Abstract
n-3 PUFAs are essential for neuronal development and brain function. However, the molecular mechanisms underlying their biological effects remain unclear. Here we examined the mechanistic action of docosahexaenoic acid (DHA), the most abundant n-3 polyunsaturated fatty acids in the brain. We found that DHA treatment of cortical neurons resulted in enhanced axon outgrowth that was due to increased axon elongation rates. DHA-mediated axon outgrowth was accompanied by the translational up-regulation of Tau and collapsin response mediator protein 2 (CRMP2), two important axon-related proteins, and the activation of Akt and p70 S6 kinase. Consistent with these findings, rapamycin, a potent inhibitor of mammalian target of rapamycin (mTOR), prevented DHA-mediated axon outgrowth and up-regulation of Tau and CRMP2. In addition, DHA-dependent activation of the Akt-mTOR-S6K pathway enhanced 5′-terminal oligopyrimidine tract-dependent translation of Tau and CRMP2. Therefore, our results revealed an important role for the Akt-mTOR-S6K pathway in DHA-mediated neuronal development.
Keywords: neurite outgrowth, neurodevelopment, neuron, PUFA, S6 kinase, translation control
Introduction
Docosahexaenoic acid (DHA,3 22:6, n-3) is an n-3 long-chain PUFA that has recently attracted attention because of its various beneficial effects on human health. DHA is particularly abundant in the central nervous system and retina and is a major n-3 lipid component in the lipid bilayer of neural cell membranes (1). DHA is essential for brain development, and DHA or its precursors, such as α-linolenic acid (18:3, n-3) must be obtained from the diet or from the maternal supply during pregnancy because α-linolenic acid cannot be synthesized de novo by animal tissues (2). DHA accumulation in neural tissue is increased greatly during the perinatal period, when the frontal lobe and hippocampus undergo intensive growth and neurogenesis, neuritogenesis, and synaptogenesis occur actively (3, 4). Therefore, an adequate supply of DHA is required for proper brain development (5). It has also been reported that n-3 PUFA deficiency in animals causes many behavioral and cognitive defects (6–8) and that DHA intake is associated significantly with visual and neural development in human infants (9, 10). The cerebral cortex and hippocampus play important roles in various higher-order brain functions such as learning, memory, and cognition, and the development of these areas in the brain is influenced by n-3 PUFA deficiency (11, 12).
Various studies have shown that DHA and/or its metabolites function not only as structural components of cellular membranes, where they affect the fluidity and sensitivity of membrane-associated proteins (receptors, ion channels, etc.), but also as extra- and intracellular signaling molecules (13, 14). However, the precise molecular mechanisms underlying the wide variety of beneficial effects of PUFAs have not been well documented.
Cultures of dissociated cortical neurons can be used to analyze the molecular mechanisms underlying neuronal development. After plating, the cortical neurons first form lamellipodia and small protrusions (stage 1) and then, within 6–12 h, extend several immature neurites (stage 2). One of the neurites elongates rapidly and forms the axon, whereas the others become dendrites (stage 3) (15). The postmitotic neurons then undergo multiple processes, including neurite outgrowth, cell polarity establishment, and axon and dendrite maturation. Accumulating evidence indicates that microtubule polymerization and stabilization play positive roles in axon and dendrite outgrowth and that microtubule-regulatory proteins such as Tau, MAP2, and CRMP2 are critically involved in this process (16).
Although DHA has been demonstrated previously to promote neurite outgrowth in primary neurons (17, 18), the underlying molecular mechanisms have remained unclear. Here we showed that the treatment of cortical neurons with low concentrations of DHA promoted axon outgrowth. This is the first report to demonstrate that Akt-mTOR-p70 S6 kinase-dependent translational control plays an important role in DHA-induced axon outgrowth.
Experimental Procedures
Materials
Docosahexaenoic acid (Enzo), vitamin E (DL-α-tocopherol acetate, Sigma), and rapamycin (Merck) were purchased. The following primary antibodies were purchased and used in this study. Anti-Tau1 (mouse, catalog no. MAB3420) and anti-microtubule-associated protein 2 (MAP2, rabbit, catalog no. AB5622) were from Chemicon. Anti-β-actin (mouse, catalog no. A5441), α-tubulin (mouse, catalog no. T9026), and anti-FLAG (rabbit, catalog no. F7425) were from Sigma. Anti-p70S6 kinase (rabbit, catalog no. Sc-230) and anti-Akt1 (goat, catalog no. Sc-1618) were from Santa Cruz Biotechnology. Anti-CRMP2 (rabbit, catalog no. 9393), anti-phospho-Akt (Ser-473, rabbit, catalog no. 4060), anti-phospho-Akt (Thr-308, rabbit, catalog no. 4056), anti-phospho-p70S6 kinase (Thr-389, rabbit, catalog no. 9205), and anti-phospho-p70S6 kinase (Thr-421/Ser-424, rabbit, catalog no. 9204) were from Cell Signaling Technology. Anti-neuronal class III β-tubulin (Tuj1, mouse, catalog no. MMS-435P) was from Covance, and anti-GAPDH (mouse, catalog no. M171-3) was from MBL. All Alexa Fluor-conjugated secondary antibodies were purchased from Life Technologies (goat, catalog nos. A11029 and A11036). HRP-conjugated anti-rabbit IgG (donkey, catalog no. NA934) and HRP-conjugated anti-mouse IgG (sheep, catalog no. NA931) were from GE Healthcare, and HRP-conjugated anti-Goat IgG (donkey, catalog no. sc-2020) was from Santa Cruz Biotechnology.
Cell Culture and Transfection
Cortical neurons were prepared from Wistar rat cortices at embryonic day 18.5. The dispersed neurons were plated on poly-l-lysine-coated coverslips and cultured in glia-conditioned minimum essential medium containing 1 mm pyruvate, 0.6% (w/v) d-glucose, and 2% B27 supplement (Life Technologies) at a density of 0.5 × 104 cells/cm2 (for endogenous Tau and MAP2 staining and time-lapse observation of axon elongation) or 1.0 × 104 cells/cm2 (for other assays). For DHA supplementation, DHA was dissolved in ethanol containing vitamin E as an antioxidant, diluted 1:500 in minimum essential medium containing 10% fatty acid-free BSA (Sigma, catalog no. A0281), and added to the culture medium (final concentrations: 1 μm, DHA; 40 μm, vitamin E). For control cells, the same procedure was performed without DHA.
