Abstract
The implications of the biologically active elements in milk for the mammalian infant are largely unknown. Animal models demonstrate that transmission of glucocorticoids through milk influences behavior and modifies brain development in offspring. The aim of this study was to determine the relation between human milk cortisol levels and temperament of the breastfed infant. Fifty-two mother and infant pairs participated when the infants were three-months old. Milk cortisol levels were assessed and each mother completed the Infant Behavior Questionnaire (IBQ), a widely used parent-report measure of infant temperament. Analyses revealed a positive association between milk cortisol and the Negative Affectivity dimension of the IBQ (partial r =.37, p < .01). No correlation was found between elevated cortisol levels and the Surgency/Extraversion or the Orienting/Regulation dimensions. Further, the positive association between increased maternal milk cortisol and Negative Affectivity was present among girls (β = .59, p < .01), but not among boys. (Although, the sex by milk cortisol interaction term was not statistically significant, suggesting that these results require replication.) Environmental factors such as maternal demographics and negative maternal affect (depression and perceived stress) at the time of assessment did not account for the positive association. The findings support the proposal that exposure to elevated levels of cortisol in human milk influences infant temperament. The findings further suggest that mothers have the ability shape offspring phenotype through the transmission of biologically active components in milk.
Keywords: Cortisol, Glucocorticoids, Temperament, Breast Milk, Human Milk, Breastfeeding, Stress, Infant Development, Fear, Behavioral Inhibition, Sex Differences
It has become increasingly clear that a wide range of early exposures to environmental influences have implications for health and development across the lifespan (Barker, 1998). Prenatal and early postnatal experiences have been linked to alterations in basic metabolic and physiological processes such as glucose metabolism, blood pressure regulation and hypothalamic-pituitary-adrenal (HPA) function (Godfrey & Barker, 2000; Phillips & Jones, 2006) and also to the development of a range of disease states including hypertension, coronary heart disease, diabetes, polycystic ovary disease, schizophrenia, depression and anxiety disorders (Van Os & Selten, 1998; Gunnell et al., 2003; Indredavik et al., 2003; Gale & Martyn, 2004; Mittendorfer-Rutz et al., 2004; Seckl & Meaney, 2006).
Despite the well-established and widespread benefits of breastfeeding on health and development (Davis, 2001; Friedman & Zeiger, 2005; Schack-Nielsen & Michaelsen, 2006), little consideration has been given to the possibility that biological components of maternal milk may be an important aspect of the early environment that shapes offspring phenotype (Glynn et al., 2007; Hinde & Capitanio, 2010). Mother's milk contains a wide variety of biologically active hormones – including glucocorticoids (GCs, cortisol in humans; Grosvenor et al., 1992; Hamosh, 2001). GCs are transferred from plasma to milk — there is no evidence for mammary synthesis (Kato et al., 1985; Hamosh, 2001). In human milk, GC levels are lower than in plasma (Pearlman, 1983), but the levels are fairly highly correlated (in the .6 to.7 range; Patacchioli et al., 1992). As such, activation of the maternal HPA-axis has implications for milk GC levels. Systemic stimulation with ACTH or exposure to stressors increase GC levels in the milk of cows and rats (Gwazdauskas et al., 1977; Paape et al., 1977; Yeh, 1984). Similarly, maternal milk GC levels have been related to affect and mood in humans (Hart et al., 2004).
GCs are believed to play a critical role in early life programming processes through transmission of signals from mother to fetus (Welberg & Seckl, 2001; Bertram & Hanson, 2002). GCs are stress responsive steroid hormones that are essential for normal development of organ systems, including the central nervous system (Meaney et al., 1996). They easily pass the blood brain barrier (Zarrow et al., 1970) and the limbic regions, such as the amygdala, involved in the regulation of fear, anxiety and behavioral inhibition, are particularly sensitive to their effects (LeDoux, 2000; Owen et al., 2005). Experimental models demonstrate that animals exposed to increased levels of GCs during the prenatal period display increased fear and greater behavioral inhibition in the face of novelty (Welberg et al., 2001; Welberg & Seckl, 2001; Weinstock, 2005). Similar findings during the pre- and early postnatal periods now have been repeatedly documented in human studies (Trautman et al., 1995; de Weerth et al., 2003; Davis et al., 2007; Glynn et al., 2007).
The possibility of lactational programming, the concept that the mother shapes offspring development through signals contained in milk, has been largely ignored. The few studies that have examined this hypothesis are consistent with the premise that milk GCs influence offspring development. Rodent models demonstrate that GCs ingested through milk readily cross the infant's intestinal epithelial barrier and are present in the neonatal plasma and brain (Angelucci et al., 1985). As adults, animals exposed to increased milk GCs during infancy, display altered HPA regulation and altered behavioral responses to stress (Catalani et al., 1993; Casolini et al., 1997; Catalani et al., 2000; Catalani et al., 2002). Further, associations between effects of naturally occurring variations in milk GCs and offspring temperament have been shown among 3–4 month old male Rhesus monkeys (Sullivan et al., 2011).
