods
In spinally transected adult rats, we compared the effects of active exercise above the level of the lesion (treadmill), passive exercise below the level of the lesion (bike), serotonergic pharmacotherapy (quipazine) and combinations of the above therapies (bike+quipazine, treadmill+quipazine, bike+treadmill+quipazine) on long term cortical reorganization (9 weeks after the spinal transection). Cortical reorganization was measured as the percent of cells recorded in the deafferented hindlimb cortex that responded to tactile stimulation of the contralateral forelimb.
Results
Bike and quipazine are ‘competing’ therapies for cortical reorganization, in the sense that quipazine limits the cortical reorganization induced by bike, whereas treadmill and quipazine are ‘collaborative’ therapies, in the sense that the reorganization induced by quipazine combined with treadmill is greater than the reorganization induced by either quipazine or treadmill.
Conclusions
These results uncover the interactive effects between active/passive exercise and serotonergic pharmacotherapy on cortical reorganization after spinal cord injury, emphasizing the importance of understanding the effects of therapeutic strategies in spinal cord injury (and in other forms of deafferentation) from an integrated system-level approach.
Keywords: spinal cord transection, somatosensory cortex, physical therapy, serotonin, cortical reorganization, electrophysiology
Introduction
The somatotopic organization of the primary somatosensory cortex is not a static map unmodifiable after development but rather a variable map that is continuously updated based on the dynamics of ongoing levels of cortical activity [1,2]: if a specific part of the body is used relatively more, its corresponding cortical area expands, if another part of the body is used relatively less, its corresponding cortical area shrinks. While this physiological process of cortical reorganization continuously takes place during sensorimotor learning [3,4], its extreme consequences can be observed in pathological conditions that lead to cortical deafferentation such as stroke, amputation, nerve injuries and spinal cord injuries: when a cortical area is deafferented, the dynamical equilibrium of the somatotopic map is disrupted, often leading to massive cortical reorganization [5–11]. From a clinical perspective, the degree of cortical reorganization after somatosensory deafferentation can be critical in the tradeoff between functional recovery [12–15] and the appearance of disabling symptoms such as neuropathic pain [16–20]. It therefore is important to gain a deep understanding of the mechanisms underlying cortical reorganization and develop optimized clinical strategies to modulate it [21].
We previously showed that in rat models of complete spinal cord transection at least three different strategies can be used to promote long-term cortical reorganization: (1) active exercise above the level of the lesion, likely through activity-dependent plasticity [22]; (2) passive exercise delivered below the level of the lesion that upregulates proteins in the cortex associated with plasticity [23]; (3) serotonergic pharmacotherapy, likely through the direct action of serotonin on sensorimotor systems [24]. From a mechanistic perspective, these findings expose the complexity in the regulation of the dynamical equilibrium underlying cortical reorganization after deafferentation. From a translational perspective, they provide the rationale for developing and optimizing both non-pharmacological (active and passive exercise) and pharmacological (serotonin) therapeutic strategies to promote or control cortical reorganization. Whether and how these potential therapeutic strategies – and their underlying mechanisms of action – interact remains unknown.
To address this issue, in the present work we compared the effects of active exercise above the level of the lesion (treadmill), passive exercise below the level of the lesion (bike), serotonergic pharmacotherapy (quipazine) and combinations of the above therapies (bike+quipazine, treadmill+quipazine, bike+treadmill+quipazine) on long term cortical reorganization after thoracic spinal cord transection. Cortical reorganization was measured as the percent of cells recorded in the deafferented hindlimb cortex that responded to tactile stimulation of the contralateral forelimb.
Methods
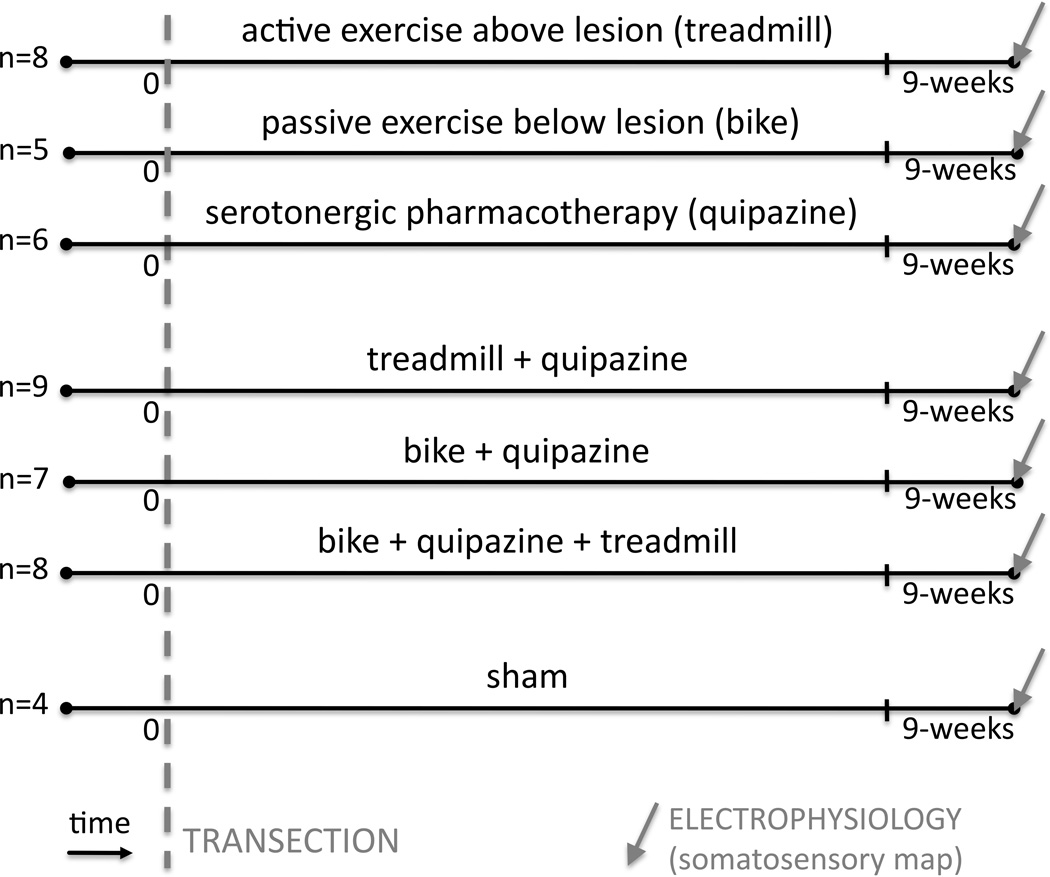
A total of 70 adult Sprague-Dawley rats were used in this study (initially 10 per group). Twenty-three animals did not survive the entire study and the remaining 47 were from the following 7 experimental groups (Fig. 1). The first three groups are animals that were spinally transected and received individual therapies after the transection: (1) treadmill animals (n=8), which received active exercise through treadmill locomotion; (2) bike animals (n=5), which received passive exercise through hindlimb bike training; (3) quipazine animals (n=6), which received serotonergic pharmacotherapy. The following three groups are animals that were spinally transected and received combinations of therapies after the transection: (4) treadmill+quipazine animals (n=9); (5) bike+quipazine animals (n=7); (6) bike+quipazine+treadmill animals (n=8). The last group (7) is a control group composed of sham animals (n=4), which were spinally transected and received sham therapies. Each animal group received its therapy (or sham) for 8 weeks after transection. Within one week after the last therapy session (i.e. week 9 after the spinal transection), animals were anesthetized to perform an acute, single-neuron mapping study in the deafferented hindlimb cortex to electrophysiologicaly quantify cortical reorganization, similarly to our previous studies [22–24]. The data for group 2 (bike) were previously published as part of another study [23] but the data were collected at the same time as the other data from the other groups. Animals were entered into the study at a rate of 2 per week, transected and assigned to one of the 7 groups in a serial fashion (animal 1 to group 1, animal 2 to group 2, …, animal 8 to group 1, etc). All procedures were performed under the guidelines of the National Institutes of Health, and approved by the Institutional Animal Care and Use Committee of Drexel University.
Fig. 1. Experimental protocol.
