Abstract
The maternal endocrine stress system is profoundly altered during the course of human pregnancy. The human placenta expresses the genes for CRH as early as the seventh week of gestation and it is the expotential increase in placental CRH (pCRH) over the course of human gestation that is responsible for the greatest modification in the maternal stress system. The bi-directional placental release of hormones into the maternal and fetal compartments has profound influences for both. The influential Fetal Programming model predicted that early or fetal exposures to maternal signals of threat or adverse conditions have lifelong consequences for health outcomes. A basic assumption of this model was that developing organisms play a dynamic role in their own construction. Data are reviewed and new data are presented that elevated pCRH over the course of human gestation plays a fundamental role in the organization of the fetal nervous system, modifies birth phenotype (the timing of the onset of spontaneous labor and delivery), and influences developmental temperamental and metabolic trajectories. Evidence for sex differences and conserved function across species is presented. Finally, a model is presented that proposes several pathways that pCRH can program risk for health and disease.
Keywords: Corticotropic-releasing-hormone, fetal programming, Stress, Human pregnancy, Fetal development, Sex differences, Child development
PRECIS
It is difficult to know where to begin to describe my relationship with Abba, Mexico City? Philadelphia? Breckenridge? Atlanta? Cozumel? Utrecht? Chicago? or perhaps at the beginning, in New Orleans. I was drawn to the Deep South in the mid-1960’s by my desire to become involved in the Civil Rights movement. I had never travelled to the Deep South from the relative tranquility of my California home and, unlike today, there was a scarcity of information available about the culture and politics of various destinations, so my decision about where to go was somewhat random. I was in my mid-twenties and made the decision that graduate school would be the vehicle to allow me and my small family to make a move. When I arrived on the Louisiana State University (LSU) campus in Baton Rouge, there were fewer than 30 African-American students enrolled among the 15,000 plus students. Martin Luther King was assassinated during my tenure at LSU and that tragedy was the impetus for a fledgling civil right movement on campus. I was a founding member of the Martin Luther King Action Movement (MLKAM) on campus and in parallel I quickly co-opted the psychology national honorary society to begin a petition against the Viet Nam war. The MLKAM remained local and contentious (and dangerous for some) but the petition against the war in Viet Nam mysteriously became national and resulted in a late Sunday night knock on my door from an agent of the US government. The faculty was passively supportive of my activities but as I found out, labeled me a “trouble-maker.”
As part of my training, the graduate program assigned me in the summers to the New Orleans Veterans Administration (NOVA) hospital. During my second summer I attended a grand round presentation related to a patient I had been working with. The patient (let’s call him Nate) was a young, very smart African American man who had recently returned from Viet Nam. Nate had taken a job at a New Orleans gas station and when an impolite customer had called him “boy” Nate promptly knocked him to the ground. Because no “sane” African American in the 1960’s would strike a white man in the Deep South, Nate was admitted to the psychiatric ward. He was perfectly sane, smart and angry and refused to comply with some of the degrading demands of the staff (such as stripping and taking medications). During the grand rounds I was more than shocked to hear an eminent consultant seriously suggest that a lobotomy might be considered a reasonable treatment. When I regained my senses and realized that this was a serious proposal, I raised my hand in protest and claimed that I could work with Nate and perhaps we could re-evaluate his case later. Without going into detail, once I told Nate what was going to happen and how he could avoid it, he was discharged within a month. Because the label of “trouble-maker” had followed me to the NOVA, coupled with my “defiance” of authority in the Grand Rounds there was an effort to re-channel my energy. The director of the psychology service, who always seemed to be terrified by me, told me about a request made by a group of researchers in a “mysterious” building for someone interested in collaborating. None of the faculty were interested and the director thought I might be (not really, he just wanted to get rid of me).
I entered Abba’s laboratory with large vats of frogs. Frogs were everywhere. He explained how frog skins were used as a bioassay for Melanocyte-Stimulating- Hormone (MSH) and that although MSH was known to influence pigmentation in amphibians, new data published by the group at the University of Utrecht indicated that it may have effects in the mammalian brain. Rats treated with MSH appeared to learn a simple discrimination faster than rats treated with placebo. The evolutionary and teleological implications regarding what was conserved and why, was captivating and we discussed possible directions for a research project. He armed me with several papers to read and asked me to return with a specific idea to test in rats. He did not seem to care that I might have been a trouble-maker or concerned that I had never worked with animals before (I probably did not tell him). The available literature at that time only examined the effects of MSH on tasks with aversive or stressful consequences so our first collaborative and published paper reported that MSH improved learning on an appetitive task (55). Many papers describing the effects of peptides on animal and human development have followed and currently I continue to examine the influence of gestational exposures to neurobiological signals of stress (including peptides and hormones) on the brain and behavior of children. The influence that Abba had on my personal and professional life cannot be overstated.
STRESS, HUMAN PREGNANCY AND FETAL EXPOSURE TO PLACENTAL CRH
Based on our initial and novel studies that described the long lasting (perhaps permanent) effects on the brain and behavior of rats exposed to stress peptides during very early-life (fetal or early infancy; 2, 6, 8, 53, 59, 60), a program of research (Figure 1) was initiated to examine of the effects of activation of the maternal endocrine stress axis during pregnancy on the human fetus. A fundamental assumption of our research program is that prenatal exposure to maternal HPA and placental hormones represent primary mechanisms underlying the effects of maternal psychological distress on subsequent infant and child development. From this program we have learned that during human pregnancy there is a complex relation between psychosocial and biological markers of prenatal stress. Both sources of stress have programming consequences for the human fetal nervous system, birth outcome and risk for subsequent health and disease (44–47).
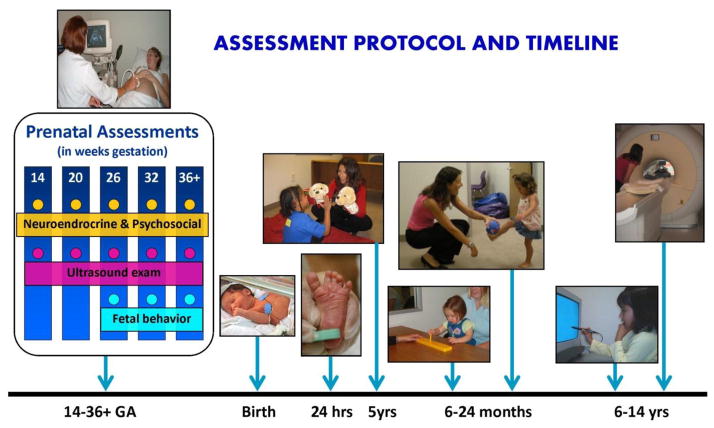
Figure 1.
The assessment protocol for research program. Pregnant women and their fetuses are initially assessed at ~14 weeks gestation. Participants are followed in current studies until they are adolescents.
