Abstract
Traumatic brain injury (TBI) is a leading cause of death and disability in the USA. Effective therapeutic strategies for TBI are needed, and increasing attention is turning toward traditional herbal medicine. Rhizoma drynariae is a traditional Chinese medicine that has immunomodulatory and anti-inflammatory effects. Here, using the controlled cortical impact model of TBI in rats, we examined whether oral administration of R. drynariae can reduce TBI-induced brain injury in rats. We also identified the likely active compound among its four major phytochemicals in decoction. We found that post-treatment with R. drynariae decreased brain lesion volume, improved neurologic and cognitive function, and reduced anxiety- and depression-like behaviors. These changes were accompanied by reduced blood levels of IL-6 and increased IL-10. R. drynariae treatment also reversed the TBI-induced decrease in blood monocyte numbers and percentage of blood CD3 and CD4 T lymphocytes while inhibiting microglial/macrophage activation. Furthermore, by using ultra performance liquid chromatography and comparing retention times with authentic standards, we identified eriodictyol as the putative active compound of R. drynariae extract in the blood of rats with TBI. These novel findings indicate that the traditional Chinese herbal medicine R. drynariae protects brain against TBI-induced brain injury, possibly via immune-promoting, anti-inflammatory, and neuroprotective effects. Eriodictyol could be its active compound.
Keywords: Microglia, Rhizoma drynariae, Traumatic brain injury, UPLC
Introduction
Mortality and permanent disability after traumatic brain injury (TBI) are major health challenges throughout the world [1–3]. Studies show that approximately 1.5 to 2 million patients suffer from TBI each year in the USA [4], and the total annual cost is estimated to be $10 billion [5]. Therefore, it is imperative to develop an effective treatment for TBI.
Unfavorable TBI outcomes result from primary mechanical injury to the brain and ensuing secondary non-mechanical injury that involves innate and adaptive immune responses. Multiple tissues and cells can be affected. Substantial amounts of research have been carried out to elucidate secondary injury mechanisms, with the goal of developing neuroprotective strategies. Although many promising pharmacologic compounds have been identified and tested in animal studies, more than 40 phase II and phase III clinical trials have failed to show efficacy of monotherapy for TBI [6, 7], including two recent phase III clinical trials for progesterone (PROTECT III and SYNAPSE) [8, 9]. The heterogeneity of the pathophysiology of TBI justifies the use of multidrug combination therapy that can act on multiple molecular targets. In this regard, Chinese herbal medicine meets the criteria required as a potential treatment for TBI because it is usually a mixture of several constituents and may act at multiple sites.
In recent years, traditional herbal medicine has gained increasing attention and become an important resource for new drug discovery [10]. Because many act at multiple targets and have strong immunomodulatory effects [11], traditional herbal medicines could represent a new therapeutic strategy for TBI. One such medicine is Rhizoma drynariae, made from the dried roots of Drynaria fortunei of the polypodiceae family. R. drynariae is a traditional Chinese herbal medicine that is able to modulate immune responses in mice [12]. It also has potent anti-inflammatory properties [13]. In a weight-drop model of TBI, we have shown previously that post-treatment of rats with R. drynariae is able to reverse the reduction in plasma level of interleukin (IL)-2 and the reduction in number of CD4-positive T lymphocytes in blood. In contrast, the number of CD8-positive T lymphocytes decreased [14]. In this study, we used the controlled cortical impact (CCI) model of TBI to evaluate whether oral R. drynariae administration after TBI provides neuroprotection in rats. We tested the hypothesis that R. drynariae has both immune-promoting and anti-inflammatory effects in the CCI model of TBI and that both effects are protective. Moreover, because R. drynariae extract contains dozens of compounds, we investigated which active compounds might be responsible for brain protection.
Materials and Methods
Animals
All animal protocols were approved by the Central South University (Changsha, China) and were implemented according to institutional guidelines of the Animal Care and Use Committee. A total of 88 adult, male Sprague–Dawley rats (250± 30 g) were purchased from the Laboratory Animal Centre of Central South University. The animals were housed under controlled conditions (12-h light/dark cycle, 25 °C, 50± 10 % relative humidity). Animal experiments were reported in accordance with the ARRIVE guidelines.
CCI Model of TBI
Rats were anesthetized with 10 % chloral hydrate via intraperitoneal injection. CCI injury was induced with a PSI TBI-0310 Impactor (Precision Systems and Instrumentation, Fairfax, VA). This device uses electromagnetic force to produce an impact velocity for which speed, depth, and dwell time can each be individually manipulated to produce injuries with different severities. The rats were placed on a stereotaxic frame with a built-in heating bed that maintains body temperature at 37 °C. The head was mounted in the stereotaxic frame. Under aseptic conditions, a midline longitudinal incision was made over the skull, and a 5-mm craniotomy was made using a portable drill and trephine over the left parietal cortex (center of the coordinates of the craniotomy relative to bregma: 1 mm posterior, 1 mm lateral). The bone flap was removed. A pneumatic cylinder with a 3-mm flat-tip impounder produced CCI in the rats at a velocity of 6 m/s, depth of 5 mm, and impact duration of 100 ms. The scalp was closed by using cyanoacrylate tissue glue. Then, anesthesia was terminated, and the rats were allowed to recover on the heating pad. After they resumed locomotor activity, the rats were returned to their home cages.
Preparation of Freeze-Dried Powder of R. drynariae Decoction
The R. drynariae herb was purchased from the pharmacy of Xiangya Hospital, Central South University, and was authenticated by Professor XZ Li. R. drynariae was boiled twice in distilled water (1:12, w/v) for 30 min. The blended supernatants were then lyophilized (yield=5.22 %, w/w). The dried extract was dissolved in sterilized saline to 1 g/mL before use.
Experimental Groups and Administration of Drugs
Eighty-eight rats were randomly divided into three groups in a blinded manner: sham (8 rats), TBI+vehicle (40 rats), and TBI+R. drynariae (40 rats). R. drynariae (20 mg/kg in sterilized saline) or vehicle (equivalent volume of sterilized saline) was administered by gavage at 4 h after TBI and then every 24 h thereafter for 14 or 28 days. The dosing, delivery route, and treatment regimens were based on our previous study [14]. Sham rats were administered an equivalent volume of saline 4 h after craniotomy alone. Investigators blinded to the treatment groups evaluated all of the study outcomes in all rats and performed calculations and analysis. All rats were included in the final blind data analysis except for those that died before the end of the study.
Blood and Plasma Sample Collection
Blood samples from six rats per group were collected on days 1, 3, 7, 14, 21, and 28 after TBI. The rats were anesthetized via intraperitoneal injection of 10 % chloral hydrate, fixed, and laparotomized. Blood was drawn through the peritoneal vein with a 5-mL vacuum heparin pipet. After performing a complete blood count, we removed 0.1 mL of blood from each sample and mixed it gently with 11.5 mL of antibody to CD3, CD4, or CD8 (eBioscience, San Diego, CA). Simultaneously, samples from the control group were mixed with 5 mL of antibody to IgG1, IgG2a, or IgG3 (eBioscience). Both sets of samples were incubated in the dark for 15 min. Next, 1× hemolysin (Becton Dickinson, Sparks, MD) was added and mixed on a vortex shaker for 5 s. The samples were again placed in the dark for 8 min and then centrifuged at 1500 rpm for 5 min. After removing the supernatant, we added 3 drops of PBS into the pellet of each sample, mixed gently, and added 1 % paraformaldehyde. The samples were subsequently stored in the dark until further testing. The remaining 4 mL of blood was centrifuged at 2000 rpm for 15 min at 4 °C. The supernatant (plasma) was then removed and stored at −80 °C for measurement of IL-6 levels. Flow cytometry was used for the detection of CD3, CD4, and CD8 T lymphocytes, and an automatic microplate reader was used for the measurement of plasma IL-6 and IL-10 levels.