We used 70 adult rats, of which 47 survived the entire study, divided in 7 experimental groups: (1) treadmill animals (n=8), which received active exercise through treadmill locomotion; (2) bike animals (n=5), which received passive exercise through hindlimb bike training; (3) quipazine animals (n=6), which received serotonergic pharmacotherapy; (4) treadmill+quipazine animals (n=9); (5) bike+quipazine animals (n=7); (6) bike+quipazine+treadmill animals (n=8); (7) sham animals (n=4), which were spinally transected and received sham therapies. Each animal group received its therapy (or sham) for 8 weeks after transection. Within one week of the last therapy session (i.e. 9 weeks after the spinal transection), animals were anesthetized to perform an acute, single-neuron mapping study in the deafferented hindlimb cortex to electrophysiologically quantify cortical reorganization,
Spinal cord transection
Animals received a complete thoracic transection of the spinal cord with procedures that are similar to our previous studies [23–25]. Adult female Sprague-Dawley (Charles River) rats were anesthetized with isoflurane (2–3% with oxygen) and the spinal cord was exposed by laminectomy at the T8/T9 level. The cord was transected with iridectomy scissors followed by aspiration of tissue within the cavity. A collagen matrix, Vitrogen, was injected into the site of the transection to fill the cavity. The muscle and skin were sutured in layers with 5-0 silk. Animals were then warmed, and when they became active, returned to their home cages. Bladders were manually expressed until the animals were able to void on their own. Animals were housed under a 12 h light/dark cycle (lights on at 07:00) with free access to food and water. All behaviors were performed in the light phase of the rats’ light/dark cycle.
Active exercise above lesion: treadmill locomotion
Treadmill therapy was chosen to be consistent with our studies in adult rats spinalized as neonates and consisted of placing the animals on a motorized treadmill apparatus for 3 min/day at a speed of 6.5 m/min, 5 days per week [22,26]. This time limit on the exercise was chosen because it is the limit of what the animals are capable of performing – after 4 minutes they become tired and stop trying to locomote. No weight support or other stimulation was applied to the rats during treadmill locomotion and the rats used their forelimb to move along the treadmill dragging their hindlimbs. Treadmill exercise began 1 week after transection and was delivered for 8 weeks.
Passive exercise below lesion: hindlimb bike
Hindlimb bike exercise [27] consisted of two 30-minute sessions with a 10-minute break, 3 days per week (M, W and F) [23,24]. This exercise regimen involved suspending the rats on a sling with the hindlimbs hanging down and the hind feet strapped onto the pedals of a bicycle-type device that was driven by a motorized belt. The exercise consisted of a pedaling motion that flexed one limb while extending the other without overstretching the limbs. Cycling speed was 0.5 Hz. This was, therefore, a passive exercise of the hindlimbs only. Passive hindlimb bike exercise or sham bike exercise started the week after the spinal transection and was delivered for 8 weeks.
Serotonergic pharmacotherapy: quipazine
Daily quipazine injections (0.075 mg/kg, IP, 5 days per week) were delivered to the animals starting 2 weeks after transection. Quipazine was chosen because it is a non-specific 5-HT agonist (i.e. it acts at both the 2A and 1 5-HT receptors) that has been shown to induce locomotor-like behaviors in the hindlimbs of spinal injured rats [28,29]. The 2-week lag post injury allowed time for 5-HT receptor upregulation in the spinal cord caudal to the lesion [30]. The dose was selected to be sufficient to elicit hindlimb locomotor-like movement but minimize tremor and/or spasticity side effects known to occur in spinal injured animals after chronic administration of 5-HT agonists (data not shown).
Combined therapies
Combined therapies were provided in the same way as individual therapies. In animals that received bike and quipazine, quipazine was always delivered 5 min before bike. In animals that received quipazine and treadmill, quipazine was always delivered 5 min before treadmill. All animals were given an injection first (either quipazine or saline). The peak effect of quipazine in the central nervous system occurs about 60 minutes after injection [31], so it was active throughout the therapy.
Sham therapy
Sham therapies were provided to maintain similar experiences across animals and ensure differences were related to the therapy and not handling or somatosensory contact with the experimental devices. Sham treadmill exercise consisted of placing the animal on the treadmill for 3 min/day but the treadmill did not move. Sham bike exercise consisted of placing the animals on the bike for 70 minutes, 3 times per week but the pedals did not move. Sham drug therapy was provided by daily injection of saline. Sham animals received all three sham therapies.
Behavioral testing
BBB scoring in the open field was used to test hindlimb behavioral recovery [32,33] during week 8. Animals that received drug therapy were tested after a 3-day washout period during which no drug was given. Spontaneous hindlimb motor activity was evaluated for 4 min in a 2.5×3 ft diameter enclosure and scored by two trained observers with an inter-rater reliability ≥95%. BBB scores of 8 or below describe various degrees of behavioral recovery of locomotor-like movements that do not include weight support. BBB scores of 9 or above (to a maximum of 21) indicate some degree of hindquarter weight support starting in stance and progressing to weight-supported stepping [32].
Electrophysiology
Acute single-neuron mapping of the deafferented hindpaw cortex was performed at the end of the study with similar techniques as in our previous studies [22–24]. Rats were anesthetized by intraperitoneal injection of urethane anesthesia (1.3 g/Kg) and placed in a stereotaxic frame. Craniotomies were performed over either the right or left cortex to expose the hindlimb representations in the primary somatosensory cortex. The stereotaxic coordinates for hindlimb craniotomy were from 0 to 3mm posterior to bregma and from 2 to 3 mm lateral (Paxinos and Watson, 1986). Electrode penetrations were defined using the stereotaxic coordinates for the hindlimb somatosensory cortex [34]. For all animals, the anesthesia level was maintained at Stage III-4 [35,36].
A tapered high impedance (10 MΩ) tungsten microelectrode (FHC, Inc, Bowdoin, ME, part no UEWSGGSE0N1E) was mounted on a stereotaxic electrode manipulator. A ground wire was inserted into the brain adjacent to the craniotomies. The microelectrode was then moved to the anterior-posterior and medial-lateral coordinates that defined a predetermined location above the hindlimb somatosensory cortex, and lowered, perpendicular to the surface of the brain, to penetrate the dura and pia. The microelectrode was then slowly inserted into the brain. Signals were amplified (10k – 15k), bandpass filtered (154Hz to 13kHz) and digitalized (40kHz) using a Multi-Neuron Acquisition Processor (MNAP) (Plexon Inc. Dallas, TX).
The signals from the microelectrode were continuously monitored on the oscilloscope and audio speakers as the electrode was lowered. When a neuron was encountered, the dorsal/ventral coordinates of the cell were noted. Two experimenters then determined whether the identified cell responded to sensory stimulation. The first experimenter, with knowledge of the electrode placement, used wooden probes to touch the hair/skin on the forelimb and shoulder. The second experimenter, blind to the position of the electrode and treatment group of the animal, determined if the cell responded to the stimulus, predominately by listening for a change in firing rate. If the cell did not modulate its firing rate in response to the stimulation, the cell was noted as negative. If the cell did modulate its firing rate, the cell was noted as positive. If the cell was noted as positive, then the receptive field of the cell was identified by tapping locations on the body rostral to the level of the injury. Stimulation of any body surface that modulated the cell’s firing rate was considered part of the cell’s receptive field. To ensure that tapping forces between animals and across sites were uniform, the responses elicited by the wooden probe were periodically compared to responses elicited by von Frey filaments to calibrate the stimulus applied by the wooden probe. The stimulation consisted of pressing a filament gently against the skin, perpendicular to its surface until the filament bent 90 degrees. This procedure was done 5 times for each filament and skin site, to ensure reproducibility of the results.
After a cell was characterized, the microelectrode was moved at least 50 microns deeper (with respect to the cortical surface) before another cell could be identified in the same penetration to ensure a new cell was encountered. Every identified cell was assigned to one of three cortical layers based on the stereotaxical depth of the microelectrode at the time the cell was recorded: supragranular: 50–700 µm (layer II/III), granular: 750–1000 µm (layer IV) or infragranular: 1050–2000 µm (layer V/VI) [22–24]. To minimize tissue damage and its possible effects on cell responsiveness during later penetrations, no more than 6 penetrations were performed per animal.