The maternal endocrine stress (hypothalamic-pituitary-adrenal; HPA) system is profoundly altered during the course of human pregnancy. The pituitary gland doubles in size increasing by several fold the synthesis, and release of pituitary peptides into the maternal circulation. Production from target tissues, such as cortisol from the adrenal gland also increases two to four-fold over the course of pregnancy. But it is the growth and development of a new fetal organ, the placenta in primates that is primarily responsible for the profound changes in the maternal/fetal stress systems (45,47). The human placenta expresses the genes for CRH (hCRHmRNA) (as well as proopiomelanocortin, the precursor for ACTH and beta-endorphin (BE)) as early as the seventh week of gestation and it is the expotential (20 to 40-fold) increase in placental CRH (pCRH) over the course of human gestation that is responsible for the greatest modification in the maternal stress system (Figure 2).
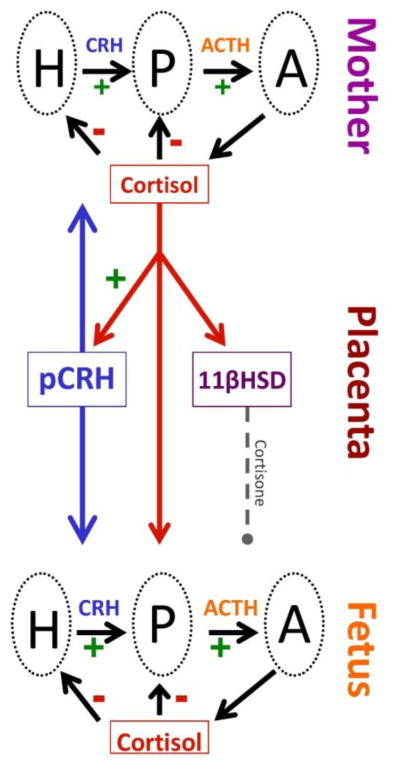
Figure 2.
During the course of human pregnancy all stress hormones rise. Placental CRH (pCRH) which is detected in maternal circulation rises exponentially over the course of gestation. In contrast to the negative feedback regulation, during pregnancy maternal cortisol increases the production of CRH from the placenta. The effects of maternal cortisol on the fetus are modulated by the presence of a placental enzyme 11βHSD2 which oxidizes it into an inactive form, cortisone. Activity of this enzyme increases as pregnancy advances, and then drops near term so that maternal cortisol is available to promote maturation of the fetal lungs, central nervous system as well as other organ systems.
Placental CRH is identical to hypothalamic CRH in structure, immunoreactivity and bioactivity (40, 63,64). However in contrast to the inhibitory influence (negative feedback) on the promoter region of the CRH gene in the hypothalamus, maternal stress signals from the adrenal glands (i.e., cortisol) activate the promoter region in the placenta and stimulate the expression of hCRHmRNA establishing a positive feedback loop that allows for the simultaneous increase of placental CRH (pCRH), ACTH and cortisol over the course of gestation (Figure 2). Thus, all of the stress related peptides/hormones levels rise as pregnancy advances, peaking during labor, and falling to basal or, in the case of pCRH, undetectable levels within 24 hours after delivery. The normative exponential increase of these stress signals, (especially pCRH) over the latter part of human gestation, (i) plays a fundamental role in the organization of the fetal nervous system (61), (ii) influences the maturation of the fetal HPA axis and other systems, (iii) modifies birth phenotype (the timing of the onset of spontaneous labor and delivery) 35, 65, 66, 68, 69, 72 and (iv) regulates maternal adaptation during pregnancy (21, 46).
The human placenta integrates numerous sources of maternal stress signals and responds with a dose dependent release of CRH. Detection by the fetal/placental unit of stress signals from the maternal environment (for instance, cortisol) “informs” the fetus that there may be a threat to survival. The placental/fetal unit responds to this by synthesizing and releasing CRH (positive feedback) and activating a cascade of consequences. Depending on the timing during gestation and the severity of the stress the myometria are activated resulting in abbreviated gestation and early fetal escape from a hostile environment. In parallel the fetus incorporates the bidirectional information to adjust its developmental trajectory and modifies its nervous system to ensure survival in a potentially hostile postpartum environment (48). Survival under these conditions can be associated with compromised growth, reproductive success, motor, cognitive and emotional function.
Moreover, it is believed that high levels of placental CRH circulating in the maternal blood stream down-regulates maternal corticotrophs blunting the communication between the hypothalamus and pituitary gland and affecting her response to the environment (21). This not only explains the decrease in maternal responses to stress as pregnancy advances but also has been proposed as a mechanism for postpartum depression (22).
The bi-directional placental release of hormones into the maternal and fetal compartments is a powerful route of communication between the fetus and mother with long term and profound influences for both. The Fetal Programming or Developmental Origins of Health and Disease Models (1) were proposed to explain the persisting influences associated with prenatal exposure to stress. This influential model predicted that early or fetal exposures to maternal signals of threat or adverse conditions have lifelong consequences for health outcomes. A basic assumption of this model was that developing organisms play a dynamic role in their own construction. (44, 46, 47). Signals from the maternal stress systems are continuous sources of information to the fetus over the course of gestation. As described above, these systems are undergoing massive but orderly changes as pregnancy advances. Deviations in these systems from normative patterns can be signals to the fetus that there is a threat to the host (mother). Depending on the severity and the timing of deviant (stressful) maternal signals the sequence of neural development can be disrupted resulting in programmed consequences for brain structure and behavior. Fetal exposure to deviant elevations of stress and placental hormones early in pregnancy have been associated with less optimal neurodevelopment, more irritable and fearful temperament, larger volumes in limbic areas of the brain and slower behavioral recovery from pain (5, 11, 12). Fetal exposure to increases in these hormones occurring very late in gestation has been associated in some instances with enhanced cognitive development (56). These influences occur against the background of the timing and sequence of fetal organ development.
The fetal period in the life cycle is unmatched by any other in growth and development, and it is the stage in the human life span that is most vulnerable to both organizing and disorganizing (programming) influences. Fetal organs develop from progenitor stem cells at precise times and in a specific sequence from conception to maturity, so the timing of maternal signals is a critical factor in determining the structure of the neurodevelopmental program. Disruption in the timing or sequence of organ development can result in tissue remodeling producing smaller organs or altered organ morphology. Remodeled tissue modifies the function and physiological capacity of the organ throughout the lifespan and is a fundamental assumption of how fetal exposures influence health and disease.
The human fetal nervous system is a primary target for these circulating programming influences because it is undergoing dramatic growth over a prolonged period of time. For instance, radial neuronal cell migration begins in the human brain around 42 days GA (71) and by 16 weeks GA, form the subplate zone. Concurrently, cells accumulating in the outer cerebral wall form the cortical plate which will become the cerebral cortex. By gestational week 20, axons form synapses with the cortical plate and there is an exponential increase in cortical thickness (22) and by gestational week 24, cortical circuits are organized (31). The human fetal brain is forming secondary and tertiary gyri, and exhibiting neuronal differentiation, dendritic arborization, axonal elongation, synapse formation and collateralization, and myelination by gestational week 28. Near term the fetal human brain contains billions of neurons and is 40% greater in number than in the adult (25). The rate of synaptogensis reaches an astonishing peak so that at gestational week 34 through 24 months postpartum, there is an increase of 40,000 synapses per minute (32).