ELISA
Plasma IL-6 and IL-10 concentrations were measured by IL-6 and IL-10 ELISA kits (Wuhan Huamei Biotechnology Company, Wuhan, Hubei, China, Catalog number: SCB-E04640r and SCB-E04595r) according to manufacturer's instructions.
Behavioral Testing
An investigator blinded to treatment group performed all behavioral tests during the light cycle phase in an enclosed behavior room. The rats were acclimatized in the behavior room for 30 min before behavioral testing.
Neurologic deficits assessment
Neurologic deficits of rats were assessed on days 1, 3, 7, 14, 21, and 28 after TBI with the modified neurologic severity score (mNSS) method based on published protocol [15–17]. The mNSS comprises tests for motor function, sensory function, reflex, and balance. These tests are sensitive to unilateral cortical injury because they reflect multiple asymmetries, including postural, sensory, and forelimb and hind limb use asymmetries.
Morris water maze
Spatial learning and memory deficits were evaluated with the Morris water maze paradigm [18, 19]. The test was conducted at 25–28 days after injury, and each rat was tested for four consecutive days with four trials per day. Formal testing was performed on day 28 after injury. During the first 4 days, we trained the rats to locate a hidden, submerged platform using peripheral visual information. Rats were introduced into the pool at one of four entry points, with every entry point used over the course of the day. The rats were given 90 s to locate the platform and then remained on the platform for 10 s before being removed. Rats that were unable to locate the platform within 90 s were placed on the platform for 10 s before being removed from the pool. Tracking software (ANYMaze; San Diego Instruments, San Diego, CA) was used to record latency to find the platform, path lengths, and swimming speed.
Elevated-plus maze
The elevated-plus maze was used to assess anxiety-like behavior on day 21 post-injury [20]. The maze consisted of a 26-in.-long cross-shaped platform made of non-porous black plastic elevated 15 in. above the ground. One set of arms was open and the other closed. A camera was positioned overhead to track the behavior of the rats. Rats have a natural preference for the closed, dark arms. Therefore, changes in behavior were evaluated by quantifying the number of entries and the time spent in open arms. Rats were placed in the center junction of the maze facing an open arm and were given 5 min to explore. The number of entries into each arm, the time spent in each arm, and the total distance traveled in the maze (and in each individual arm) were tracked by the video camera and analyzed with ANYMaze software. The software tracked the center point of the rat's body. Rats were considered to have entered an arm when their center point crossed into the arm.
Forced swim test
Depression-like behavior was assessed on day 21 after injury with the forced swim test (FST) [21, 22]. In the FST, the rat was placed in a 5000-mL glass beaker containing 30 cm of water (25 °C). It remained in the water for 5 min with no means of escape. The absence of escape-oriented behaviors such as swimming, jumping, rearing, sniffing, or diving is indicative of behavioral despair. The duration of immobility was recorded during the last 4 min of the test by an investigator blinded to experimental groups. ANYMaze software was used to track and analyze the data. The rat was judged to be immobile when it ceased struggling and remained floating motionless in an upright position in the water, making only small movements to keep its head above the water [21, 22].
Lesion Volume Analysis
After the completion of behavioral testing on day 3 post-TBI, rats were anesthetized with 10 % chloral hydrate via intraperitoneal injection and perfused with ice-cold saline. Brains were extracted and stored in 4 % paraformaldehyde overnight and cryoprotected in serial phosphate-buffered sucrose solutions (20, 30, and 40 %) at 4 °C [23, 24]. Brains were frozen and sliced into 40-μm-thick coronal sections on a freezing microtome. Every 12 sections from bregma 1 mm to bregma 6 mm were mounted on glass slides, stained with Gill's hematoxylin and eosin (H&E; 2.5 %), and covered with a cover slip. We assessed lesion volume with the Cavalieri method of unbiased stereology using Stereologer software [25]. The area was multiplied by the known distance (0.48 mm) between the sections to obtain the total lesion volume [26, 27].
Immunohistochemistry
Based on our established protocol of brain tissue processing for immunohistochemistry [28], 20-μm-thick frozen sections were placed on slides, dried completely, and immersed three times in PBS for 3 min each. To quench endogenous peroxidase activity, the sections were immersed in 3 % hydrogen peroxide for 15 min. Sections were then washed with PBS as before, 3 times for 3 min each. Next, CD68 antibody (a marker for activated microglia/macrophages, Maixin-Bio, Fuzhou, Fujian, China, Catalog number: 130617408C) was added onto every section. The sections were then kept overnight at 4 °C and washed again three times in PBS. MaxVision reagent (Maixin-Bio, Catalog number: 130617408C) was added and incubated for 15 min at room temperature. Lastly, the slides were developed with DAB (3,3′ diaminobenzidine) chromogen (MaxVision, Batch number: 130618003).
Preparation of Plasma Sample After Oral Administration of R. drynariae to Rats
One milliliter of plasma was collected from sham rats and rats treated with vehicle or R. drynariae. Then, 950 μL of methanol was added to the plasma, and the solution was vortexed for 3 min. The denatured protein precipitate was separated by centrifugation at 3000×g for 15 min at 4 °C [29].
Identification of Active Phytochemicals in Decoction and Plasma by Ultra Performance Liquid Chromatography
Phytochemicals of R. drynariae extract or R. drynariae in plasma were identified by comparing retention time in a Waters ACQUITY UPLC BEH 2.1×100 mm 1.7 μm C18 column system (Waters Corporation, Milford, MA, USA). The system consisted of a quaternary pump solvent management system, an on-line degasser, and an autosampler. The raw data were detected, acquired, and processed with Empower software. The mobile phase was composed of (a) acetonitrile and (b) 0.1 % acetic acid, with gradient elution as follows: 0–5 min, 10–20 % A; 5–12 min, 20–35 % A; 12–15 min, 35–10 % A. The flow rate of the mobile phase was 0.3 mL/min, and the temperature was maintained at 25 °C [22, 30, 31].
A standard stock solution of each of the following components was prepared directly in methanol: naringin (Shanghai, CAS#: 10236-47-2, Batch: 20120221), eriodictyol (Shanghai, CAS#: 552-58-9, Batch: 20120807), trans-cinnamic acid (Shanghai, CAS#: 140-10-3, Batch: 20130323), and P-coumaric acid (Sigma, CAS#: 501-98-4, Batch:1001285623). Working standard solutions containing the four compounds were prepared and diluted with methanol to appropriate concentrations for establishment of calibration curves. The standard stock solutions and working solutions were all prepared in dark brown calibrated flasks and stored at 4 °C. The linearity of the responses was determined for seven concentrations. Empower software was used to prepare the standard curves from the peak area of each compound. The contents of these constituents in the test samples were calculated by using the regression parameters obtained from the standard curves.
Statistics
Data are presented as mean±SD. We evaluated all behavioral tests by two-way repeated measures ANOVA or one-way ANOVA followed by post hoc test to detect differences between groups. In anatomical and biochemical studies, one-way or two-way ANOVA followed by post hoc test was used for comparisons among multiple groups. Differences between two groups were tested with the Student's t test (SPSS). The criterion for statistical significance was p<0.05.
