Abstract
Atrial fibrillation (AF) is an extremely common clinical problem associated with increased morbidity and mortality. Current antiarrhythmic options include pharmacological, ablation, and surgical therapies, and have significantly improved clinical outcomes. However, their efficacy remains suboptimal, and their use is limited by a variety of potentially serious adverse effects. There is a clear need for improved therapeutic options. Several decades of research have substantially expanded our understanding of the basic mechanisms of AF. Ectopic firing and re-entrant activity have been identified as the predominant mechanisms for arrhythmia initiation and maintenance. However, it has become clear that the clinical factors predisposing to AF and the cellular and molecular mechanisms involved are extremely complex. Moreover, all AF-promoting and maintaining mechanisms are dynamically regulated and subject to remodelling caused by both AF and cardiovascular disease. Accordingly, the initial presentation and clinical progression of AF patients are enormously heterogeneous. An understanding of arrhythmia mechanisms is widely assumed to be the basis of therapeutic innovation, but while this assumption seems self-evident, we are not aware of any papers that have critically examined the practical contributions of basic research into AF mechanisms to arrhythmia management. Here, we review recent insights into the basic mechanisms of AF, critically analyse the role of basic research insights in the development of presently used anti-AF therapeutic options and assess the potential value of contemporary experimental discoveries for future therapeutic innovation. Finally, we highlight some of the important challenges to the translation of basic science findings to clinical application.
Keywords: Ablation, Antiarrhythmic drugs, Atrial fibrillation, Cellular electrophysiology, Ectopic activity, Imaging, Re-entry
1. Introduction
Atrial fibrillation (AF) has received widespread attention from both clinicians and scientists for over a century.1,2 AF is the commonest arrhythmia in clinical practice, with an incidence that is rising, and significantly affects morbidity and mortality.3 Current therapeutic options have limited efficacy and substantial adverse effects,4,5 and there is a clear need for further therapeutic innovation. Basic research into AF pathophysiology has been extensive over the past 100 years;1,2 one of the major underlying assumptions has been that improved insights into fundamental arrhythmic and antiarrhythmic mechanisms will help to improve AF management.1,4,5 While this assumption seems self-evident, we are not aware of any papers that have specifically and critically examined the practical contributions of basic research in AF to its management. Here, we provide a brief conceptual overview of predominant AF mechanisms and describe recent insights, evaluate the role of basic research knowledge in the development of current AF therapy, assess the potential value of recently obtained information in developing new treatment approaches, and consider the challenges in translating basic mechanisms to therapeutic innovation.
2. Conceptual framework and basic mechanisms underlying AF
2.1. Conceptual framework
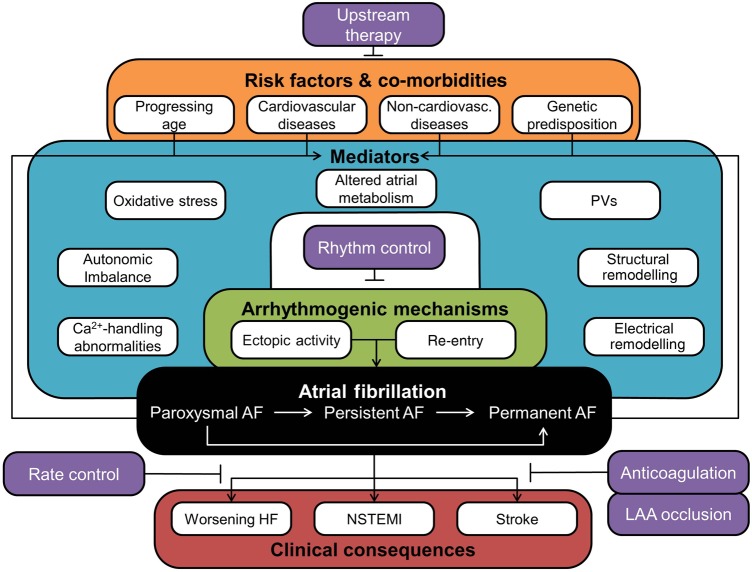
All forms of AF arise from interactions between genetic predisposition, advancing age, environmental factors, and cardiovascular/non-cardiovascular diseases,3,6 which disturb normal atrial electrophysiology, promoting focal ectopic activity and re-entry, the fundamental arrhythmogenic mechanisms underlying AF initiation and maintenance (Figure 1). Of note, these components are also strongly modulated by AF itself. AF is classified into paroxysmal (pAF, converting spontaneously within 7 days to sinus rhythm), persistent (persAF, >7 days), long-standing persistent (>6 months, abbreviated as ‘chronic’ AF; cAF), and permanent forms, for which no further attempts are made to restore sinus rhythm.7 The interactions between AF-related remodelling, progression of co-morbidities, and dynamic environmental factors contribute to AF progression to more advanced forms.
