Abstract
Background. Limited data exist on human immunodeficiency virus (HIV)-infected individuals' ability to work after receiving combination antiretroviral therapy (cART). We aimed to investigate predictors of regaining full ability to work at 1 year after starting cART.
Methods. Antiretroviral-naive HIV-infected individuals <60 years who started cART from January 1998 through December 2012 within the framework of the Swiss HIV Cohort Study were analyzed. Inability to work was defined as a medical judgment of the patient's ability to work as 0%.
Results. Of 5800 subjects, 4382 (75.6%) were fully able to work, 471 (8.1%) able to work part time, and 947 (16.3%) were unable to work at baseline. Of the 947 patients unable to work, 439 (46.3%) were able to work either full time or part time at 1 year of treatment. Predictors of recovering full ability to work were non-white ethnicity (odds ratio [OR], 2.06; 95% confidence interval [CI], 1.20–3.54), higher education (OR, 4.03; 95% CI, 2.47–7.48), and achieving HIV-ribonucleic acid <50 copies/mL (OR, 1.83; 95% CI, 1.20–2.80). Older age (OR, 0.55; 95% CI, .42–.72, per 10 years older) and psychiatric disorders (OR, 0.24; 95% CI, .13–.47) were associated with lower odds of ability to work. Recovering full ability to work at 1 year increased from 24.0% in 1998–2001 to 41.2% in 2009–2012, but the employment rates did not increase.
Conclusions. Regaining full ability to work depends primarily on achieving viral suppression, absence of psychiatric comorbidity, and favorable psychosocial factors. The discrepancy between patients' ability to work and employment rates indicates barriers to reintegration of persons infected with HIV.
Keywords: ability to work, antiretroviral therapy, disability, HIV/AIDS, employment
Over the past 20 years, combination antiretroviral therapy (cART) has improved the prognosis for a close to normal life expectancy in a considerable proportion of individuals with human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS) [1]. As a result, individuals previously unable to work due to HIV-related morbidity are able to return to work in light of their improved health. Benefits of employment include economic well being, decreased symptoms of depression, and improved social support [2–6]. However, barriers to workforce re-entry have been reported for HIV-infected individuals, side effects of cART, and discrimination [7–11]. In 2004, a cross-sectional study within the Swiss HIV Cohort Study (SHCS) estimated an annual productivity loss of 121.9 million Swiss francs (95.2 million US dollars) for approximately 5000 HIV-infected individuals living in Switzerland [4]. Inability to work was independently associated with older age, AIDS-defining disease, history of using of intravenous drugs, and lower CD4 cell counts, whereas a higher level of education and a stable partnership were associated with greater ability to work, suggesting that socioeconomic factors may influence the cost-effectiveness of healthcare interventions in individuals infected with HIV. The arrival of new antiretroviral drugs with improved potency, safety, tolerability, and convenience, and starting cART earlier in the course of the disease may have affected the level of the ability to work [12, 13]. However, the employment rates of adults with HIV infection who are living in high-income countries are still lower compared with those of the general population [14, 15].
The overall aim of this study was to investigate the ability to work in HIV-1-infected adults receiving cART. In particular, the specific objectives were to (1) explore predictors of patients regaining the ability to work full time at 1 year after starting cART, (2) compare time trends in the ability to work and in the primary sources of income over a 5-year period in treated individuals, and (3) investigate the risk factors for developing a new disability that impaired a patient's ability to work.
METHODS
Study Design
This study is a subset of the prospective SHCS, with continuous enrollment of individuals infected with HIV ≥18 years of age observed in the HIV outpatient clinics of 7 Swiss hospitals (Basel, Bern, Geneva, Lausanne, Lugano, St. Gallen, Zurich) and associated smaller hospitals' and physicians' practices [16]. Data were prospectively collected at time of patients' enrollment in the study and every 6 months thereafter on standardized data collection forms and include basic sociodemographic characteristics and information about the clinical course of the disease, coinfection with hepatitis B and hepatitis C, psychiatric comorbidity, antiretroviral treatment, comedication, and immunological and virological parameters. The patient's ability to work in the month preceding a follow-up visit was assessed, independent of the patient's current employment status or the patient's own opinion, by the treating physician using a scale of 0% to 100% (0% = total disability; 100% = no disability). In the instruction materials, there were no details specifying which medicals factors should be calculated in assessing each patient's ability to work. The patient's primary source of income was recorded every 6 months according to 5 main categories (job, insurance, unemployment benefits, relatives, savings). For the present analysis, we used the SHCS database extract of June 2014. The local ethical committees of all participating study sites (Ethics Committee northwest/central Switzerland; Ethics Committee Bern, Ethics Committee Geneva; Ethics Committee St. Gallen, Ethics Committee Ticino; Ethics Committee Vaud; Ethics Committee Zurich) approved the study, and written informed consent was obtained from all participants.
Study Population
Antiretroviral-naive HIV-infected individuals below 60 years who were participating in the SHCS and started cART between January 1, 1998 and December 31, 2012, and for whom data on their ability to work at baseline and after 1 year of treatment were available, were eligible for this study. Similar to Switzerland, the retirement age in our cohort was 65, and we restricted our analysis to patients younger than 60, accounting for a potential follow-up of 5 years.
Definitions
“Inability to work” was defined as a medical judgment that the patient could not pursue any income-producing activities due to his or her health and was reported as 0% ability. “Partial ability to work” was defined as a medical judgment of the patient's ability to work part time, reported as 1%–99% ability. “Full work ability” was defined as a medical judgment of the patient's ability to work full time, reported as 100% ability. Finally, “cART” was defined as an antiretroviral regimen containing at least 3 drugs, ie, 2 nucleos(t)ide reverse-transcriptase inhibitors in combination with either a nonnucleoside reverse-transcriptase inhibitor, an integrase inhibitor, or a protease inhibitor. Level of formal education was noted as 1 of 3 categories: “low” (mandatory education of up to 9 years), “medium” (mandatory and vocational education of 9–12 years), or “high” (formal education of more than 12 years).