Only two studies have investigated the role of milk GCs in human development. First, Hart et al. (2004) showed that higher levels of milk GCs were predictive of enhanced performance on the autonomic stability cluster on the Neonatal Behavioral Assessment Scale among neonates. A second study using maternal plasma GCs as a surrogate measure for milk GC levels (by relying on the high correlation between plasma and milk GCs), found that higher levels of maternal GCs were predictive of increased fearful temperament at 2-months of age among breastfed infants, but no relation was evident among the non-breastfed infants (Glynn et al., 2007). Although in this second study, GCs levels were not assessed directly in milk, the presence of a relation between maternal cortisol and temperament among the breastfed group only, strongly suggests that milk GCs have the potential to affect fearful temperament in humans.
The purpose of the present study was to assess for the first time, whether direct exposure to milk GCs is associated with temperament in human infants. Cortisol levels in breast milk and infant temperament were assessed when the infants were 3-months-old. Because as described above, the limbic regions of the brain that are involved in the regulation of fear, anxiety and behavioral inhibition, represent a primary target for GC exposure (Owen et al., 2005), it was anticipated that these aspects of temperament would be most likely to show associations with milk GCs. Further, because sex differences in early influences on development are common and present as early as the prenatal period (Bernardes et al., 2008; Buss et al., 2009; DiPietro et al., 2009) and because sex appears to be a moderating factor in animal models of the effects of milk GC exposure (Angelucci et al., 1983; Sullivan et al., 2011), we anticipated that the associations between milk cortisol and temperament would differ by infant sex.
Methods
Study Overview
The relation between milk GCs and infant temperament was examined among breastfed infants. At 3-months postpartum cortisol levels were determined in milk collected from breastfeeding mothers. Each mother completed the Revised Infant Behavior Questionnaire, a widely used and well-validated measure of infant temperament (Gartstein & Rothbart, 2003), and measures of maternal perceived stress and depression.
Participants
Fifty-two mother and infant pairs who were enrolled in a larger longitudinal study of early development at a large university medical center participated when the infants were three-months old. The Institutional Review Board approved the study procedures and all participants provided written informed consent. Exclusion criteria for enrollment in the current study included: infants whose mothers were taking corticosteroid medications during the postpartum period and admittance to the Neonatal Intensive Care Unit at birth because of compromised health (e.g. intrauterine growth restriction and respiratory distress syndrome). Characteristics of the mother-infant pairs are presented in Table 1.
Table 1.
Sample characteristics and associations between characteristics and temperament.
| Mother/Infant Pair (n=52) | Association with Temperament Dimensions (p-value) | |||
|---|---|---|---|---|
|
|
||||
| Surgency/Extraversion | Negative Affectivity | Orienting/Regulation | ||
| Ethnicity (%) | .37 | .33 | .61 | |
| Latina | 21 | |||
| Non-Hispanic White | 55 | |||
| Asian | 12 | |||
| Other | 12 | |||
| Average Maternal Age (years) | 29.0 | .86 | .35 | .39 |
| Education (%) | .20 | .35 | .98 | |
| High School or Less | 9 | |||
| Associates or Technical | 33 | |||
| College | 31 | |||
| Graduate | 27 | |||
| Annual Household Income (dollars) | 68,950 | .08 | .11 | .20 |
| Marital Status (% married) | 75 | .36 | .06 | .67 |
| Employment (% currently working) | 29 | .43 | .99 | .43 |
| Sex of infant (% male) | 48 | .35 | .64 | .10 |
| Birth order (% first born) | 52 | .53 | .19 | .54 |
| 5-minute Apgar Score | 9.0 | .91 | .86 | .74 |
| Birth Weight (grams) | 3413 | .05 | .72 | .61 |
| Gestational Age at Birth (weeks) | 39.6 | .47 | .56 | .85 |
| Infant Age at Assessment (weeks) | 13.0 | .48 | .67 | .88 |
|
| ||||
| Milk Cortisol (ug/dL) | 0.22 | |||
| Temperament | ||||
| Surgency/Extraversion | 3.95 | |||
| Negative Affectivity | 3.07 | |||
| Orienting/Regulation | 5.05 | |||
Infant Temperament
Infant temperament was assessed using the Rothbart Revised Infant Behavior Questionnaire (IBQ-R; Gartstein & Rothbart, 2003). The IBQ-R includes specific questions addressing concrete behaviors such as, “During a peek-a-boo game, how often did the baby smile?” and “How often during the last week did the baby startle to a sudden or loud noise?” and only inquires about recently occurring events to prevent errors in recall. Parent responses are reported using a 7-point, Likert-type scale (1-never to 7-always). The IBQ-R measures three broad dimensions of temperament: Negative Affectivity, Surgency/Extraversion and Orienting/Regulation. The instrument has been shown to be both a reliable and valid measure of infant temperament (Worobey & Blajda, 1989; Goldsmith & Campos, 1990; Gartstein & Rothbart, 2003).
Determination of Milk Cortisol Levels
The mother cleaned the breast and nipple area with an antibacterial wipe and allowed the area to air dry. Following this, she emptied the contents of one breast with an electric breast pump into a sterile plastic container (Medela, Inc., McHenry, IL). The sample was then pipetted directly into polypropylene tubes. All of the aliquots were stored at 70° C until assayed.