Figure 1.
Conceptual overview of the major components of AF pathophysiology. AF is a progressive disease (black box) with important clinical consequences (red box). AF is initiated and maintained by two major arrhythmogenic mechanisms (green box) that are modulated by numerous mediators (teal box). Several risk factors and co-morbidities (orange box) as well as AF itself promote AF development and progression by acting upon these mediators. A number of therapeutic strategies (purple boxes) have been developed to treat AF and/or its clinical consequences. HF, heart failure; LAA, left-atrial appendage; NSTEMI, non-ST segment elevation myocardial infarction; PV, pulmonary vein.
2.2. Basic mechanisms of atrial electrical activity and arrhythmogenesis
2.2.1. Atrial action potential and calcium handling
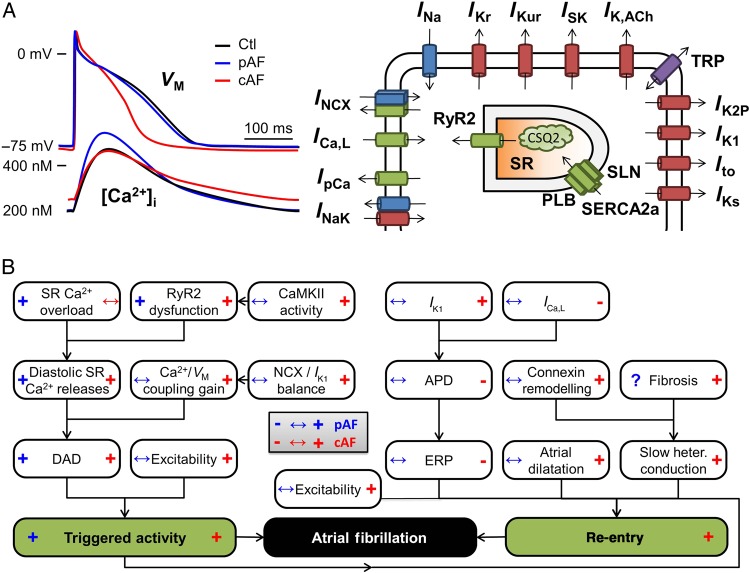
The main properties of atrial cellular electrophysiology and Ca2+ handling under physiological conditions, including differences between atrial and ventricular cardiomyocytes, have been discussed in detail elsewhere.6,8 In brief, the Na+ current (INa) is responsible for the action potential (AP) upstroke. Repolarization is controlled by the balance between numerous ion-currents, each with their specific kinetic characteristics and regulation, enabling precise control over AP morphology and duration (APD; Figure 2A). These include L-type Ca2+ current (ICa,L), responsible for initiating Ca2+ release from the sarcoplasmic reticulum (SR) through type-2 ryanodine receptor (RyR2) channels, producing the systolic Ca2+ transient and activating excitation–contraction coupling, as well as a wide range of K+ channels. The K+ channels mediate inward-rectifier K+ currents that are activated under basal conditions (IK1) or in response to vagal stimulation (acetylcholine-activated IK,ACh), and multiple delayed rectifier K+ currents distinguished by their kinetics (e.g. slow IKs, rapid IKr, and ultra-rapid IKur). Each type of K+ channel is encoded by a distinct pore-forming alpha-subunit and contains various accessory and regulatory subunits, allowing control through numerous signalling pathways.9 Recent work has identified additional ion channels that can influence atrial electrophysiology and arrhythmogenesis, including two-pore-domain K+ (K2P) channels,10 Kv1.1-channels,11 SK channels,12 and transient-receptor potential (TRP) channels,13 which present novel potential therapeutic targets (Figure 2A). Haemodynamic factors that induce atrial stretch affect a variety of stretch-sensitive channels that can modulate APD and various forms of spontaneous activity.14
Figure 2.