Statistical Analysis
The primary endpoint was the proportion of individuals unable to work at baseline who recovered sufficiently to be able to work full time at the end of the first year of treatment. Secondary endpoints were the proportion of individuals able to work at baseline who became unable to work at 1 year and at 5 years of cART, and the changes over time in patients' ability to work and in their primary sources of income. Basic sociodemographic characteristics, comorbidities (including current use of intravenous drugs, psychiatric comorbidity), CD4 cell count, HIV viral load, and ART were compared with respect to the ability to work at baseline using the χ2 test for categorical variables and the Mann–Whitney U or the Kruskal-Wallis test for continuous variables. Logistic regression models were used (1) to investigate predictors of achieving full ability to work at 1 year in patients who were unable to work at baseline and (2) to explore risk factors for a patient's developing a new disability to work. We introduced mixed-effect models to adjust for the variability of outcome assessment by physicians. All analyses were performed using Stata software (version 11 for Windows; StataCorp, College Station, TX).
RESULTS
Study Population
Among the more than 18 000 SHCS participants, 6782 antiretroviral-naive patients started cART from January 1, 1998 through December 31, 2012. Of these, we excluded 291 who were more than 60 years old and 691 for whom no data were available with respect to their ability to work at baseline or after the first year of treatment. The final study population comprised 5800 persons. At baseline, 4382 individuals (75.6%) were fully able to work, 471 (8.1%) were only able to work part time, and 947 (16.3%) were unable to work at all. Among 471 patients who were classified as partially able to work, only 12 patients were judged to be unable to work in a proportion of 10% and 6 patients were assessed to be 90% able to work; all other patients were able to work between 20% and 80%. Nobody was assessed to be able to work below 10% or above 90%. Baseline characteristics according to the ability to work at starting cART are shown in Table 1.
Table 1.
Characteristics of HIV-Infected Study Population With Respect to Patients' Ability to Work at the Start of cART (n = 5800)
| Characteristic at Baseline | 100% Able to Work (N = 4382) |
10%–90% Able to Work (N = 471) |
0% Able to Work (N = 947) |
P Value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Median age, IQR | 37 | 31–43 | 38 | 33–45 | 39 | 34–45 | <.001 |
| Male gender | 3201 | 73.1 | 293 | 62.2 | 618 | 65.3 | <.001 |
| Transmission | |||||||
| MSM | 2129 | 48.6 | 111 | 23.6 | 181 | 19.1 | <.001a |
| Heterosexual | 1812 | 41.4 | 201 | 42.7 | 372 | 39.3 | |
| PWID | 267 | 6.1 | 138 | 29.3 | 346 | 36.5 | |
| Other | 174 | 4.0 | 21 | 4.5 | 48 | 5.1 | |
| White ethnicity | 3210 | 73.3 | 379 | 80.5 | 773 | 81.6 | <.001 |
| Low educational attainment | 994 | 22.9 | 163 | 35.9 | 325 | 36.1 | <.001 |
| Hepatitis C coinfection | 786 | 17.9 | 191 | 40.6 | 452 | 47.3 | <.001 |
| Hepatitis B coinfection (antigen HBs positive) | 224 | 5.1 | 20 | 4.3 | 62 | 6.6 | .117 |
| Prior AIDS-defining condition | 532 | 12.1 | 120 | 25.5 | 354 | 37.8 | <.001 |
| Active injecting drug use | 78 | 1.8 | 60 | 12.7 | 154 | 16.3 | <.001 |
| Opiate substitution program | 280 | 6.4 | 83 | 17.6 | 249 | 26.3 | <.001 |
| Psychiatric disorder | 229 | 5.2 | 66 | 14.0 | 186 | 19.6 | <.001 |
| Median HIV-RNA, IQR | 4.7 | 4.1–5.2 | 4.8 | 3.9–5.3 | 4.9 | 4.2–5.5 | <.001 |
| Median CD4 cell count, IQR | 263 | 160–372 | 196 | 65–325 | 157 | 54–291 | <.001 |
| CD4 cell count | |||||||
| <50 | 386 | 8.9 | 101 | 21.4 | 233 | 24.6 | <.001a |
| 50–99 | 276 | 6.3 | 42 | 8.9 | 108 | 11.4 | |
| 100–199 | 828 | 18.8 | 94 | 20.0 | 213 | 22.5 | |
| 200–349 | 1621 | 37.0 | 135 | 28.7 | 225 | 23.7 | |
| >350 | 1271 | 29.0 | 99 | 21.0 | 168 | 17.8 | |
| cART | |||||||
| PI | 2354 | 53.7 | 273 | 58.0 | 598 | 63.1 | <.001a |
| NNRTI | 1735 | 39.6 | 155 | 32.9 | 284 | 30.0 | |
| Other | 293 | 6.7 | 43 | 9.1 | 65 | 6.9 | |
| Time period | |||||||
| 1998–2000 | 855 | 19.5 | 144 | 30.6 | 330 | 34.9 | <.001a |
| 2001–2005 | 1133 | 25.9 | 164 | 34.8 | 292 | 30.8 | |
| 2006–2012 | 2394 | 54.6 | 163 | 34.6 | 325 | 34.3 | |
Abbreviations: AIDS, acquired immune deficiency syndrome; cART, combination antiretroviral therapy; HB, hepatitis B; HIV, human immunodeficiency virus; IQR, interquartile range; MSM, men who have sex with men; NNRTI, nonnucleoside reverse-transcriptase inhibitors; PI, boosted protease inhibitors; PWID, people who inject drugs; RNA, ribonucleic acid.
a χ2 test.