PRENATAL EXPOSURE TO pCRH AND FETAL DEVELOPMENT
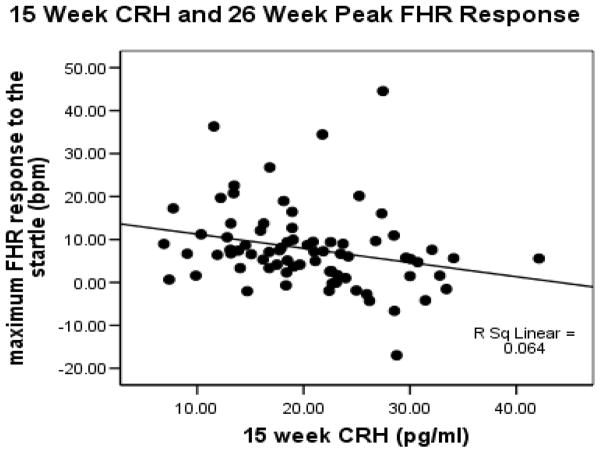
Our program of research has been focused on the influence of fetal exposure to maternal psychobiological markers of stress on fetal development. As discussed above, we particularly have been interested in variations in the concentrations of pCRH (i) because it is a major stress peptide with direct effects on the nervous system and (ii) because deviations in pCRH provides objective evidence that the fetus has been exposed, and is responding, to maternal signals of stress. Studies of human fetal heart rate in response to stimulation provide a model for assessing neurological integrity before there are postpartum influences on development such as socialization and parenting (62). In a prospective longitudinal study, we assessed fetal nervous system maturation at 26, 31 and 37 weeks gestation by monitoring fetal heart rate (FHR) responses to vibroacoustic stimulation (VAS) in 191 maternal/fetal dyads (4). The VAS is used routinely to awake a sleeping fetus and consists of a tone and vibration from a device placed on the mother’s abdomen. Reliable startle responses were not detected in all fetuses until 31 weeks gestational age. There was, however, evidence that some fetuses did respond at 26 weeks, so we considered that there may be individual differences in response patterns that could be explained by exposures to placental markers of stress. To examine this we (8) evaluated in 138 maternal/fetal dyads the association between startle responses at 26 weeks and pCRH levels collected at 15, 20 and 26 weeks of gestation. Elevated placental CRH levels at 15 weeks of gestation, but not later points, predicted (r (137)=.24, p<.01) smaller peak FHR responses to the VAS (FIGURE 3).
Figure 3.
Scatterplot that shows fetal exposure to elevated pCRH at 15 weeks gestation is associated with dampened heart rate responses to an external stimulus.
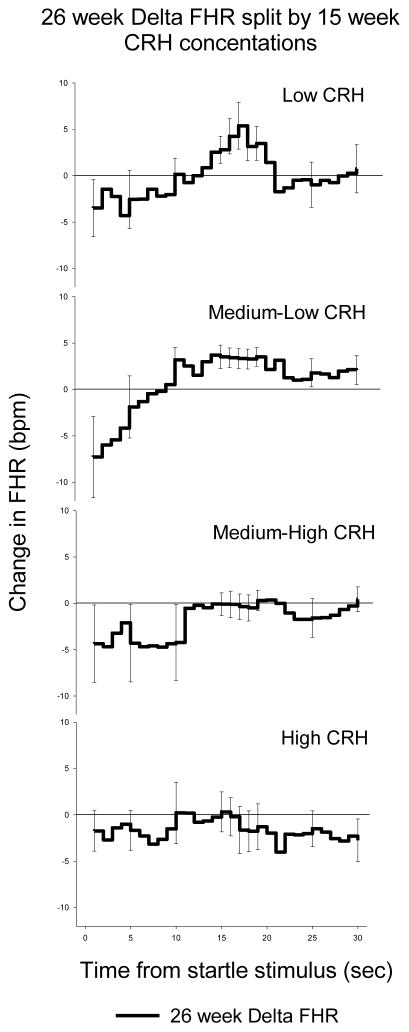
Figure 4 shows that fetal exposure to the lowest levels of CRH at 15 weeks gestation is associated with a clear response and recovery pattern to the VAS at 26 weeks gestation. Fetal exposure to the highest level of CRH is associated with failure to respond. These findings suggested that fetal exposure to the lowest concentrations of pCRH (optimal, low stress exposure) early in gestation reflected greater fetal maturity and accelerated neurological development. In an earlier study we had assessed effects of fetal exposure to pCRH on fetal memory and attention by presenting a series of repeated VAS, interrupted by a novel VAS. In this model, improved memory and attention is reflected in the extent to which the novel stimulation disrupts the pattern of habituation. We reported that lower placental CRH during the third trimester was associated with an improved ability to habituate to repeated presentations of the same VAS stimulus and to identify a novel stimulus (61).
Figure 4.
Changes (delta) in fetal heart rate associated with low to high levels of pCRH. The greatest change in response (heart rate acceleration above basline) was obseved in conditions of exposure to low levels of pCRH. No respone was observed at median-high or high levels of pCRH.