Atrial electrophysiology and basic arrhythmogenic mechanisms. (A) Representative atrial action potentials (APs) and Ca2+ transients from sinus rhythm (Ctl), paroxysmal AF (pAF) and long-standing persistent (chronic) AF (cAF) patients (left), and schematic overview of the major atrial ion channels and Ca2+-handling proteins (right). (B) Electrophysiological mechanisms of AF-promoting triggered activity (left part) and re-entry (right part). Blue and red symbols in boxes indicate changes in AF-promoting factors observed in pAF (blue, left side) and cAF (red, right side) patients. APD, action-potential duration; DAD, delayed after-depolarization; ERP, effective refractory period; SR, sarcoplasmic reticulum. See text for further abbreviations.
SR Ca2+ release via RyR2 channels is strongly modulated by their subcellular environment. Atrial cardiomyocytes have a less well-developed T-tubular structure than ventricular cardiomyocytes, producing a centripetal Ca2+ wave from the sarcolemma to the cell centre during SR Ca2+ release. Ca2+ homeostasis is maintained through re-uptake into the SR by the SR Ca2+-ATPase (SERCA2a) and extrusion via the type-1 Na+/Ca2+-exchanger (NCX1). SERCA2a is inhibited by dephosphorylated phospholamban and sarcolipin, the latter being atrial-specific, and their phosphorylation disinhibits SERCA2a, allowing for the regulation of SR Ca2+ uptake under the control of various kinases and phosphatases. Na+ homeostasis is maintained through Na+-K+-ATPase and is closely coupled to atrial Ca2+ handling through NCX1.15 Different AF forms have been associated with distinct remodelling patterns, producing altered APs and Ca2+ handling (Figure 2A) that predispose to AF initiation and maintenance through triggered activity and/or re-entry.
2.2.2. Triggered activity
Triggered activity can result from early- or delayed after-depolarizations (EADs and DADs, respectively). EADs occur with prolonged repolarization, allowing L-type Ca2+ channels to recover from voltage/Ca2+-dependent inactivation and produce secondary depolarizations before full AP-repolarization. DADs result from spontaneous diastolic SR Ca2+ release events (SCaEs) that activate NCX, producing a depolarizing transient-inward current. If membrane depolarization is sufficiently large to reach threshold, a triggered AP ensues, which can produce focal ectopic firing. These ectopic foci can trigger AF-maintaining re-entry in a vulnerable substrate or, if they fire repetitively, act as AF-maintaining drivers.
2.2.3. Re-entry
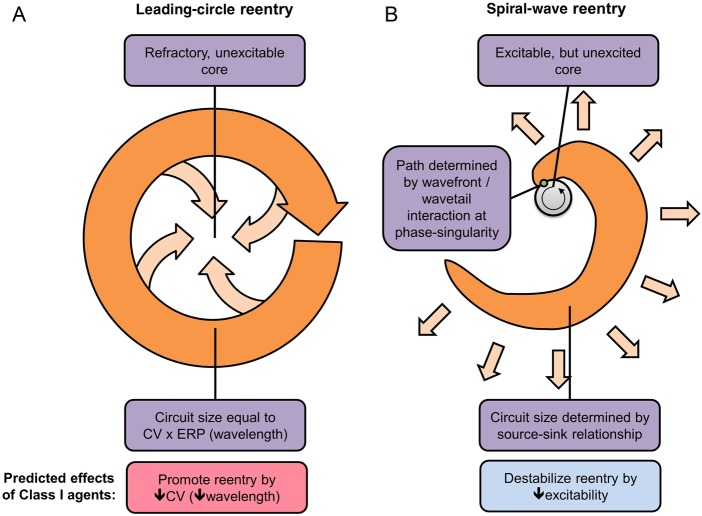
Re-entry can occur around a fixed anatomical obstacle when the balance between conduction velocity (CV) and effective refractory period (ERP) allows atrial tissue to become re-excitable before the re-entrant impulse arrives.16 Re-entry can also occur with a purely functional substrate, conceptually described by leading-circle and spiral-wave theories (Figure 3).16,17 In leading-circle re-entry, activity occurs around a refractory core in a circuit with a size at least equal to the wavelength (CV × ERP, Figure 3A). Spiral-wave re-entry depends on wave curvature and sink-to-source relationships between the wavefront's excitatory current and the current drawn off to excite neighbouring tissue (Figure 3B).16,17
Figure 3.