Results
During this study, the mortality was 10 % (4/40) in the vehicle group and 10 % (4/40) in the R. drynariae-treated group. The 8 rats that died prematurely were excluded from the final blind data analysis.
R. drynariae Treatment Decreases CCI-Induced Lesion Volume
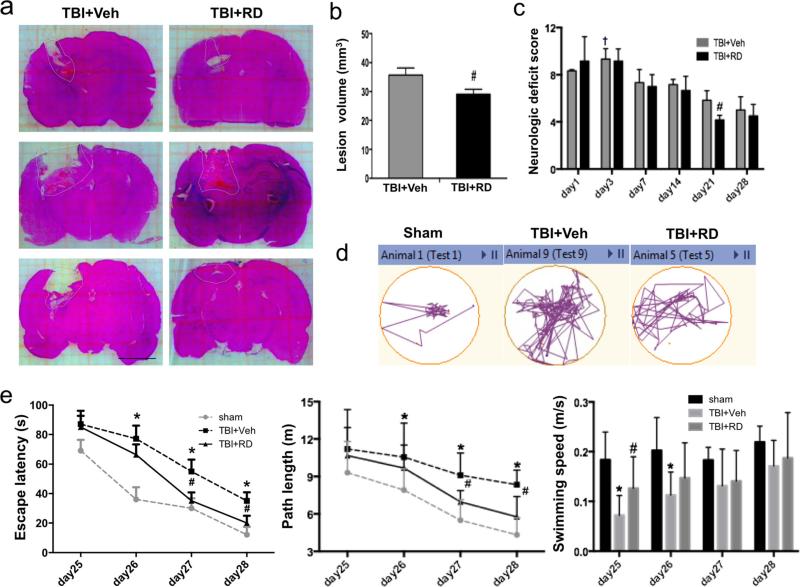
On day 3 after CCI, H&E-stained [32] brain slices showed that lesion volume was smaller in R. drynariae-treated rats than in vehicle-treated rats (n=6 rats/group, p<0.05; Fig. 1a, b). No brain lesion was observed in the sham-operated rats.
Fig. 1.
Effects of Rhizoma drynariae (RD) on lesion volume, neurologic deficits, and learning/memory function in rats subjected to CCI. a Representative H&E-stained brain sections on day 3. Lesion areas are circled in white dotted lines. b Quantification analysis shows that brain lesion volume was smaller in the R. drynariae-treated group than in the vehicle-treated group on day 3 after CCI. Scale bar: 5 mm. c Rats subjected to CCI exhibited neurologic deficits from days 1 to 28. Maximal neurologic deficit score is 10; normal score is 0. The maximum neurologic deficit score was observed on day 3 after CCI. The R. drynariae-treated group had significantly lower scores than the vehicle-treated group on day 21 after CCI. †p<0.05 vs. all other time points in the vehicle group. #p<0.05 vs. vehicle-treated group. d Representative images of the swim paths on day 28 show that rats treated with R. drynariae were able to find the hidden platform more easily than rats treated with vehicle. e Escape latency and path length are the time and distance that rats swim to reach the target platform. Rats treated with R. drynariae had shorter escape latencies and path lengths than did rats treated with vehicle. The swimming speed was impaired in rats on days 25 and 26 after CCI but was increased by R. drynariae treatment. On days 27 and 28, the swimming speed did not differ significantly between the two groups. All values are mean±SD; n=6 rats/group; *p<0.01 vs. sham group, #p<0.05 vs. vehicle group
R. drynariae Treatment Decreases CCI-Induced Neurologic Deficit Score
All rats exhibited neurologic deficits on days 1, 3, 7, 14, 21, and 28 after CCI. The maximum deficit was seen on day 3. The R. drynariae-treated group had lower neurologic deficit scores than did the vehicle-treated group on day 21, but not on day 28, after CCI (n=6 rats/group, F= 5.76 for day 3, F=5.324 for day 21, both p<0.05; Fig. 1c).
R. drynariae Treatment Improves CCI-Induced Cognitive Deficits
Rats that underwent CCI exhibited cognitive impairment in the Morris water maze on day 28. The escape latency and length of the swim path to find the platform were both longer in the vehicle-treated TBI rats than in the sham-operated rats on days 26 to 28 (n=6 rats/group; F=19.59, all p<0.01 for latency; F=6.31, all p<0.05 for path length); however, R. drynariae treatment reduced the time and swim path length on days 27 and 28 compared to those of the vehicle-treated group (n=6 rats/group; F=5.16, both p<0.05 for latency; F=5.49, p<0.05 for path length); the swimming speed did not differ between R. drynariae- and vehicle-treated groups on days 26 to 28 (n=6 rats/group, F=3.611, F=1.585, F=2.774 for days 26, 27, and 28, respectively, all p>0.05; Fig. 1d, e).
R. drynariae Treatment Reduces CCI-Induced Anxiety-Like Behavior
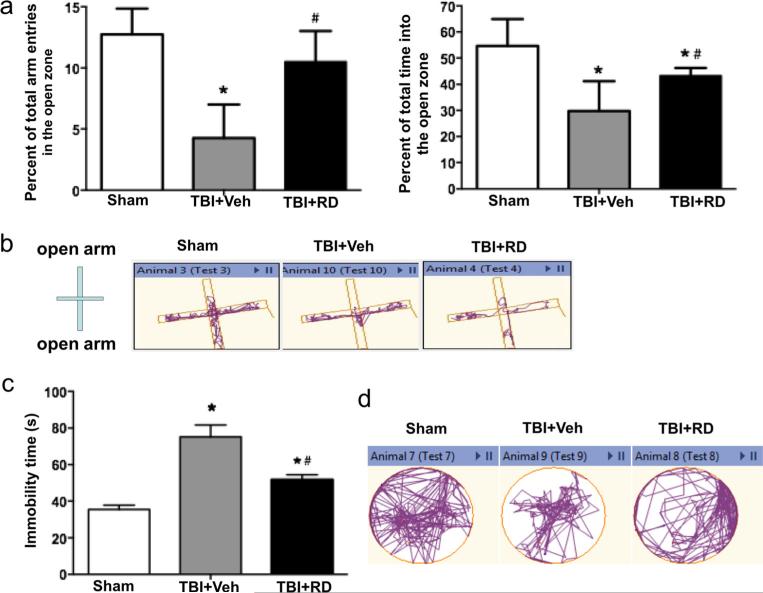
The elevated-plus maze was used to assess anxiety-like behavior in rats on day 21 after CCI [33]. Compared with sham-operated rats, vehicle-treated TBI rats had fewer entries into the open arms and spent less time in the open arms (n=6 rats/group, F=11.295, p<0.01; Fig. 2a, b). Rats treated with R. drynariae had more entries into the open arms and spent more time in the open arms than rats treated with vehicle (n=6 rats/group, F=5.461, p<0.05; Fig. 2a, b).
Fig. 2.