Milk cortisol concentrations were determined by chemiluminescent immunoassay (IBL Immuno-Biological Laboratories, Hamburg, Germany). Thawed samples (100 μl in duplicate; non-spiked and spiked sample for extraction efficiency determination) were extracted with chilled dichloromethane (500 μl) in capped polypropylene tubes, vortexed, and allowed to stand in an ice bath for 10 minutes. After centrifugation at 1500 ×g for 5 minutes, the top aqueous phase was removed. Extracts (100μl) were transferred into tubes and evaporated to dryness at room temperature (in fume hood). Diluent (50 μl) was added to each dried tube and allowed to sit for 10 minutes at room temperature. Aliquots of extracted milk (20 μl) were incubated with enzyme conjugate solution for 3 hours at room temperature in sealed antibody-coated microtiter strips. After aspirating and washing each well four times with wash buffer (250 μl) and blotting dry, chemiluminescence substrate solution mixture (50 μl) was added. Relative luminescence units were measured with a microplate luminometer between 10 to 40 minutes after addition of substrate solution. The cross-reactivity of the assay for 11-deoxycortisol is 12%, and for cortisone, corticosterone, and other naturally occurring steroids is <2.5%. The intra- and inter-assay coefficients of variances are <8% and <12% respectively with a minimal detectable dose of 0.015 μg/dL (95% confidence). Data reduction for the milk assay was done by an automated four-parameter logistics computer program (software Mikro Win 2000; Berthold Microplate Luminometer) and the results were corrected by the recovery calculation of the extraction procedure for each sample. One cortisol value was more than 3 standard deviations above the group mean and was assigned the next highest value, bringing it within the continuous portion of the distribution.
Maternal Demographics and Psychological State
Maternal reports of ethnicity, age, educational level, income, marital and employment status were collected by structured interview. Information about the infants' peri- and neonatal health and Apgar scores was abstracted from medical records. At the time of the cortisol collection, the mothers completed Cohen's Perceived Stress Scale (Cohen et al., 1983) and the Edinburgh Postnatal Depression Scale (Cox et al., 1987). These measures of postnatal affect were included to rule out the possible reporting bias on the part of the mother (Youngstrom et al., 1999). For example, to address the possibility that mothers who report more stress or depression, also have elevated levels of milk cortisol and report that their infants have more difficult or fearful temperaments.
Analysis Plans
First, the relation between milk cortisol levels and infant temperament was assessed with partial correlations adjusting only for the time of sample collection. Then, in the case in which a statistically significant relation was revealed, a hierarchical regression analysis was used to assess whether milk cortisol levels predicted variance in infant temperament above and beyond maternal postpartum psychological distress (depression and perceived stress). In this regression model maternal affect, and any maternal demographic characteristics such as race/ethnicity, maternal age or parity, that showed a relation at a significance level of less than 0.1 with infant temperament, were entered first, and then time of collection and milk cortisol were entered into the second step of the model. This regression model then was repeated to examine whether any relation between milk cortisol and infant temperament differed by infant sex.
Results
Milk cortisol and infant temperament
Initial analyses of the association between milk cortisol and infant temperament revealed a positive correlation between cortisol and the Negative Affectivity dimension of the IBQ after adjusting for time of sample collection (partial r =.37, p < .01; see Figure 1). The association between milk cortisol and the Orienting/Regulation dimension approached significance (partial r = −.25, p = .07). No association was found between milk cortisol and the Surgency/Extraversion dimension (partial r = .14, p = .32). No association was found between milk cortisol and the Surgency/Extraversion dimension (partial r = .14, p = .32).
Figure 1.
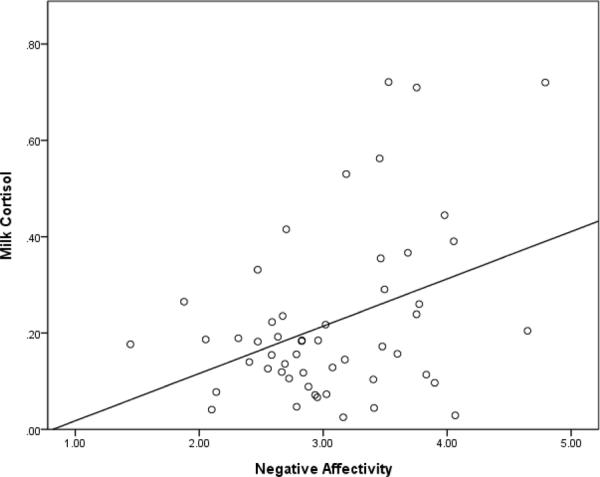
Scatterplot of milk cortisol and infant negative affectivity.
The hierarchical regression model revealed that the relation between milk cortisol and Negative Affectivity remained statistically significant after adjusting for the contributions of maternal psychological distress and also maternal demographic factors predictive of infant temperament (See Table 2). Repeating this regression analysis for each of the Negative Affectivity subscales showed that the overall positive association between Negative Affectivity and milk cortisol was due to the fear (β = .47, p < .01) and sadness (β = .36, p < .05) subscales, and less to the falling reactivity (β = −.29, p = .06) and distress to limitations (β = .20, p = .20) subscales.
Table 2.