Leading-circle (A) and spiral-wave (B) models of re-entry. (A) Maintenance of the leading circle depends on there being a zone of tissue large enough to accommodate a re-entry circuit of the dimension (wavelength) travelled by the cardiac impulse in one effective refractory period (ERP), given by the product of conduction velocity (CV) and ERP. Importantly, drugs (like Class I antiarrhythmics) that reduce CV should favour leading-circle re-entry by reducing the wavelength. (B) Maintenance of spiral-wave re-entry depends on current source/tissue excitability (favouring propagation) and current sink (impairing propagation). In this paradigm, Class I agents destabilize spiral-wave sources by reducing current source/excitability.
2.3. Factors promoting atrial arrhythmogenesis
2.3.1. Ca2+-handling abnormalities
Increased SR Ca2+ leak with enhanced SCaE incidence has been observed in both pAF and cAF, albeit with distinct underlying molecular mechanisms (Figure 2B, left).6,18,19 In cAF, SCaEs are primarily due to RyR2 hyperphosphorylation caused by increased Ca2+/calmodulin-dependent protein kinase-II (CaMKII) activity.18 In contrast, in pAF, CaMKII activity is unchanged and SCaEs are due to phosphorylation-independent RyR2 dysregulation and increased SR Ca2+ load resulting from increased SERCA2a activity (Figure 2B, left).19 RyR2 dysregulation in pAF involves both increased channel open probability and increased protein expression levels, likely due to a reduction in the inhibitory microRNA-106b-25 cluster.20 Although Ca2+-handling abnormalities/DADs occur in atrial cardiomyocytes from cAF patients,18,19 atrial after contractions are reduced in atrial trabeculae from AF patients,21 and there is recent evidence that rapid atrial rates may cause Ca2+ silencing.22 More work is clearly needed to clarify the role of abnormal Ca2+ handling in the generation of atrial ectopic activity and the initiation/maintenance of AF in specific patient populations.
Atrial Ca2+-handling abnormalities associated with catecholaminergic polymorphic ventricular tachycardia (CPVT) reduce atrial CV by inhibiting INa.23 Computational modelling has also shown that subthreshold DADs can cause local conduction slowing and/or block by reducing INa.24 Finally, Ca2+-dependent signalling can contribute to atrial electrical and structural remodelling (discussed below). Thus, in addition to focal ectopy, Ca2+-handling abnormalities can contribute to the re-entry-promoting substrate facilitating AF maintenance.