Cortical neurons were transfected by calcium phosphate precipitation as described previously (19). To obtain higher transfection efficiencies for the exogenous S6K expression and reporter assay, nucleofection was performed using a rat neuron nucleofector kit (Lonza). All procedures involving animals and their care were approved by the Animal Care Committees of Iwate Medical University and were carried out according to their guidelines.
Plasmids
EGFP (enhanced GFP), CA-S6K (ΔCT(T389)), DN-S6K (T229A), Tau, and CRMP2 were expressed in the pCAGGS expression vector and have been described previously (19). The reporter plasmids pGL4.14-Tau (WT 5′-TOP), pGL4.14-Tau (mutated 5′-TOP), pGL4.14-CRMP2 (WT 5′-TOP), and pGL4.14-CRMP2 (mutated 5′-TOP) have been described previously (19). The coding region of β-galactosidase was amplified by PCR and cloned into pGL3 (pGL3-β-gal).
Luciferase Reporter Assay
The above-mentioned reporter plasmids were transfected into cortical neurons by nucleofection along with pGL3-β-gal, which was used to normalize the transfection efficiency. The transfected neurons were treated with 1 μm DHA or left untreated and lysed with passive lysis buffer (Promega) after 3 days of in vitro culture (3 DIV). Luciferase and β-galactosidase activities were measured using the ONE-Glo luciferase assay system (Promega) and the luminescence β-galactosidase detection kit II (Clontech), respectively.
Quantitative Real-time RT-PCR
The neurons were lysed using the RealTime Ready cell lysis kit (Roche) and reverse-transcribed with the Transcriptor Universal cDNA Master (Roche). The cDNA was amplified with gene-specific primer pairs using the SYBR FAST quantitative PCR kit (Kapa Biosystems). The primer sequences used in this study were as follows: GAPDH forward, CTCCCTTTCTTCCACCTTTGATG;GAPDH reverse, CCACCACCCTGTTGCTGTAG; tau forward, GATTGGCTCCTTGGATAACATCAC; tau reverse, GCGGACACTTCATCGGCTAA; CRMP2 forward, GCACAAGGGCATCCAGGAG; CRMP2 reverse, AACCGATCTTTGAAAGCCATGTAC; MAP2 forward, CTTGCCTATGTCTTGCCTTGATTC; MAP2 reverse, GCTTGCTCTGTCTTATCTTCTTCCT; Tuj1 forward, CGTCTCTAGCCGAGTGAAGTC; and Tuj1 reverse, GTAGTAGACACTGATGCGTTCC. Gene expression measured by real-time PCR was normalized to the GAPDH expression in each experiment. For evaluation of the reporter assay, the following primer pairs were used: β-galactosidase forward, ACTATCCCGACCGCCTTACT; β-galactosidase reverse, TAGCGGCTGATGTTGAACTG; luciferase forward, GCTCAGCAAGGAGGTAGGTG; andluciferase reverse, TCTTACCGGTGTCCAAGTCC.
Imaging
Neurons cultured on coverslips were fixed using 4% paraformaldehyde and then incubated with primary antibody (1:200 dilution), followed by the appropriate Alexa Fluor-conjugated secondary antibody (1:200 dilution). The stained samples were observed under a Biorevo BZ-9000 (Keyence) fluorescence microscope with a ×10 (numerical aperture 0.45) dry lens or ×60 (numerical aperture 1.4) oil immersion lens or under an Axiovert 200M fluorescence microscope (Carl Zeiss) with a ×63 (numerical aperture 1.4) oil immersion lens. For time-lapse imaging, neurons were observed under an Axiovert 200M microscope with a ×20 (numerical aperture 0.8) lens. The images were captured over a 180-min time span with intervals of 10 min. The captured images were contrast-enhanced using Adobe Photoshop software.
Western Blotting Analysis
Western blotting analysis was performed as described previously (20). The detected band intensities were quantified using ImageJ software.
Statistical Analysis
All values represent mean ± S.E. All experiments were performed with at least three independent replicates. We used Excel Statistics add-in software (Social Survey Research Information Co., Ltd.) for statistical analyses. Statistical differences between pairs of values were analyzed by Mann-Whitney U test or Student's t test depending on the sample size. One-way analysis of variance (ANOVA) with Tukey-Kramer post hoc test was used for group comparisons. p < 0.05 was considered significant.
Results
DHA Treatment Promotes Axon Outgrowth in Cortical Neurons
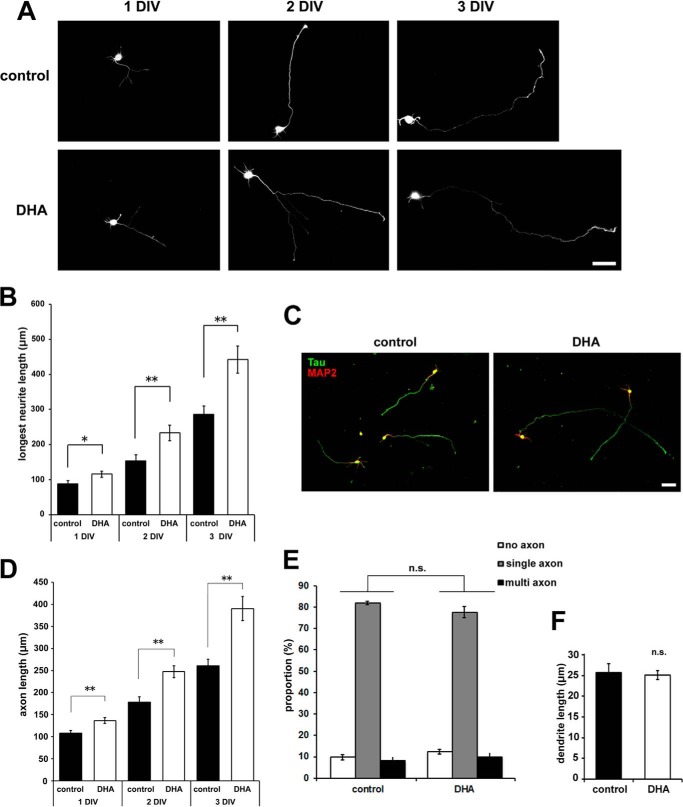
Cultured cortical neurons prepared from rat embryos were plated and treated with 1 μm DHA on the same day. Because DHA was dissolved in ethanol supplemented with vitamin E, the control samples were treated with the same mixture lacking DHA. To visualize the cell morphology, the neurons were transfected with EGFP before plating. We found that cortical neurons treated with DHA exhibited a 1.52-fold increase in neurite outgrowth compared with controls at 3 DIV (Fig. 1, A and B). The DHA-mediated effect was observed at 1 DIV and became more apparent with increased treatment time. The longest neurites were identified as axons at 3 DIV by immunostaining with antibodies against Tau, an axon marker, and MAP2, a dendrite marker (Fig. 1C). Although axon length was increased significantly by DHA treatment (Fig. 1D), axon numbers were not affected significantly (Fig. 1E). In addition, there was no significant effect of DHA on dendrite outgrowth, at leastuntil 3 DIV (Fig. 1F).