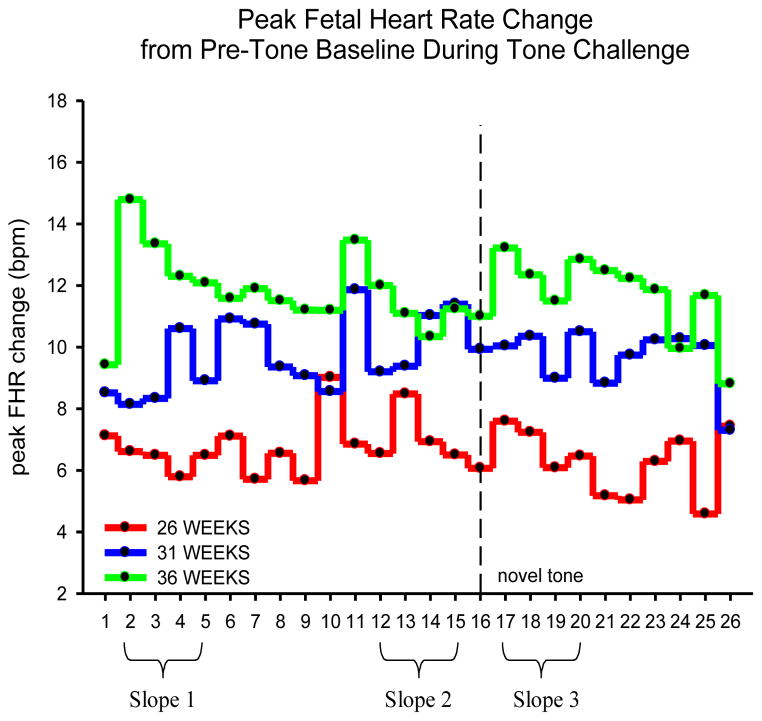
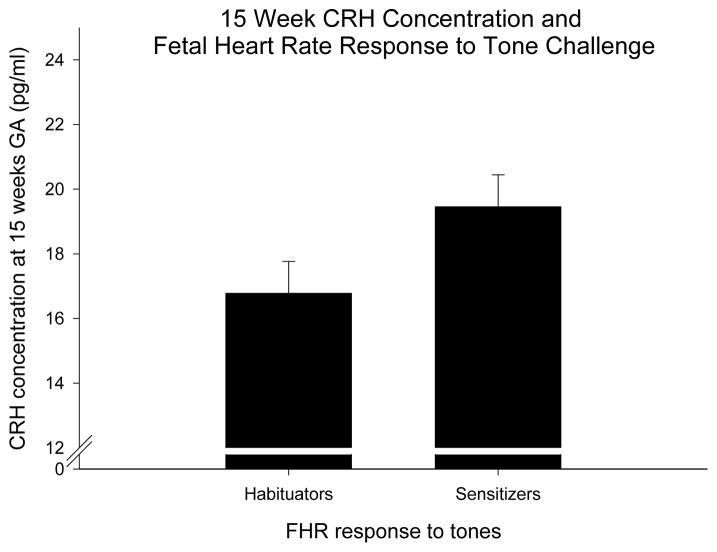
We repeated the habituation experiment by presenting pure tone stimulation to the fetus delivered by speakers attached to the mother’s abdomen. Following previously reported methods (52,62) we compared the slopes of the FHR response to the last four stimuli of the initial trials (trials 12–15, slope2) with the first four trials after the novel, dishabituating stimulus (trials 17–20, slope 3) (Figure 5). It is apparent that fetal responses at 36 weeks are characterized by a large initial FHR response followed by a progressive decrease in response (habituation). At 30 weeks fetuses show sensitization (progressive FHR response to subsequent stimulation) and there is no evidence of a response at 26 weeks GA. Moreover, at 36 weeks there is a fetal heart rate response following the novel stimulus (tone sixteen) and then a trend toward dishabituation. We found that dishabituation increases with age in both groups however fetuses exposed to low concentrations of pCRH show a greater dishabituation response (better learning) than fetuses exposed to high levels of CRH (F(1,130)= 5.3, p= 0.02). Based on the first four tones, we divided the groups who decrease (habituators) and those who increase (sensitizers) heart rate over these four trials. There was a trend suggesting that fetuses classified as habituators at 36 weeks gestation were exposed to lower CRH concentrations at 15 weeks gestation than the sensitizing fetuses (Figure 6). These findings suggested that exposure to CRH early in pregnancy influenced fetal maturation as measured by startle and habituation: high concentrations “retard” maturation and low levels accelerate it.
Figure 5.
Specific age-related patterns in peak fetal heart rate changes to tone stimulation. The oldest fetuses 36 weeks gestation) have the largest response followed by a progressive decrease in response (habituation). Slopes of the last four stimuli of the initial trials (trials 12–15, slope2) were compared with the first four trials after the novel, dishabituating stimulus (trials 17–20, slope 3) providing support for more pronounced dishabituation in the oldest fetuses.
Figure 6.
Slopes of the FHR response to the first four tones at 36 weeks of gestation (slope 1), were divided into those who decreased (habituators) and those who increased (sensitizers). There was a trend that fetuses classified as habituators were exposed to lower CRH concentrations at 15 weeks gestation than the sensitizing fetuses.
FETAL EXPOSURE TO pCRH AND BIRTH PHENOTYPE
Biological markers of stress consistently have been associated with adverse birth outcomes. The general findings among methodologically sound studies are that women reporting elevated levels of psychosocial stress during pregnancy are at significant risk for adverse birth outcomes (19, 33). Within the stress pathway, pCRH is most strongly linked to gestational length. Placental CRH has been characterized as controlling a “placental clock” that determines or alters the timing of onset of parturition (35, 65). Elevated levels and steeper trajectories of pCRH over the course of gestation initiate a cascade of events resulting in myometrial activation and in extreme cases preterm birth (35, 67). Because, it is the trajectory of pCRH production (i.e., the rate of acceleration), rather than the absolute hormone concentration that best predicts preterm birth it suggests that target cells are highly responsive to relative changes in pCRH concentrations (67).
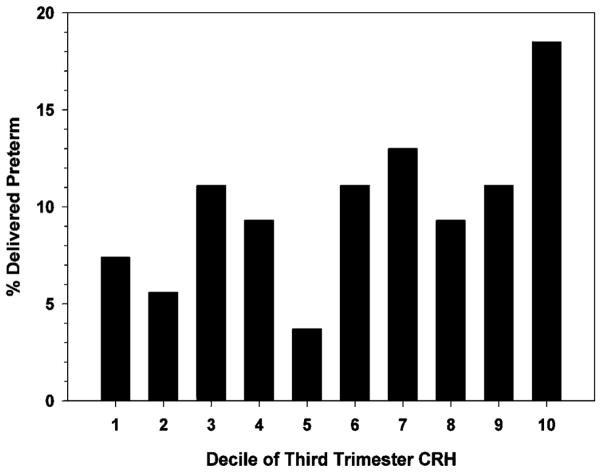
In a study published in Peptides to examine the association between birth outcome and a panel of biological stress markers, maternal levels of B-endorphin, ACTH, cortisol and pCRH were assessed at regular intervals from 15 to 36 weeks gestation in 203 pregnant women (49). Consistent with previous studies, pCRH levels in women destined to deliver preterm (before 37 weeks) had faster rates of increase and significantly higher levels of pCRH confined to the beginning of the early third trimester than women who subsequently delivered at term. Of the other maternal measures, only cortisol, as early as 15 weeks gestation, was elevated in women delivering preterm. Models that accounted for the independent and shared variance of pCRH and cortisol indicated that only pCRH between 26 and 31 weeks gestation predicted gestational length (Figure 7). However, we found that the best predictor of elevated pCRH at 31 weeks was elevated maternal cortisol at 15 weeks. The findings from this study indicated that a plausible stress-related endocrine signal, elevated cortisol from the mother very early in pregnancy, predicted the precocious rise in CRH leading to an abbreviated gestation. The pattern of findings supported the argument that the effect of elevated cortisol early in pregnancy reflected priming or programming effects on the eventual fetal/placental CRH response.
Figure 7.
The percentage of women delivering preterm (before 37 weeks gestation) was greatest who were among the highest decile (90th %tile) in third trimester CRH.
In addition to the regulation of gestational length, pCRH also plays a key role in the regulation of fetal maturation with consequences for birth phenotype. Neonatal evaluations of neuromuscular and physical characteristics of the newborn that develop over the course of gestation were done within 24 hours of birth to assess developmental maturation. In our study of 158 newborns within 24 hours after birth, fetal exposure to increased levels of maternal cortisol at 15 and at 19 weeks gestation and increased levels of pCRH at 31 weeks gestation were associated with significant decreases in newborn physical and neuromuscular maturation (20). These effects remained significant after adjusting for length of gestation.