Perfusion and histological processing of the spinal cord
At the end of the mapping sessions, the rats were perfused transcardially with buffered saline, followed by buffered 2% paraformaldehyde, and then by buffered 2% paraformaldehyde containing 10% sucrose. Spinal cords were removed and placed in phosphate buffer containing 30% sucrose for 72 h. Specimens were frozen in OCT and sectioned on a freezing microtome at 20 µm. The transection segments of the spinal cords were sectioned parasagitally, and alternate sections were Nissl-myelin stained. The resulting sections were examined under a microscope to confirm completeness of the transection.
Statistical analyses
To assess the effects of individual therapies and combined therapies after spinal transection on the electrophysiological reorganization of the deafferented cortex, we performed several types of analyses. First, the number recorded cells per track per animal were entered into separate one-way or two-way ANOVAs for individual therapies or for combined therapies. Second, the proportion of cells recorded in the deafferented hindlimb cortex responding to forelimb stimuli was analyzed by entering the raw binary neural data (responding/non-responding neurons, n=cells) into generalized linear models (GZLM) with binomial distribution and logit link function. GZLMs allow binary data to be rigorously analyzed with ANOVA-like designs. Post-hoc comparisons were performed with Tukey Honest Significant Difference Test or less conservative two-proportion tests. Third, the percentage of cells per track stereotaxically located in the deafferented hindlimb cortex that responded to stimulation of the intact forelimb were entered into independent-measures ANOVAs, considering each track and layer as independent samples. Tukey’s test were used for post-hoc comparisons. Using similar statistical designs, we verified that between-group differences in percentage of responsive cells were not due to between-group biases in recording locations (antero-posterior and medial-lateral coordinates were analyzed separately) or number of tracks per animals (in this case we used non-parametric statistics). All results were considered significant at p<0.05.
Results
Each animal group received its therapy (or sham) for 8 weeks after complete thoracic transection of the spinal cord. Within one week of the last therapy session (i.e. during week 9 after the spinal transection), animals were anesthetized to perform an acute, single-neuron mapping study in the deafferented hindlimb cortex (2–6 tracks per animal). A total of 2697 single neurons from the supragranular, granular, and infragranular layers were isolated and identified as either responsive or not responsive to cutaneous stimulation of the forelimbs (Table 1). Animals were behaviorally evaluated with BBB scores at the end of therapy (week 8).
Table 1.
Electrophysiological data and BBB scores
| Responsive tracks per animal | Responsive cells per animal | BBB | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SG | G | IG | TOT | SG | G | IG | TOT | week 8 | ||
| SHAM 1 | 0 of 4 | 0 of 4 | 0 of 4 | 0 of 4 | 0 of 13 | 0 of 20 | 0 of 30 | 0 of 63 | 0 | |
| SHAM 2 | 0 of 5 | 0 of 5 | 0 of 5 | 0 of 5 | 0 of 21 | 0 of 21 | 0 of 28 | 0 of 70 | 1 | |
| SHAM 3 | 0 of 4 | 0 of 4 | 0 of 4 | 0 of 4 | 0 of 16 | 0 of 7 | 0 of 28 | 0 of 51 | 2 | |
| SHAM 4 | 0 of 4 | 2 of 4 | 1 of 4 | 2 of 4 | 0 of 17 | 2 0f 17 | 1 of 35 | 3 of 69 | 1 | |
| TOT | 0 of 17 | 2 of 17 | 1 of 17 | 2 of 17 | 0 of 67 | 2 of 65 | 1 of 121 | 3 of 253 | MEAN | 1,0 |
| % | 0,0 | 11,8 | 5,9 | 11,8 | 0,0 | 3,1 | 0,8 | 1,2 | SD | 0,8 |
| TREADMILL (T) 1 | 0 of 3 | 0 of 3 | 0 of 3 | 0 of 3 | 0 of 12 | 0 of 8 | 0 of 21 | 0 of 41 | 4 | |
| TREADMILL (T) 2 | 0 of 3 | 0 of 3 | 0 of 3 | 0 of 3 | 0 of 11 | 0 of 6 | 0 of 16 | 0 of 33 | 2 | |
| TREADMILL (T) 3 | 1 of 5 | 1 of 5 | 0 of 5 | 2 of 5 | 1 of 31 | 1 of 10 | 0 of 28 | 2 of 69 | 0,5 | |
| TREADMILL (T) 4 | 0 of 4 | 0 of 4 | 0 of 4 | 0 of 4 | 0 of 17 | 0 of 14 | 0 of 17 | 0 of 48 | 0,5 | |
| TREADMILL (T) 5 | 0 of 4 | 0 of 4 | 0 of 4 | 0 of 4 | 0 of 18 | 0 of 8 | 0 of 32 | 0 of 58 | 2 | |
| TREADMILL (T) 6 | 0 of 4 | 0 of 4 | 0 of 4 | 0 of 4 | 0 of 10 | 0 of 13 | 0 of 15 | 0 of 38 | 1 | |
| TREADMILL (T) 7 | 0 of 3 | 0 of 3 | 0 of 3 | 0 of 3 | 0 of 4 | 0 of 13 | 0 of 22 | 0 of 39 | 2,5 | |
| TREADMILL (T) 8 | 1 of 3 | 1 of 3 | 1 of 3 | 2 of 3 | 1 of 16 | 1 of 11 | 1 of 25 | 3 of 52 | 1,5 | |
| TOT | 2 of 29 | 2 of 29 | 1 of 29 | 4 of 29 | 2 of 119 | 2 of 83 | 1 of 176 | 5 of 378 | 1,8 | |
| % | 6,9 | 6,9 | 3,4 | 13,8 | 1,7 | 2,4 | 0,6 | 1,3 | 1,2 | |
| BIKE (B) 1 | 6 of 6 | 6 of 6 | 5 of 6 | 6 of 6 | 13 of 54 | 10 of 30 | 12 of 54 | 35 of 138 | 0 | |