2.3.2. Electrical remodelling
Re-entry-promoting shortening of APD seen with cAF (Figure 2B, right) is due to reduced ICa,L and increased repolarizing currents, particularly IK1 and agonist-independent ‘constitutive’ IK,ACh, all of which are in part Ca2+ regulated.4,6 Increased inward-rectifier K+ currents also produce resting membrane potential hyperpolarization that enhances excitability and stabilizes spiral-wave re-entry.25 Up-regulation of other K+ currents like two-pore K+ currents10 may contribute to APD shortening. Qualitatively similar atrial electrical remodelling occurs in animal models.26 In contrast, APD of pAF patients is unchanged (Figure 2B, right),10,19 suggesting that APD shortening is a consequence of AF. Finally, electrical remodelling can change the expression, function, or localization of connexins, altering cell-to-cell electrical coupling and resulting in re-entry-promoting slow and/or heterogeneous conduction.27
2.3.3. Atrial structural remodelling
Atrial structural remodelling is a major re-entry-promoting factor.1,3 Atrial fibrosis produces heterogeneous pathways of slow conduction and atrial dilatation provides larger pathways that more readily sustain (multiple) re-entrant circuits.28 Many co-morbidities and risk factors for AF cause atrial structural remodelling.3 Furthermore, AF can promote atrial fibrosis, contributing to AF progression.28,29 Fibrosis is caused by activation of cardiac (myo)fibroblasts in response to a wide range of growth factors, cytokines, hormones, and stress signals.28,29 The atria are more susceptible than ventricles to develop fibrosis.30 Recent work suggests that structural remodelling might be Ca2+ dependent. Genetic ablation of RyR2 hyperphosphorylation reduces SR Ca2+ leak, suppresses atrial dilatation, forestalls atrial conduction abnormalities, and prevents AF progression in mice with cardiac-restricted overexpression of a repressor form of cAMP-response element modulator,31 whereas increased fibroblast Ca2+ entry through TRP canonical-3 channels promotes fibroblast proliferation and AF-promoting remodelling.32 Atrial dilation is closely related to AF risk, and atrial stretch is known to promote AF.33
2.3.4. Autonomic imbalance
Increased activity of both sympathetic and parasympathetic components of the autonomic nervous system (ANS) can promote AF.34 Vagal stimulation activates G-protein-coupled muscarinic receptors, causing Gβγ-subunit-mediated activation of IK,ACh, reduced atrial ERP, and enhanced re-entrant activity/AF maintenance. The effects of acetylcholine are strongly localized via efficient degradation by acetylcholinesterases, causing heterogeneous effects on repolarization that contribute to arrhythmogenesis.34 Sympathetic stimulation engages numerous signalling pathways through G-protein-coupled receptors, activating PKA and downstream CaMKII, producing phosphorylation of many ion channels and Ca2+-handling proteins, including phospholamban, RyR2, and L-type Ca2+ channels.34 The resulting increase in cardiomyocyte Ca2+ cycling mediates the positive inotropic effects of sympathetic stimulation but may also promote SCaEs, DADs, and triggered activity. Simultaneous sympathovagal activity commonly precedes atrial arrhythmias.34 Combined sympathetic/parasympathetic stimulation causes IK,ACh-mediated APD shortening along with large Ca2+ transients, creating a substrate for late Phase 3 EADs.32 Finally, chronic sympathetic hyperactivity can promote AF maintenance via CaMKII and calcineurin-mediated structural remodelling.34
3. What has basic research contributed to present state-of-the-art AF therapy?
3.1. Pharmacological therapy
Because of the large size of the AF population, pharmacological approaches will likely remain important for AF therapy. Class I and Class III antiarrhythmic drugs like flecainide, sotalol, and amiodarone are currently the most commonly used options for pharmacological rhythm control. Basic and clinical sciences have grown together over the past three decades and have interacted frequently. Initially, the role of basic science was primarily to understand the mechanisms underlying therapeutic efficacy and toxicity of empirically developed antiarrhythmic drugs. As our understanding of fundamental pharmacological and physiological processes developed, basic science contributed increasingly to the discovery of new therapeutic targets, approaches, and agents.
3.1.1. Class I antiarrhythmic drugs
Class I antiarrhythmic agents have been used to treat AF since the early 1920s.35 Class Ic agents were developed in the course of a concerted search for potent suppressors of ectopic activity, with the assumption that sudden cardiac death results from critically timed premature ventricular extrasystoles falling on the vulnerable phase of the cardiac cycle.36–38 AF-suppressing activity of Class Ic agents was subsequently noted in clinical39 and experimental40 models, and Class Ic agents are now widely used to treat AF.
Basic research led to the development of Class Ic agents; however, the clinical observation that they are effective in AF challenged the then-prevailing theory of re-entry (the leading-circle hypothesis, Figure 3A), according to which drugs that slow conduction should decrease the wavelength and thereby promote AF rather than suppress it.41 This apparent paradox led to extensive theoretical analysis and basic research, which ultimately provided a satisfactory explanation for Class I drug efficacy in AF based on the spiral-wave concept (Figure 3B).16,42 The extensive development of Class I antiarrhythmic drugs in the 1980s also led to improved understanding of the fundamental biophysical determinants of state-dependent Na+-channel-blocking action.43,44 These advances led to the subsequent systematic development of novel Na+-channel blockers to treat AF; like vernakalant, a highly effective drug for rapid termination of recent-onset AF.45–47 The main limitation to the wider development and the use of Class I agents for AF is the risk of pro-arrhythmic and other side effects of ventricular Na+-channel blockade. A potentially promising approach to minimizing this risk is the development of AF-selective agents,48 discussed further below.
