Abstract
Aim:
The aim was to detect virulence gene associated with the Salmonella serovars isolated from pork and Slaughterhouse environment.
Materials and Methods:
Salmonella isolates (n=37) used in this study were isolated from 270 pork and slaughter house environmental samples collected from the Ahmedabad Municipal Corporation Slaughter House, Ahmedabad, Gujarat, India. Salmonella serovars were isolated and identified as per BAM USFDA method and serotyped at National Salmonella and Escherichia Centre, Central Research Institute, Kasauli (Himachal Pradesh, India). Polymerase chain reaction technique was used for detection of five genes, namely invA, spvR, spvC, fimA and stn among different serovars of Salmonella.
Results:
Out of a total of 270 samples, 37 (13.70%) Salmonella were isolated with two serovars, namely Enteritidis and Typhimurium. All Salmonella serovars produced 284 bp invA gene, 84 bp fimA and 260 bp amplicon for enterotoxin (stn) gene whereas 30 isolates possessed 310 bp spvR gene, but no isolate possessed spvC gene.
Conclusion:
Presence of invA, fimA and stn gene in all isolates shows that they are the specific targets for Salmonella identification and are capable of producing gastroenteric illness to humans, whereas 20 Typhimurium serovars and 10 Enteritidis serovars can able to produce systemic infection.
Keywords: pork, Salmonella, slaughterhouse environment, virulence genes
Introduction
Pork is one of the most widely eaten meats in the world, accounting for about 38% of meat production worldwide, although consumption varies widely from place to place [1]. Most of the pork consumer’s peoples are from tribal areas and pork is mainly consumed in the northeastern states of India. The present production of meat in India is estimated at 6.27 million tons in 2013 [2], which are 2.21% of the world’s meat production. The meat production has increased from 764,000 tons in 1970-71 to 6.27 million tons in 2010 in India, which is 2.21% of the world’s meat production. The contribution of meat from a pig is 5.31% [2]. According to the Food and Agriculture Organization of the United Nations, world’s pork production reached 114.2 million tons in 2012. Asia is the principal region, accounting for almost 60% of world pig meat production, World meat production is anticipated to expand modestly in 2013 to reach 308.3 million tons, an increase of 4.2 million tones or 1.4%, compared with 2012 [3].
Food safety hazards caused by food-borne pathogens such as Salmonella remain a major problem for the food industry. Salmonellosis is an important health problem and a major challenge worldwide having greater significance in developing countries [4]. Pork and pork products are recognized as an important source of human salmonellosis [5]. Salmonella is an important cause of food-borne (alimentary) health problems in humans [6]. The risk of Salmonella might differ between the production systems, caused by components of the husbandry systems affecting disease development and pathogen shedding or differences in the level of resistance to the pathogen [7]. The increased consumption of pork coupled with the high prevalence of enteropathogens in the swine industry suggests a rise in food-borne illness cases which can lead to human food-borne illness and loss of product shelf-life.
The virulence of Salmonella is linked to a combination of chromosomal and plasmid factors. Different genes such as inv, spv, fimA and stn have been identified as major virulence genes responsible for salmonellosis. Salmonella pathogenicity islands (SPIs) are large gene cassettes within the Salmonella chromosome that encode determinants responsible for establishing specific interactions with the host, and are required for bacterial virulence in a given animal like other pathogenicity islands. More than 20 SPIs have been described [8]. The chromosomally located invasion gene invA codes for a protein in the inner membrane of bacteria that is necessary for invasion of epithelial cells [9]. Whereas, an operon (spvRABCD), containing five genes, is present on plasmids commonly associated with some serotypes. One main function of the spv operon is to potentiate the systemic spread of the pathogen [10]. The spvC is virulence-related gene on the plasmid required for survival within host cell [11]. Some studies have provided evidence that the virulence plasmid plays a significant role in human disease [12]. Salmonella induced diarrhea is a complex phenomen on involving several pathogenic mechanisms, including production of enterotoxin. This enterotoxin production is mediated by the stn thus it plays a significant role in causing gastroenteritis by producing enterotoxin [13].
The purpose of this study was to evaluate the potential virulence of Salmonella isolates from eggs and poultry house environment by detecting the presence of the invA, spvR, spvC, fimA and stn virulence genes using the polymerase chain reaction (PCR).