Ability to Work and the Main Source of Income at 1 Year of Treatment
Of the 947 individuals unable to work at baseline, 508 (53.6%) were still unable to work at 1 year after starting cART, 310 (32.8%) were assessed as fully able to work, and 129 (13.6%) were assessed as able to work part time (Figure 1). In contrast, of the 4382 individuals who were 100% able to work at baseline, only 217 (5.0%) had developed a disability that interfered with their ability to work at 1 year of cART. When the analysis was restricted to 580 of 947 (61.2%) patients who were unable to work at baseline and did not change their ART regime during the first year of treatment, similar results were observed at 1 year (50.9% were unable to work, 34.8% were fully able to work, and 14.3% were able to work part time).
Figure 1.
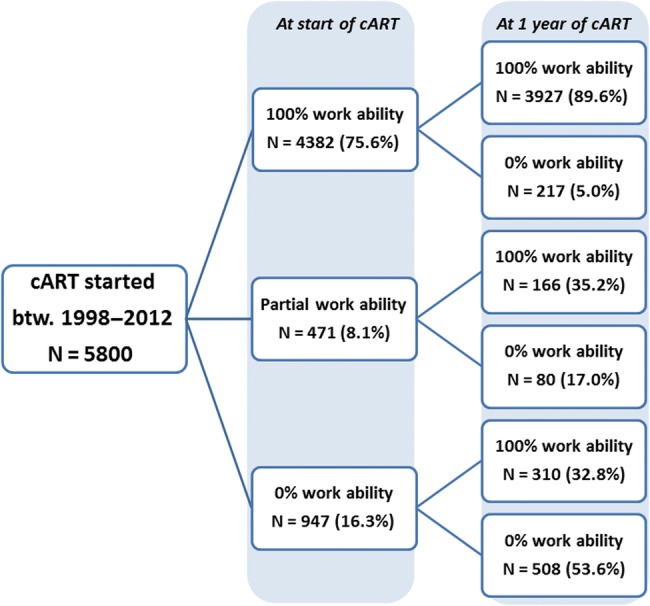
Medical assessment of patients' ability to work at the start of antiretroviral therapy and after 1 year of treatment (n = 5800). btw., between; cART, combination antiretroviral therapy.
In multivariate analysis (Table 2), predictors of recovering full ability to work at 1 year were non-white ethnicity (odds ratio [OR], 2.04; 95% confidence interval [CI], 1.20–3.44), a higher level of education (OR, 4.03; 95% CI, 2.47–7.48), CD4 cell counts at 1 year of at least 500 cells/µL (OR, 2.53; 95% CI, 1.27–5.04 for CD4 cell count >500 compared with <200 cells/µL), HIV-ribonucleic acid (RNA) of <50 copies/mL at 1 year (OR, 2.06; 95% CI, 1.20–3.54), and having started cART later in the study period (OR, 2.11; 95% CI, 1.30–3.44, for the period 2006–2012 compared with 1998–2000). In contrast, older age (OR, 0.55; 95% CI, .42–.72, per 10 years older), a psychiatric disorder (OR, 0.24; 95% CI, .13–.47), and participating in an opiate substitution program (OR, 0.41; 95% CI, .23–.73) were associated with lower odds of ability to work at 1 year. Risk factors for developing a new disability to work were older age (OR, 1.37; 95% CI, 1.20–1.58, per 10 years older), female gender (OR, 1.74; 95% CI, 1.32–2.29), coinfection with hepatitis C (OR, 1.76; 95% CI, 1.36–2.29), a prior AIDS-defining disease (OR, 1.58; 95% CI, 1.16–2.46), active intravenous drug use at baseline (OR, 4.15; 95% CI, 2.02–8.53), and psychiatric disorder (OR, 2.70; 95% CI, 1.78–4.11) (Table 3). Individuals of non-white ethnicity (OR, 0.66; 95% CI, .51–.93), with a higher level of education (OR, 0.46; 95% CI, .32–.66), higher CD4 cell count (OR, 0.54; 95% CI, .33–.86 for CD4 cell count >350 compared with <200 cells/µL), viral suppression (OR, 0.63; 95% CI, .47–.83), or starting cART later in the study period (OR, 0.41; 95% CI, .31–.55, for 2006–2012 compared with 1998–2000) were less likely to be unable to work after 1 year of treatment.
Table 2.
Predictors of Full Ability to Work After 1 Year of Treatment in Patients Who Were 0% Able to Work at the Start of cART (n = 947)a
| Characteristic at Baseline | Univariate Analysis |
Multivariate Analysis |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | ORb | 95% CI | P Value | |
| Age, per 10 y older | 0.73 | .62–.87 | <.001 | 0.55 | .42–.72 | <.001 |
| Female gender | 0.85 | 0.64–1.13 | .263 | 0.74 | .45–1.22 | .238 |
| Non-white ethnicity | 3.12 | 2.23–4.37 | <.001 | 2.04 | 1.20–3.44 | .008 |
| Education | ||||||
| Low | Ref. | – | – | Ref. | – | – |
| Medium | 1.02 | .75–1.40 | .881 | 2.01 | 1.22–3.33 | .007 |
| High | 2.56 | 1.70–3.83 | <.001 | 4.03 | 2.47–7.48 | <.001 |
| Hepatitis C coinfection | 0.28 | .21–.37 | <.001 | 0.65 | .41–1.05 | .077 |
| Prior AIDS-defining condition | 1.53 | 1.16–2.02 | .003 | 1.59 | .83–1.78 | .213 |
| CD4 cells at baseline | ||||||
| <100 | Ref. | – | – | Ref. | – | – |
| 100–199 | 1.67 | 1.39–2.02 | <.001 | 0.69 | .40–1.19 | .186 |
| 200–349 | 2.04 | 2.02–2.85 | <.001 | 0.55 | .30–1.02 | .058 |
| >350 | 2.43 | 2.02–2.92 | <.001 | 0.52 | .25–1.04 | .064 |
| CD4 cells at 1 y | ||||||
| <200 | Ref. | – | – | Ref. | – | – |
| 200–349 | 1.97 | 1.62–2.40 | <.001 | 1.41 | .86–2.32 | .176 |
| 350–499 | 3.33 | 2.70–4.11 | <.001 | 1.11 | .60–2.08 | .741 |