Hierarchical regression model examining whether milk cortisol accounts for unique variance in infant Negative Affectivity beyond postnatal maternal affect and maternal demographic characteristics (n=52).
| R2 | ΔR2 | β | Partial r | |
|---|---|---|---|---|
| Model 1 | .14* | |||
| Depression | −.24 | −.20 | ||
| Perceived Stress | .47** | .36** | ||
| Model 2 | .29** | .15* | ||
| Depression | −.29† | −.26† | ||
| Perceived Stress | .48** | .41** | ||
| Sample Collection Time | .06 | .04 | ||
| Milk Cortisol | .42** | .40** | ||
| Model 3 | .33** | .04 | ||
| Depression | −.35† | −.24 | ||
| Perceived Stress | .53** | .44** | ||
| Sample Collection Time | .04 | .04 | ||
| Milk Cortisol | .53** | 44** | ||
| Infant Sex | −.15 | −.17 | ||
| Sex by Milk Cortisol | −.19 | −.17 |
Note: Infant sex coded 0 = female.
p<.01,
p<.05,
p<.10
Sex Differences
Model 3 (Table 2) includes infant sex and the infant sex X milk cortisol interaction term. Separate hierarchical regressions examining the relations between milk cortisol and Negative Affectivity within infant sex suggested that females account for the positive association between increased milk cortisol and Negative Affectivity (β = .59, p < .01). There was no significant relation between milk cortisol and Negative Affectivity among the male infants (β = .14, p =.58). Milk cortisol levels (ANCOVA; F = .18, n.s.), variance in milk cortisol (Levene's Test; F=.31, p=.58) and the infant temperament dimensions (t-test; all t's < −1.6, p's > .10) did not differ between the male and female infants.
Discussion
These data are among the first to suggest that one avenue through which the human mother may influence offspring phenotype is by the transmission of biologically active hormones in her milk. Specifically, the data demonstrate that infant consumption of maternal milk with higher levels of cortisol is associated with more negative infant temperament. Confidence is increased in these findings because the association persisted even after taking into account maternal demographic characteristics and psychological distress, suggesting the link was due to milk GC exposures and not to other maternal characteristics known to influence infant temperament such as maternal education level, stress and depression.
The findings are consistent with the single other human study that examined the link between maternal cortisol and infant temperament in breastfed infants (Glynn et al., 2007). However, the current study represents an important advance because milk cortisol levels were directly measured, whereas the other study relied on plasma cortisol as a proxy for milk cortisol. More broadly, the data also are consistent with literature documenting the lasting effects of maternal GCs on emotion and stress regulation of the offspring. In humans, increased levels of maternal cortisol during the prenatal period are associated with increased negative reactivity and fearful temperament and also to altered HPA axis regulation in infants and toddlers (de Weerth et al., 2003; Davis et al., 2007; Davis et al., 2011). Further, infants who had been exposed prenatally to synthetic GCs exhibit increased shyness and emotionality during the first 5 years of life (Trautman et al., 1995; Davis et al., 2004).
The relation between milk cortisol and negative temperament was only observed among the female infants, and not among males. Neither the mean nor the variance in milk cortisol differed between the mothers of males and females, suggesting that differences in exposures to milk cortisol do not account for the effect. With both rodent and non-human primate models, sex differences in the effects of milk GCs also have been reported. In rodents, elevated milk GCs resulted in increased HPA-axis activity in female offspring, but decreased HPA activity in males (Angelucci, et al., 1983). Sullivan et al. (2011), have shown that higher levels of milk cortisol are associated with increased confident temperament among 3–4 month old male rhesus monkeys, but not among the female offspring. In the present study the sex by milk cortisol interaction term did not reach statistical significance. Further, because of the small number of studies, species differences, differences in timing of assessment of outcomes (i.e., infant, adult), differences in outcomes under study (i.e. HPA-axis function, temperament dimensions), and potential sex-dependent differences in milk composition (Hinde & Capatanio, 2010), it would be premature to draw conclusions regarding sex-dependent differential sensitivity to milk GCs. However, given that it now has been repeatedly shown that these effects do appear to be moderated by sex, further exploration of these differences is clearly warranted.
It is possible that the sex differences observed in the current study may be due in part to the differing developmental trajectories of human males and females. Additionally, there is some evidence that male and female fetuses are differentially affected by prenatal cortisol exposures (DiPietro et al., 2009; Clifton, 2010; Glynn & Sandman, in press), and that these differential sensitivities may extend into the postnatal period (Zuloaga et al., in press). It also is possible that enhanced sensitivity to GC exposures in females during early life may help illuminate underlying causes of the increased vulnerability to and prevalence of certain psychiatric disorders among females later in life (Jacobi et al., 2004; Hyde et al., 2008; McLean & Anderson, 2009). Children classified as having a negative or difficult temperament during infancy and early childhood are at risk of developing psychiatric conditions including eating disorders, depression, and anxiety during later childhood and adolescence (Kagan et al. 1999; Schwartz et al. 1999; Martin et al. 2000; Prior et al. 2000; De Pauw and Mervielde 2010; Dougherty and Klein 2010).
The specific mechanisms through which exposure to milk GCs affects development are unknown. GC receptors are highly expressed in the developing brain and GC exposure impacts the development of neural systems involved in the regulation of emotion and behavioral stress responses (Nagano et al., 2008; Lee et al., 2011). The amygdala is the primary brain structure responsible for the experience of emotion (McDonald, 1998; LeDoux, 2000) and GC exposures early in life result in alterations in the developmental trajectory of the structure of the amygdala (Salm et al., 2004; Kraszpulski et al., 2006). Further, findings from animal models illustrate that prenatal stress exposures, including excess GCs, both alter the density of GC receptors (Kapoor et al., 2006) and increase the production of CRH in the amygdala (Cratty et al., 1995; Mueller & Bale, 2008).