FETAL EXPOSURE TO pCRH AND INFANT DEVELOPMENT
Research from our laboratory as well as from several international laboratories provides support for the influence of fetal exposures on developmental trajectories. It has become clear that one of the most consistent findings is that fetal exposure to psychological and biological stress signals is associated with more reactive physiological and behavioral responses to challenge during infancy perhaps reflecting more fearful temperament (11,15,16, 38, 73). In a sample of 247 mothers and their full term infants, we previously had examined the possible influence of the stress hormone pathway on infant temperament. We reported that elevated maternal cortisol at 30 gestational weeks (12) and elevated concentrations of pCRH at 25 gestational weeks each were significantly associated with greater maternal report of infant negative reactivity (11). Importantly these findings were independent of birth outcome, sociodemographic factors and postnatal maternal psychological measures. These novel findings were the first reports in infants/children of programming consequences of prenatal exposure to pCRH and suggested a biological pathway for the effects of maternal stress on the behavior of infants.
In addition to the effects on behavior and the central nervous system, there is evidence that prenatal maternal stress signals influence fetal growth, child obesity, and metabolic risk. In a recent study of 246 women and their healthy children, we (70) evaluated the association between fetal exposure to pCRH (from 15 to 37 weeks gestation) and child body mass index (BMI) in infants from 3 to 24 months of age. First, after adjusting for length of gestation, elevated pCRH at 30 weeks gestation was associated with both BMI and weight at birth. Second, four profiles of childhood growth were identified during early childhood development. One of the growth profiles was characterized by early small body size followed by rapid catch-up growth. This pattern of catch-up growth has been associated with increased risk for obesity and was associated with elevated pCRH at 30 gestation. These data suggested that fetal exposure to pCRH contributes to programming of metabolic risk.
SEX DIFFERENCES AND FETAL PROGRAMMING
Under certain conditions secondary sex ratio (number of males to females born) is associated with, and perhaps programmed by, preconceptional and maternal/fetal exposures to signals of stress and adversity. Compared with females, more males (i) are born preterm (10), (ii) have poorer neonatal and infant health outcomes (39), (iii) have higher risk for motor and cognitive outcomes, and (iv) are less likely to survive in intensive care. It is believed that the differential mortality, “programming” of morbidity and developmental impairments in males occurs early in gestation (or even preconceptionally) so that a new reproductive cycle can be initiated and that parental resources can be directed to another pregnancy (75). For example, sex differences have been observed in mammalian animal models as early as meisois (24). There is further evidence that within weeks of implantation the female placenta is more responsive than the male placenta to changes in stress signals including detection and response to maternal glucocorticoid concentration (9). In a recent review (50) we addressed sex differences by reviewing and reanalyzing data from previously published findings. We reasoned that if biological sex itself was influenced by early environmental exposures, then the influence of intrauterine exposures on males and females would be programmed differently.
As reviewed above, fetal exposure to cortisol early in gestation and pCRH at 31 weeks gestation was associated with delayed neuromuscular and motor maturation (20). Supplementary analyses indicated that these effects were significant only among males. Male fetuses exposed to elevated levels of cortisol early in pregnancy and CRH late in pregnancy exhibited delayed physical and neuromuscular maturation in very early infancy.
We previously had reported (and reviewed above) that fetal exposure to elevated pCRH at 25 gestational weeks in women was a risk factor for fearful or reactive temperament during infancy (11). New analyses illustrated that these effects were observed only among females. Specifically, elevated placental CRH at 25 gestational weeks was significantly associated with more fearful temperament and higher levels of distress behavior among female infants, but not male infants.
CONCLUSION
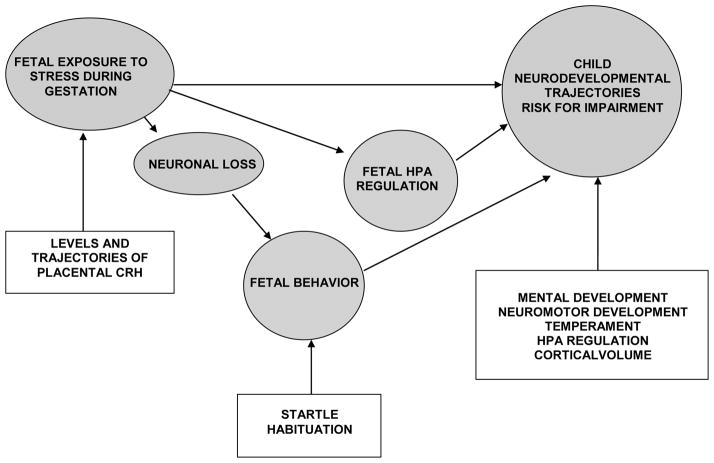
Figure 8 summarizes several pathways by which fetal exposure to stress generally, and pCRH specifically, can influence development and program risk for health and disease. Studies conducted (45, 47, 51) and underway in our program have been, and continue to be, designed to examine these possible pathways. One promising pathway is related to neuronal loss. Convincing evidence in animal models indicates that early life exposure to stress, specifically including CRH, decreases dendritic spine density in areas of brain related to cognitive and emotional regulation (7, 34). There is new evidence that fetal exposure to biological and psychological markers of stress is associated with patterns of cortical and subcortical development in children (5, 43) that may reflect dendritic arborization. We have found that the patterns of nervous system remodeling in children mediate the association between fetal exposure to adversity and subsequent behavioral trajectories and risk for impairment. It will be a challenge to follow these findings to basic mechanisms of neuronal loss.
Figure 8.
Model that describes our reseach program to assess plausible pathways between fetal exposure to adversity (including pCRH) and developmental outcomes.
In our initial study of peptide effects on behavior, indeed in the primary rationale for examining the behavioral properties of MSH, we assumed that there must be conserved functions of peptides across species. MSH and its fragments promotes escape behavior of the amphibian by altering pigmentation (27) and in the mammal, including humans, by increasing attention (26–30, 41,42, 53–59, 74). In both cases awareness of the environment is essential suggesting a unifying evolutionary purpose.
In studies of early life exposures to CRH there is strong evidence for conserved function across species that result in programming consequences for later life history outcomes. For instance, surveillance and response systems have evolved and are conserved so that many species including the desert dwelling Western Spadefoot tadpole can detect threats to survival and adjust their developmental trajectory (3). In rapidly evaporating pools of desert water with life-threatening consequences an elevation of CRH in the median eminence of the tadpole precipitates metamorphosis (17,18). If the CRH response is blocked during environmental desiccation, the rate of development is arrested and the tadpole’s survival is compromised. There are long-term (life history) consequences for the surviving toad exposed to this stress because it is smaller and is at a disadvantage against normally developing toads in foraging for food and reproducing. As reviewed above, the human fetus exposed to elevated pCRH may escape the inhospitable maternal host with abbreviated gestation and/or suffer with temperamental or behavioral impairment. Just as the toad may struggle to survive after early adversity, so will the developing child who has been exposed to maternal signals of adversity including pCRH.