| >500 | 3.84 | 3.16–4.67 | <.001 | 2.53 | 1.27–5.04 | .008 |
| HIV-RNA >100 000 copies/mL at baseline | 1.53 | 1.15–2.02 | .003 | 1.31 | .89–1.92 | .170 |
| HIV-RNA <50 copies/mL at 1 y | 2.17 | 1.54–3.07 | <.001 | 2.06 | 1.20–3.54 | .002 |
| Active injecting drug use | 0.21 | .13–.36 | <.001 | 0.63 | .29–1.36 | .239 |
| Opiate substitution program | 0.18 | .12–.27 | <.001 | 0.41 | .23–.73 | .003 |
| Psychiatric disorder | 0.44 | .30–.66 | <.001 | 0.24 | .13–.47 | <.001 |
| cART class | ||||||
| PI | Ref. | – | – | Ref. | – | – |
| NNRTI | 1.10 | .81–1.48 | .540 | 0.78 | .54–1.15 | .217 |
| Other | 0.62 | .34–1.12 | .114 | 0.63 | .30–1.35 | .237 |
| Time period | ||||||
| 1998–2000 | Ref. | – | – | Ref. | – | – |
| 2001–2005 | 1.53 | 1.08–2.16 | .017 | 1.57 | .98–2.51 | .059 |
| 2006–2012 | 2.02 | 1.44–2.82 | <.001 | 2.11 | 1.30–3.44 | .003 |
Abbreviations: AIDS, acquired immune deficiency syndrome; cART, combination antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; NNRTI, nonnucleoside reverse-transcriptase inhibitors; OR, odds ratio; PI, boosted protease inhibitors; Ref., reference; RNA, ribonucleic acid.
a Uni- and multivariate ORs using logistic regression.
b Odds ratios adjusted for all variables listed.
Table 3.
Predictors for Developing a 0% Ability to Work During First Year of Treatment in Patients Who Were 100% Able to Work at the Start of cART (n = 4382)a
| Characteristic at Baseline | Univariate Analysis |
Multivariate Analysis |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | ORb | 95% CI | P Value | |
| Age, per 10 y older | 1.17 | 1.05–1.29 | .004 | 1.37 | 1.20–1.58 | <.001 |
| Female gender | 1.44 | 1.17–1.78 | <.001 | 1.74 | 1.32–2.29 | <.001 |
| Non-white ethnicity | 0.73 | .58–.92 | .008 | 0.66 | .48–.91 | .012 |
| Education | ||||||
| Low | Ref. | – | – | Ref. | – | – |
| Medium | 0.75 | .60–.94 | .013 | 0.69 | .51–.93 | .015 |
| High | 0.40 | .30–.52 | <.001 | 0.46 | .32–.66 | <.001 |
| Hepatitis C coinfection | 2.83 | 2.29–3.49 | <.001 | 1.76 | 1.36–2.29 | <.001 |
| Hepatitis B coinfection | 1.59 | 1.09–2.32 | .017 | 1.59 | 1.04–2.46 | .034 |
| Prior AIDS-defining condition | 2.01 | 1.57–2.58 | <.001 | 1.58 | 1.16–2.17 | .004 |
| CD4 cells at baseline | ||||||
| <100 | Ref. | – | – | Ref. | – | – |
| 100–199 | 0.40 | .65–.75 | .002 | 0.52 | .33–.80 | <.001 |
| 200–349 | 0.37 | .56–.94 | .003 | 0.66 | .42–1.04 | .076 |
| >350 | 0.39 | .13–.54 | <.001 | 0.69 | .41–1.17 | .172 |
| CD4 cells at 1 y | ||||||
| <200 | Ref. | – | – | Ref. | – | – |
| 200–349 | 0.50 | .36–.68 | <.001 | 0.81 | .54–1.20 | .289 |
| 350–499 | 0.28 | .20–.40 | <.001 | 0.54 | .33–1.34 | .163 |
| >500 | 0.31 | .23–.42 | <.001 | 0.60 | .36–1.08 | .058 |
| CD4 cell count at the start of cART, per 100 cells/µL increase | 0.90 | .84–.97 | .004 | 1.00 | .90–1.10 | .951 |
| CD4 cell count at 1 y, per 100 cells/µL increase | 0.88 | .83–.94 | <.001 | 0.92 | .85–.99 | .040 |
| HIV-RNA >100 000 copies/mL at starting cART | 1.18 | .96–1.44 | .119 | 1.11 | .86–1.44 | .412 |
| HIV-RNA <50 copies/mL at 1 y | 0.50 | .39–.63 | <.001 | 0.63 | .47–.83 | .001 |
| Active injecting drug use | 8.39 | .32–13.2 | <.001 | 4.15 | 2.02–8.53 | <.001 |
| Opiate substitution program | 2.15 | 1.57–2.95 | <.001 | 1.15 | .71–1.89 | .564 |
| Psychiatric disorder | 2.59 | 1.86–3.60 | <.001 | 2.70 | 1.78–4.11 | <.001 |
| cART class | ||||||
| PI | Ref. | – | – | Ref. | – | – |
| NNRTI | 0.81 | .65–1.00 | .049 | 1.05 | .82–1.36 | .684 |
| Other | 1.37 | .96–1.94 | .083 | 1.28 | .83–1.96 | .263 |
| Time period | ||||||
| 1998–2000 | Ref. | – | – | Ref. | – | – |
| 2001–2005 | 0.55 | .43–.71 | <.001 | 0.48 | .35–.66 | <.001 |
| 2006–2012 | 0.31 | .25–.40 | <.001 | 0.41 | .31–.55 | <.001 |
Abbreviations: AIDS, acquired immune deficiency syndrome; cART, combination antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; NNRTI, nonnucleoside reverse-transcriptase inhibitors; OR, odds ratio; PI, boosted protease inhibitors; Ref., reference; RNA, ribonucleic acid.
a Uni- and multivariate ORs using logistic regression.
b Odds ratios adjusted for all variable listed.