The strengths of our study include the direct assessment of milk cortisol and a carefully characterized cohort of mother-infant pairs. It could be argued that our study was limited by maternal report of infant temperament, which may be subject to bias. However, with the IBQ, the potential for bias is reduced through addressing concrete infant behaviors so the parent does not have to make comparative judgments involving other infants and by inquiring only about recently occurring events to prevent errors in recall. Further, the primary caregiver has extensive and unique insight into the behavior of the infant that cannot be approximated by most other observers. In addition, we took the further conservative step of assessing maternal negative affect to address the possibility of reporting bias, and this did not alter the association between infant temperament and milk cortisol. Because our study relied on naturally occurring levels of milk cortisol instead of controlled manipulations, there is the possibility for a more complex relation between cortisol levels and infant temperament. For example, the postnatal environment including maternal psychological state and shared genetic effects could contribute to the findings. However, when adjusting for maternal affect, the positive association between milk cortisol and temperament still remained. Additionally, if genetic factors accounted for the findings, it would suggest an implausibly complex relationship between genetics, milk cortisol and infant temperament. We have shown previously that among formula-fed infants, maternal cortisol does not predict temperament. This argues against a genetic explanation for the current study's findings because the genetic influences underlying the relations would be unique to breastfeeding mothers, an unlikely selectivity.
Findings from experimental animal models demonstrate that exposure to milk-borne GCs affects development, and that these influences persist across the lifespan (Angelucci et al., 1983; Catalani et al., 2000). Further, excesses or deficits in other milk proteins and hormones also appear to exert lasting effects on development (Ellis et al., 1996; Sullivan et al., 2003). Biochemical communication through milk represents one potentially important maternal signal that offspring may incorporate to maximize adaptation to their environments. This possibility is consistent with developmental models in which the quality of adaptation to the early environment is dependent in part on the nature and continuity of maternal signals and the early attachment relationship (e.g. Sroufe, 1979; Sroufe et al., 2010) and also with even more broad theoretical frameworks describing the contributions of the early environment to lifespan health and development (c.f. Hales & Barker, 2001; Gluckman & Hanson, 2004; Ellis et al., 2011). These new data contribute to the small but accumulating literature suggesting that these perspectives might be usefully expanded to include lactational programming effects.
Acknowledgements
The authors thank the families who participated in this project and the staff at the UCI Women and Children's Health and Well-Being project for their excellent work. This research was supported by grants from the National Institutes of Health (HD-40967 to LMG and NS-41298) and by a grant from the University of Califorina, Irvine (CORCLR to LMG). The breast pumps were donated by Medela.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest The authors have no conflicts of interest to disclose.
Contributors Laura Glynn designed and funded the study and oversaw the statistical analyses. Katherine Grey assisted with the statistical analyses and wrote the first draft of the manuscript. Elysia Davis consulted on study design and infant data collection. Curt Sandman provided partial funding for the study. All authors contributed to and have approved the final manuscript.
References
- Angelucci L, Patacchioli FR, Chierichetti C, Laurenti S. Perinatal mother-offspring pituitary adrenal interrelationship in rats: Corticosterone in milk may affect adult life. Endocrinologia Experimentalis. 1983;17:191–205. [PubMed] [Google Scholar]
- Angelucci L, Patacchioli FR, Scaccianoce S, Di Sciullo A, Cardillo A, Maccari S. A model for later-life effects of perinatal drug exposure: maternal hormone mediation. Neurobehavioral Toxicology and Teratology. 1985;7:511–517. [PubMed] [Google Scholar]
- Barker DJP. Mothers, babies and health in later life. Harcourt Brace & Co. Ltd.; Edinburgh: 1998. [Google Scholar]
- Bernardes J, Goncalves H, Ayres-de-Campos D, Rocha AP. Linear and complex heart rate dynamics vary with sex in relation to fetal behavioural states. Early Human Development. 2008;84:433–439. doi: 10.1016/j.earlhumdev.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Bertram CE, Hanson MA. Prenatal programming of postnatal endocrine responses by glucocorticoids. Reproduction. 2002;124:459–467. doi: 10.1530/rep.0.1240459. [DOI] [PubMed] [Google Scholar]
- Buss C, Davis EP, Class QA, Gierczak M, Patillo C, Glynn LM, Sandman CA. Maturation of the human fetal startle response: evidence for sex-specific maturation of the human fetus. Early Human Development. 2009;85:633–638. doi: 10.1016/j.earlhumdev.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casolini P, Cigliana G, Alemà GS, Ruggieri V, Angelucci L, Catalani A. Effect of increased maternal corticosterone during lactation on hippocampal corticosteroid receptors, stress response and learning in offspring in the early stages of life. Neuroscience. 1997;79:1005–1012. doi: 10.1016/s0306-4522(96)00668-9. [DOI] [PubMed] [Google Scholar]