Effects of Rhizoma drynariae (RD) on anxiety- and depression-like behavior in rats subjected to CCI. Anxiety- and depression-like behavior were assessed by the elevated-plus maze (a, b) and the forced swim test (c, d), respectively, on day 21 after CCI. a The total number of entries into the open arms and the total time spent in the open arms were significantly less in R. drynariae-treated rats than in vehicle-treated rats over the duration of the test. b Representative activity traces from sham, vehicle-, and R. drynariae-treated rats in the elevated-plus maze test. c R. drynariae treatment decreased the immobility time in the forced swim test compared to that of vehicle-treated rats. d Representative swim paths of sham, vehicle-, and R. drynariae-treated rats in the forced swim test. Values are mean±SD; n=6 rats/group; *p<0.05 vs. sham group, #p<0.05 vs. vehicle group
R. drynariae Treatment Relieves CCI-Induced Depression-Like Behavior
On day 21 after CCI, immobility time in the FST was 40 % longer for TBI rats than for sham rats (n=6 rats/group, F=7.345, p<0.05; Fig. 2c, d). R. drynariae treatment reduced the immobility time by 21 % compared with vehicle treatment (n=6 rats/group, F=6.237, p<0.05; Fig. 2c, d).
R. drynariae Treatment Reduces IL-6 Levels and Increases IL-10 Levels in the Peripheral Blood
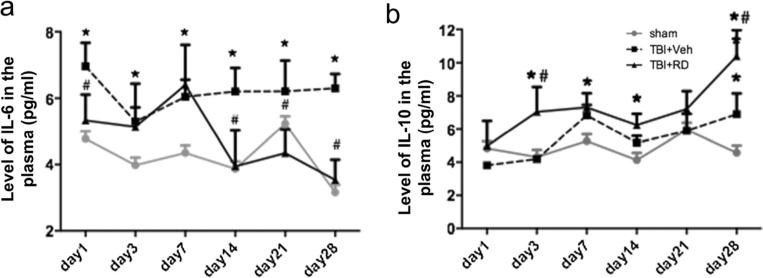
We measured IL-6 and IL-10 levels in the peripheral blood of rats on days 1, 3, 7, 14, 21, and 28 after CCI. Compared to that of the sham group, blood IL-6 level was elevated from day 1 to day 28 after CCI (n=6 rats/group, F=12.352, all p<0.01; Fig. 3a). R. drynariae treatment decreased the blood IL-6 level on days 1, 14, 21, and 28 (all p<0.05) compared with vehicle treatment (n=6 rats/group, F=9.624, p<0.05; Fig. 3a). In contrast, blood IL-10 level was not significantly elevated on days 1, 3, 7, 14, and 21 after CCI compared to that in the sham group (n=6 rats/group, F=0.898, p>0.05). However, IL-10 was significantly increased on day 28 (n=6 rats/group, F=4.981, p<0.05; Fig. 3b). R. drynariae increased blood IL-10 level at all time points compared with that in the sham group, with significant differences on days 3, 7, 14, and 28 (all p<0.05). Blood IL-10 level was also significantly higher in the R. drynariae-treated group than in the vehicle-treated group on days 3 and 28 (n=6 rats/group, F=11.058, p<0.01 for day 3; F=9.114, p<0.01 for day 28; Fig. 3b).
Fig. 3.
Effects of Rhizoma drynariae (RD) on blood IL-6 and IL-10 levels in rats subjected to CCI. The IL-6 and IL-10 levels in blood were measured by ELISA on days 1, 3, 7, 14, 21, and 28 after CCI. a R. drynariae treatment decreased blood IL-6 level on days 14, 21, and 28 compared with that of vehicle-treated rats. b R. drynariae increased blood IL-10 level on days 3 and 28 compared with that of vehicle-treated rats. Values are mean±SD; n=6 rats/group; *p<0.05 vs. sham group, #p<0.01 vs. vehicle group
R. drynariae Treatment Reverses the CCI-Induced Decrease in Blood Monocytes
We counted white blood cells, neutrophils, lymphocytes, and monocytes in the peripheral blood on day 3 after CCI. CCI did not change the number of white blood cells, neutrophils, or lymphocytes (n=6 rats/group, F=0.461, F=1.297, F=0.936, respectively, all p>0.05; Supplementary Fig. 1a, b, d); however, it significantly reduced the number of monocytes. R. drynariae reversed this decrease (n=6 rats/group, F=5.293, p< 0.05; Supplementary Fig. 1c).
R. drynariae Treatment Reverses the CCI-Induced Decrease in Blood CD3 and CD4 T Lymphocytes
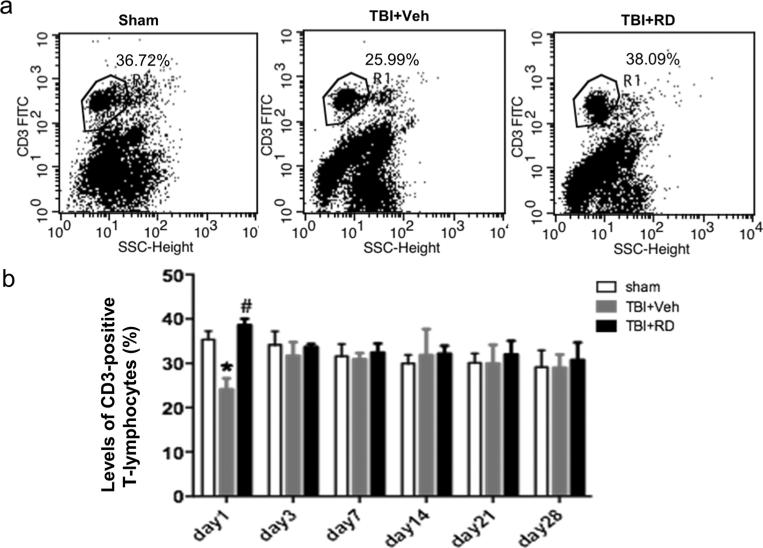
We further measured CD3, CD4, and CD8 T lymphocytes in the peripheral blood by flow cytometry on days 1, 3, 7, 14, 21, and 28 after CCI. A two-way ANOVA was used to determine the changes in T lymphocyte subpopulations. The percentage of CD3-positive T lymphocytes was significantly lower in the vehicle-treated CCI group than in the sham group on day 1 after CCI, but R. drynariae reversed this decrease (n=6 rats/group, F=6.428, p<0.05; Fig. 4a, b). On day 3 after CCI, the percentage of CD3 T lymphocytes returned to normal, and R. drynariae had no further effect (all p>0.05, Fig. 4b).
Fig. 4.