At the start of cART, the primary source of income was a job for 3397 individuals (58.6%), insurance payments for 1198 (20.7%), unemployment benefits for 101 (1.7%), relatives for 432 (7.5%), and savings for 46 (0.8%), whereas 626 individuals (10.8%) reported 2 equivalent sources of income, such as job and assistance from relatives. After 1 year of treatment, 3287 individuals (56.7%) reported living on their job income, 1374 (23.7%) from insurance payments, 110 (1.9%) from unemployment benefits, 441 (7.6%) on income from relatives, and 45 (0.8%) from savings, whereas 543 individuals (9.4%) reported 2 equivalent sources of income. In the group of 947 individuals who were unable to work at baseline, of the 310 (51.9%) who had recovered a full ability to work, 161 reported that they were living on their job income after 1 year of treatment, and of the 508 (84.8%) with a persistent disability, 431 were living on income from insurance payments.
Time Trends in Ability to Work and the Main Source of Income
At starting cART, the overall proportion of individuals fully able to work increased from 64.4% in the years 1998–2001 to 85.9% in the years 2009–2012 (test for trend P < .001) (Figure 2A). A job as the patient's main source of income increased from 50% in the years 1998–2001 to 62.4% in the years 2009–2012 (test for trend P < .001), whereas insurance payments as the primary source of income decreased from 27.4% in the years 1998–2001 to 13.5% in the years 2009–2012 (test for trend P < .001). Of the 947 people unable to work at baseline, recovering a full ability to work at 1 year increased from 24.0% in the years 1998–2001 to 41.2% in the years 2009–2012 (test for trend P = .001) (Figure 2B). In contrast, the proportion of people earning their living exclusively from their jobs remained stable (17.0% in the years 1998–2001 vs 18.9% in the years 2009–2012 [P = .094]), despite the fact that insurance payments decreased from 64.4% in the years 1998–2001 to 59.2% in the years 2009–2012 (P = .052).
Figure 2.
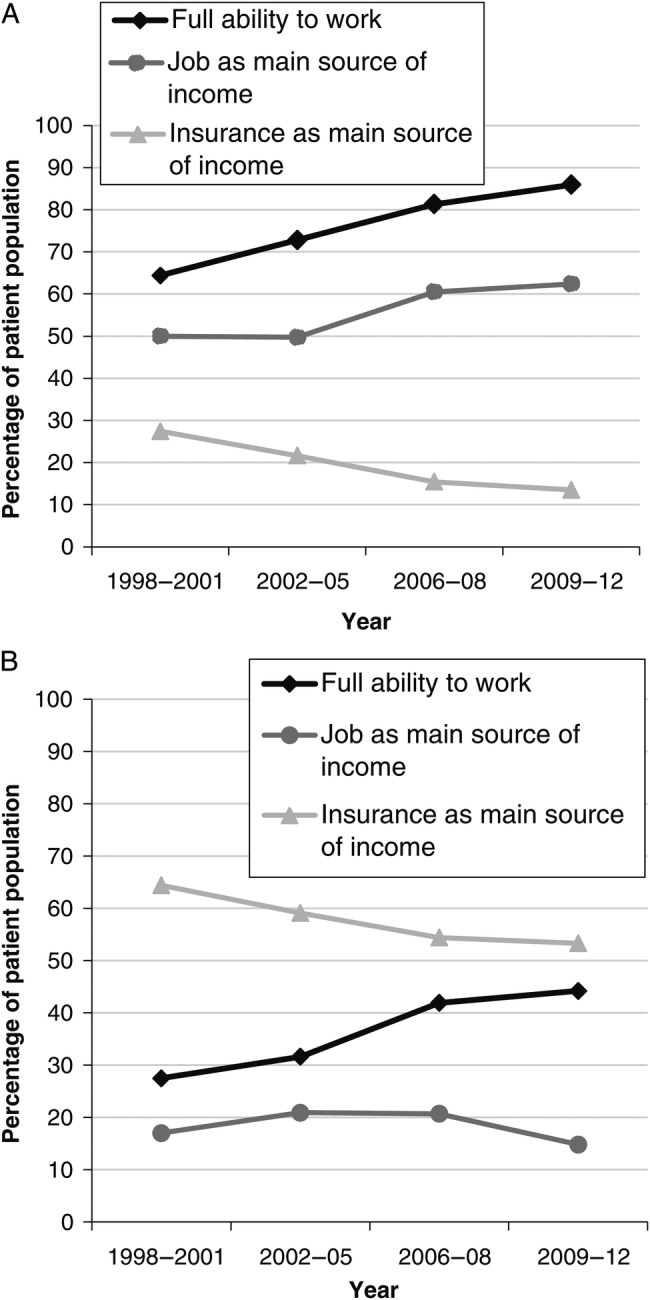
Full ability to work and primary sources of income over time (A) at baseline in the study population (n = 5800) and (B) at 1 year of combination antiretroviral therapy in patients who were unable to work at baseline (n = 947).
In a more detailed analysis, taking into account the patient's previous occupation type, we observed that patients reporting to have previously been self-employed or having worked in a relatives business were more likely to be unemployed when regained their full ability to work compared with those who had worked as manager, middle or lower staff, or had been an employee (P < .001).
Changes in Ability to Work and the Main Source of Income Over a 5-Year Period
Data on ability to work at 5 years after starting cART were available for 3029 (52.2%) of the 5800 patients included in the study. Of this group, 2187 (72.2%) were assessed to be fully able to work, 392 (12.9%) were able to work part time, and 450 (14.9%) were unable to work at 5 years. Of the 2264 individuals who were able to work at 1 year, 1980 (87.5%) were still able to work at 5 years, and only 131 (5.8%) had developed a new disability that impaired their ability to work. Only 71% of the individuals fully able to work at 5 years reported living on income from their jobs. Among those unable to work at 5 years, 89.1% reported living from insurance payments. The main reasons of the missing data on the ability to work at 5 years in 2771 patients were the short potential follow-up in 1892 (68.3%) patients who started cART in the last study period (between 2006 and 2012), lost to follow-up in 574 (20.7%), and death in 17 (6.1%) patients.