- Catalani A, Casolini P, Cigliana G, Scaccianoce S, Consoli C, Cinque C, Zuena AR, Angelucci L. Maternal corticosterone influences behavior, stress response and corticosteroid receptors in the female rat. Pharmacology, Biochemistry and Behavior. 2002;73:105–114. doi: 10.1016/s0091-3057(02)00755-4. [DOI] [PubMed] [Google Scholar]
- Catalani A, Casolini P, Scaccianoce S, Patacchioli FR, Spinozzi P, Angelucci L. Maternal corticosterone during lactation permanently affects brain corticosteroid receptors, stress response and behaviour in rat progeny. Neuroscience. 2000;100:319–325. doi: 10.1016/s0306-4522(00)00277-3. [DOI] [PubMed] [Google Scholar]
- Catalani A, Marinelli M, Scaccianoce S, Nicolai r., Muscolo LA, Porcu A, Koranyi L, Piazza PV, Angelucci L. Progeny of mothers drinking corticosterone during lactation has lower stress-induced corticosterone secretion and better cognitive performance. Brain Research. 1993;8:209–215. doi: 10.1016/0006-8993(93)90079-3. [DOI] [PubMed] [Google Scholar]
- Clifton VL. Sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;24:S33–S39. doi: 10.1016/j.placenta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R. Detection of posnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. British Journal of Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Cratty MS, Ward HE, Johnson EA, Azzaro AJ, Birkle DL. Prenatal stress increases corticotropin-releasing factor (CRF) content and release in rat amygdala minces. Brain Research. 1995;675:297–302. doi: 10.1016/0006-8993(95)00087-7. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-DeMet A, Sandman CA. Prenatal exposure to maternal depression and cortisol influences infant temperament. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:737–746. doi: 10.1097/chi.0b013e318047b775. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Waffarn F, Sandman CA. Prenatal maternal stress programs infant stress regulation. Journal of Child Psychology and Psychiatry. 2011;52:119–129. doi: 10.1111/j.1469-7610.2010.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Townsend EL, Gunnar MR, Georgieff MK, Guiang SF, Ciffuentes RF, Lussky RC. Effects of prenatal betamethasone exposure on regulation of stress physiology in healthy premature infants. Psychoneuroendocrinology. 2004;29:1028–1036. doi: 10.1016/j.psyneuen.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Davis MK. Breastfeeding and chronic disease in childhood and adolescence. Pediatric Clinics of North America. 2001;48:125–141. doi: 10.1016/s0031-3955(05)70289-3. [DOI] [PubMed] [Google Scholar]
- De Pauw SS, Mervielde I. Temperament, personality and developmental psychopathology: a review based on the conceptual dimensions underlying childhood traits. Child Psychiatry and Human Development. 2010;41:313–329. doi: 10.1007/s10578-009-0171-8. [DOI] [PubMed] [Google Scholar]
- de Weerth C, van Hees Y, Buitelaar JK. Prenatal maternal cortisol levels and infant behavior during the first 5 months. Early Human Development. 2003;74:139–151. doi: 10.1016/s0378-3782(03)00088-4. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Kivlighan KT, Costigan KA, Laudenslager ML. Fetal motor activity and maternal cortisol. Developmental Psychobiology. 2009;51:505–512. doi: 10.1002/dev.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty L, Klein D. Temperamental positive and negative emotionality and children's depressive symptoms: A longitudinal prospective study from age three to age ten. Journal of Social and Clinical Psychology. 2010;29:462–488. [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, Van Ijzendoorn MH. Differential susceptibility to the environment: an evolutionary-neurodevelopmental theory. Development and Psychopathology. 2011;23:7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Ellis LA, Mastro AM, Picciano MF. Milk-borne prolactin and neonatal development. Journal of Mammary Gland Biology and Neoplasia. 1996;1:259–269. doi: 10.1007/BF02018079. [DOI] [PubMed] [Google Scholar]
- Friedman NJ, Zeiger RS. The role of breast-feeding in the development of allergies and asthma. Journal of Allergy and Clinical Immunology. 2005;115:1238–1248. doi: 10.1016/j.jaci.2005.01.069. [DOI] [PubMed] [Google Scholar]
- Gale CR, Martyn CN. Birth weight and later risk of depression in a national birth cohort. British Journal of Psychiatry. 2004;184:28–33. doi: 10.1192/bjp.184.1.28. [DOI] [PubMed] [Google Scholar]
- Gartstein MA, Rothbart MK. Studying infant temperament via the Revised Infant Behavior Questionnaire. Infant Behavior and Development. 2003;26:64–86. [Google Scholar]
- Gluckman PD, Hanson MA. Living with the past: Evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Davis EP, Dunkel Schetter C, Chicz-DeMet A, Hobel CJ, Sandman CA. Postnatal maternal cortisol levels predict temperament in healthy breastfed infants. Early Human Development. 2007;83:675–681. doi: 10.1016/j.earlhumdev.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Sandman CA. Sex moderates associations between prenatal glucocorticoid exposure and human fetal neurological development. Developmental Science. doi: 10.1111/j.1467-7687.2012.01159.x. in press. [DOI] [PubMed] [Google Scholar]