FIGURE 1.
DHA treatment promotes axon outgrowth in cortical neurons. A, EGFP-transfected cortical neurons were treated with or without 1 μm DHA for the indicated number of days. Representative images are shown. Scale bar = 50 μm. B, measurement of the longest neurite (axon) length at 1, 2, and 3 DIV (n = 3). 1 DIV: control, 41 neurons; DHA, 41 neurons. 2 DIV: control 66 neurons; DHA, 51 neurons. 3 DIV: control, 61 neurons; DHA, 52 neurons. C, cortical neurons were treated with or without 1 μm DHA fixed at 3 DIV, and then stained with anti-Tau1 (green) and anti-MAP2 (red) antibodies. Representative images are shown. Scale bar = 50 μm. D, measurement of Tau-positive axon length at 1, 2, and 3 DIV (n = 4). 1 DIV: control, 75 neurons; DHA, 67 neurons. 2 DIV: control, 68 neurons; DHA, 73 neurons. 3 DIV: control, 60 neurons; DHA, 60 neurons. E, neurons were classified according to their polarity: no axon, single axon, or multiple axons (n = 4; control, 227 neurons; DHA, 213 neurons). F, measurement of MAP2-positive dendrite length at 3 DIV (n = 4; control, 25 neurons; DHA, 51 neurons). Error bars indicate mean ± S.E., Student's t test. *, p < 0.05; **, p < 0.01; n.s., not significant.
DHA Treatment Enhances Axon Elongation Rates
The DHA-induced axon outgrowth could be a result of various cellular processes, including neuronal differentiation, polarity determination, and/or increased axon elongation rates. We examined the developmental process of axon outgrowth by time-lapse imaging. Our observations revealed that DHA treatment enhanced the axon elongation rate 1.41-fold compared with the control at 3 DIV (Fig. 2, A–C).
FIGURE 2.
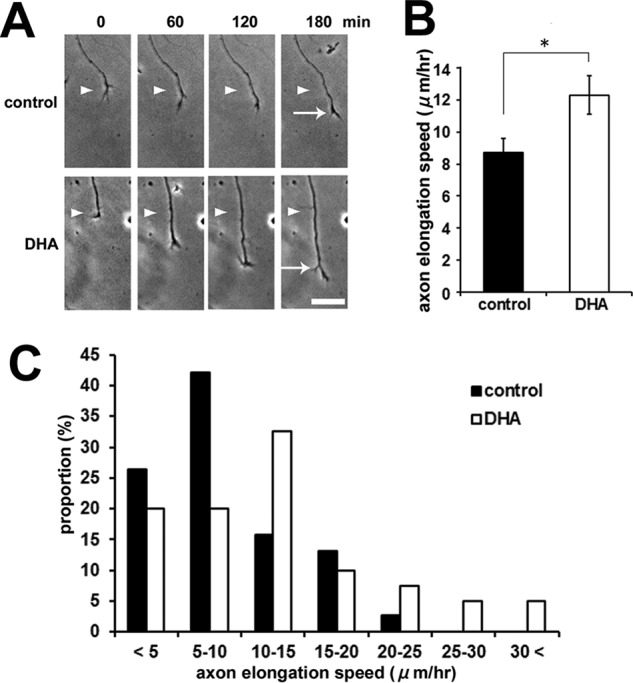
DHA treatment enhances the axon elongation rate. A, DHA enhances the axon elongation rate in cortical neurons. Representative time-lapse images are shown. The neurons were imaged at 0, 60, 120, and 180 min. The positions of the growth cone of the axon at 0 min (arrowhead) and at 180 min (arrow) are marked. Scale bar = 25 μm. B, quantification of axon elongation rates on the basis of the time lapse images shown in A. Error bars indicate mean ± S.E., Student's t test. *, p < 0.05. C, distribution of axon elongation speed. The sum of three independent experiments is shown (n = 3; control, 38 neurons; DHA, 40 neurons).
DHA Treatment Activates Akt and S6K Activities and Up-regulates Tau and CRMP2 Expression
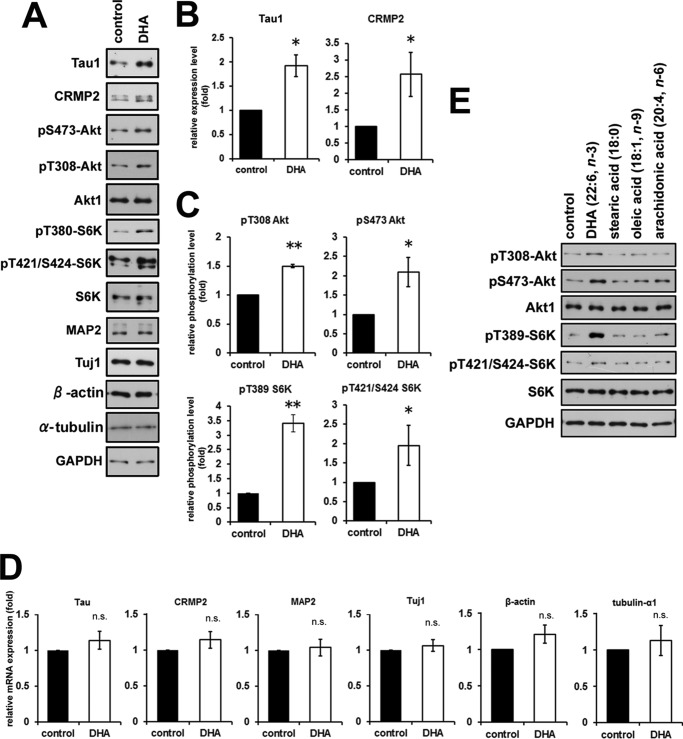
To elucidate the molecular mechanisms underlying DHA-induced axon outgrowth, we analyzed the expression of several neuronal proteins whose functions are known to be related to neurite outgrowth and polarity determination. Among them, we found that the expression levels of Tau and CRMP2 were up-regulated markedly in DHA-treated neurons (Fig. 3, A and B). In contrast, the expression levels of Tau and CRMP2 mRNAs were not up-regulated significantly in response to DHA (Fig. 3D). These results suggested that the increased expression of Tau and CRMP2 was regulated at the translational level. We have reported previously that the expression of Tau and CRMP2 is regulated translationally by the p70 S6 kinase (S6K)-dependent pathway during neuronal polarity determination (19). The Akt-mTOR-S6K signaling pathway is a critical regulator of translational activity and plays important roles in regulating neuronal polarity and neurite elongation (19, 21–23). Here we found that S6K phosphorylation at Thr-381 and Thr-421/Ser-424, which correlates with S6K activation, was increased in DHA-treated neurons at 3 DIV (Fig. 3, A and C). Similarly, Akt phosphorylation at Thr-308 and Ser-473 was also increased in DHA-treated neurons at 3 DIV (Fig. 3, A and C). The up-regulated phosphorylation levels of S6K and Akt were evoked by DHA treatment only. It was not affected significantly by treatment with other fatty acids, such as stearic acid (18:0, saturated), oleic acid (18:1, monounsaturated, n-9), and arachidonic acid (20:4, polyunsaturated, n-6) at the same concentration of DHA (1 μm) (Fig. 3E). The promoting effect on axon outgrowth was also evoked by DHA only but not by other fatty acids (data not shown). These results strongly suggest that DHA treatment of cortical neurons activates the Akt-mTOR-S6K signaling pathway, which, in turn, up-regulates the expression of translational target proteins.