| BIKE (B) 2 | 1 of 5 | 1 of 5 | 2 of 5 | 3 of 5 | 1 of 22 | 1 of 18 | 3 of 55 | 5 of 95 | 4,5 | |
| BIKE (B) 3 | 1 of 4 | 1 of 4 | 4 of 4 | 4 of 4 | 1 of 12 | 1 of 7 | 4 of 56 | 6 of 75 | 3 | |
| BIKE (B) 4 | 2 of 5 | 2 of 5 | 2 of 5 | 2 of 5 | 6 of 30 | 7 of 22 | 8 of 48 | 21 of 100 | 4 | |
| BIKE (B) 5 | 0 of 4 | 0 of 4 | 0 of 4 | 0 of 4 | 0 of 24 | 0 of 18 | 0 of 40 | 0 of 82 | 2 | |
| TOT | 10 of 24 | 10 of 24 | 13 of 24 | 15 of 24 | 21 of 142 | 19 of 95 | 27 of 253 | 67 of 490 | 2,7 | |
| % | 41,7 | 41,7 | 54,2 | 62,5 | 14,8 | 20,0 | 10,7 | 13,7 | 1,8 | |
| QUIPAZINE (Q) 1 | 1 of 2 | 0 of 2 | 0 of 2 | 1 of 2 | 1 of 12 | 0 of 7 | 0 of 14 | 1 of 33 | 1 | |
| QUIPAZINE (Q) 2 | 0 of 2 | 0 of 2 | 0 of 2 | 0 of 2 | 0 of 8 | 0 of 4 | 0 of 13 | 0 of 25 | 0,5 | |
| QUIPAZINE (Q) 3 | 1 of 4 | 1 of 4 | 0 of 4 | 1 of 4 | 1 of 18 | 1 of 11 | 0 of 28 | 2 of 57 | 1 | |
| QUIPAZINE (Q) 4 | 1 of 4 | 0 of 4 | 1 of 4 | 1 of 4 | 1 of 12 | 0 of 4 | 3 of 14 | 4 of 30 | 2 | |
| QUIPAZINE (Q) 5 | 0 of 3 | 0 of 3 | 1 of 3 | 1 of 3 | 0 of 14 | 0 of 8 | 1 of 24 | 1 of 46 | 1 | |
| QUIPAZINE (Q) 6 | 2 of 5 | 2 of 5 | 1 of 5 | 2 of 5 | 2 of 21 | 2 of 24 | 1 of 48 | 5 of 93 | 1,5 | |
| TOT | 5 of 20 | 3 of 20 | 3 of 20 | 6 of 20 | 5 of 85 | 3 of 58 | 5 of 141 | 13 of 284 | 1,2 | |
| % | 25,0 | 15,0 | 15,0 | 30,0 | 5,9 | 5,2 | 3,5 | 4,6 | 0,5 | |
| T+Q1 | 2 of 4 | 2 of 4 | 2 of 4 | 2 of 4 | 4 of 17 | 3 of 13 | 7 of 21 | 14 of 51 | 1 | |
| T+Q2 | 0 of 3 | 0 of 3 | 1 of 3 | 1 of 3 | 0 of 16 | 0 of 2 | 1 of 20 | 1 of 38 | 3 | |
| T+Q3 | 2 of 3 | 2 of 3 | 2 of 3 | 3 of 3 | 6 of 25 | 3 of 8 | 6 of 20 | 15 of 53 | 2 | |
| T+Q4 | 0 of 3 | 0 of 3 | 1 of 3 | 1 of 3 | 0 of 17 | 0 of 8 | 1 of 10 | 1 of 35 | 1 | |
| T+Q5 | 0 of 2 | 0 of 2 | 0 of 2 | 0 of 2 | 0 of 1 | 0 of 0 | 0 of 7 | 0 of 8 | 6 | |
| T+Q6 | 2 of 3 | 0 of 3 | 0 of 3 | 2 of 3 | 2 of 15 | 0 of 9 | 0 of 22 | 2 of 46 | 7,5 | |
| T+Q7 | 0 of 3 | 0 of 3 | 0 of 3 | 0 of 3 | 0 of 15 | 0 of 5 | 0 of 19 | 0 of 39 | 2,5 | |
| T+Q8 | 0 of 3 | 0 of 3 | 0 of 3 | 0 of 3 | 0 of 13 | 0 of 5 | 0 of 11 | 0 of 29 | 0,5 | |
| T+Q9 | 1 of 4 | 1 of 4 | 1 of 4 | 2 of 4 | 1 of 15 | 2 of 17 | 2 of 29 | 5 of 61 | 2 | |
| TOT | 7 of 28 | 5 of 28 | 7 of 28 | 11 of 28 | 13 of 134 | 8 of 67 | 17 of 159 | 38 of 360 | 2,8 | |
| % | 25,0 | 17,9 | 25,0 | 39,3 | 9,7 | 11,9 | 10,7 | 10,6 | 2,4 | |
| B+Q1 | 2 of 3 | 2 of 3 | 3 of 3 | 3 of 3 | 4 of 23 | 2 of 13 | 4 of 37 | 10 of 73 | 7 | |
| B+Q2 | 1 of 5 | 1 of 5 | 3 of 5 | 3 of 5 | 1 of 22 | 1 of 18 | 6 of 55 | 8 of 95 | 4 | |
| B+Q3 | 0 of 3 | 0 of 3 | 3 of 3 | 3 of 3 | 0 of 7 | 0 of 6 | 7 of 43 | 7 of 56 | 4 | |
| B+Q4 | 2 of 4 | 2 of 4 | 3 of 4 | 3 of 4 | 2 of 14 | 2 of 20 | 5 of 59 | 9 of 93 | 2,5 | |
| B+Q5 | 0 of 4 | 0 of 4 | 0 of 4 | 0 of 4 | 0 of 18 | 0 of 17 | 0 of 33 | 0 of 68 | 1 | |
| B+Q6 | 0 of 4 | 0 of 4 | 1 of 4 | 1 of 4 | 0 of 13 | 0 of 19 | 1 of 30 | 1 of 62 | 4 | |
| TOT | 6 of 27 | 6 of 27 | 14 of 27 | 14 of 27 | 8 of 106 | 12 of 127 | 30 of 308 | 50 of 541 | 4,0 | |
| % | 22,2 | 22,2 | 51,9 | 51,9 | 7,5 | 9,4 | 9,7 | 9,2 | 1,9 | |
| B+Q+T1 | 1 of 3 | 2 of 3 | 2 of 3 | 3 of 3 | 1 of 3 | 3 of 9 | 3 of 19 | 7 of 31 | 4 | |
| B+Q+T2 | 1 of 3 | 1 of 3 | 1 of 3 | 1 of 3 | 1 of 2 | 1 of 5 | 2 of 12 | 4 of 19 | 3 | |
| B+Q+T3 | 2 of 3 | 2 of 3 | 1 of 3 | 2 of 3 | 4 of 19 | 11 of 26 | 14 of 44 | 29 of 89 | 1 | |
| B+Q+T4 | 1 of 2 | 1 of 2 | 1 of 2 | 1 of 2 | 2 of 11 | 2 of 14 | 2 of 11 | 6 of 36 | 1 | |
| B+Q+T5 | 0 of 2 | 1 of 2 | 1 of 2 | 1 of 2 | 0 of 7 | 2 of 15 | 1 of 25 | 3 of 47 | 5 | |
| B+Q+T6 | 2 of 4 | 1 of 4 | 1 of 4 | 2 of 4 | 3 of 20 | 1 of 14 | 1 of 19 | 5 of 53 | 3,5 | |
| B+Q+T7 | 1 of 4 | 2 of 4 | 2 of 4 | 2 of 4 | 2 of 20 | 3 of 13 | 3 of 26 | 8 of 59 | 4 | |
| B+Q+T8 | 1 of 5 | 1 of 5 | 0 of 5 | 1 of 5 | 1 of 23 | 1 of 14 | 0 of 20 | 2 of 57 | 4 | |
| TOT | 9 of 26 | 11 of 26 | 9 of 26 | 13 of 26 | 14 of 105 | 24 of 110 | 26 of 176 | 64 of 391 | 3,2 | |
| % | 34,6 | 42,3 | 34,6 | 50,0 | 13,3 | 21,8 | 14,8 | 16,4 | 1,5 | |
SG = supragranular, G = granular, IG = infragranular. n.a. = not available
Individual therapies
We first compared the ability of individual therapies – active exercise above the level of the lesion (treadmill), passive exercise below the level of the lesion (bike) or serotonergic pharmacotherapy (quipazine) – to induce neurophysiological reorganization of the deafferented hindpaw cortex after complete thoracic transection of the spinal cord, compared with transected animals that received sham therapy (sham). Even though the scope of the present work was not to optimize individual therapies, these initial comparisons represent an important basis to understand the interactions between therapies, which will be assessed in the next section.