- Godfrey KM, Barker DJP. Fetal nutrition and adult disease. American Journal of Clinical Nutrition. 2000;71:1344S. doi: 10.1093/ajcn/71.5.1344s. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Campos JJ. The structure of temperamental fear and pleasure in infants: a psychometric perspective. Child Development. 1990;61:1944–1964. [PubMed] [Google Scholar]
- Grosvenor CE, Picciano MF, Baumrucker CR. Hormones and growth factors in milk. Endocrine Reviews. 1992;14:710–728. doi: 10.1210/edrv-14-6-710. [DOI] [PubMed] [Google Scholar]
- Gunnell D, Rasmussen F, Fouskakis D, Tynelius P, Harrison G. Patterns of fetal and childhood growth and the development of psychosis in young males: a cohort study. American Journal of Epidemiology. 2003;4:291–300. doi: 10.1093/aje/kwg118. [DOI] [PubMed] [Google Scholar]
- Gwazdauskas FC, Paape MJ, McGilliard ML. Milk and plasma glucocorticoid alterations after injections of hydrocortisone and adrenocorticotropin. Proceedings of the Society for Experimental Biology and Medicine. 1977;154:543–545. doi: 10.3181/00379727-154-39714. [DOI] [PubMed] [Google Scholar]
- Hales CN, Barker DJ. The thrifty phenotype hypothesis. British Medical Bulletin. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- Hamosh M. Bioactive factors in human milk. Pediatric Clinics of North America. 2001;48:69–86. doi: 10.1016/s0031-3955(05)70286-8. [DOI] [PubMed] [Google Scholar]
- Hart S, Boylan LM, Border B, Carroll SR, McGunegle D, Lampe RM. Breast milk levels of cortisol and Secretory Immunoglobulin A (SIgA) differ with maternal mood and infant neuro-behavioral functioning. Infant Behavior and Development. 2004;27:101–106. [Google Scholar]
- Hinde K, Capatanio JP. Lactational programming? Mother's milk energy predicts infant behavior and temperament in rhesus macaques (Macaca mulatta) American Journal of Primatology. 2010;72:522–529. doi: 10.1002/ajp.20806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JS, Mezulis AH, Abramson LY. The ABCs of depression: integrating affective, biological, and cognitive models to explain the emergence of the gender difference in depression. Psychological Review. 2008;115:291–313. doi: 10.1037/0033-295X.115.2.291. [DOI] [PubMed] [Google Scholar]
- Indredavik MS, Vik T, Heyerdahl S, Kulseng S, Fayers P, Brubakk A-M. Psychiatric symptoms and disorders in adolescents with low birth weight. Archives of Disease in Childhood: Fetal-Neonatal Edition. 2003;89:F445–F450. doi: 10.1136/adc.2003.038943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobi C, Hayward C, de Zwaan M, Kraemer HC, Agras WS. Coming to terms with risk factors for eating disorders: application of risk terminology and suggestions for a general taxonomy. Psychological Bulletin. 2004;130:19–65. doi: 10.1037/0033-2909.130.1.19. [DOI] [PubMed] [Google Scholar]
- Kagan J, Snidman N, Zentner M, Peterson E. Infant temperament and anxious symptoms in school age children. Developmental Psychopathology. 1999;11:209–224. doi: 10.1017/s0954579499002023. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J Physiol. 2006;572:31–44. doi: 10.1113/jphysiol.2006.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato EA, Hsu BR, Raymoure WJ, Kuhn RW. Evidence for the direct transfer of corticosteroid-binding globulin from plasma to whey in the guinea pig. Endocrinology. 1985;117:1404–1408. doi: 10.1210/endo-117-4-1404. [DOI] [PubMed] [Google Scholar]
- Kraszpulski M, Dickerson PA, Salm AK. Prenatal stress affects the developmental trajectory of the rat amygdala. Stress. 2006;9:85–95. doi: 10.1080/10253890600798109. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Son GH, Chung S, Lee S, Kim J, Choi S, Kim K. Impairment of fear memory consolidation in maternally stressed male mouse offspring: evidence for nongenomic glucocorticoid action on the amygdala. Journal of Neuroscience. 2011;11:7131–7140. doi: 10.1523/JNEUROSCI.4692-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GC, Wertheim EH, Prior M, Smart D, Sanson A, Oberklaid F. A longitudinal study of the role of childhood temperament in the later development of eating concerns. International Journal of Eating Disorders. 2000;27:150–162. doi: 10.1002/(sici)1098-108x(200003)27:2<150::aid-eat3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Progress in Neurobiology. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McLean CP, Anderson ER. Brave men and timid women? A review of the gender differences in fear and anxiety. Clinical Psychology Reviews. 2009;29:496–505. doi: 10.1016/j.cpr.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, Sharma S, Seckl JR, Plotsky PM. Early environmental regulation of the forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Developmental Neuroscience. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- Mittendorfer-Rutz E, Rasmussen F, Wasserman D. Restricted fetal growth and adverse maternal psychosocial and socioeconomic conditions as risk factors for suicidal behavior of offspring: a cohort study. Lancet. 2004;364:1135–1140. doi: 10.1016/S0140-6736(04)17099-2. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano M, Ozawa H, Suzuki H. Prenatal dexamethasone exposure affects anxiety-like behaviour and neuroendocrine systems in an age-dependent manner. Neuroscience Research. 2008;60:364–371. doi: 10.1016/j.neures.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Owen D, Andrews MH, Matthews SG. Maternal adversity, glucocorticoids and programming of neuroendocrine function and behavior. Neuroscience and Biobehavioral Reviews. 2005;29:209–226. doi: 10.1016/j.neubiorev.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Paape MJ, Desjardins C, Guidry MS, Miller RH, Smith VR. Response of plasma corticosteroids and circulating leukocytes in cattle following intravenous injection of different doses of adrenocorticotropin. American Journal of Veterinary Research. 1977;38:1345–1348. [PubMed] [Google Scholar]