Materials and Methods
Approximately, a total of 270 samples of pork and slaughterhouse environment will be collected from the Ahmedabad Municipal Corporation Slaughterhouse, Ahmedabad, Gujarat under aseptic precautions. The samples were collected in sterilized polyethylene bags and transported to the departmental P.G. Research Laboratory in an icebox for further processing and microbiological analysis. All the samples collected are shown in Table-1.
Table-1.
Number of samples collected from different sources for isolation of Salmonella spp.
| Type of sample | Number of samples |
|---|---|
| Muscles | 30 |
| Tonsils | 30 |
| Rectal swabs | 30 |
| Intestine | 30 |
| Lymph node | 30 |
| Water | 30 |
| Liver | 30 |
| Knife swab | 30 |
| Butchers hand swab | 30 |
| Total | 270 |
Our study used Salmonella isolates (n=37) recovered from pork and Slaughterhouse environmental samples collected from the Ahmedabad Municipal Corporation Slaughterhouse, Ahmedabad, (Gujarat), India. 13 Salmonella enteritidis and 24 Salmonella typhimurium Salmonella serovars were isolated and identified as per BAM USFDA method [14] and serotyped at National Salmonella and Escherichia Centre, Central Research Institute, Kasauli (Himachal Pradesh, India). The DNA of isolates of Salmonella was prepared by boiling method. Approximately, loop full of culture was taken in microcentrifuge in 100 µl of sterilized DNAse and RNAse-free milliQ water (Millipore, USA). Then, vortexed and samples were heated at 95°C for 10 min, cell debris was removed by centrifugation and 3 μl of the supernatant was used as a DNA template in PCR reaction mixture. PCR was performed with four sets of primer pairs specific for the invasion gene invA, spvR gene, spvC gene, fimA gene and stn gene as shown in Table-2.
Table-2.
Primer pairs used for virulence characterization of Salmonella isolates.
| Primer pair target | Primer sequence (5’→3’) | Annealing temp (°C) | Length (bp) | Reference |
|---|---|---|---|---|
| invA | F: GTG AAA TTA TCG CCA CGT TCG GGC AA R: TCA TCG CAC CGT CAA AGG AAC C |
63 | 284 | [15] |
| spvR | F: CAG GTT CCT TCA GTA TCG CA R: TTT GGC CGG AAA TGG TCA GT |
57 | 310 | [16] |
| spvC | F: ACT CCT TGC ACA ACC AAA TGC GGA R: TGT CTT CTG CAT TTC GCC ACC ATC A |
63 | 571 | [17] |
| fimA | F: CCT TTC TCC ATC GTC CTG AA R: TGG TGT TAT CTG CCT GAC CA |
56 | 85 | [18] |
| stn | F: CTT TGG TCG TAA AAT AAG GCG R: TGC CCA AAG CAG AGA GAT TC |
55 | 260 | [20] |
PCR amplifications were performed in a final volume of 25 µl containing DNA template (3 µl), ×2 PCR Mastermix (MBI Fermentas) (12.5 µl), 10 pmol/µl of each primer (MWG-Biotech AG, Germany) (1 µl) and 5.5 µl nuclease-free water. Amplification for invA gene was carried out as described by Kumar et al. [15] with minor modifications. The reaction conditions involved initial denaturation at 94°C for 3 min, followed by 35 cycles of 94°C for 30 s, 63°C for 30 s, and 72°C for 30 s. A final extension of 5 min at 72°C was employed. The amplification for spvR gene was carried out similarly by employing standardized annealing temperature. The fimA gene fragment was amplified at annealing temperature of 56°C and extension for 30 s. The spvC gene fragment was amplified at annealing temperature of 63°C and extension for 1 min. The amplification for stn gene was carried out employing same conditions as invA except annealing at 55°C. Amplification products were separated by electrophoresed on 2% agarose gel stained with 5 µg/ml of ethidium bromide with a 100 bp DNA ladder as molecular weight marker.
Results and Discussion
All 37 Salmonella isolates (13 of which belonged to serovar Enteritidis and 24 belonged to Typhimurium) contained the invasion gene invA, other studies having reported similar results [17,21-24], which was expected since the invA is an invasion gene conserved among Salmonella serotypes.
Similar to invA gene all isolates produced 260 bp DNA fragment specific for stn gene which was in agreement with other authors [25-27]. Thus, all the Salmonella isolates were found highly invasive and enterotoxigenic.