3.1.2. Class III antiarrhythmic drugs
APD prolongation as a distinct mechanism of antiarrhythmic efficacy was identified in basic studies with sotalol and labelled Class III action by Singh and Vaughan Williams in 1970.49 Sotalol was first shown to suppress AF recurrences by Prakash et al.50 The benefit of Class III action in AF fits with the suppressant effect of APD prolongation in both leading-circle and spiral-wave paradigms.16 The Class III principle was rapidly identified as central to the antiarrhythmic effects of amiodarone51 and applied to develop new antiarrhythmic agents like ibutilide by molecular design based on the structures of sotalol and clofilium.52 The main limitation to the use of Class III drugs in AF is the risk of Torsades de Pointes due to excess ventricular APD prolongation.53 One way to preserve effective Class III action in AF, while minimizing ventricular pro-arrhythmic liability is to develop agents with atrial/AF-selective actions,54 as discussed further below.
3.1.3. Multiple ion-channel blockers
Amiodarone was initially considered a Class III antiarrhythmic drug and was noted to be remarkably effective against a wide range of arrhythmias, including AF.55 It was subsequently found to have clinically relevant state-dependent blocking properties on both Na+ and Ca2+ channels56,57 and recognized to have actions of all antiarrhythmic classes.58 Amiodarone has about twice the anti-AF efficacy of Class Ic and Class III agents.59 Its unique effectiveness and low pro-arrhythmic potential have been attributed to its broad spectrum of ion-channel-blocking action. However, amiodarone is plagued by a host of extracardiac adverse effects with long-term therapy, which limit its clinical applicability.
The pharmaceutical industry therefore searched for compounds that would retain amiodarone's advantages without its liabilities. One approach was to apply molecular engineering to the amiodarone molecule. A prominent result of this effort was dronedarone,60 with structural similarities to amiodarone but lacking its iodine moieties (eliminating troublesome thyroid toxicity) and possessing an added methanesulphonyl group (reducing lipophilicity, creating more tractable pharmacokinetics). Dronedarone showed surprising benefits against cardiovascular events in the ATHENA trial.61 However, the drug also proved to enhance mortality in some patient populations, particularly those with structural heart disease and significant left ventricular dysfunction.62–64
An alternative, and perhaps superior approach, lies in the identification of specific combinations of ion-channel blockade needed to optimize antiarrhythmic drug efficacy and safety and then producing them with modern biopharmaceutical technology.65 The main challenge to this approach has been the accurate pinpointing of the requisite channel-blocking profile.