DISCUSSION
This study illustrates that the ability of treated patients to engage in full- or part-time employment has improved significantly over time. Up to 50% of those unable to work at the start of cART were able to work either full time or part time 1 year later, and the ability to work that was regained was sustained after 5 years of treatment. However, even in a country with very low unemployment rates such as Switzerland, the percentage of fully capable HIV-infected patients for whom a job is the primary source of income has not increased over time, which suggests that barriers for work reintegration of persons with HIV/AIDS still exist.
Our results considerably extend those of earlier studies that have reported increasing employment rates of treated HIV-infected adults over time in both high- and low-income countries [3, 7, 8, 14, 15, 17, 18], mainly as a result of the improved access to cART, potency, tolerability, and safety of newer antiretroviral drugs. Indeed, our study shows substantial improvement in the ability to work in HIV-infected individuals starting cART and also a parallel increase in the proportion of individuals reporting their jobs as their primary source of income, and an overall decrease in disability payments.
In the group of individuals who were unable to work at baseline, their regaining the ability to work also has increased considerably over time. However, the improvement in the patients' ability to work has not been accompanied by an increase in their employment rates; the proportion of individuals living on their job income and the proportion living on income from disability payments both remained stable over time. These results are consistent with recent studies that indicates lower employment rates of HIV-infected individuals compared with the background population [14, 19–23]. The discrepancy between patients' ability to work full time and their current employment rates, even in a country with low unemployment rates, may indicate barriers to work reintegration for HIV-infected persons that can include concerns over uncertain future health, possible loss of benefits, outdated job skills, side effects of cART, and discrimination [7–11]. Moreover, non-HIV comorbidities such as cardiovascular disease, cancer, liver disease, and neurocognitive impairment might have negatively affected re-employment rates in an aging HIV population [14, 15, 24].
In our study, recovering a full ability to work was affected primarily by whether viral suppression was achieved, as well as by psychiatric comorbidity, current intravenous drug use, and by psychosocial factors. Mental health is a known key mediator for employment-related outcomes, influencing also neurocognitive performance and adherence to cART [5, 23, 25, 26]. Among psychosocial factors, a higher level of formal education was strongly associated with greater odds of being able to work after disability. It is of interest to note that the probability of workforce re-entry in more highly educated individuals increased over time, which was also observed in other studies [5, 14, 27–30], possibly because of increased job opportunities and higher occupational positions. In this view, persons who had previously worked as a manager or middle/lower staff had a higher probability of returning to work after regaining full ability to work.
In our study, non-white ethnicity was associated with recovering from disability after starting cART. Because most non-white people in Switzerland are immigrants from sub-Saharan Africa, recovery of their ability to work may reflect improvements in health after treatment of opportunistic infections such as tuberculosis, which is not necessarily associated with sequelae causing long-term disability; however, it also may reflect a “healthy immigrant effect”, because immigrants are generally healthy young persons without comorbidities [31]. Nonetheless, only few immigrants reported earning a living from a job, most likely because working in Switzerland is allowed only after residence authorization has been obtained.
In patients fully able to work at baseline, cART increases the probability of remaining employed [5, 14, 15, 29]. Factors associated with a new disability were either related to HIV disease, namely having had a prior AIDS-defining condition and not achieving viral suppression, or to specific comorbidities such as psychiatric disorders, current intravenous drug use, and coinfection with hepatitis B and hepatitis C. Prior studies have shown that non-HIV comorbidities in HIV-infected individuals are associated with a higher risk of hospitalization and death as well as with a loss of employment [5, 14, 15, 32]. Hepatitis C coinfection was associated with unemployment in many studies, mainly due to its symptoms such as fatigue, probably contributing to lower productivity and higher risk of job loss in these patients [33].
We acknowledge some limitations. First, information bias is possible because assessment of a person's ability to work relies on the physician's perception, and this perception depends on the physician's experience as well as on other comorbidities and the nature of the patient's profession. However, a very good correlation was found between the validated instrument “work ability index” and the simple item question “current ability to work compared with the lifetime best” that is close to the physician's assessment in the SHCS [34]. Moreover, medical judgment of the ability to work correlated very well with the main source of income. Second, selection bias may have occurred because healthy individuals who are less prone to being lost to follow-up may be overrepresented, leading to an overestimation of the full capability of people with an HIV infection. We mentioned in particular a healthy immigrant effect in people of non-white ethnicity who are generally younger and without comorbidities.
Our study has several strengths. To our knowledge, this is the largest comprehensive study investigating the effect of cART on HIV-infected individuals' ability to work over a long follow-up period, comparing time trends and the primary sources of income. More importantly, the study population included a high proportion of women, patients who use intravenous drugs, patients at an advanced HIV clinical stage, and patients with comorbidities, all of which reflects the real-life scenarios for HIV-infected individuals in many countries. The available literature to date is based on the employment rates of HIV-infected adults, not on medical judgments of patients' ability to work. Data on employment do not mirror the ability to work because of several factors: some patients continue to work despite a disability due to a fear of losing their jobs, cannot find a job, do not have permission to work, or decide not to work due to family duties. The situation in Switzerland may be particularly meaningful for assessing the ability to work, because the labor market was not as deeply affected by the international financial crisis and the unemployment rates remained very low (between 3.5% and 4.2% in the years investigated) [35]. Hence, unemployment rates were unlikely to affect the employment status of HIV-infected individuals.