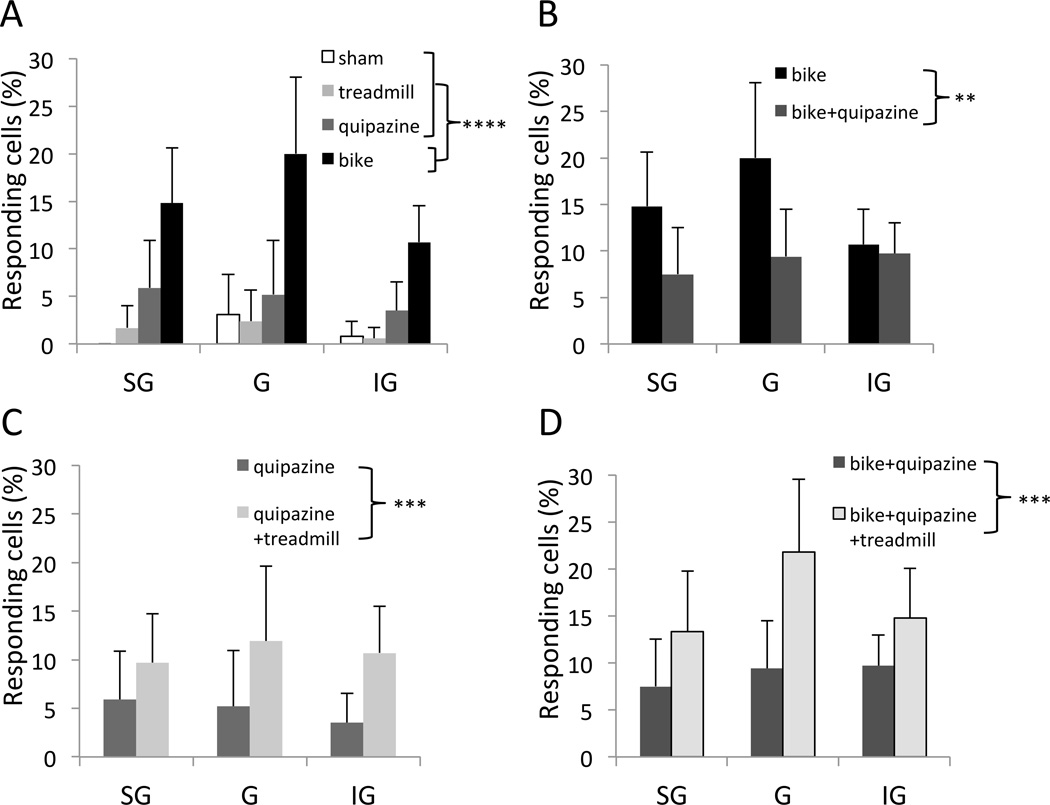
The average number of cells recorded per track per animal was significantly higher in the bike group (20.3±1.7) compared to other individual therapies (one-way ANOVA, factor therapy [sham, treadmill, bike, quipazine], F(3,19)=7.9, p=0.0013; Tukey, bike vs quipazine, 14.1±3.8, p=0.0090; bike vs treadmill, 13.1±2.4, p=0.0025; bike vs sham, 14.9±2.0, p=0.0506). The percentage of cells stereotaxically located in the deafferented hindlimb cortex that responded to tactile stimulation of the contralateral forelimb was significantly higher in the bike group (13.7% of 490 cells) compared to other individual therapies (two-way GZLM, factor therapy, Wald chi2(3)=37.6, p<0.0001; Tukey p<0.0001; Fig. 2A). The difference between bike and other individual therapies was not layer-specific (interaction therapy × layer, Wald chi2(6)=1.0, p=0.98). A less conservative post-hoc analysis revealed that, compared to the low percentage of responding cells in sham animals (1.2% of 253 cells), quipazine induced only a small increase (4.6% of 284 cells; two-proportion test: p=0.0080), whereas treadmill did not have any effect (1.3% of 378 cells; p=0.73). Essentially the same results were obtained considering the percentage of responding cells in each track as independent samples (two-way ANOVA, factor therapy: F(3,258)=16.9, p<0.0001; interaction therapy × layer; F(6,258)=0.55, p=0.76). These results were not due to possible between-group biases in the recording locations (group average ranges: 1.2–1.4 mm posterior, factor therapy, F(3,86)=0.7, p=0.55; 2.5–2.6 mm lateral, F(3,86)=1.6, p=0.19). From a behavioral perspective, no individual therapies was able to significantly improve the BBB scores of the animals (one-way ANOVA, factor therapy: F(3,20)=1.19, p=0.40; Table 1).
Fig. 2.
Neurophysiological reorganization of the somatosensory cortex. (A–D) Percentage of responding cells in the different animal groups, separated by cortical layer: supragranular (SG), granular (G) and infragranular (IG). (A) Individual therapies. (B–D) Combined therapies. Error bars indicate 95% confidence intervals. **p<0.01, ***p<0.001, ****p<0.0001.
Combined therapies
We then investigated how the above therapies interact when combined (i.e. when two or more different therapies were provided to same animal).
When bike was combined with quipazine (bike+quipazine), the average number of cells recorded per track (20.3±3.5) per animal did not change compared to bike alone (F(1,10)=0.01, p=0.97). Surprisingly, however, the percentage of responding cells significantly decreased with bike+quipazine compared to bike alone (from 13.7% of 490 cells, reported above, to 9.2% of 541 cells; two-way GZLM, factor therapy [bike, bike+quipazine], Wald chi2(1)=7.0, p=0.0081; Fig. 2B), without a clear layer-specificity (therapy × layer, Wald chi2(2)=3.2, p=0.20). This result was confirmed when considering the percentage of responding cells in each track as independent samples (two-way ANOVA, factor therapy: F(1,147)=9.0, p=0.0032; therapy × layer: F(2,147)=1.2, p=0.30). These results were not due to possible between-group biases in the recording locations (group averages: 1.0–1.2 mm posterior, t(49)=0.96, p=0.34; 2.6–2.6 mm lateral, t(49)=0.59, p=0.55). BBB scores did not change in animals that received bike+quipazine compared to animals that received bike alone (Table 1; t-test: p=0.26). These results suggests that bike and quipazine are competing therapies for cortical reorganization, in the sense that quipazine interferes with the cortical reorganization induced by bike.
Even though treadmill alone did not induce a measurable increase in cortical responsiveness in our experiments (see above), treadmill combined with quipazine (treadmill+quipazine) produced a higher percentage of responding cells compared to quipazine alone (from 4.6% of 284 cells, reported above, to 10.6% of 360; two-proportion test: p=0.0044). This positive effect of treadmill was also evident in the combination of the three therapies (bike+quipazine+treadmill), which again increased the proportion of responding cells compared to bike+quipazine (from 9.2% of 541 cells, reported above, to 16.4% of 391 cells; p=0.0012). This result was confirmed by more rigorous statistical analysis on the percentage of responding cells (three-way GZLM, factor treadmill [yes or no], Wald chi2(1)=14.8, p=0.0001; interactions with layers and therapy Wald chi2<0.98, p>0.61; Fig. 2C,D), as well as when considering the percentage of responding cells in each track as independent samples (three-way ANOVA, factor treadmill [yes or no]: F(1,97)=4.1, p=0.0451; interaction treadmill × therapy [quipazine, bike+quipazine]: p=0.63). The effect of treadmill did not depend on cortical layer (interaction treadmill × layer: p=0.77; interaction treadmill × therapy × layer: p=0.29). These results were not due to possible between-group biases in the recording locations (group average ranges: 1.0–1.4 mm posterior [factor therapy, F(1,97)=1.6, p=0.21; factor treadmill, F(1,97)=3.8, p=0.054; interaction treadmill × therapy, F(1,97)=0.11, p=0.73]; 2.5–2.6 mm lateral, p>0.19). Note that there was a tendency for recording locations to be slightly more medial in animals that receive treadmill therapy as a combined therapy compared to animals that did not. Because more medial implies more far away from the forelimb cortex, this tendency is conservative for our results. From a behavioral perspective, BBB scores tended to increase in animals that received quipazine+treadmill compared to animals that received quipazine or treadmill alone (Table 1; t-test: p=0.0732). BBB scores did not change in animals that receive bike+quipazine+treadmill compared to animals that received bike+quipazine (Table 1; t-test: p=0.37).
These results suggests that treadmill and quipazine were collaborative therapies for cortical reorganization, in the sense that the percentage of responding cells obtained with treadmill+quipazine (with or without bike) was greater than percentage of responding cells obtained with either treadmill alone or quipazine alone (with or without bike).
Discussion
The main result of the present work is that exercise and serotonergic pharmacotherapy interact in their effects on cortical reorganization after spinal cord injury: passive exercise below the level of the lesion (bike) and serotonergic pharmacotherapy (quipazine) are ‘competing’ therapies (i.e. quipazine limits the cortical reorganization induced by bike), whereas active exercise above the level of the lesion (treadmill) and serotonergic pharmacotherapy (quipazine) are ‘collaborative’ therapies (i.e. the reorganization induced by quipazine combined with treadmill is greater than the reorganization induced by either quipazine or treadmill). From a mechanistic perspective, these findings expose the complex interactions between different pathways to cortical reorganization after massive deafferentation. From a translational perspective, they emphasize the importance of understanding the neuroplasticity effects of therapeutic strategies in spinal cord injury (or in other forms of deafferentation) from an integrated system-level approach.
Methodological considerations
The main measure we used to assess cortical reorganization after complete spinal cord transection is the percentage of neurons recorded in the deafferented hindlimb cortex that responded to light tactile stimulation to body areas above the level of the lesion. This binary measure, which consists of empirically categorizing each recorded neuron as either ‘responder’ or ‘non-responder’ is particularly suitable for our purpose because of the following reasons: (i) in our experimental conditions, the great majority of recorded neurons are non-responders (i.e. their response magnitude, if quantified, would be approximately zero at all locations); (ii) from a biological perspective, the transition from non-responder to responder is particularly relevant and is often used as electrophysiological evidence of cortical reorganization after deafferentation [22–24]; (iii) from an experimental perspective, the binary categorization allows a high number of neurons to be sampled per rat, maximizing statistical power. Importantly, the rigor of the empirical measurement was guaranteed by having two neurophysiologists always involved in the experiments, the one responsible for the binary categorization being blind to both the location of stimulation and the animal group.