The fimA gene was detected in all 37 isolate produced 85 bp DNA fragment. Which is similar to that of Naravaneni and Jamil [18,19] and this demonstrated that fimA gene has a high degree of sequence conservation among Salmonella serovars. This is very useful in the diagnosis of Salmonella organisms at the genus level.
The spvR gene was detected in 30 isolates belonged to Typhimurium and Enteritidis, which is similar to that of Araque [23] and this shows that the strains have the plasmid borne virulence characters that have ability to cause the systemic infection while spvC was not detected in any isolates, which is in contrast to that of Soto et al. [27] who found presence of spvC in all the isolates (Table-3). Electrophoreses results of invA, spvR, fimA and stn gene are shown in Figures-1-4, respectively.
Table-3.
Virulence genes present in different serovars of Salmonella.
| Serotype | Virulence genes | ||||
|---|---|---|---|---|---|
| invA | spvR | spvC | fimA | stn | |
| Enteritidis (13) | 13 | 10 | 13 | 13 | |
| Typhimurium (24) | 24 | 30 | 24 | 24 | |
Figure-1.
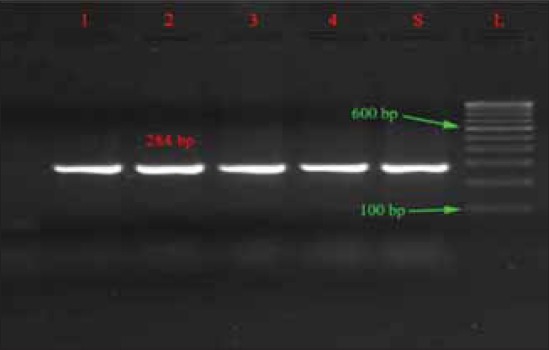
Agarose gel showing polymerase chain reaction amplification products of invA gene (284 bp).
Figure-2.
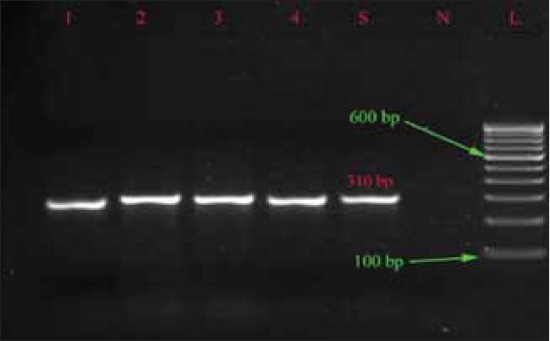
Agarose gel showing polymerase chain reaction amplification products of spvR gene (310 bp).
Figure-3.
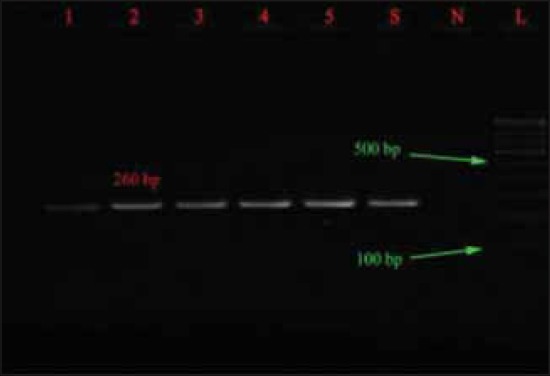
Agarose gel showing polymerase chain reaction amplification products of stn gene (260 bp).
Figure-4.
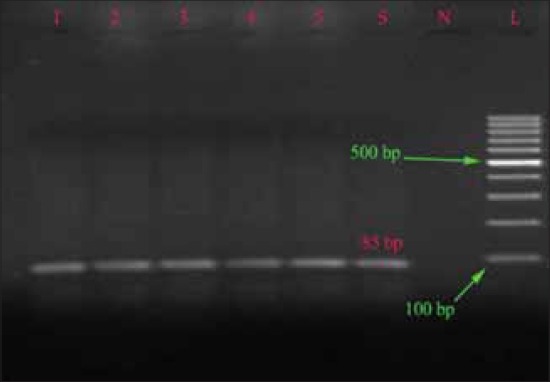
Agarose gel showing polymerase chain reaction amplification products of fimA gene (85 bp).
Conclusion
We can conclude that the invA and stn genes can be used as specific targets for detection of Salmonella as they are conserved among the Salmonella irrespective of serotype and plasmid-borne genes (spv) are not specific targets for the same.