Effects of Rhizoma drynariae (RD) on CD3-positive T lymphocytes in the blood of rats subjected to CCI. CD3-positive T lymphocytes in blood were measured by flow cytometry on days 1, 3, 7, 14, 21, and 28 after CCI. a Representative flow cytometric dot plots show the percentage of CD3-positive T lymphocytes in sham, vehicle-treated, and R. drynariae-treated groups on day 1 after CCI. b Histograms show that the percentage of CD3-positive T lymphocytes was lower in the vehicle-treated group than in the sham group on day 1 after CCI and that R. drynariae treatment reversed this decrease. On days 3, 7, 14, 21, and 28 after CCI, the percentage of CD3 T lymphocytes had returned to normal, and R. drynariae treatment had no further effect. Values are mean±SD; n=6 rats/group; *p<0.05 vs. sham group, #p<0.05 vs. vehicle group
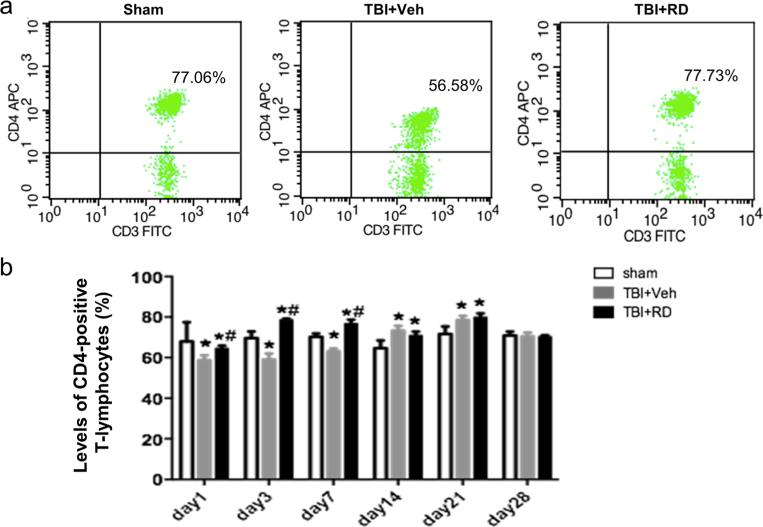
The percentage of CD4-positive T lymphocytes was significantly lower in the vehicle-treated group than in the sham group on days 1, 3, and 7 after CCI, but R. drynariae reversed this decrease at all three time points (n=6 rats/group, F=6.913, F=9.3491, F=7.564, respectively, all p<0.05; Fig. 5a, b). Compared with that in the sham group, the percentage of CD4-positive T lymphocytes in the vehicle-treated group was increased on days 14 and 21 after CCI (n=6 rats/group, F=8.742, F=7.966, respectively, both p<0.05), and R. drynariae did not further increase it (n=6 rats/group, both p>0.05, Fig. 5a, b).
Fig. 5.
Effects of Rhizoma drynariae (RD) on CD4-positive T lymphocytes in the blood of rats subjected to CCI. CD4-positive T lymphocytes were measured in blood by flow cytometry on days 1, 3, 7, 14, 21, and 28 after CCI. (a) Representative flow cytometric dot plots show the percentage of CD4-positive T lymphocytes in sham, vehicle- and R. drynariae-treated rats on day 3 after CCI. b Histograms show that the percentage of CD4-positive T lymphocytes was significantly lower in the vehicle-treated group than in the sham group on days 1, 3, and 7 after CCI and that R. drynariae treatment reversed these decreases. The percentage of CD4-positive T lymphocytes was significantly higher in the vehicle- and R. drynariae-treated groups than in the sham group on days 14 and 21 after CCI but did not differ between the two treatment groups. Values are mean±SD; n=6 rats/group; *p<0.05 vs. sham group, #p<0.05 vs. vehicle group
The percentage of CD8-positive T lymphocytes was similar in the vehicle-treated and sham groups on days 1, 3, 7, 14, 21, and 28 after CCI and was unaltered by R. drynariae treatment (n=6 rats/group, all p>0.05; Supplementary Fig. 2a, b).
R. drynariae Treatment Inhibits CCI-Induced Microglia/Macrophage Activation
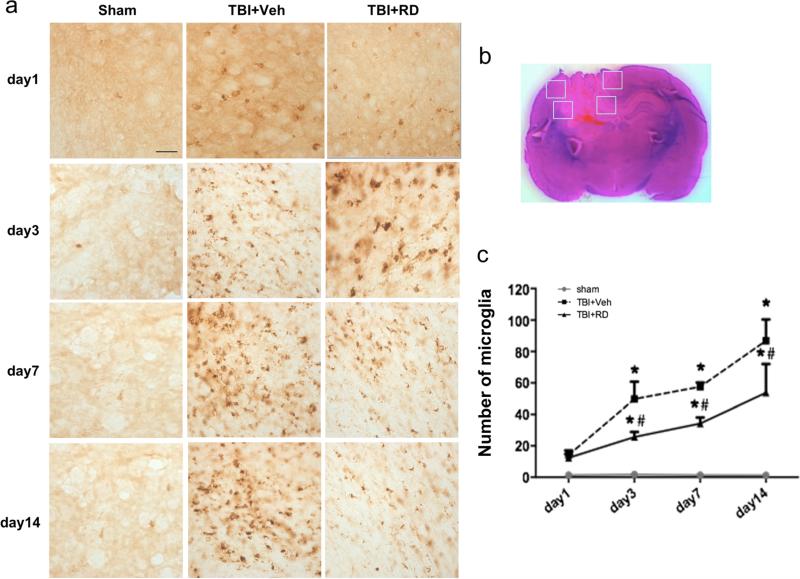
Immunohistochemical staining of CD68 was used to assess microglia/macrophage activation on days 1, 3, 7, and 14 after CCI. The sham group did not show microglial/macrophage activation (Fig. 6). In the TBI rats, microglial activation appeared on day 1 and continued to increase on days 3, 7, and 14 in four pre-selected areas of the lesion border (Fig. 6b). R. drynariae treatment inhibited microglial activation on days 3, 7, and 14 compared with that in the vehicle-treated TBI group (n=6 rats/group, F=11.29, all p<0.05; Fig. 6a, c).
Fig. 6.
Effects of Rhizoma drynariae (RD) on microglial/macrophage activation in rats subjected to CCI. Immunohistochemical staining of CD68 was used to assess microglial/macrophage activation on days 1, 3, 7, and 14 after CCI. a Microglial/macrophage activation was not observed in sham rats. Microglial activation began on day 1 and continued to increase on days 3, 7, and 14 after CCI; R. drynariae treatment inhibited microglial activation on days 3, 7, and 14 compared with that in vehicle-treated rats. b In this H&E-stained brain section, the white boxes indicate the four preselected areas of the lesion border used for counting CD68-positive activated microglia/macrophages. c Bar graph shows quantification analysis of CD68-positive microglia/macrophages. Values are mean±SD; n=6 rats/group; *p<0.01 vs. sham group, #p<0.05 vs. vehicle group
Identification of R. drynariae Active Compound
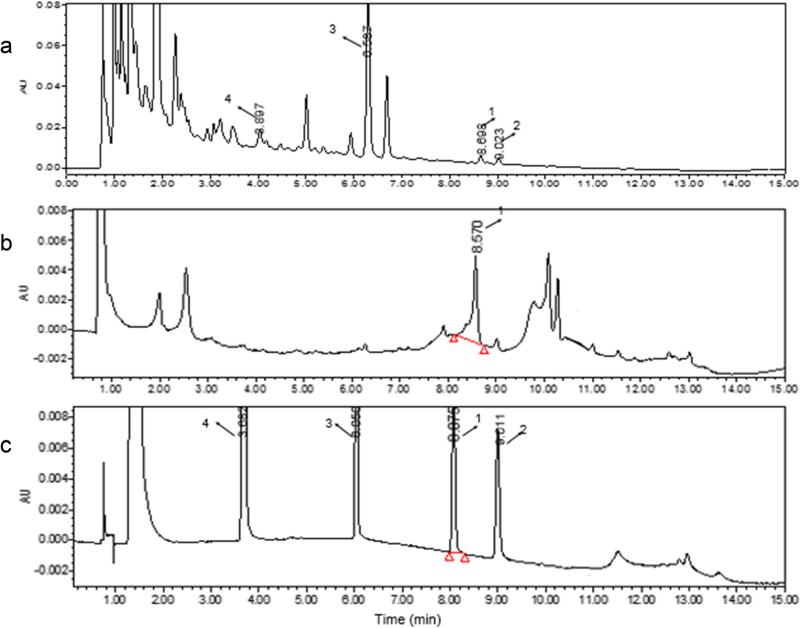
Chromatographic analysis of the R. drynariae decoction revealed four typical peaks numbered 1, 2, 3, and 4 (Fig. 7a). Their retention times were 8.698, 9.023, 6.597, and 3.987 min, respectively. The plasma of rats treated with R. drynariae exhibited one peak with a retention time of 8.570 min (Fig. 7b). These data indicated that peak 1 from the R. drynariae decoction and the plasma peak were from the same compound. To identify the active compound found in the R. drynariae decoction and in the plasma of R. drynariae-treated rats, we selected four standards based on literature [34]. Peaks 1–4 were eriodictyol, trans-cinnamic acid, naringin, and P-coumaric acid, which had retention times of 8.101, 9.011, 6.050, and 3.682 min, respectively (Fig. 7c). Eriodictyol had a retention time closest to that of the R. drynariae decoction and R. drynariae-treated rat plasma.