FIGURE 3.
DHA treatment enhances Akt and S6K activities and up-regulates Tau and CRMP2 expression. A, Western blotting analysis of cell lysates prepared from neurons treated with or without DHA for 3 DIV. B, quantified values of expression levels of Tau and CRMP2. The values were normalized to GAPDH expression (Tau, n = 5; CRMP2, n = 6). C, quantified values of phosphorylation levels of Ser(P)-473 Akt, Thr(P)-308 Akt, Thr(P)-389 S6K, and Thr(P)-421/Ser-424 S6K. The values were normalized to each expression level of the total protein, respectively (n > 3). D, mRNA expression quantified by real-time quantitative PCR. The values were normalized to GAPDH expression (n = 5). E, Western blotting analysis of cell lysates prepared from neurons treated with 1 μm DHA, stearic acid, oleic acid, or arachidonic acid for 3 DIV. Error bars indicate mean ± S.E., Mann-Whitney U test. *, p < 0.05; **, p < 0.01; n.s., not significant.
Rapamycin Abrogates the Effect of DHA on Axon Outgrowth
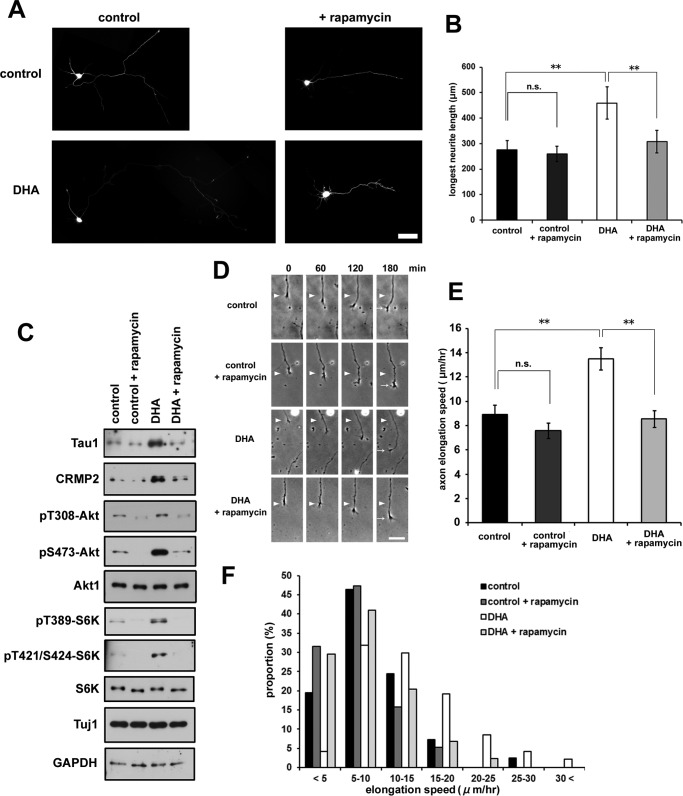
We next examined the effect of rapamycin, a small molecule inhibitor of the mTOR signaling pathway. Rapamycin treatment of cortical neurons clearly abrogated the DHA-induced enhancement of axon outgrowth (Fig. 4, A and B). DHA-induced phosphorylation of Akt and S6K was also prevented by rapamycin treatment (Fig. 4C), resulting in reduced protein expression of Tau and CRMP2 (Fig. 4C). Furthermore, DHA-mediated enhancement of the axon elongation rate was also inhibited by rapamycin treatment (Fig. 4, D–F).
FIGURE 4.
Rapamycin abrogates DHA-induced axon outgrowth. A, EGFP-transfected neurons were treated with or without 1 μm DHA in the presence of rapamycin and fixed at 3 DIV. Representative images are shown. Scale bar = 50 μm. B, longest neurite (axon) measurement (n = 4; control, 55 neurons; control +rapamycin, 72 neurons; DHA, 53 neurons; DHA + rapamycin, 48 neurons). Error bars indicate mean ± S.E., one-way ANOVA with Tukey-Kramer post hoc test. *, p < 0.05; **, p < 0.01; n.s., not significant. C, Western blotting analysis of cell lysates prepared from neurons treated with or without DHA in the presence of rapamycin for 3 DIV. D, rapamycin abrogated the enhanced elongation rate of axons by DHA. Representative time-lapse images are shown. The neurons were imaged at 0, 60, 120, and 180 min. The positions of the growth cone of the axon at 0 min (arrowheads) and at 180 min (arrows) are marked. Scale bar = 25 μm. E, quantification of axon elongation rates on the basis of the time-lapse images shown in D. Error bars indicate mean ± S.E. One-way ANOVA with Tukey-Kramer post hoc test. *, p < 0.05; **, p < 0.01. F, distribution of axon elongation speed. The sum of five independent experiments is shown (n = control, 41 neurons; control + rapamycin, 38 neurons; DHA, 47 neurons; DHA + rapamycin, 44 neurons).