Several points should be mentioned to raise caution about the possible translational value of our findings. First, our experiments were not designed to perform analyses with N=animals, so any analysis/inference using N=animals is not reliable. Nonetheless, at a qualitative level, it is interesting to note that some animals appeared to be ‘reorganizers’ with other animals being ‘non-reorganizers’. This observation will deserve further investigation. Second, all behaviors were performed in the light phase of the rats’ light/dark cycle, i.e. during the rat's inactive phase (note that is common practice in the field). Third, our behavioral data (BBB scores) are reported for completeness; it is premature to imply any causal relationship between reorganization of the somatosensory cortex and possible functional recovery in this complete transection model. Cortical reorganization could be either “adaptive”, i.e. contributing to functional recovery, or “maladaptive”, i.e. contributing to neuropathic pain [37]. In fact, allodynia was recently reported in the same rat model of spinal cord transection [38], which encourages further work to clarify the behavioral impact of therapies and the role of cortical reorganization in this model.
Individual therapies
We previously showed that passive bike exercise of the hind limbs after complete thoracic transection of the spinal cord promotes reorganization of the deafferented hind limb cortex, at least in part related to the ability of bike exercise to increase cortical levels of ADCY1 and BNDF [23]. Increased level of BDNF could explain the intriguing present finding of higher number of recorded cells per track when animals received bike compared to other individual therapies.
Passive bike exercise was the therapy that induced the greatest cortical reorganization when provided alone, but this greater impact is not conclusive, because we did not fully optimize each individual therapy – which is beyond the scope of the present work. Furthermore, we specifically tested cortical reorganization using light tactile stimuli that reach the brain through the dorsal column pathway. Different reorganization profiles might be observed using stimuli of higher intensity that maximize dorsal column inputs and also activate the spinothalamic tract [10,11,39–42]. Nonetheless, because passive exercise of the hind limbs is a common rehabilitation practice in patient with spinal cord injury [43–49], spinal transection with passive bike exercise in rats provides a clinically relevant model of cortical reorganization after spinal cord injury [24].
We previously showed that serotonergic pharmacotherapy (quipazine combined with 8-OH-DPAT) promotes cortical reorganization after complete spinal cord transection [24]. Here we showed that quipazine alone induces a small yet significant reorganization of the deafferented hindlimb cortex. Quipazine is well known to improve functional recovery after spinal cord injury, which has been documented in cats [50–51], rats [28,29] and mice [52–55]. The suggested mechanism mediating this functional improvement was the excitation of neurons in the central pattern generator below the lesion that have been deprived of their normal descending 5-HT input from the raphe nucleus. However, this mechanism is unlikely to explain our increase in cortical reorganization, which instead could be related to the ability of 5-HT to promote cortical plasticity after sensory deafferentation, as previously described both in the visual cortex [56,57] and in the barrel cortex [58]. Therefore, the results of the present study and of our previous work [24] collectively suggest a supraspinal action of serotonergic therapy that supports cortical reorganization after spinal cord injury.
We previously showed that treadmill therapy promotes cortical reorganization and functional recovery in adult rats spinalized as neonates [22]. In the present study, the same treadmill therapy alone was not sufficient to promote cortical reorganization or to induce functional recovery in adult rats spinalized as adults, which identifies an important comparative difference between spinal cord injury models. Even though no individual therapy was able to induce significant functional recovery as measured by BBB scores, we cannot exclude that more subtle improvements might be detected with finer behavioral measures.
Combined therapies
The treadmill therapy used here is in line with previous strategies of increasing the activity of the intact cortex to maximize cortical reorganization by actively exercising the non-affected body or the residual functions of the affected body, investigated both in animals models [26,59,60] and in patients with spinal cord injury [12,61–63]. Even though treadmill therapy alone was not sufficient to promote cortical reorganization or to induce functional recovery in adult spinalized rats, it did promote cortical reorganization and tended to induce functional recovery when combined with quipazine compared to quipazine alone. This collaborative interaction of treadmill and quipazine could have at least two possible non-exclusive explanations: (1) behavioral ‘collaboration’, i.e. because quipazine was always given before the treadmill session in our experiments, the acute effects of quipazine might have helped the rat performing a more efficient treadmill exercise; (2) mechanistic ‘collaboration’, i.e. treadmill exercise might ultimately act on the same pathway to cortical reorganization as quipazine. Interestingly, the cortical reorganization induced by quipazine combined with treadmill was actually greater than the sum of the reorganization induced by quipazine plus the reorganization induced by treadmill. Even though it is tempting to speculate a synergic interaction between quipazine and treadmill, this synergy could also be explained by a non-linearity in the dose response of individual therapies (e.g. a threshold effect).
Quipazine combined with passive bike exercise decreased cortical reorganization compared to bike alone. This result is in contrast with our previous study, in which quipazine+8-OH-DPAT combined with bike increased cortical reorganization compared to bike alone [24]. The mechanisms underlying these complex interactions between serotonergic pharmacotherapy and passive exercise remain unclear, but differences among specific 5-HT receptors seem to be critical. At the molecular level, an intriguing possibility is that quipazine (but not quipazine+8-OH-DPAT) might interfere with the up-regulation of brain-derived neurotrophic factor (BDNF) and/or adenylate cyclase 1 (ADCY1) induced at cortical level by passive bike exercise [23]. Future investigations on the effects of 8-OH-DPAT alone (and of 8-OH-DPAT + bike) on cortical reorganization after spinal cord injury will be necessary to obtain a complete mechanistic picture.
Overall, the present results have several important translational implications: (1) different therapies that individually promote cortical reorganization do not necessarily benefit from each other when combined (e.g. bike and quipazine); (2) the same serotonergic therapy (e.g. quipazine) can either promote brain plasticity or limit brain plasticity depending on the model of sensory deafferentation (e.g. spinal transection vs. spinal transection + bike); (3) different serotonergic therapies (e.g quipazine vs. quipazine + 8-OH-DPAT) can induce opposite effects on brain plasticity in the same animal model of sensory deafferentation (e.g. spinal transection + bike). In any case, particular caution should be adopted when attempting to translate results and therapies from animal models to patients.
In conclusion, our findings uncover the interactive effects between active/passive exercise and serotonergic pharmacotherapy on cortical reorganization after spinal cord injury, emphasizing the importance of understanding the effects of therapeutic strategies in spinal cord injury (and in other forms of deafferentation) from an integrated system-level approach.