3.1.4. Substrate-targeting/upstream therapy
The concept of ‘atrial remodelling’ arose from basic research in animal models.28 The prevention of AF-promoting remodelling is a potentially interesting therapeutic approach that was initially proposed based on experimental observations66 and has the advantage of targeting the arrhythmic substrate rather than the final electrical end product. The notion of directing antiarrhythmic therapy to underlying cardiovascular disease processes was first suggested 25 years ago67 and termed ‘upstream therapy’ by the Sicilian Gambit group 8 years later.68 While there is extensive experimental evidence for the prevention of AF by upstream therapy with such agents as statins, renin-angiotensin-aldosterone system (RAAS) inhibitors and anti-inflammatory agents, solid clinical confirmation has been much harder to obtain.69,70 Part of the reason may be the slow development of atrial remodelling and its limited reversibility once established. The only upstream therapy presently conditionally recommended by European Society of Cardiology guidelines is addition of a RAAS-antagonist after cardioversion. However, basic studies have provided insights into the mechanisms by which conditions like obesity71 and obstructive sleep apnoea72 promote AF. Encouraging data have been presented for the clinical targeting of these AF risk factors,73,74 indicating that basic research may help to identify targets for effective AF prevention interventions.75
3.2. Non-pharmacological therapies
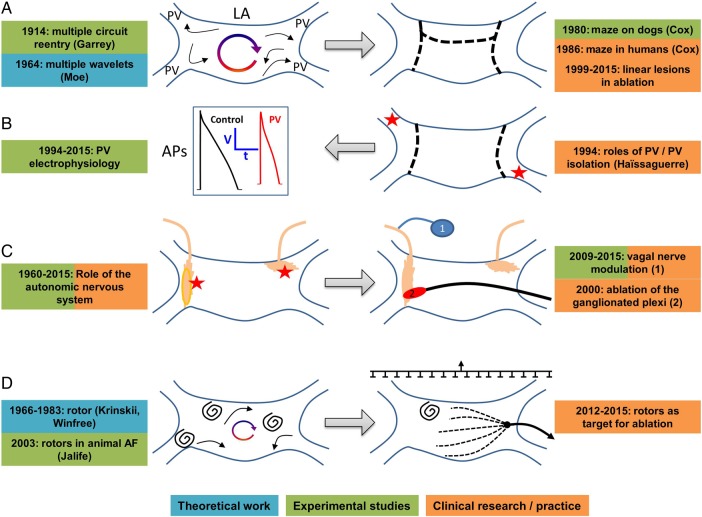
The development of surgical/ablation approaches to AF management has been an iterative process between basic and clinical research, with key discoveries in either sphere leading to extensive progress in the other (Figure 4).76 The first, and arguably most successful, non-pharmacological procedure for AF was the maze operation,77 based on the classical multiple-circuit/multiple-wavelet basic mechanism initially described by Walter Garrey and later refined by Gordon Moe.1 With the widespread, largely successful application of ablation to other arrhythmias, the first attempts at rhythm control for AF patients by ablation mimicked the surgical maze.76 These efforts were largely unsuccessful because of inability to create transmural linear atrial lesion sets and complications of left-atrial thrombogenesis.
Figure 4.
Development of non-pharmacological AF therapies based on basic clinical research interaction. (A) Development of surgical maze procedure based on basic theory and observation. (B) Clinical recognition of role of pulmonary veins (PVs) led to experimental definition of mechanisms. (C) Experimental studies of role of cardiac ganglionated plexuses in AF led to clinical approaches. (D) Experimental and theoretical work on rotors and mapping led to improved methods for persistent AF ablation.
A paradigm shift in AF ablation came from the purely clinical observation of the important role of pulmonary vein (PV) cardiomyocyte sleeves by Haissaguerre and colleagues.78 Subsequent basic research provided insight into the complex mechanisms underlying the participation of the PVs, which are particularly important in pAF.79 Clinical observations indicated that recurrences of pAF are generally due to resumption of conduction from PV to left atrium, and that purinergic agonists can identify ‘dormant’ PVs at risk of reconnection.80 Experimental work identified the mechanisms underlying dormant conduction,81 and a subsequent multicentre clinical trial confirmed the ability of adenosine-guided PV ablation to reduce pAF recurrence rates.82
The next major challenge has been the successful ablation of persAF, which has proved more challenging than pAF.76 Empirical clinical methods like the targeting of complex fractionated atrial electrograms and the stepped application of additional linear lesions have not proved terribly successful, and a recent controlled trial suggests that they may even be of less value for persAF than PV isolation alone.83 Recent basic research developments may help to improve the success of persAF ablation. The potential role of spiral-wave rotors in arrhythmia was first described in the Russian literature in the mid-1960s,84 and the concept applied to AF by the Jalife lab.85 Subsequent developments in non-linear dynamic theory and intracavitary basket electrode technology led to proprietary software for the mapping and ablation of AF drivers, including both stationary rotors and focal sources (‘Focal Impulse and Rotor Modulation’ or FIRM). Initial work by Narayan et al.86 has suggested that this approach may greatly improve the success of persAF ablation. Another important advance has been the development of non-invasive electrocardiographic imaging by body surface-potential recording and computational inverse solution, which can identify AF sources.87 This method has been used to localize driver regions and guide persAF-ablation.88 Rotors were also evident in the latter work, but were much more evanescent than seen with FIRM and not amenable to direct targeting. Other recent studies have emphasized the importance of transmural transmission in multiplying the number of active waves89 and/or re-entry circuits in persAF,90 particularly the long-standing variety; thus, the precise mechanisms maintaining persAF remain controversial. Notwithstanding the controversies, the evolving concepts and methodologies emerging from this basic work promise to lead to more successful approaches to persAF ablation and are presently being evaluated in various clinical trials (ClinicalTrials.gov numbers NCT02101541, NCT02274857, NCT01924377, NCT02386345, NCT02497248, NCT02113761).