HIGHLIGHTS.
Placental CRH influences human fetal heart rate
Placental CRH organizes human neurobehavioral development
Elevated placental CRH is associated with preterm birth
Elevated placental CRH is associated with fearful temperament in children
Fetal exposure to CRH remodels the nervous system
Conservation of function across species
Acknowledgments
Portions of the research reported here was supported by National Institute of Health grants NS-41298, HD-51852, HD-28413 HD-40967, HD-50662, HD-65823 and Conte Center award MH-96889.
Particular gratitude is expressed to the families (mothers and children) who have continued in our longitudinal studies. I am grateful for the expert contributions of students, post-doctoral fellows and collaborators, most of whom are referenced in this manuscript. Special appreciation is extended to long-time and current collaborators, Laura M. Glynn, PhD and Elysia Poggi Davis, PhD. Of course none of the research reported here and the many other research projects that have made my career and life a special blessing would be (or have been) possible if it were not for the mentoring and life-long support of Abba Kastin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barker DJP. Mothers babies and health in later life. 2. Edinburgh: Churchill Livingstone; 1998. [Google Scholar]
- 2.Beckwith BE, Sandman CA, Hothersall D, Kastin AJ. The influence of neonatal injections of alpha-MSH on learning, memory and attention in rats. Physiology and Behavior. 1977;18:63–71. doi: 10.1016/0031-9384(77)90095-6. [DOI] [PubMed] [Google Scholar]
- 3.Boorse GC, Denver RJ. Acceleration of Ambystoma tigrinum metamorphosis by corticotropin-releasing hormone. J Exp Zool. 2002;29:94–98. doi: 10.1002/jez.10115. [DOI] [PubMed] [Google Scholar]
- 4.Buss C, Davis EP, Class QA, Gierczak M, Patillo C, Glynn LM, Sandman CA. Maturation of the human fetal startle response: Evidence for sex-specific maturation of the human fetus. Early Human Development. 2009;85:633–638. doi: 10.1016/j.earlhumdev.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(20):E1312–9. doi: 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Champney T, Kastin AJ, Sandman CA. Effects of neonatal crebral ventricular injection of ACTH 4-9 and subsequent adult injections on learning in male and female albino rats. In: Sandman CA, Miller LH, Kastin AJ, editors. The Neuropeptides: Pharmacology, Physiological Substrates and Behavioral Effects: Pharmacology, Biochemistry and Behavior Monograph Supplement. Vol. 5. 1976. pp. 3–10. [DOI] [PubMed] [Google Scholar]
- 7.Chen YC, Rex CS, Rice CJ, et al. Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(29):13123–8. doi: 10.1073/pnas.1003825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Class QA, Buss C, Davis EP, Gierczak M, Pattillo C, Chicz-DeMet A, Sandman CA. Low levels of corticotrophin-releasing hormone during early pregnancy are associated with precocious maturation of the human fetus. Developmental Neuroscience. 2008;30:419–426. doi: 10.1159/000191213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clifton VL. Review: Sex and the human placenta: Mediating differential strategies of fetal growth and survival. Placenta. 2010;31:S33–39. doi: 10.1016/j.placenta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Cooperstock M, Campbell J. Excess males in preterm birth: Interactions with gestational age, race, and multiple birth. Obstet Gynecol. 1996;88:189–193. doi: 10.1016/0029-7844(96)00106-8. [DOI] [PubMed] [Google Scholar]
- 11.Davis EP, Glynn LM, Dunkel Schetter C, Hobel C, Chicz-Demet A, Sandman CA. Corticotropin-releasing hormone during pregnancy is associated with infant temperament. Dev Neurosci. 2005;27(5):299–305. doi: 10.1159/000086709. [DOI] [PubMed] [Google Scholar]
- 12.Davis EP, Glynn LM, Dunkel Schetter C, Hobel C, Chicz-DeMet A, Sandman CA. Prenatal exposure to maternal depression and cortisol influences infant temperament. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(6):737–46. doi: 10.1097/chi.0b013e318047b775. [DOI] [PubMed] [Google Scholar]
- 13.Davis EP, Glynn LM, Waffarn F, Sandman CA. Prenatal maternal stress programs infant stress regulation. Journal of Child Psychology and Psychiatry. 2011;52:119–129. doi: 10.1111/j.1469-7610.2010.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev. 2010;81(1):131–48. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis EP, Sandman CA. Prenatal psychobiological predictors of anxiety risk in preadolescent children. Psychoneuroendocrinology. 2012;37:1224–1233. doi: 10.1016/j.psyneuen.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis EP, Sandman C, Buss AC, Wing DA, Head K. Fetal glucocorticoid exposure is associated with preadolescent brain development. Biol Psychiatry. 2013;74:647–655. doi: 10.1016/j.biopsych.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denver RJ. Environmental stress as a developmental cue: Corticotropin-releasing hormone is a proximate mediator of adaptive phenotypic plasticity in amphibian metamorphosis. Hormones & Behavior. 1997;31:169–179. doi: 10.1006/hbeh.1997.1383. [DOI] [PubMed] [Google Scholar]
- 18.Denver RJ. Evolution of the corticotropin-releasing hormone signaling system and its role in stress-induced phenotypic plasticity. In: Sandman CA, Strand FL, Beckwith B, Chronwall BM, Flynn FW, Nachman RJ, editors. Neuropeptides. Structure and Function in Biology and Behavior. Vol. 897. New York, NY: The New York Academy of Sciences; 1999. pp. 46–53. Annals of the New York Academy of Sciences. [DOI] [PubMed] [Google Scholar]
- 19.Dunkel Schetter C, Glynn LM. Stress in pregnancy: empirical evidence and theoretical issues to guide interdisciplinary research. In: Contrada R, Baum A, editors. Handbook of Stress. New York: Springer; 2010. [Google Scholar]
- 20.Ellman LM, Dunkel-Schetter C, Hobel CJ, Chicz-DeMet A, Glynn LM, Sandman CA. Timing of fetal exposure to stress hormones: Effects on newborn physical and neuromuscular maturation. Dev Psychobiol. 2008;50:232–241. doi: 10.1002/dev.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glynn LM, Sandman CA. Prenatal origins of neurological development: A critical period for fetal and mother. Current Directions in Psychological Science. 2011;20:384–389. [Google Scholar]