Fig. 7.
Identification of the active compound of Rhizoma drynariae extract in the blood of rats subjected to CCI. The Waters Acquity UPLC was used to analyze R. drynariae extract and the active compound in the blood of CCI rats. a R. drynariae decoction chromatography. Retention times of peaks 1, 2, 3, and 4 were 8.698, 9.023, 6.597, and 3.987 min, respectively. b Chromatographic peak in the plasma of rats treated with R. drynariae. The retention time of peak 1 was 8.570 min. c Chromatographic peaks generated from the standards. Peaks 1–4 are eriodictyol, trans-cinnamic acid, naringin, and P-coumaric acid, and their retention times were 8.101, 9.011, 6.050, and 3.682 min, respectively
Discussion
In our previous work, we used a weight-drop model of TBI and found that the traditional Chinese herbal medicine R. drynariae had immune-promoting effects [14]. In the present study, we used a CCI model of TBI in rats and found that R. drynariae treatment (1) decreased brain lesion volume, improved neurologic and cognitive function, and reduced anxiety- and depression-like behaviors, (2) reduced blood levels of IL-6 and increased blood levels of IL-10, (3) reversed the decrease in blood monocyte numbers and the percentage of blood CD3 and CD4 T lymphocytes, and (4) inhibited microglial/macrophage activation. Additionally, we found that eriodictyol could be the active compound of R. drynariae extract that is absorbed into and present in the blood.
TBI is characterized by direct damage to brain tissue and impairment in regulation of cerebral blood flow and metabolism [35]. Secondary injury after TBI involves brain and systemic immune activation. Victims of TBI often suffer from chronic symptoms such as motor and sensory deficits; cognitive impairments such as problems in memory, learning, and attention; and neuropsychiatric symptoms such as depression, anxiety, irritability, aggression, and suicidal rumination [36]. Currently, no available drug is effective for clinical treatment of TBI. However, our finding that R. drynariae improves histologic and functional outcomes supports the idea that it could be considered as a potential therapeutic strategy for TBI.
Blood immune cells, such as monocytes, macrophages, and lymphocytes, change after various diseases in the central nervous system. Here, we found that blood monocyte counts significantly decreased after CCI, a finding also reported by Wang et al. [37] and Schwulst et al. [38]. Vermeij et al. [39] reported recently that TBI produced systemic immune suppression in rats. Using flow cytometry, we measured blood CD3, CD4, and CD8 T lymphocyte levels of rats on days 1, 3, 7, 14, 21, and 28 after CCI. We found that while CD8 level was unchanged, CD3 level was decreased on day 1 and CD4 level continuously decreased from days 1 to 7. Interestingly, R. drynariae treatment was able to reverse these decreases and reduce TBI-induced early immunosuppression, further supporting its immune-promoting effect [12, 14]. Immuno-suppression increases the risk of infection after TBI. R. drynariae might be useful for preventing infection in such patients via its immune-promoting effect. R. drynariae had no further effect when CD4 level was increased from days 14 to 21 after CCI. Interestingly, a recent study showed that circulating regulatory T cells (CD4+CD25+FoxP3+) were positively correlated with clinical outcome of TBI [40]. We still need to determine whether R. drynariae increases the number of regulatory T cells in the blood and brain after TBI.
Inflammation is recognized as an important contributor to secondary brain injury after various central nervous system diseases. TBI results in a rapid and robust cellular inflammatory response characterized in part by activation of microglia [41]. In our study, using CD68 as a marker, we demonstrated a continued increase in microglial/macrophage activation in the perilesional region from days 1 to 14 after TBI. R. drynariae treatment decreased the number of CD68-positive activated microglia and macrophages from days 3 to 14. In addition to brain inflammation, TBI also produces systemic inflammatory responses. In the brain, neurons, astrocytes, and microglia have been shown to express proinflammatory cytokine IL-6. It has been argued that these cells are the main source of IL-6 in the serum after TBI [42]. Elevated serum IL-6 has been shown to correlate with multi-organ failure and death after TBI [43, 44]. In this study, we found that blood levels of IL-6 continued to increase from days 1 to 28 after TBI, whereas the anti-inflammatory cytokine IL-10 did not increase significantly until 28 days after TBI. R. drynariae treatment significantly reduced the blood IL-6 level on days 1, 14, 21, and 28 and increased blood IL-10 level on days 3 and 28 after TBI. These results, together with the inhibitory effect on microglial/macrophage activation, indicate that R. drynariae has anti-inflammatory effects, which may lead to reduced brain lesion volume and improvements in the neurobehavioral tests.
R. drynariae contains 9 to 10 components that have been separated [45, 46]; however, the active compound that enters the blood is unknown. In our study, we used UPLC to analyze the active compounds of R. drynariae in the blood of TBI rats. By comparing retention times with authentic standards, we demonstrated for the first time that eriodictyol might be the active compound of R. drynariae that is responsible for its multiple biologic properties. Interestingly, eriodictyol was shown to be protective against ischemic brain injury both in vitro and in vivo, possibly through activation of the nuclear factor erythroid-2-related factor 2 (Nrf2) and antioxidant response element (ARE) pathway [47]. It also can protect rat pheochromocytoma cells (PC12 cells) against hydrogen peroxide-induced oxidative stress through the same Nrf2/ARE signaling pathway [48]. These findings further support the possibility that eriodictyol is the component of R. drynariae that exerts neuroprotective effects in the rat TBI model. Additional work is ongoing to examine the neuroprotective effects of eriodictyol in TBI models and to assess whether eriodictyol provides neuroprotection similar to that of R. drynariae.
Conclusion
With immune-promoting, anti-inflammatory, and neuroprotective effects, R. drynariae might have therapeutic potential for the treatment of TBI. The putative active compound eriodictyol might be responsible for multiple biologic properties of R. drynariae.
Supplementary Material
Acknowledgments
This work was supported by grants from NSFC (No.81072967), the fund from National Key Clinical Specialist Vocational School of TCM encephalopathy, and NIH (R01NS078026, R01AT007317). Wenzhu Wang is the recipient of the China Scholarship Council Joint PhD Training award (CSC NO.201406370078). We thank Jiarui Wang and Claire Levine for assistance with this manuscript.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s12035-015-9385-x) contains supplementary material, which is available to authorized users.
Compliance with Ethical Standards
Conflict of Interest The authors declare no conflict of interest.