S6 Kinase Is Critical for DHA-dependent Enhancement of Axon Outgrowth
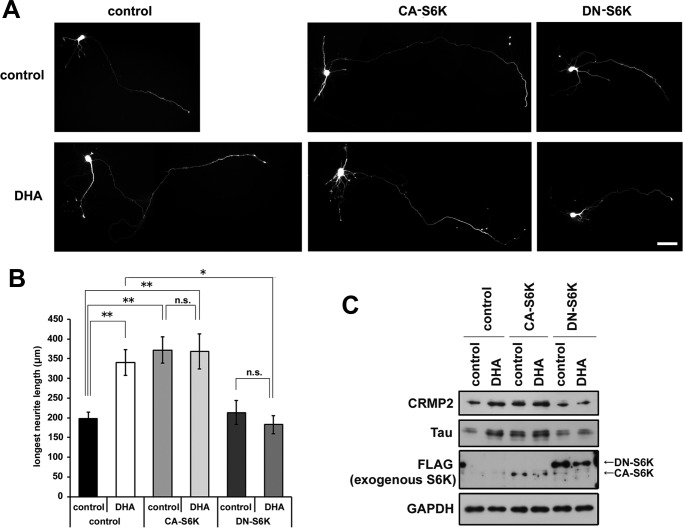
S6K phosphorylates ribosomal protein S6 at several residues, including Ser-235, and activates protein synthesis (21). We examined the contribution of S6K activity to DHA-induced axon outgrowth. A constitutively active form of S6K (CA-S6K) and an inactive dominant-negative form of S6K (DN-S6K) were co-transfected with an EGFP-expressing plasmid into cortical neurons before plating. CA-S6K expression enhanced axon outgrowth irrespective of DHA treatment (Fig. 5, A and B). CA-S6K-transfected neurons also exhibited an increase in axon number (data not shown) in contrast to DHA-treated neurons (Fig. 1E). Notably, DN-S6K expression abrogated DHA-mediated enhancement of axon outgrowth (Fig. 5, A and B) but did not inhibit the basal axon outgrowth observed under control conditions. Furthermore, we examined the effect of exogenous expression of S6K on Tau and CRMP2 expression. Cortical neurons were transfected with CA-S6K or DN-S6K with a high transfection efficiency (up to ∼50%) by nucleofection and treated with or without DHA. Similar to the result of neurite length with DHA, CA-S6K expression up-regulated Tau and CRMP2 without DHA treatment, and DN-S6K expression suppressed DHA-dependent up-regulation of Tau and CRMP2 (Fig. 5C). Combined with results showing that CA-S6K and DN-S6K did not significantly affect the mRNA expression levels of Tau and CRMP2 by real-time quantitative PCR (data not shown), our data indicate that DHA-dependent activation of Akt-S6K translational control is responsible for DHA-induced axonal elongation.
FIGURE 5.
S6 kinase is critical for DHA-dependent enhanced axon outgrowth. A, cortical neurons were transfected with plasmids expressing CA-S6K or DN-S6K or with empty plasmid in combination with EGFP, cultured with or without DHA, and fixed at 3 DIV. Representative images are shown. Scale bar = 50 μm. B, the longest neurite (axon) measurement (n = 4; control-control, 41 neurons; control + DHA, 48 neurons; CA-S6K-control, 29 neurons; CA-S6K + DHA, 32 neurons; DN-S6K-control, 28 neurons; DN-S6K + DHA, 25 neurons). Error bars indicate mean ± S.E., one-way ANOVA with Tukey-Kramer post hoc test. *, p < 0.05; **, p < 0.01; n.s., not significant. C, Western blotting analysis of cell lysates prepared from CA-S6K or DN-S6K-transfected neurons treated with or without DHA for 3 DIV.
Up-regulation of Tau and CRMP2 Enhances Axon Outgrowth
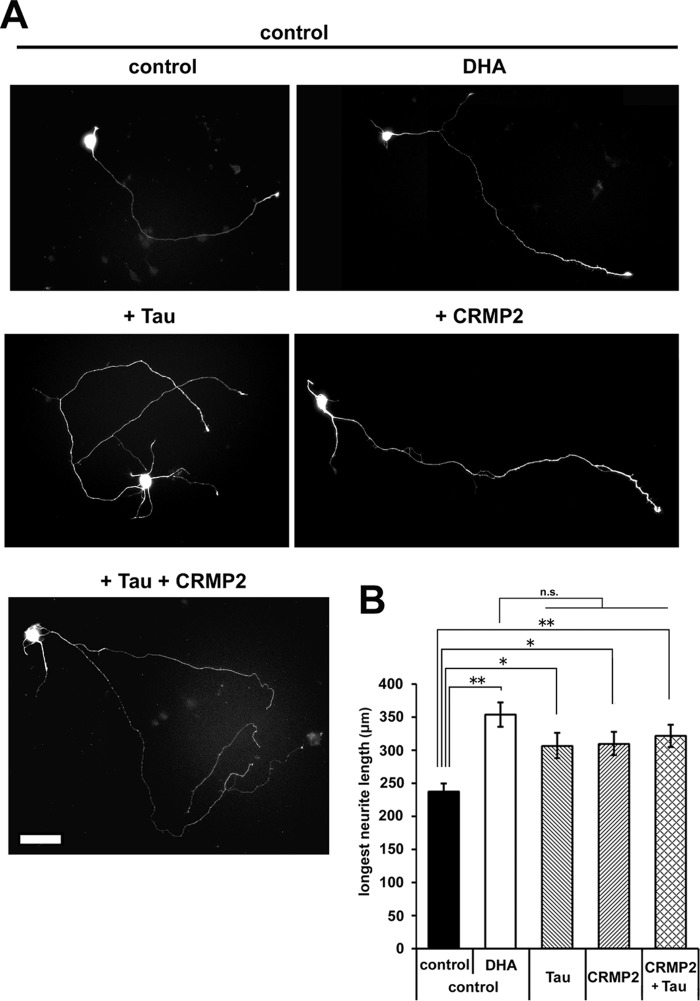
To determine the contribution of up-regulated Tau and CRMP2 to axon elongation, cortical neurons were transfected with exogenous Tau and/or CRMP2. Either or both Tau and CRMP2 expression enhanced axon elongation (Fig. 6, A and B). Tau and CRMP2, particularly Tau, promoted increased axon numbers as well as axon elongation (data not shown), whereas DHA did not significantly promote the formation of multiple axons (Fig. 1E).
FIGURE 6.
Up-regulation of Tau and CRMP2 enhances axon outgrowth. A, cortical neurons were transfected with plasmids expressing Tau and/or CRMP2 or with empty plasmid in combination with EGFP, cultured with or without DHA, and fixed at 3 DIV. Representative images are shown. Scale bar = 50 μm. B, the longest neurite (axon) measurement (n = 5; control-control, 127 neurons; control + DHA, 93 neurons; Tau, 107 neurons; CRMP2, 125 neurons; Tau + CRMP2, 140 neurons). Error bars indicate mean ± S.E., one-way ANOVA with Tukey-Kramer post hoc test. *, p < 0.05; **, p < 0.01; n.s., not significant.