Authors’ Contributions
JBN and MNB planned and designed the study. JHC collected and processed samples, the experiment was conducted, and laboratory work was done by JHC and PPM. All authors participated in the preparation of draft of the manuscript and read and approved the final manuscript.
Acknowledgments
The authors are grateful to the Department of Veterinary Public Health, Anand Agricultural University for providing financial support to the present investigation.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Jeffries W. Mother Earth News-What Good Is a Pig, Homesteading and livestock. 2012. [Accessed on 05-05-2012]. Available from: http://www.motherearthnews.com/homesteading-andlivestock/what-good-is-a-pig-cuts-of-pork-nose-to-tail .
- 2.Agriculture and Processed Food Export Development Agency (APEDA) 2013. [Accessed on 25-08-2013]. Available from: http://www.apeda.gov.in/apedawebsite/SubHead_Products/Sheep_Goat_Meat.htm .
- 3.FAO (Food and Agriculture Organization) Food Outlook. Global Market Analysis. 2013. [Last accessed on 14-12-2014]. pp. 51–54. Available from: http://www.fao.org/giews/
- 4.Wang L, Shi L, Alam M.J, Geng Y, Li L. Specific and rapid detection of foodborne Salmonella by loop-mediated isothermal amplification method. Food Res. Int. 2008;41:69–74. [Google Scholar]
- 5.Smith R.P, Clough H.E, Cook A.J. Analysis of meat juice ELISA results and questionnaire data to investigate farm-level risk factors for Salmonella infection in UK pigs. Zoonoses Public Health. 2010;57(1):39–48. doi: 10.1111/j.1863-2378.2010.01362.x. [DOI] [PubMed] [Google Scholar]
- 6.Hernandeza M, Gomez J, Luqueb I, Herrera S, Maldonadob A, Reguillob L, Astorgab R.J. Salmonella prevalence and characterization in a free-range pig processing plant: Tracking in trucks, lairage, slaughter line and quartering. Int. J. Food Microbiol. 2013;162(1):48–54. doi: 10.1016/j.ijfoodmicro.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 7.Zheng D.M, Bonde M, Sorensen J.T. Associations between the proportion of Salmonella seropositive slaughter pigs and the presence of herd level risk factors for introduction and transmission of Salmonella in 34 Danish organic, outdoor (non-organic) and indoor finishing-pig farms. Livest. Sci. 2007;106:189–199. [Google Scholar]
- 8.Sabbagh S.C, Forest C.G, Lepage C, Leclerc J.M, Daigle F. Uncovering distinctive features in the genomes of Salmonella enterica serovars Typhimurium and typhi. FEMS. Microbiol. Lett. 2010;305(1):1–13. doi: 10.1111/j.1574-6968.2010.01904.x. [DOI] [PubMed] [Google Scholar]
- 9.Darwin K.H, Miller V.L. Molecular basis of the interaction of Salmonella with the intestinal mucosa. Clin. Microbiol. Rev. 1999;12(3):405–428. doi: 10.1128/cmr.12.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heithoff D.M, Shimp W.R, Lau P.W, Badie G, Enioutina E.Y, Daynes K, Barbara R.A, Byrne A, House J, Mahan M.J. Human Salmonella clinical isolates distinct from those of animal origin. Appl. Environ. Microbiol. 2008;10:1757–1766. doi: 10.1128/AEM.02740-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiu C, Ou J.T. Rapid identification of salmonella serovars in feces by specific detection of virulence genes inv Aand spv C, by an enrichment broth culture-multiplex PCR combination assay. J. Clin. Microbiol. 1996;34(10):2619–2622. doi: 10.1128/jcm.34.10.2619-2622.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guiney D, Fang F, Krause M, Libby S. Plasmid mediated virulence genes in non-typhoid Salmonella serovares. FEEMS Microbiol. Lett. 1994;124(1):1–9. doi: 10.1111/j.1574-6968.1994.tb07253.x. [DOI] [PubMed] [Google Scholar]