References
- 1.Thal SC, Neuhaus W. The blood–brain barrier as a target in traumatic brain injury treatment. Arch Med Res. 2014;45(8):698–710. doi: 10.1016/j.arcmed.2014.11.006. doi:10.1016/j.arcmed.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Scrimgeour AG, Condlin ML. Nutritional treatment for traumatic brain injury. J Neurotrauma. 2014;31(11):989–999. doi: 10.1089/neu.2013.3234. doi:10.1089/neu.2013.3234. [DOI] [PubMed] [Google Scholar]
- 3.Warner N, Eggenberger E. Traumatic optic neuropathy: a review of the current literature. Curr Opin Ophthalmol. 2010;21(6):459–462. doi: 10.1097/ICU.0b013e32833f00c9. doi:10.1097/ICU.0b013e32833f00c9. [DOI] [PubMed] [Google Scholar]
- 4.Taylor AE, Fuller CW, Molloy MG. Injury surveillance during the 2010 IRB Women's Rugby World Cup. Br J Sports Med. 2011;45(15):1243–1245. doi: 10.1136/bjsports-2011-090024. doi:10.1136/bjsports-2011-090024. [DOI] [PubMed] [Google Scholar]
- 5.Andelic N, Sigurdardottir S, Brunborg C, Roe C. Incidence of hospital-treated traumatic brain injury in the Oslo population. Neuroepidemiology. 2008;30(2):120–128. doi: 10.1159/000120025. doi:10.1159/000120025. [DOI] [PubMed] [Google Scholar]
- 6.Stein DG, Geddes RI, Sribnick EA. Recent developments in clinical trials for the treatment of traumatic brain injury. Handb Clin Neurol. 2015;127:433–451. doi: 10.1016/B978-0-444-52892-6.00028-3. doi:10.1016/B978-0-444-52892-6.00028-3. [DOI] [PubMed] [Google Scholar]
- 7.McConeghy KW, Hatton J, Hughes L, Cook AM. A review of neuroprotection pharmacology and therapies in patients with acute traumatic brain injury. CNS Drugs. 2012;26(7):613–636. doi: 10.2165/11634020-000000000-00000. doi:10. 2165/11634020-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Wright DW, Yeatts SD, Silbergleit R, Palesch YY, Hertzberg VS, Frankel M, Goldstein FC, Caveney AF, et al. Very early administration of progesterone for acute traumatic brain injury. N Engl J Med. 2014;371(26):2457–2466. doi: 10.1056/NEJMoa1404304. doi:10.1056/NEJMoa1404304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skolnick BE, Maas AI, Narayan RK, van der Hoop RG, MacAllister T, Ward JD, Nelson NR, Stocchetti N, et al. A clinical trial of progesterone for severe traumatic brain injury. N Engl J Med. 2014;371(26):2467–2476. doi: 10.1056/NEJMoa1411090. doi:10.1056/NEJMoa1411090. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Fan X, Qu H, Gao X, Cheng Y. Strategies and techniques for multi-component drug design from medicinal herbs and traditional Chinese medicine. Curr Top Med Chem. 2012;12(12):1356–1362. doi: 10.2174/156802612801319034. [DOI] [PubMed] [Google Scholar]
- 11.Ma HD, Deng YR, Tian Z, Lian ZX. Traditional Chinese medicine and immune regulation. Clin Rev Allergy Immunol. 2013;44(3):229–241. doi: 10.1007/s12016-012-8332-0. doi:10.1007/s12016-012-8332-0. [DOI] [PubMed] [Google Scholar]
- 12.An HJ, Lee GG, Lee KT. Drynariae rhizoma increases immune response in mice. Nat Prod Commun. 2012;7(7):905–908. [PubMed] [Google Scholar]
- 13.Anuja GI, Latha PG, Suja SR, Shyamal S, Shine VJ, Sini S, Pradeep S, Shikha P, et al. Anti-inflammatory and analgesic properties of Drynaria quercifolia (L.) J. Smith. J Ethnopharmacol. 2010;132(2):456–460. doi: 10.1016/j.jep.2010.08.038. doi:10.1016/j.jep.2010.08.038. [DOI] [PubMed] [Google Scholar]
- 14.Wang WZ, Pan YZ, Wei JB, Huang LP, Huang X, Li K. The effects of rhizoma drynariae on interleukin-2 and T-lymphocyte levels in rats after severe head injury. J Ethnopharmacol. 2012;142(1):300–304. doi: 10.1016/j.jep.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Lomnitski L, Michaelson DM, Shohami E. Motor and cognitive deficits in apolipoprotein E-deficient mice after closed head injury. Neuroscience. 1997;80(4):1255–1262. doi: 10.1016/s0306-4522(97)00007-9. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Liu X, Lu H, Jiang C, Cui X, Yu L, Fu X, Li Q, et al. CXCR4(+)CD45(−) BMMNC subpopulation is superior to unfractionated BMMNCs for protection after ischemic stroke in mice. Brain Behav Immun. 2015;45:98–108. doi: 10.1016/j.bbi.2014.12.015. doi:10.1016/j.bbi.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Yu L, Jiang C, Fu X, Liu X, Wang M, Ou C, Cui X, et al. Cerebral ischemia increases bone marrow CD4+CD25+ FoxP3+ regulatory T cells in mice via signals from sympathetic nervous system. Brain Behav Immun. 2015;43:172–183. doi: 10.1016/j.bbi.2014.07.022. doi:10.1016/j.bbi.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox-Robichaud A, Payne D, Hasan SU, Ostrovsky L, Fairhead T, Reinhardt P, Kubes P. Inhaled NO as a viable antiadhesive therapy for ischemia/reperfusion injury of distal microvascular beds. J Clin Invest. 1998;101(11):2497–2505. doi: 10.1172/JCI2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Fu X, Jiang C, Yu L, Wang M, Han W, Liu L, Wang J. Bone marrow mononuclear cell transplantation promotes therapeutic angiogenesis via upregulation of the VEGF-VEGFR2 signaling pathway in a rat model of vascular dementia. Behav Brain Res. 2014;265:171–180. doi: 10.1016/j.bbr.2014.02.033. doi:10.1016/j.bbr.2014.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fogaca MV, Sonego AB, Rioli V, Gozzo FC, Dale CS, Ferro ES, Guimaraes FS. Anxiogenic-like effects induced by hemopressin in rats. Pharmacol Biochem Behav. 2015;129:7–13. doi: 10.1016/j.pbb.2014.11.013. doi: 10.1016/j.pbb.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Zhu W, Gao Y, Chang CF, Wan JR, Zhu SS, Wang J. Mouse models of intracerebral hemorrhage in ventricle, cortex, and hippo-campus by injections of autologous blood or collagenase. PLoS One. 2014;9(5):e97423. doi: 10.1371/journal.pone.0097423. doi:10.1371/journal.pone.0097423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie Y, Huang X, Hu SY, Qiu XJ, Zhang YJ, Ren P, Wang Y, Ji H, et al. Meranzin hydrate exhibits anti-depressive and prokinetic-like effects through regulation of the shared alpha2-adrenoceptor in the brain-gut axis of rats in the forced swimming test. Neuropharmacology. 2013;67:318–325. doi: 10.1016/j.neuropharm.2012.10.003. doi:10.1016/j.neuropharm. 2012.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Wu H, Wu T, Xu X, Wang J, Wang J. Iron toxicity in mice with collagenase-induced intracerebral hemorrhage. J Cereb Blood Flow Metab. 2011;31(5):1243–1250. doi: 10.1038/jcbfm.2010.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu H, Wu T, Li M, Wang J. Efficacy of the lipid-soluble iron chelator 2,2′-dipyridyl against hemorrhagic brain injury. Neurobiol Dis. 2012;45(1):388–394. doi: 10.1016/j.nbd.