CONCLUSIONS
In conclusion, the ability to work of treated HIV-infected individuals has increased over time. Ability to work in this population is mainly dependent on achieving viral suppression and beneficial psychosocial factors as well as on the absence of specific comorbidities. The overall proportion of individuals reporting a job as the main source of their income has also increased. Our study reflects a better prognosis for individuals with HIV infection who are treated with cART. Further studies will have to address whether specific measures, such as offer of modified work (work accommodation) to sick workers, training of supervisors, and communication between employer and healthcare providers, might support reintegration and increase employment rates of treated HIV-infected persons.
Acknowledgments
M. B. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial support. This study was funded in the framework of the Swiss HIV Cohort Study (SHCS), supported by the Swiss National Science Foundation (SHCS project 708). The members of the Swiss HIV Cohort Study (J. Barth, M. Battegay, E. Bernasconi, J. Böni, H. C. Bucher, P. Bürgisser, C. Burton-Jeangros, A. Calmy, M. Cavassini, R. Dubs, M. Egger, L. Elzi, J. Fehr, M. Fischer, M. Flepp, H. Furrer [Chairman of the Clinical and Laboratory Committee], C. A. Fux, M. Gorgievski, H. Günthard [President of the SHCS], B. Hasse, H. H. Hirsch, B. Hirschel, I. Hösli, C. Kahlert, L. Kaiser, O. Keiser, C. Kind, T. Klimkait, H. Kovari, B. Ledergerber, G. Martinetti, B. Martinez de Tejada, N. Müller, D. Nadal, G. Pantaleo, A. Rauch [Chairmann of the Scientific Board], S. Regenass, M. Rickenbach [Head of Data Center], C. Rudin [Chairman of the Mother & Child Substudy], P. Schmid, D. Schultze, F. Schöni-Affolter, J. Schüpbach, R. Speck, P. Taffé, P. Tarr, A. Telenti, A. Trkola, P. Vernazza, V. von Wyl, R. Weber, S. Yerly, L. Elzi, and A. Conen) have been supported by an unrestricted grant from the Stiftung Forschung Infektionskrankheiten (project no. 39).
Potential conflicts of interest. L. E. has received travel grants from Abbvie, Bristol-Myers Squibb, Janssen-Tibotec, Gilead Sciences, ViiV Healthcare. A. C. received travel grants from Merck Sharp & Dohme, Gilead Sciences, Bristol-Myers Squibb, and ViiV Healthcare. J. F. has received travel, educational, and research grants from Abbvie, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Merck Sharp & Dohme, Janssen-Tibotec, and ViiV Healthcare. M. C. has received travel grants from Abbvie, Boehringer-Ingelheim, Bristol-Myers Squibb, and Gilead Sciences. A. C. has received travel grants from Boehringer-Ingelheim, Gilead Sciences, and educational grants for her institution from Abbvie, Gilead Sciences, Janssen-Tibotec, ViiV Healthcare, and Merck Sharp & Dohme. E. B. has received travel grants or speakers' honoraria from Abbvie, Bristol-Myers Squibb, Gilead, Janssen-Tibotec, Merck Sharp & Dohme, Pfizer, and ViiV Healthcare. The institution of H. F. has received consultancy honoraria and grants from Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead, Merck Sharp & Dohme, ViiV Healthcare, and Abbvie. M. B. has received research grants or speakers' honoraria from Abbvie, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead, Merck Sharp & Dohme, Janssen-Tibotec, and ViiV Healthcare. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Collaborators: the Swiss HIV Cohort Study Group, J. Barth, M. Battegay, E. Bernasconi, J. Böni, H.C. Bucher, P. Bürgisser, C. Burton-Jeangros, A. Calmy, M. Cavassini, R. Dubs, M. Egger, L. Elzi, J. Fehr, M. Fischer, M. Flepp, H. Furrer, C.A. Fux, M. Gorgievski, H. Günthard, B. Hasse, H.H. Hirsch, B. Hirschel, I. Hösli, C. Kahlert, L. Kaiser, O. Keiser, C. Kind, T. Klimkait, H. Kovari, B. Ledergerber, G. Martinetti, B. Martinez de Tejada, N. Müller, D. Nadal, G. Pantaleo, A. Rauch, S. Regenass, M. Rickenbach, C. Rudin, P. Schmid, D. Schultze, F. Schöni-Affolter, J. Schüpbach, R. Speck, P. Taffé, P. Tarr, A. Telenti, A. Trkola, P. Vernazza, V. von Wyl, R. Weber, and S. Yerly
References
- 1.May MT, Gompels M, Delpech V et al. . Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy . AIDS 2014; 28:1193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson LA, Milloy MJ, Kerr TH et al. . Employment predicts decreased mortality among HIV-seropositive illicit drug users in a setting of universal HIV care. J Epidemiol Community Health 2014; 68:93–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosen S, Larson B, Rohr J et al. . Effect of antiretroviral therapy on patients' economic well being: five-year follow-up . AIDS 2014; 28:417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sendi P, Schellenberg F, Ungsedhapand C et al. . Productivity costs and determinants of productivity in HIV-infected patients. Clin Ther 2004; 26:791–800. [DOI] [PubMed] [Google Scholar]
- 5.McGoldrick C. HIV and employment. Occup Med (Lond) 2012; 62:242–53. [DOI] [PubMed] [Google Scholar]
- 6.Rueda S, Raboud J, Mustard C et al. . Employment status is associated with both physical and mental health quality of life in people living with HIV. AIDS Care 2011; 23:435–43. [DOI] [PubMed] [Google Scholar]
- 7.Barkey V, Watanabe E, Solomon P, Wilkins S. Barriers and facilitators to participation in work among Canadian women living with HIV/AIDS. Can J Occup Ther 2009; 76:269–75. [DOI] [PubMed] [Google Scholar]
- 8.Goldman DP, Bao Y. Effective HIV treatment and the employment of HIV(+) adults . Health Serv Res 2004; 39 (6 Pt 1):1691–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thirumurthy H, Galarraga O, Larson B, Rosen S. HIV treatment produces economic returns through increased work and education, and warrants continued US support. Health Aff (Millwood) 2012; 31:1470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torres-Madriz G, Lerner D, Ruthazer R et al. . Work-related barriers and facilitators to antiretroviral therapy adherence in persons living with HIV infection. AIDS Behav 2011; 15:1475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagener MN, van Opstal SE, Miedema HS et al. . Employment-related concerns of HIV-positive people in the Netherlands: input for a multidisciplinary guideline. J Occup Rehabil 2014; 24:790–7. [DOI] [PubMed] [Google Scholar]
- 12.AIDSinfo: DHHS. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available at: http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf Accessed 14 July 2014.