DHA Promotes the 5′-TOP-dependent Translational Up-regulation of Tau and CRMP2
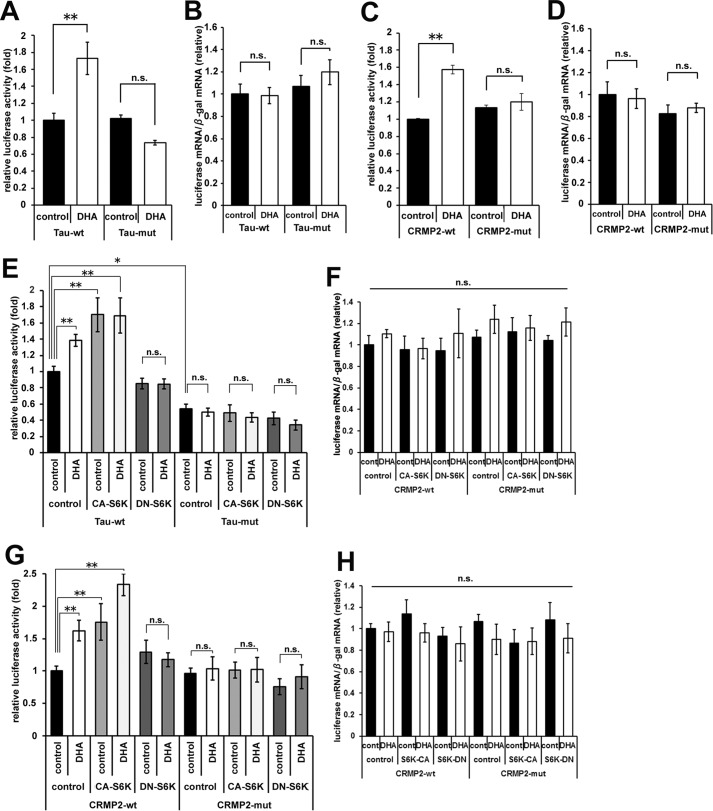
The activation of S6K induces the translational activation of a series of 5′-TOP-containing mRNAs. Both Tau and CRMP2 mRNAs have been identified as targets of 5′-TOP-dependent translational regulation (19). We therefore examined the translational regulation of Tau and CRMP2 by using 5′ UTR-containing luciferase reporter constructs (19). DHA significantly enhanced the luciferase activity of the wild-type 5′-TOP-containing Tau reporter but not that of a Tau reporter with a mutated 5′-TOP (Fig. 7A). The transcription level of luciferase mRNA was not altered in response to DHA (Fig. 7B). Similar results were obtained using CRMP2 reporter constructs (Fig. 7, C and D). Furthermore, it was confirmed that exogenous CA-S6K expression up-regulated both Tau and CRMP2 reporter activities without DHA and that DN-S6K expression suppressed the DHA-induced reporter activities (Fig. 7, E–H). These results indicated that the DHA treatment of cortical neurons resulted in the 5′-TOP-dependent translational up-regulation of Tau and CRMP2.
FIGURE 7.
DHA promotes the 5′-TOP-dependent translational up-regulation of Tau and CRMP2. A, luciferase reporter assays were performed using pGL4.14-Tau (WT 5′-TOP) or pGL4.14-Tau (mutated (mut) 5′-TOP) vectors. The transfected neurons were treated with or without DHA for 1 day (2–3 DIV). Luciferase activities were measured at 3 DIV and normalized to β-gal activity (n = 3). B, the expression levels of luciferase and β-gal mRNAs were detected by real-time quantitative PCR. Luciferase mRNA expression was normalized to β-gal mRNA. C, luciferase reporter assays were performed using pGL4.14-CRMP2 (WT 5′-TOP) or pGL4.14-CRMP2 (mutated 5′-TOP) vectors. The transfected neurons were processed as in A (n = 3). D, expression of luciferase was normalized to β-gal mRNA as in B. E, the effect of S6K activity on luciferase reporter assays using Tau reporter vectors. The neurons were transfected with CA-S6K, DN-S6K, or empty plasmid with reporter plasmids and treated with or without DHA for 1 day (2–3 DIV). The transfected neurons were processed as in A (n = 4). F, expression of luciferase was normalized to β-gal mRNA as in B. G, the effect of S6K activity on luciferase reporter assays using CRMP2 reporter vectors. The neurons were processed as in E (n = 4). H, expression of luciferase was normalized to β-gal mRNA as in B. Error bars indicate mean ± S.E., one-way ANOVA with Tukey-Kramer post hoc test. *, p < 0.05; **, p < 0.01; n.s., not significant.
Discussion
Recent studies have reported that DHA has various beneficial effects for patients with cardiovascular diseases and those with psychiatric, neurodevelopmental, and neurodegenerative disorders (24). Although there is controversy regarding the clinical significance of DHA supplementation, n-3 PUFAs are thought to be important for the development and maintenance of learning and memory functions throughout life. However, the molecular mechanisms underlying the effects of DHA on neuronal function have been unclear.
The results of this study revealed that DHA promoted axon but not dendrite outgrowth by increasing the axon elongation rate during the early stages of neuronal development (Figs. 1 and 2). We also found that DHA, an n-3 PUFA, specifically activated the Akt-mTOR-S6K signaling pathway, resulting in increased translational activity and up-regulation of Tau and CRMP2 expression (Figs. 3–7). This is the first report to demonstrate the importance of translational control in DHA-mediated axon outgrowth.