- 22.Glynn LM, Davis EP, Sandman CA. New insights into the role of perinatal HPA-axis dysregulation in postpartum depression. Neuropeptides. 2013;47:363–370. doi: 10.1016/j.npep.2013.10.007. http://dx.doi.org/10.1016/j.npep.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Huang H, Xue R, Zhang JY, et al. Anatomical Characterization of Human Fetal Brain Development with Diffusion Tensor Magnetic Resonance Imaging. Journal of Neuroscience. 2009;29(13):4263–73. doi: 10.1523/JNEUROSCI.2769-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunt PA, Hassold TJ. Sex matters in meiosis. Science. 2002;296:2181–2183. doi: 10.1126/science.1071907. [DOI] [PubMed] [Google Scholar]
- 25.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(SICI)1096-9861(19971020)387:2<167::AID-CNE1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 26.Kastin AJ, Miller LH, Sandman CA, Schally AV, Plotnikoff NP. Essays in Neuropharmacology and Neurophysiology. Vol. 1. Wiley Press; New York: 1977. CNS and pituitary effects of hypothalamic peptides and MSH; pp. 139–176. [PubMed] [Google Scholar]
- 27.Kastin AJ, Plotnikoff N, Schally AV, Sandman CA. Endocrine and central nervous system effects of hypothalamic Peptides and MSH. In: Ehrenpreis S, Kopin IJ, editors. Reviews of Neuroscience. Vol. 2. Raven Press; New York: 1976. pp. 111–148. [Google Scholar]
- 28.Kastin AJ, Sandman CA, Stratton LO, Schally AV, Miller LH. Behavioral and electrographic changes in rat and man after MSH. In: DeWeid D, et al., editors. Hormones. Vol. 42. Homeostasis and the Brain Elsevier Press; Amsterdam: 1975. pp. 143–150. [DOI] [PubMed] [Google Scholar]
- 29.Kastin AJ, Sandman CA, Schally AV. Effects of MSH on the brain. In: Riley V, editor. Mechanisms in Pigmentation. Vol. 3. Basal: S Karger; 1976. pp. 228–236. [Google Scholar]
- 30.Kastin AJ, Zadina JE, Coy DH, Schally AV, Sandman CA. Hypothalamic peptides affect behavior after systemic injection. In: Beers RF, Bassett EG, editors. Polypeptide Hormones. Raven Press; New York: 1980. pp. 223–233. [Google Scholar]
- 31.Kostovic I, Judas M, Rados M, Hrabac P. Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex. 2002;12(5):536–44. doi: 10.1093/cercor/12.5.536. [DOI] [PubMed] [Google Scholar]
- 32.Levitt P. Structural and functional maturation of the developing primate brain. J Pediatr. 2003;143:S35–45. doi: 10.1067/S0022-3476(03)00400-1. [DOI] [PubMed] [Google Scholar]
- 33.Lobel M. Conceptualizations, measurement, and effects of prenatal maternal stress on birth outcomes. Journal of Behavioral Medicine. 1994;17:225–272. doi: 10.1007/BF01857952. [DOI] [PubMed] [Google Scholar]
- 34.Maras PM, Baram TZ. Sculpting the hippocampus from within: stress, spines, and CRH. Trends Neurosci. 2012;35(5):315–24. doi: 10.1016/j.tins.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R. A placental clock controlling the length of human pregnancy. Nat Med. 1995;1:460–3. doi: 10.1038/nm0595-460. [DOI] [PubMed] [Google Scholar]
- 36.McMullen S, Langley-Evans SC, Gambling L, Lang C, Swali A, McArdle HJ. A common cause for a common phenotype: The gatekeeper hypothesis in fetal programming. Med Hypotheses. 2012;78(1):88–94. doi: 10.1016/j.mehy.2011.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moldow RL, Kastin AJ, Hollander CS, Coy DH, Sandman CA. Brain beta-endorphin-like immunoreactivity in adult rats given beta-endorphin neonatally. Brain Research Bulletin. 1981;7:683–686. doi: 10.1016/0361-9230(81)90118-0. [DOI] [PubMed] [Google Scholar]
- 38.O’Connor TG, Bergman K, Sarkar P, Glover V. Prenatal cortisol exposure predicts infant cortisol response to acute stress. Dev Psychobiol. 2013;55:145–155. doi: 10.1002/dev.21007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peacock JL, Marston L, Marlow N, Calvert SA, Greenough A. Neonatal and infant outcome in boys and girls born very prematurely. Pediatr Res. 2012;71:305–310. doi: 10.1038/pr.2011.50. [DOI] [PubMed] [Google Scholar]
- 40.Petraglia F, Sutton S, Vale W. Neurotransmitters and peptides modulate the release of immunoreactive corticotropin-releasing factor from cultured human placental cells. Am J Obstet Gynecol. 1989;160(1):247–251. doi: 10.1016/0002-9378(89)90130-0. [DOI] [PubMed] [Google Scholar]
- 41.Sandman CA, Beckwith BE, Kastin AJ. Are learning and attention related to the sequence of amino acids in ACTH/MSH peptides? Peptides. 1980;1:277–280. doi: 10.1016/0196-9781(80)90002-9. [DOI] [PubMed] [Google Scholar]
- 42.Sandman CA, Beckwith W, Gittis MM, Kastin AJ. Melanocyte- stimulating hormone (MSH) and overtraining effects on extradimensional shift (EDS) learning. Physiology and Behavior. 1974;13:163–166. doi: 10.1016/0031-9384(74)90320-5. [DOI] [PubMed] [Google Scholar]
- 43.Sandman CA, Buss C, Head K, Davis EP. Fetal exposure to maternal depressive symptoms is associated with cortical thickness in late childhood. Biological Psychiatry. 2015;77:324–334. doi: 10.1016/j.biopsych.2014.06.025. NIHMS613398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sandman CA, Davis EP. Gestational stress influences cognition and behavior. Future Neurology. 2010;5(5):675–90. [Google Scholar]
- 45.Sandman CA, Davis EP. Neurobehavioral risk is associated with gestational exposure to stress hormones. Expert Review of Endocrinology & Metabolism. 2012;7:445–459. doi: 10.1586/eem.12.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sandman CA, Davis EP, Buss C, Glynn LM. Prenatal programming of human neurological function. International journal of peptides. 2011;2011:837596. doi: 10.1155/2011/837596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sandman CA, Davis EP, Buss C, Glynn LM. Exposure to prenatal psychobiological stress exerts programming influences on the mother and her fetus. Neuroendocrinology. 2012;95:8–21. doi: 10.1159/000327017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sandman CA, Davis EP, Glynn LM. Prescient human fetuses thrive. Psychological Science. 2012;23:93–100. doi: 10.1177/0956797611422073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sandman CA, Glynn L, Schetter CD, et al. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides. 2006;27(6):1457–63. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 50.Sandman CA, Glynn LM, Davis EP. Is there a viability-vulnerability tradeoff? Sex differences in fetal programming Journal of Psychosomatic Research. 2013;75:327–335. doi: 10.1016/j.jpsychores.2013.07.009. http://dx.doi.org/10.1016/j.jpsychores.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sandman CA, Glynn LM, Davis EP. Neurobehavioral consequences of fetal exposure to gestational stress. In: Kisilevsky BS, Reissland N, editors. Advancing research on fetal development. New York, NY: Springer; in press. [Google Scholar]