- Patacchioli FR, Cigliana G, Cilumbriello A, Perrone G, Capri O, Alemà GS, Zichella L, Angelucci L. Maternal plasma and milk free cortisol during the first 3 days of breast-feeding following spontaneous delivery or elective cesarean section. Gynecologic and Obstetric Investigations. 1992;34:159–163. doi: 10.1159/000292751. [DOI] [PubMed] [Google Scholar]
- Pearlman WH. Glucocorticoids in milk: a review. Endocrinologia Experimentalis. 1983;17:165–174. [PubMed] [Google Scholar]
- Phillips DI, Jones A. Fetal programming of autonomic and HPA function: do people who were small babies have enhanced stress responses? Journal of Physiology. 2006;572:45–50. doi: 10.1113/jphysiol.2005.104695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior M, Smart D, Sanson A, Oberklaid F. Does shy-inhibited temperament in childhood lead to anxiety problems in adolescence? Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:461–468. doi: 10.1097/00004583-200004000-00015. [DOI] [PubMed] [Google Scholar]
- Salm AK, Pavelko M, Krouse EM, Webster W, Kraszpulski M, Birkle DL. Lateral amygdaloid nucleus expansion in adult rats is associated with exposure to prenatal stress. Brain Research. Developmental Brain Research. 2004;128:159–167. doi: 10.1016/j.devbrainres.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Schack-Nielsen L, Michaelsen KF. Breast feeding and future health. Current Opinion in Clinical Nutrition and Metabolic Care. 2006;9:289–296. doi: 10.1097/01.mco.0000222114.84159.79. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:1008–1015. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Meaney ML. Glucocorticoid “programming” and PTSD risk. Annals of the New York Academy of Sciences. 2006;1071:351–378. doi: 10.1196/annals.1364.027. [DOI] [PubMed] [Google Scholar]
- Sroufe LA. The coherence of individual development. Early care, attachment, and subsequent developmental issues. American Psychologist. 1979;34:834–841. [Google Scholar]
- Sroufe LA, Coffino B, Carlson EA. Conceptualizing the role of early experience: lessons from the Minnesota longitudinal study. Developmental Review. 2010;30:36–51. doi: 10.1016/j.dr.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EC, Dubé C, Dorenbos K, Steward O, Baram TZ. Mitochondrial uncoupling protein-2 protects the immature brain from exocitotoxic neuronal death. Annals of Neuroscience. 2003;53:711–717. doi: 10.1002/ana.10543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EC, Hinde K, Mendoza SP, Capitanio JP. Cortisol concentrations in the milk of the rhesus monkey mothers are associated with confident temperament in sons, but not daughters. Developmental Psychobiology. 2011;53:96–104. doi: 10.1002/dev.20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautman PD, Meyer-Bahlburg HFL, Postelnek J, New MI. Effects of early prenatal dexamethasone on the cognitive and behavioral development of young children: results of a pilot study. Psychoneuroendocrinology. 1995;20:439–449. doi: 10.1016/0306-4530(94)00070-0. [DOI] [PubMed] [Google Scholar]
- Van Os J, Selten JP. Prenatal exposure to maternal stress and subsequent schizophrenia. The May 1940 invasion of The Netherlands. British Journal of Psychiatry. 1998;172:324–326. doi: 10.1192/bjp.172.4.324. [DOI] [PubMed] [Google Scholar]
- Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain, Behavior, and Immunity. 2005;19:196–308. doi: 10.1016/j.bbi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR, Holmes MC. Prenatal glucocorticoid programming of the brain corticosteroid receptors and corticotrophin-releasing hormone: possible implications for behavior. Neuroscience. 2001;104:71–79. doi: 10.1016/s0306-4522(01)00065-3. [DOI] [PubMed] [Google Scholar]
- Welberg LAM, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. Journal of Neuroendocrinology. 2001;13:113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- Worobey J, Blajda VM. Temperament ratings at 2 weeks, 2 months, and 1 year: differential stability of activity and emotionality. Developmental Psychology. 1989;25:257–263. [Google Scholar]
- Yeh K-Y. Corticosterone concentrations in the serum and milk of lactating rats: parallel changes after induced stress. Endocrinology. 1984;115:1364–1370. doi: 10.1210/endo-115-4-1364. [DOI] [PubMed] [Google Scholar]
- Youngstrom E, Izard C, Ackerman B. Dysphoria-related bias in maternal ratings of children. Journal of Consulting and Clinical Psychology. 1999;67:905–916. doi: 10.1037//0022-006x.67.6.905. [DOI] [PubMed] [Google Scholar]
- Zarrow MX, Philpott JE, Denenberg VH. Passage of 14C-4-corticosterone from the rat mother to the foetus and neonate. Nature. 1970;13:1058–1059. doi: 10.1038/2261058a0. [DOI] [PubMed] [Google Scholar]
- Zuloaga DG, Carbone DL, Hiroi R, Chong DL, Handa RJ. Dexamethasone induces apoptosis in the developing rat amygdala in an age-, reion-, and sex-specific manner. Neuroscience. doi: 10.1016/j.neuroscience.2011.09.052. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