Translational control is thought to be important for the spatiotemporal expression of neuronal target genes. Because neurons have polarized and extended cell structures, rapid protein synthesis in response to extracellular stimuli is often required at sites that are distal to the cell body (25). Tau is a major microtubule-associated protein that is predominantly expressed in axons and promotes microtubule assembly and stability in growing axons (26). CRMP2 is a microtubule-binding protein that regulates microtubule dynamics and promotes the differentiation of axons from immature neurites (27, 28). Notably, the forced expression of either Tau or CRMP2 promotes axon outgrowth in cortical neurons (Fig. 6), as reported previously in other experimental systems (29, 30). Both Tau and CRMP2 are microtubule-associated proteins and regulate microtubule dynamics, although their binding properties to tubulin are different (26, 28). Tau binds preferentially to microtubules, but CRMP2 prefers tubulin dimers to microtubules. Tau stimulates tubulin polymerization and the stability of microtubules (29, 31), and CRMP2 promotes microtubule assembly and stability (28). Both of them may contribute to axon elongation via regulation of microtubule dynamics. We have demonstrated previously that Tau and CRMP2 are important for axon development and that their expression is regulated translationally by the mTOR-S6K signaling pathway (19). Tau and CRMP2 mRNAs possess 5′-TOP sequences in their 5′ UTRs that are crucial for mediating selective translational control (Fig. 7) (19). The expression of CA-S6K promoted axon elongation (Fig. 5) and axon formation (data not shown). This result is consistent with our previous findings indicating that CA-S6K expression activates 5′-TOP-dependent translation and up-regulates Tau and CRMP2 expression, which leads to axon formation and elongation (Fig. 6). Although our previous study has reported the importance of the mTOR-S6K translational regulation in cell-autonomous establishment of cell polarity (19), this study revealed DHA-dependent Akt activation as the upstream signaling for the mTOR-S6K translational pathway. Our findings will shed new light on the significance of the regulatory mechanism in DHA-mediated neuronal function.
Here we found that DHA treatment did not induce multiple axon formation (Fig. 1E) but did induce Akt and S6K activation and the translational up-regulation of CRMP2 and Tau. Other fatty acids, such as saturated, monounsaturated, or n-6 polyunsaturated fatty acids, did not activate Akt and S6K (Fig. 3). Akt is targeted to the plasma membrane by its pleckstrin homology domain and plays an important role in directional cell movement (32). Akt and its localization are also critical for establishing neuronal polarity and neurite outgrowth. Akt is activated by its phosphorylation on Thr-308 by 3-phosphoinositide-dependent protein kinase 1 (PDK1) and on Ser-473 by either TOR complex 2 (TORC2) or integrin-linked kinase (33). Notably, DHA treatment promoted Akt activation but did not disturb neuronal polarity (Fig. 1). These results suggest that DHA may lower the threshold for Akt activation. This hypothesis is supported by the findings that DHA increases phosphatidylserine levels in the cell membrane and that phosphatidylserine accumulation in the membrane causes the translocation and activation of Akt (34, 35). As shown by our data (Fig. 3E), Akt activation is promoted in an n-3 PUFA-specific manner (34). In contrast, DHA does not induce activation of PI3K or production of phosphatidylinositol 3,4,5-trisphosphate (33). Taken together, these findings suggest that Akt may be targeted more accurately to the tips of neurites and activated more effectively by the presence of phosphatidylserine in DHA-treated neurons. Exogenous CA-S6K, Tau, or CRMP2 expression increased multiple axon formation as well as axon elongation (Fig. 6). Because of the ectopic and strong expression, multiple axon formation might be promoted. It is noteworthy that both Tau and CRMP2 are phosphorylated by GSK3β and inhibited their activities for microtubule stabilization (36). It is also known that GSK3β is inhibited by activated Akt (37). In growing axons, DHA-dependent Akt activation may synergistically enhance the function of Tau and CRMP2 in the regulation of microtubule dynamics and translational up-regulation of their expression. Alternatively, DHA and/or its metabolites may function as signaling molecules that interact with cell surface or intracellular receptors. Future studies are required to determine the precise mechanism of DHA-induced Akt activation.
In conclusion, our study demonstrated that DHA promoted axon outgrowth through the activation of Akt-mTOR-S6K-dependent translational control in cortical neurons. Tau and CRMP2, which play important roles in axon differentiation and elongation, were shown to be regulated translationally by this DHA-affected pathway through the 5′-TOP sequences in their mRNA. Our findings revealed a novel molecular mechanism for the pleiotropic effects of n-3 PUFAs on brain development.
Author Contributions
T. Mayanagi, K. O., A. S., and K. S. designed the research. T. Mita, T. Mayanagi, H. I., and K. F. performed the experiments and analyzed the data. T. Mayanagi and K. S. wrote the manuscript. All authors reviewed the results and approved the final version of the manuscript.
This work was supported by Japan Society for the Promotion of Science KAKENHI Grants 20240038, 23110510, and 25110728 (to K. S.); 21700352 and 15K21319 (to T. M.); and 22791124 and 24791229 (to K. F.). The authors declare that they have no conflicts of interest with the contents of this article.
- DHA
- docosahexaenoic acid
- EGFP
- enhanced GFP
- TOP
- terminal oligopyrimidine
- DIV
- day(s) in vitro
- ANOVA
- analysis of variance
- CA
- constitutively active
- DN
- dominant-negative.
References
- 1. Aveldaño M. I., and Bazán N. G. (1973) Fatty acids composition and level of diacylglycerols and phosphoglycerides in brain and retina. Biochim. Biophys. Acta 296, 1–9 [DOI] [PubMed] [Google Scholar]
- 2. Sinclair A. J. (1975) Incorporation of radioactive polyunsaturated fatty acids into liver and brain of developing rat. Lipids 10, 175–184 [DOI] [PubMed] [Google Scholar]
- 3. Dobbing J., and Sands J. (1973) Quantitative growth and development of human brain. Arch. Dis. Child. 48, 757–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Green P., and Yavin E. (1998) Mechanisms of docosahexaenoic acid accretion in the fetal brain. J. Neurosci. Res. 52, 129–136 [DOI] [PubMed] [Google Scholar]
- 5. Morse N. L. (2012) Benefits of docosahexaenoic acid, folic acid, vitamin D and iodine on foetal and infant brain development and function following maternal supplementation during pregnancy and lactation. Nutrients 4, 799–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wainwright P. E., Huang Y. S., Coscina D. V., Lévesque S., and McCutcheon D. (1994) Brain and behavioral effects of dietary n-3 deficiency in mice: a three generational study. Dev. Psychobiol. 27, 467–487 [DOI] [PubMed] [Google Scholar]