- 13.European Clinical AIDS Society. European Treatment Guidelines. Version 7.02, June 2014. Available at: http://www.eacsociety.org/files/guidelines-7.1-english.pdf. Accessed 14 July 2014.
- 14.Legarth R, Omland LH, Kronborg G et al. . Employment status in persons with and without HIV infection in Denmark: 1996–2011 . AIDS 2014; 28:1489–98. [DOI] [PubMed] [Google Scholar]
- 15.Dray-Spira R, Legeai C, Le Den M et al. . Burden of HIV disease and comorbidities on the chances of maintaining employment in the era of sustained combined antiretoviral therapies use . AIDS 2012; 26:207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoeni-Affolter F, Ledergerber B, Rickenbach M et al. . Cohort profile: the Swiss HIV Cohort study. Int J Epidemiol 2010; 39:1179–89. [DOI] [PubMed] [Google Scholar]
- 17.Bor J, Tanser F, Newell ML, Barnighausen T. In a study of a population cohort in South Africa, HIV patients on antiretrovirals had nearly full recovery of employment. Health Aff (Millwood) 2012; 31:1459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beard J, Feeley F, Rosen S. Economic and quality of life outcomes of antiretroviral therapy for HIV/AIDS in developing countries: a systematic literature review. AIDS Care 2009; 21:1343–56. [DOI] [PubMed] [Google Scholar]
- 19.Braveman B, Levin M, Kielhofner G, Finlayson M. HIV/AIDS and return to work: a literature review one-decade post-introduction of combination therapy (HAART) . Work 2006; 27:295–303. [PubMed] [Google Scholar]
- 20.Dray-Spira R, Lert F. Living and working with HIV in France in 2003: results from the ANRS-EN12-VESPA Study . AIDS 2007; 21 (Suppl 1):S29–36. [DOI] [PubMed] [Google Scholar]
- 21.Worthington C, O'Brien K, Zack E et al. . Enhancing labour force participation for people living with HIV: a multi-perspective summary of the research evidence. AIDS Behav 2012; 16:231–43. [DOI] [PubMed] [Google Scholar]
- 22.Lem M, Moore D, Marion S et al. . Back to work: correlates of employment among persons receiving highly active antiretroviral therapy. AIDS Care 2005; 17:740–6. [DOI] [PubMed] [Google Scholar]
- 23.Rabkin JG, McElhiney M, Ferrando SJ et al. . Predictors of employment of men with HIV/AIDS: a longitudinal study. Psychosom Med 2004; 66:72–8. [DOI] [PubMed] [Google Scholar]
- 24.Dray-Spira R, Persoz A, Boufassa F et al. . Employment loss following HIV infection in the era of highly active antiretroviral therapies . Eur J Public Health 2006; 16:89–95. [DOI] [PubMed] [Google Scholar]
- 25.Martin DJ, Steckart MJ, Arns PG. Returning to work with HIV/AIDS: a qualitative study . Work 2006; 27:209–19. [PubMed] [Google Scholar]
- 26.Fogarty AS, Zablotska I, Rawstorne P et al. . Factors distinguishing employed from unemployed people in the Positive Health Study . AIDS 2007; 21 (Suppl 1):S37–42. [DOI] [PubMed] [Google Scholar]
- 27.Dray-Spira R, Gueguen A, Ravaud JF, Lert F. Socioeconomic differences in the impact of HIV infection on workforce participation in France in the era of highly active antiretroviral therapy. Am J Public Health 2007; 97:552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Legarth R, Omland LH, Kronborg G et al. . Educational attainment and risk of HIV infection, response to antiretroviral treatment, and mortality in HIV-infected patients . AIDS 2014; 28:387–96. [DOI] [PubMed] [Google Scholar]
- 29.Dray-Spira R, Gueguen A, Lert F. Disease severity, self-reported experience of workplace discrimination and employment loss during the course of chronic HIV disease: differences according to gender and education. Occup Environ Med 2008; 65:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibrahim F, Anderson J, Bukutu C, Elford J. Social and economic hardship among people living with HIV in London . HIV Med 2008; 9:616–24. [DOI] [PubMed] [Google Scholar]
- 31.McDonald JT, Kennedy S. Insights into the ‘healthy immigrant effect’: health status and health service use of immigrants to Canada. Soc Sci Med 2004; 59:1613–27. [DOI] [PubMed] [Google Scholar]
- 32.Murray M, Hogg RS, Lima VD et al. . The effect of injecting drug use history on disease progression and death among HIV-positive individuals initiating combination antiretroviral therapy: collaborative cohort analysis . HIV Med 2012; 13:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Groß M, Herr A, Hower M et al. . Unemployment, health, and education of HIV-infected males in Germany [Epub ahead of print]. Int J Public Health 2015; doi number 10.1007/s00038-015-0750-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahlstrom L, Grimby-Ekman A, Hagberg M, Dellve L. The work ability index and single-item question: associations with sick leave, symptoms, and health--a prospective study of women on long-term sick leave. Scand J Work Environ Health 2010; 36:404–12. [DOI] [PubMed] [Google Scholar]
- 35.Swiss employment statistics. Swiss Federal Office of Statistics. Available at: http://www.bfs.admin.ch/bfs/portal/en/index/themen/03.html Accessed 14 July 2014.