Acknowledgements
We thank Dr. Elizabeth Dugan for assistance with the transection surgery, animal care and bicycle therapy, Jennifer Garcia for performing the spinal surgeries, managing the therapy protocols and caring for the animals. We would also like to thank Dr. Tim Himes and Theresa Connors and the Spinal Cord Research Center at Drexel University for assistance with the therapy protocols, animal care, transection surgery and histological analysis and Dr. Robert Flint and Matt Cozon for their assistance during the electrophysiological recordings. This work was supported by Grant 36206 from the Neilsen Foundation and Grants P01 NS 055976 and R01 NS05741 from the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Erzurumlu RS, Kind PC. Neural activity: sculptor of 'barrels' in the neocortex. Trends Neurosci. 2001 Oct;24(10):589–595. doi: 10.1016/s0166-2236(00)01958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldman DE, Brecht M. Map plasticity in somatosensory cortex. Science. 2005 Nov 4;310(5749):810–815. doi: 10.1126/science.1115807. [DOI] [PubMed] [Google Scholar]
- 3.Barnes SJ, Finnerty GT. Sensory experience and cortical rewiring. Neuroscientist. 2010 Apr;16(2):186–198. doi: 10.1177/1073858409343961. [DOI] [PubMed] [Google Scholar]
- 4.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009 Sep;10(9):647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 5.Wall PD, Egger MD. Formation of new connexions in adult rat brains after partial deafferentation. Nature. 1971 Aug 20;232(5312):542–545. doi: 10.1038/232542a0. [DOI] [PubMed] [Google Scholar]
- 6.Calford MB, Tweedale R. Immediate and chronic changes in responses of somatosensory cortex in adult flying-fox after digit amputation. Nature. 1988 Mar 31;332(6163):446–448. doi: 10.1038/332446a0. [DOI] [PubMed] [Google Scholar]
- 7.Pons TP, Garraghty PE, Ommaya AK, Kaas JH, Taub E, Mishkin M. Massive cortical reorganization after sensory deafferentation in adult macaques. Science. 1991 Jun 28;252(5014):1857–1860. doi: 10.1126/science.1843843. [DOI] [PubMed] [Google Scholar]
- 8.Jain N, Catania KC, Kaas JH. Deactivation and reactivation of somatosensory cortex after dorsal spinal cord injury. Nature. 1997 Apr 3;386(6624):495–498. doi: 10.1038/386495a0. [DOI] [PubMed] [Google Scholar]
- 9.Florence SL, Taub HB, Kaas JH. Large-scale sprouting of cortical connections after peripheral injury in adult macaque monkeys. Science. 1998 Nov 6;282(5391):1117–1121. doi: 10.1126/science.282.5391.1117. [DOI] [PubMed] [Google Scholar]
- 10.Endo T, Spenger C, Tominaga T, Brene S, Olson L. Cortical sensory map rearrangement after spinal cord injury: fMRI responses linked to Nogo signalling. Brain. 2007 Nov;130(Pt 11):2951–2961. doi: 10.1093/brain/awm237. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh A, Haiss F, Sydekum E, Schneider R, Gullo M, Wyss MT, et al. Rewiring of hindlimb corticospinal neurons after spinal cord injury. Nat Neurosci. 2010 Jan;13(1):97–104. doi: 10.1038/nn.2448. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman LR, Field-Fote EC. Cortical reorganization following bimanual training and somatosensory stimulation in cervical spinal cord injury: a case report. Phys Ther. 2007 Feb;87(2):208–223. doi: 10.2522/ptj.20050365. [DOI] [PubMed] [Google Scholar]
- 13.Lotze M, Laubis-Herrmann U, Topka H. Combination of TMS and fMRI reveals a specific pattern of reorganization in M1 in patients after complete spinal cord injury. Restor Neurol Neurosci. 2006;24(2):97–107. [PubMed] [Google Scholar]
- 14.Cramer SC, Lastra L, Lacourse MG, Cohen MJ. Brain motor system function after chronic, complete spinal cord injury. Brain. 2005 Dec;128(Pt 12):2941–2950. doi: 10.1093/brain/awh648. [DOI] [PubMed] [Google Scholar]
- 15.Curt A, Bruehlmeier M, Leenders KL, Roelcke U, Dietz V. Differential effect of spinal cord injury and functional impairment on human brain activation. J Neurotrauma. 2002 Jan;19(1):43–51. doi: 10.1089/089771502753460222. [DOI] [PubMed] [Google Scholar]
- 16.Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N, et al. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995 Jun 8;375(6531):482–484. doi: 10.1038/375482a0. [DOI] [PubMed] [Google Scholar]
- 17.Lotze M, Grodd W, Birbaumer N, Erb M, Huse E, Flor H. Does use of a myoelectric prosthesis prevent cortical reorganization and phantom limb pain? Nat Neurosci. 1999 Jun;2(6):501–502. doi: 10.1038/9145. [DOI] [PubMed] [Google Scholar]
- 18.Klit H, Finnerup NB, Jensen TS. Central post-stroke pain: clinical characteristics, pathophysiology, and management. Lancet Neurol. 2009 Sep;8(9):857–868. doi: 10.1016/S1474-4422(09)70176-0. [DOI] [PubMed] [Google Scholar]
- 19.Wrigley PJ, Press SR, Gustin SM, Macefield VG, Gandevia SC, Cousins MJ, et al. Neuropathic pain and primary somatosensory cortex reorganization following spinal cord injury. Pain. 2009 Jan;141(1–2):52–59. doi: 10.1016/j.pain.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Gustin SM, Peck CC, Cheney LB, Macey PM, Murray GM, Henderson LA. Pain and plasticity: is chronic pain always associated with somatosensory cortex activity and reorganization? J Neurosci. 2012 Oct 24;32(43):14874–14884. doi: 10.1523/JNEUROSCI.1733-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, et al. Reversing pathological neural activity using targeted plasticity. Nature. 2011 Feb 3;470(7332):101–104. doi: 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kao T, Shumsky JS, Murray M, Moxon KA. Exercise induces cortical plasticity after neonatal spinal cord injury in the rat. J Neurosci. 2009 Jun 10;29(23):7549–7557. doi: 10.1523/JNEUROSCI.2474-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graziano A, Foffani G, Knudsen EB, Shumsky J, Moxon KA. Passive exercise of the hind limbs after complete thoracic transection of the spinal cord promotes cortical reorganization. PLoS One. 2013;8(1):e54350. doi: 10.1371/journal.pone.0054350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganzer PD, Moxon KA, Knudsen EB, Shumsky JS. Serotonergic pharmacotherapy promotes cortical reorganization after spinal cord injury. Exp Neurol. 2013 Mar;241:84–94. doi: 10.1016/j.expneurol.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moxon KA, Kao T, Shumsky JS. Role of cortical reorganization on the effect of 5-HT pharmacotherapy for spinal cord injury. Exp Neurol. 2013 Feb;240:17–27. doi: 10.1016/j.expneurol.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 26.Kao T, Shumsky JS, Knudsen EB, Murray M, Moxon KA. Functional role of exercise-induced cortical organization of sensorimotor cortex after spinal transection. J Neurophysiol. 2011 Nov;106(5):2662–2674. doi: 10.1152/jn.01017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skinner RD, Houle JD, Reese NB, Berry CL, Garcia-Rill E. Effects of exercise and fetal spinal cord implants on the H-reflex in chronically spinalized adult rats. Brain Res. 1996 Aug 5;729(1):127–131. [PubMed] [Google Scholar]
- 28.Feraboli-Lohnherr D, Barthe JY, Orsal D. Serotonin-induced activation of the network for locomotion in adult spinal rats. J Neurosci Res. 1999 Jan 1;55(1):87–98. doi: 10.1002/(SICI)1097-4547(19990101)55:1<87::AID-JNR10>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 29.Antri M, Orsal D, Barthe JY. Locomotor recovery in the chronic spinal rat: effects of long-term treatment with a 5-HT2 agonist. Eur J Neurosci. 2002 Aug;16(3):467–476. doi: 10.1046/j.1460-9568.2002.02088.x. [DOI] [PubMed] [Google Scholar]
- 30.Kim D, Adipudi V, Shibayama M, Giszter S, Tessler A, Murray M, et al. Direct agonists for serotonin receptors enhance locomotor function in rats that received neural transplants after neonatal spinal transection. J Neurosci. 1999 Jul 15;19(14):6213–6224. doi: 10.1523/JNEUROSCI.19-14-06213.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freo U, Ricchieri GL, Holloway HW, Soncrant TT. Time- and dose-dependent effects of the serotonergic agent quipazine on regional cerebral metabolism in rats. Brain Res. 1993 Jan 15;600(2):249–256. doi: 10.1016/0006-8993(93)91380-b. [DOI] [PubMed] [Google Scholar]