- 13.Chopra A.K, Houston C.W, Peterson J.W, Prasad R, Mekalanos J.J. Cloning and expression of the Salmonella enterotoxin gene. J. Bacteriol. 1987;169(11):5095–5100. doi: 10.1128/jb.169.11.5095-5100.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrews W.H, Andrew J, Hammack T. Salmonella. In: Bacteriological Analytical Manual. 8th ed. Ch. 5. Gaithersburg, MD: Revision A. U.S. Food and Drug Administration, AOAC International; 2011. [Google Scholar]
- 15.Kumar K, Saklaini A.C, Singh S, Singh V.P. Evaluation of specificity for inv A gene PCR for detection of Salmonella spp. [November 07-09-2008];Proceeding of VIIth Annual Conference of Indian Association of Veterinary Public Health Specialists (IAVPHS) 2008 [Google Scholar]
- 16.Pasmans F, Van Immerseel F, Heyndrickx M, Godard C, Wildemauwe C, Ducatelle R, Haesebrouck F. Host adaptation of pigeon isolates of Salmonella serovar Typhimurium var. Copenhagen PT99 is associated with macrophage cytotoxicity. Infect. Immunol. 2003;71(10):6068–6074. doi: 10.1128/IAI.71.10.6068-6074.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliveira S.D, Rodenbusch C.R, Michae G.B, Cardoso M.I, Canal C.W, Brandelli A. Detection of virulence genes in Salmonella Enteritidis isolated from different sources. Braz. J. Microbiol. 2003;34(1):123–124. [Google Scholar]
- 18.Naravaneni R, Jamil K. Rapid detection of food-borne pathogens by using molecular techniques. J. Med. Microbiol. 2005;54:51–54. doi: 10.1099/jmm.0.45687-0. [DOI] [PubMed] [Google Scholar]
- 19.Alphons J.A.M.V, Jaap E.V.D. Distribution of “classic” virulence factors among Salmonella spp. FEMS. Immunol. Med. Microbiol. 2005;44:251–259. doi: 10.1016/j.femsim.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Makino S, Kurazono H, Chongsanguam M, Hayashi H, Cheun H, Suzuki S, Shirahata T. Establishment of the PCR system specific to Salmonella spp. and its application for the inspection of food and fecal samples. J. Vet. Med. Sci. 1999;61(11):1245–1247. doi: 10.1292/jvms.61.1245. [DOI] [PubMed] [Google Scholar]
- 21.Swamy S.C, Barnhart H.M, Lee M.D, Dreesen D.W. Virulence determinants inv Aand spv Cin salmonellae isolated from poultry products, wastewater, and human sources. Appl. Environ. Microbiol. 1996;62(10):3768–3771. doi: 10.1128/aem.62.10.3768-3771.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhatta D.R, Bangtrakulnonth A, Tishyadhigama P, Saroj S.D, Bandekar J.R, Hendriksen R.S, Kapadnis B.P. Serotyping, PCR, phage-typing and antibiotic sensitivity testing of Salmonella serovars isolated from urban drinking water supply systems of Nepal. Lett. Appl. Microbiol. 2007;44:588–594. doi: 10.1111/j.1472-765X.2007.02133.x. [DOI] [PubMed] [Google Scholar]
- 23.Araque M. Nontyphoid Salmonella gastroenteritis in pediatric patients from urban areas in the city of Mérida, Venezuela. J. Infect. Dev. Control. 2009;3(1):28–34. doi: 10.3855/jidc.102. [DOI] [PubMed] [Google Scholar]
- 24.Amini K, Salehi T.Z, Nikbakht G, Ranjbar R, Amini J, Ashrafganjooei S.B. Molecular detection of inv A and spv virulence genes in Salmonella enteritidis isolated from human and animals in Iran. Afr. J. Microbiol. Res. 2010;4(21):2202–2210. [Google Scholar]
- 25.Dinjus U, Hanvel I, Muller W, Bauerfeind R, Helmuth R. Detection of the induction of Salmonella enterotoxin gene expression by contact with epithelial cells with RT-PCR. FEMS Microbiol. Lett. 1997;146(2):175–178. doi: 10.1111/j.1574-6968.1997.tb10189.x. [DOI] [PubMed] [Google Scholar]
- 26.Rahman H. Prevalence of enterotoxin gene (stn) among different serovars of Salmonella. Indian J. Med. Res. 1999;110:43–46. [PubMed] [Google Scholar]
- 27.Soto S.M, Rodriguez I, Rodicio M.R, Vila J, Mendoza M.C. Detection of virulence determinants in clinical strains of Salmonella enterica serovar Enteritidis and mapping on macrorestriction profiles. J. Med. Microbiol. 2006;55:365–373. doi: 10.1099/jmm.0.46257-0. [DOI] [PubMed] [Google Scholar]