- 7. Yoshida S., Yasuda A., Kawazato H., Sakai K., Shimada T., Takeshita M., Yuasa S., Kobayashi T., Watanabe S., and Okuyama H. (1997) Synaptic vesicle ultrastructural changes in the rat hippocampus induced by a combination of α-linolenate deficiency and a learning task. J. Neurochem. 68, 1261–1268 [DOI] [PubMed] [Google Scholar]
- 8. Moriguchi T., Greiner R. S., and Salem N. Jr. (2000) Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J. Neurochem. 75, 2563–2573 [DOI] [PubMed] [Google Scholar]
- 9. Innis S. M., Gilley J., and Werker J. (2001) Are human-milk long-chain poly-unsaturated fatty acids related to visual and neural development in breast-fed infants? J. Pediatr. 139, 532–538 [DOI] [PubMed] [Google Scholar]
- 10. Jørgensen M. H., Hernell O., Hughes E., and Michaelsen K. F. (2001) Is there a relation between docosahexaenoic acid concentration in mothers' milk and visual development in term infants? J. Pediatr. Gastroenterol. Nutr. 32, 293–296 [DOI] [PubMed] [Google Scholar]
- 11. Xiao Y., Huang Y., and Chen Z. Y. (2005) Distribution, depletion and recovery of docosahexaenoic acid are region-specific in rat brain. Br. J. Nutr. 94, 544–550 [DOI] [PubMed] [Google Scholar]
- 12. Chung W. L., Chen J. J., and Su H. M. (2008) Fish oil supplementation of control and (n-3) fatty acid-deficient male rats enhances reference and working memory performance and increases brain regional docosahexaenoic acid levels. J. Nutr. 138, 1165–1171 [DOI] [PubMed] [Google Scholar]
- 13. Salem N. Jr., Litman B., Kim H. Y., and Gawrisch K. (2001) Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids 36, 945–959 [DOI] [PubMed] [Google Scholar]
- 14. Gawrisch K., Eldho N. V., and Holte L. L. (2003) The structure of DHA in phospholipid membranes. Lipids 38, 445–452 [DOI] [PubMed] [Google Scholar]
- 15. Kaech S., and Banker G. (2006) Culturing hippocampal neurons. Nat. Protoc. 1, 2406–2415 [DOI] [PubMed] [Google Scholar]
- 16. Dehmelt L., and Halpain S. (2004) Actin and microtubules in neurite initiation: are MAPs the missing link? J. Neurobiol. 58, 18–33 [DOI] [PubMed] [Google Scholar]
- 17. Calderon F., and Kim H. Y. (2004) Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J. Neurochem. 90, 979–988 [DOI] [PubMed] [Google Scholar]
- 18. Cao D., Xue R., Xu J., and Liu Z. (2005) Effects of docosahexaenoic acid on the survival and neurite outgrowth of rat cortical neurons in primary cultures. J. Nutr. Biochem. 16, 538–546 [DOI] [PubMed] [Google Scholar]
- 19. Morita T., and Sobue K. (2009) Specification of neuronal polarity regulated by local translation of CRMP2 and Tau via the mTOR-p70S6K pathway. J. Biol. Chem. 284, 27734–27745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fukumoto K., Morita T., Mayanagi T., Tanokashira D., Yoshida T., Sakai A., and Sobue K. (2009) Detrimental effects of glucocorticoids on neuronal migration during brain development. Mol. Psychiatry 14, 1119–1131 [DOI] [PubMed] [Google Scholar]
- 21. Ruvinsky I., and Meyuhas O. (2006) Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends. Biochem. Sci. 31, 342–348 [DOI] [PubMed] [Google Scholar]
- 22. Read D. E., and Gorman A. M. (2009) Involvement of Akt in neurite outgrowth. Cell Mol. Life. Sci. 66, 2975–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zoncu R., Efeyan A., and Sabatini D. M. (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 12, 21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McNamara R. K., Able J., Jandacek R., Rider T., Tso P., Eliassen J. C., Alfieri D., Weber W., Jarvis K., DelBello M. P., Strakowski S. M., and Adler C. M. (2010) Docosahexaenoic acid supplementation increases prefrontal cortex activation during sustained attention in healthy boys: a placebo-controlled, dose-ranging, functional magnetic resonance imaging study. Am. J. Clin. Nutr. 91, 1060–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Costa-Mattioli M., Sossin W. S., Klann E., and Sonenberg N. (2009) Translational control of long-lasting synaptic plasticity and memory. Neuron 61, 10–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Black M. M., Slaughter T., Moshiach S., Obrocka M., and Fischer I. (1996) Tau is enriched on dynamic microtubules in the distal region of growing axons. J. Neurosci. 16, 3601–3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Inagaki N., Chihara K., Arimura N., Ménager C., Kawano Y., Matsuo N., Nishimura T., Amano M., and Kaibuchi K. (2001) CRMP-2 induces axons in cultured hippocampal neurons. Nat. Neurosci. 4, 781–782 [DOI] [PubMed] [Google Scholar]
- 28. Fukata Y., Itoh T. J., Kimura T., Ménager C., Nishimura T., Shiromizu T., Watanabe H., Inagaki N., Iwamatsu A., Hotani H., and Kaibuchi K. (2002) CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nat. Cell Biol. 4, 583–591 [DOI] [PubMed] [Google Scholar]
- 29. Esmaeli-Azad B., McCarty J. H., and Feinstein S. C. (1994) Sense and antisense transfection analysis of tau function: tau influences net microtubule assembly, neurite outgrowth and neuritic stability. J. Cell Sci. 107, 869–879 [DOI] [PubMed] [Google Scholar]
- 30. Cole A. R., Knebel A., Morrice N.A., Robertson L. A., Irving A. J., Connolly C. N., and Sutherland C. (2004) GSK-3 phosphorylation of the Alzheimer epitope within collapsin response mediator proteins regulates axon elongation in primary neurons. J. Biol. Chem. 279, 50176–50180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Garcia M. L., and Cleveland D. W. (2001) Going new places using an old MAP: tau, microtubules and human neurodegenerative disease. Curr. Opin. Cell Biol. 13, 41–48 [DOI] [PubMed] [Google Scholar]
- 32. Merlot S., and Firtel R. A. (2003) Leading the way: directional sensing through phosphatidylinositol 3-kinase and other signaling pathways. J. Cell Sci. 116, 3471–3478 [DOI] [PubMed] [Google Scholar]
- 33. Yoshimura T., Arimura N., and Kaibuchi K. (2006) Signaling networks in neuronal polarization. J. Neurosci. 26, 10626–10630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Akbar M., Calderon F., Wen Z., and Kim H. Y. (2005) Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc. Natl. Acad. Sci. U.S.A. 102, 10858–10863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang B. X., Akbar M., Kevala K., and Kim H. Y. (2011) Phosphatidylserine is a critical modulator for Akt activation. J. Cell Biol. 192, 979–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ye T., Fu A. K., and Ip N. Y. (2015) Emerging roles of Axin in cerebral cortical development. Front. Cell Neurosci. 9, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arimura N., and Kaibuchi K. (2005) Key regulators in neuronal polarity. Neuron 48, 881–884 [DOI] [PubMed] [Google Scholar]