- 32.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995 Feb;12(1):1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 33.Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996 Jun;139(2):244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 34.Chapin JK, Lin CS. Mapping the body representation in the SI cortex of anesthetized and awake rats. J Comp Neurol. 1984 Oct 20;229(2):199–213. doi: 10.1002/cne.902290206. [DOI] [PubMed] [Google Scholar]
- 35.Friedberg MH, Lee SM, Ebner FF. Modulation of receptive field properties of thalamic somatosensory neurons by the depth of anesthesia. J Neurophysiol. 1999 May;81(5):2243–2252. doi: 10.1152/jn.1999.81.5.2243. [DOI] [PubMed] [Google Scholar]
- 36.Erchova IA, Lebedev MA, Diamond ME. Somatosensory cortical neuronal population activity across states of anaesthesia. Eur J Neurosci. 2002 Feb;15(4):744–752. doi: 10.1046/j.0953-816x.2002.01898.x. [DOI] [PubMed] [Google Scholar]
- 37.Moxon KA, Oliviero A, Aguilar J, Foffani G. Cortical reorganization after spinal cord injury: always for good? Neuroscience. 2014 Dec 26;283:78–94. doi: 10.1016/j.neuroscience.2014.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.M'Dahoma S, Bourgoin S, Kayser V, Barthelemy S, Chevarin C, Chali F, Orsal D, Hamon M. Spinal cord transection-induced allodynia in rats--behavioral, physiopathological and pharmacological characterization. PLoS One. 2014 Jul 14;9(7):e102027. doi: 10.1371/journal.pone.0102027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aguilar J, Humanes-Valera D, Alonso-Calvino E, Yague JG, Moxon KA, Oliviero A, et al. Spinal cord injury immediately changes the state of the brain. J Neurosci. 2010 Jun 2;30(22):7528–7537. doi: 10.1523/JNEUROSCI.0379-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yague JG, Foffani G, Aguilar J. Cortical hyperexcitability in response to preserved spinothalamic inputs immediately after spinal cord hemisection. Exp Neurol. 2011 Feb;227(2):252–263. doi: 10.1016/j.expneurol.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 41.Humanes-Valera D, Aguilar J, Foffani G. Reorganization of the intact somatosensory cortex immediately after spinal cord injury. PLoS One. 2013 Jul 29;8(7):e69655. doi: 10.1371/journal.pone.0069655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yagüe JG, Humanes-Valera D, Aguilar J, Foffani G. Functional reorganization of the forepaw cortical representation immediately after thoracic spinal cord hemisection in rats. Exp Neurol. 2014 Jul;257:19–24. doi: 10.1016/j.expneurol.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 43.Hangartner TN, Rodgers MM, Glaser RM, Barre PS. Tibial bone density loss in spinal cord injured patients: effects of FES exercise. J Rehabil Res Dev. 1994;31(1):50–61. [PubMed] [Google Scholar]
- 44.De Mello MT, Esteves AM, Tufik S. Comparison between dopaminergic agents and physical exercise as treatment for periodic limb movements in patients with spinal cord injury. Spinal Cord. 2004 Apr;42(4):218–221. doi: 10.1038/sj.sc.3101575. [DOI] [PubMed] [Google Scholar]
- 45.Kiser TS, Reese NB, Maresh T, Hearn S, Yates C, Skinner RD, et al. Use of a motorized bicycle exercise trainer to normalize frequency-dependent habituation of the H-reflex in spinal cord injury. J Spinal Cord Med. 2005;28(3):241–245. doi: 10.1080/10790268.2005.11753818. [DOI] [PubMed] [Google Scholar]
- 46.Phadke CP, Flynn SM, Thompson FJ, Behrman AL, Trimble MH, Kukulka CG. Comparison of single bout effects of bicycle training versus locomotor training on paired reflex depression of the soleus H-reflex after motor incomplete spinal cord injury. Arch Phys Med Rehabil. 2009 Jul;90(7):1218–1228. doi: 10.1016/j.apmr.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 47.Rayegani SM, Shojaee H, Sedighipour L, Soroush MR, Baghbani M, Amirani OB. The effect of electrical passive cycling on spasticity in war veterans with spinal cord injury. Front Neurol. 2011;2:39. doi: 10.3389/fneur.2011.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lauer RT, Smith BT, Mulcahey MJ, Betz RR, Johnston TE. Effects of cycling and/or electrical stimulation on bone mineral density in children with spinal cord injury. Spinal Cord. 2011 Aug;49(8):917–923. doi: 10.1038/sc.2011.19. [DOI] [PubMed] [Google Scholar]
- 49.Phillips AA, Cote AT, Warburton DE. A systematic review of exercise as a therapeutic intervention to improve arterial function in persons living with spinal cord injury. Spinal Cord. 2011 Jun;49(6):702–714. doi: 10.1038/sc.2010.193. [DOI] [PubMed] [Google Scholar]
- 50.Barbeau H, Rossignol S. The effects of serotonergic drugs on the locomotor pattern and on cutaneous reflexes of the adult chronic spinal cat. Brain Res. 1990 Apr 23;514(1):55–67. doi: 10.1016/0006-8993(90)90435-e. [DOI] [PubMed] [Google Scholar]
- 51.Brustein E, Rossignol S. Recovery of locomotion after ventral and ventrolateral spinal lesions in the cat. II. Effects of noradrenergic and serotoninergic drugs. J Neurophysiol. 1999 Apr;81(4):1513–1530. doi: 10.1152/jn.1999.81.4.1513. [DOI] [PubMed] [Google Scholar]
- 52.Guertin PA. Role of NMDA receptor activation in serotonin agonist-induced air-stepping in paraplegic mice. Spinal Cord. 2004 Mar;42(3):185–190. doi: 10.1038/sj.sc.3101580. [DOI] [PubMed] [Google Scholar]
- 53.Fong AJ, Cai LL, Otoshi CK, Reinkensmeyer DJ, Burdick JW, Roy RR, et al. Spinal cord-transected mice learn to step in response to quipazine treatment and robotic training. J Neurosci. 2005 Dec 14;25(50):11738–11747. doi: 10.1523/JNEUROSCI.1523-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ziegler MD, Zhong H, Roy RR, Edgerton VR. Why variability facilitates spinal learning. J Neurosci. 2010 Aug 11;30(32):10720–10726. doi: 10.1523/JNEUROSCI.1938-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ziegler MD, Hsu D, Takeoka A, Zhong H, Ramon-Cueto A, Phelps PE, et al. Further evidence of olfactory ensheathing glia facilitating axonal regeneration after a complete spinal cord transection. Exp Neurol. 2011 May;229(1):109–119. doi: 10.1016/j.expneurol.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O'Leary OF, et al. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008 Apr 18;320(5874):385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- 57.Maya Vetencourt JF, Tiraboschi E, Spolidoro M, Castren E, Maffei L. Serotonin triggers a transient epigenetic mechanism that reinstates adult visual cortex plasticity in rats. Eur J Neurosci. 2011 Jan;33(1):49–57. doi: 10.1111/j.1460-9568.2010.07488.x. [DOI] [PubMed] [Google Scholar]
- 58.Sheikhkanloui-Milan H, Sheibani V, Afarinesh M, Esmaeili-Mahani S, Shamsizadeh A, Sepehri G. Effects of electrical stimulation of dorsal raphe nucleus on neuronal response properties of barrel cortex layer IV neurons following long-term sensory deprivation. Neurosci Bull. 2010 Oct;26(5):388–394. doi: 10.1007/s12264-010-0412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Friel KM, Heddings AA, Nudo RJ. Effects of postlesion experience on behavioral recovery and neurophysiologic reorganization after cortical injury in primates. Neurorehabil Neural Repair. 2000;14(3):187–198. doi: 10.1177/154596830001400304. [DOI] [PubMed] [Google Scholar]
- 60.Girgis J, Merrett D, Kirkland S, Metz GA, Verge V, Fouad K. Reaching training in rats with spinal cord injury promotes plasticity and task specific recovery. Brain. 2007 Nov;130(Pt 11):2993–3003. doi: 10.1093/brain/awm245. [DOI] [PubMed] [Google Scholar]
- 61.Thomas SL, Gorassini MA. Increases in corticospinal tract function by treadmill training after incomplete spinal cord injury. J Neurophysiol. 2005 Oct;94(4):2844–2855. doi: 10.1152/jn.00532.2005. [DOI] [PubMed] [Google Scholar]
- 62.Winchester P, McColl R, Querry R, Foreman N, Mosby J, Tansey K, et al. Changes in supraspinal activation patterns following robotic locomotor therapy in motor-incomplete spinal cord injury. Neurorehabil Neural Repair. 2005 Dec;19(4):313–324. doi: 10.1177/1545968305281515. [DOI] [PubMed] [Google Scholar]
- 63.Knikou M. Plasticity of corticospinal neural control after locomotor training in human spinal cord injury. Neural Plast. 2012;2012:254948. doi: 10.1155/2012/254948. [DOI] [PMC free article] [PubMed] [Google Scholar]