- 52.Sandman C, Glynn AL, Wadhwa PD, Chicz-DeMet A, Porto M, Garite T. Maternal hypothalamic-pituitary-adrenal disregulation during the third trimester influences human fetal responses. Developmental Neuroscience. 2003;25:41–49. doi: 10.1159/000071467. [DOI] [PubMed] [Google Scholar]
- 53.Sandman CA, Kastin AJ. The influence of fragments of the LPH chain on learning, memory and attention in animals and man. Pharmacology and Therapeutics. 1981;13:39–60. doi: 10.1016/0163-7258(81)90066-8. [DOI] [PubMed] [Google Scholar]
- 54.Sandman CA, Kastin AJ, Miller LH. Central nervous system actions of MSH and related pituitary peptides. In: Besser GM, Martini L, editors. Clinical Neuroendocrinology. Academic Press; New York: 1977. pp. 443–470. [Google Scholar]
- 55.Sandman CA, Kastin AJ, Schally AV. Melanocyte-stimulating hormone and learned appetitive behavior. Experientia. 1969;25:1001–1002. doi: 10.1007/BF01898118. [DOI] [PubMed] [Google Scholar]
- 56.Sandman CA, Kastin AJ, Schally AV. Behavioral inhibition as modified by melanocyte stimulating hormone (MSH) and light-dark conditions. Physiology and Behavior. 1972;1:45–48. doi: 10.1016/0031-9384(71)90012-6. [DOI] [PubMed] [Google Scholar]
- 57.Sandman CA, Kastin AJ, Schally AV. Neuropeptide influences on the central nervous system: A psychobiological perspective. In: Hrdina PS, Singhal RL, editors. Neuroendocrine Regulation and Altered Behavior. Croom Helm Lts; London: 1981. pp. 5–27. [Google Scholar]
- 58.Sandman CA, Miller LH, Kastin AJ, Schally AV. A neuroendocrine influence on attention and memory. Journal of Comparative and Physiological Psychology. 1972;80:54–58. doi: 10.1037/h0032827. [DOI] [PubMed] [Google Scholar]
- 59.Sandman CA, O’Halloran JP. Pro-opiomelanocortin, learning, memeory and attention. In: DeWied D, Gispen WH, van Wimersma Greidanus TjB, editors. Enclyclopedia on Pharmacology and Therapeutics. Pergamon Press; 1986. pp. 397–420. [Google Scholar]
- 60.Sandman CA, Yessian N. Persisting subsensitivity of the striatal dopamine system after fetal exposure to B-endorphin. Life Sciences. 1986;39:1755–1763. doi: 10.1016/0024-3205(86)90095-0. [DOI] [PubMed] [Google Scholar]
- 61.Sandman CA, Wadhwa PD, Chicz-DeMet A, Porto M, Garite TJ. Maternal corticotropin-releasing hormone and habituation in the human fetus. Developmental psychobiology. 1999;34(3):163–73. doi: 10.1002/(sici)1098-2302(199904)34:3<163::aid-dev1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 62.Sandman CA, Wadhwa PD, Hetrick WP, Porto M, Peeke HVS. Human fetal heart rate dishabituation at 32 weeks gestation. Child Development. 1997;68:1031–1040. [PubMed] [Google Scholar]
- 63.Sasaki A, Shinkawa O, Margioris AN, Liotta AS, Sato S, Murakami O, Yoshinaga K. Immunoreactive corticotropin-releasing hormone in human plasma during pregnancy, labor, and delivery. J Clin Endocrinol Metab. 1987;64:224–229. doi: 10.1210/jcem-64-2-224. [DOI] [PubMed] [Google Scholar]
- 64.Sasaki A, Tempst P, Liotta AS, Margioris AN, Hood LE, Kent SB, Krieger DT. Isolation and characterization of a corticotropin-releasing hormone-like peptide from human placenta. J Clin Endocrinol Metab. 1988;67:768–773. doi: 10.1210/jcem-67-4-768. [DOI] [PubMed] [Google Scholar]
- 65.Smith R, Mesiano S, McGrath S. Hormone trajectories leading to human birth. Regulatory peptides. 2002;108(2–3):159–64. doi: 10.1016/s0167-0115(02)00105-2. [DOI] [PubMed] [Google Scholar]
- 66.Smith R, Nicholson RC. Corticotrophin releasing hormone and the timing of birth. Frontiers in bioscience: a journal and virtual library. 2007;12:912–8. doi: 10.2741/2113. [DOI] [PubMed] [Google Scholar]
- 67.Smith R, Smith JI, Shen X, Engel PJ, Bowman ME, McGrath SA, Smith DW. Patterns of plasma corticotropin-releasing hormone, progesterone, estradiol, and estriol change and the onset of human labor. J Clin Endocrinol Metab. 2009;94:2066–2074. doi: 10.1210/jc.2008-2257. [DOI] [PubMed] [Google Scholar]
- 68.Smith R, Mesiano S, McGrath S. Hormone trajectories leading to human birth. Regulatory peptides. 2002;108(2–3):159–64. doi: 10.1016/s0167-0115(02)00105-2. [DOI] [PubMed] [Google Scholar]
- 69.Smith R, Nicholson RC. Corticotrophin releasing hormone and the timing of birth. Frontiers in bioscience: a journal and virtual library. 2007;12:912–8. doi: 10.2741/2113. [DOI] [PubMed] [Google Scholar]
- 70.Stout SA, Espel EV, Sandman CA, Glynn LM, Davis EP. Fetal programming of children’s obesity risk. Psychoneuroendocrinology. 2015;53:29–39. doi: 10.1016/j.psyneuen.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takahashi E, Folkerth RD, Galaburda AM, Grant PE. Emerging Cerebral Connectivity in the Human Fetal Brain: An MR Tractography Study. Cereb Cortex. 2012;22(2):455–64. doi: 10.1093/cercor/bhr126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tyson EK, Smith R, Read M. Evidence that corticotropin-releasing hormone modulates myometrial contractility during human pregnancy. Endocrinology. 2009;150(12):5617–25. doi: 10.1210/en.2009-0348. [DOI] [PubMed] [Google Scholar]
- 73.Van den Bergh BR, Van Calster B, Smits T, Van Huffel S, Lagae L. Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: A prospective study on the fetal origins of depressed mood. Neuropsychopharmacology. 2008;33:536–545. doi: 10.1038/sj.npp.1301450. [DOI] [PubMed] [Google Scholar]
- 74.Walker BB, Sandman CA. Influences of an analog of the neuropeptide ACTH 4-9 on mentally retarded adults. American Journal of Mental Deficiency. 1979;83:346–352. [PubMed] [Google Scholar]
- 75.Wells JC. Natural selection and sex differences in morbidity and mortality in early life. J Theor Biol. 2000;202:65–76. doi: 10.1006/jtbi.1999.1044. [DOI] [PubMed] [Google Scholar]