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Fields J, Zhao C, Langer J, Thimmulappa RK, Kensler TW, Yamamoto M, Biswal S, et al. Role of Nrf2 in protection against intracerebral hemorrhage injury in mice. Free Radic Biol Med. 2007;43(3):408–414. doi: 10.1016/j.freeradbiomed.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu H, Wu T, Hua W, Dong X, Gao Y, Zhao X, Chen W, Cao W. PGE2 receptor agonist misoprostol protects brain against intracerebral hemorrhage in mice. Neurobiol Aging. 2015;36(3):1439–1450. doi: 10.1016/j.neurobiolaging.2014.12.029. doi:10.1016/j.neurobiolaging.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao X, Wu T, Chang CF, Wu H, Han X, Li Q, Gao Y, Li Q, et al. Toxic role of prostaglandin E2 receptor EP1 after intracerebral hemorrhage in mice. Brain Behav Immun. 2015;46:293–310. doi: 10.1016/j.bbi.2015.02.011. doi:10.1016/j.bbi.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu T, Wu H, Wang J, Wang J. Expression and cellular localization of cyclooxygenases and prostaglandin E synthases in the hemorrhagic brain. J Neuroinflammation. 2011;8:22. doi: 10.1186/1742-2094-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiu XJ, Huang X, Chen ZQ, Ren P, Huang W, Qin F, Hu SH, Huang J, et al. Pharmacokinetic study of the prokinetic compounds meranzin hydrate and ferulic acid following oral administration of chaihu-shugan-San to patients with functional dyspepsia. J Ethnopharmacol. 2011;137(1):205–213. doi: 10.1016/j.jep.2011.05.009. doi:10.1016/j.jep.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Huang X, Liang QH, Fan R, Qin F, Guo Y, Yan KP, Liu W, et al. A strategy for detecting absorbed bioactive compounds for quality control in the water extract of rhubarb by ultra performance liquid chromatography with photodiode array detector. Chin J Integr Med. 2012;18(9):690–698. doi: 10.1007/s11655-012-1053-7. doi:10.1007/s11655-012-1053-7. [DOI] [PubMed] [Google Scholar]
- 31.Huang W, Xiong ZH, Huang X, Chen X, Liu WP, Wang Y, Ren P. Simultaneous UPLC analysis of three major flavonoids in granule decoctions of Fructus aurantii-type formulae. Pharmazie. 2012;67(7):586–589. [PubMed] [Google Scholar]
- 32.Mahmood A, Lu D, Wang L, Chopp M. Intracerebral transplantation of marrow stromal cells cultured with neurotrophic factors promotes functional recovery in adult rats subjected to traumatic brain injury. J Neurotrauma. 2002;19(12):1609–1617. doi: 10.1089/089771502762300265. doi:10.1089/089771502762300265. [DOI] [PubMed] [Google Scholar]
- 33.Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14(3):149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 34.Zhang YJ, Huang X, Wang Y, Xie Y, Qiu XJ, Ren P, Gao LC, Zhou HH, et al. Ferulic acid-induced anti-depression and prokinetics similar to Chaihu-Shugan-San via polypharmacology. Brain Res Bull. 2011;86(3–4):222–228. doi: 10.1016/j.brainresbull.2011.07.002. doi:10.1016/j.brainresbull.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99(1):4–9. doi: 10.1093/bja/aem131. doi:10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 36.Malkesman O, Tucker LB, Ozl J, McCabe JT. Traumatic brain injury—modeling neuropsychiatric symptoms in rodents. Front Neurol. 2013;4:157. doi: 10.3389/fneur.2013.00157. doi:10.3389/fneur.2013.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang HC, Yang TM, Lin YJ, Chen WF, Ho JT, Lin YT, Kwan AL, Lu CH. Serial serum leukocyte apoptosis levels as predictors of outcome in acute traumatic brain injury. Biomed Res Int. 2014;2014:720870. doi: 10.1155/2014/720870. doi:10.1155/2014/720870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwulst SJ, Trahanas DM, Saber R, Perlman H. Traumatic brain injury-induced alterations in peripheral immunity. J Trauma Acute Care Surg. 2013;75(5):780–788. doi: 10.1097/TA.0b013e318299616a. doi:10.1097/TA. 0b013e318299616a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vermeij JD, Aslami H, Fluiter K, Roelofs JJ, van den Bergh WM, Juffermans NP, Schultz MJ, Van der Sluijs K, et al. Traumatic brain injury in rats induces lung injury and systemic immune suppression. J Neurotrauma. 2013;30(24):2073–2079. doi: 10.1089/neu.2013.3060. doi:10.1089/neu.2013.3060. [DOI] [PubMed] [Google Scholar]
- 40.Li M, Lin YP, Chen JL, Li H, Jiang RC, Zhang JN. Role of regulatory T cell in clinical outcome of traumatic brain injury. Chin Med J. 2015;128(8):1072–1078. doi: 10.4103/0366-6999.155094. doi:10.4103/0366-6999.155094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang B, West EJ, Van KC, Gurkoff GG, Zhou J, Zhang XM, Kozikowski AP, Lyeth BG. HDAC inhibitor increases his-tone H3 acetylation and reduces microglia inflammatory response following traumatic brain injury in rats. Brain Res. 2008;1226:181–191. doi: 10.1016/j.brainres.2008.05.085. doi:10.1016/j.brainres.2008.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hergenroeder GW, Moore AN, McCoy JP, Jr, Samsel L, Ward NH, 3rd, Clifton GL, Dash PK. Serum IL-6: a candidate biomarker for intracranial pressure elevation following isolated traumatic brain injury. J Neuroinflammation. 2010;7:19. doi: 10.1186/1742-2094-7-19. doi:10.1186/1742-2094-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arand M, Melzner H, Kinzl L, Bruckner UB, Gebhard F. Early inflammatory mediator response following isolated traumatic brain injury and other major trauma in humans. Langenbeck's Arch Surg. 2001;386(4):241–248. doi: 10.1007/s004230100204. [DOI] [PubMed] [Google Scholar]
- 44.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, Heimovitz H, Cohen HJ, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106(5):506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 45.Shang Z, Zhao Q, Tian J, Yang L, Yang M, Shi G. Chemical constituents from rhizomes of Drynaria fortunei. Pract Pharm Clin Rem. 2010;13(4):262–277. [Google Scholar]
- 46.Gao Y, Wang X, Wang N, Yao X. Chemical constituents from Drynaria fortunei. Chin J Med Chem. 2008;8(4):284–287. [Google Scholar]
- 47.Jing X, Ren D, Wei X, Shi H, Zhang X, Perez RG, Lou H, Lou H. Eriodictyol-7-O-glucoside activates Nrf2 and protects against cerebral ischemic injury. Toxicol Appl Pharmacol. 2013;273(3):672–679. [PubMed] [Google Scholar]
- 48.Lou H, Jing X, Ren D, Wei X, Zhang X. Eriodictyol protects against H(2)O(2)-induced neuron-like PC12 cell death through activation of Nrf2/ARE signaling pathway. Neurochem Int. 2012;61(2):251–257. doi: 10.1016/j.neuint.2012.05.013. doi:10.1016/j.neuint.2012.05.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.