A final potential application of basic research to improve AF ablation has been increasing awareness of the role of the cardiac ANS.34 Enhancement of vagal and/or sympathetic tone can promote AF,34 and remodelling of atrial autonomic innervation occurs with AF-promoting pathology or AF itself.91 The location of autonomic ganglia near the PV ostia makes their modulation a potential contributor to the effectiveness of PV ablation.92 Ablation targeting atrial ganglionated plexuses has shown promise in improving the success of PV isolation procedures.93 Creative new approaches to non-invasive modulation of autonomic tone are being developed to manage AF.34
4. Mechanism-based therapeutic innovation
The information presented above clearly indicates that basic research has played a major role in developing present state-of-the-art therapy for AF. It is reasonable to wonder whether the extensive basic science advances over the past 10–20 years will lead to practical new developments in AF management.
4.1. Pharmacological therapies
4.1.1. Novel ion-channel targets
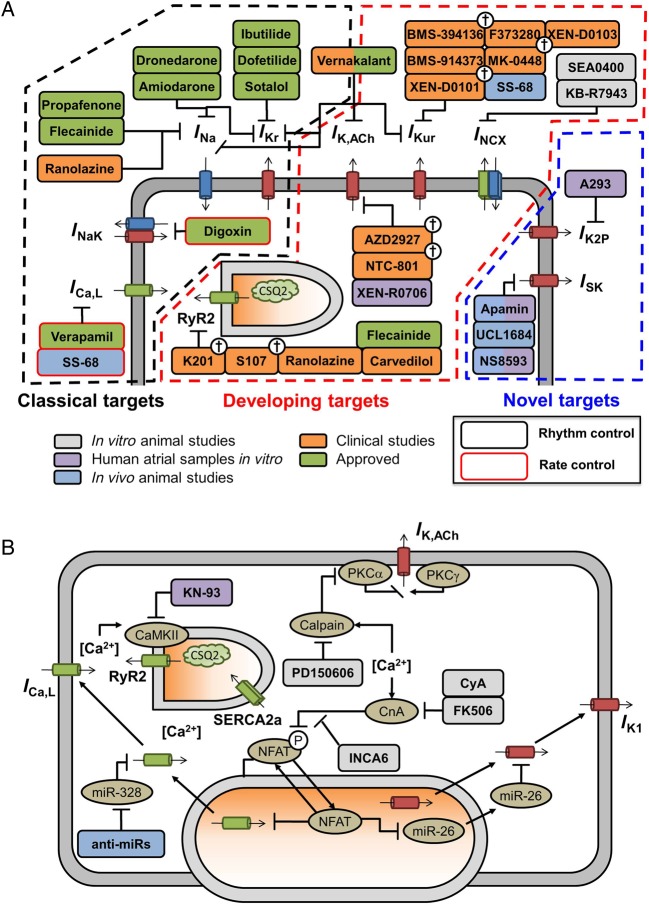
In addition to the ‘classical’ targets for both cardioversion and rhythm control of AF (INa, IKr, and ICa,L), a range of novel potential targets have been identified through fundamental research into basic AF mechanisms (Figure 5A). There is particular interest in K+ channels with atrial-predominant expression such as IKur and constitutive IK,ACh to avoid ventricular pro-arrhythmic side effects.94 SK channels represent another novel K+ channel target with relative atrial predominance, albeit with a still incompletely understood role in cardiac electrophysiology.12,94 Since K2P channels show atrial-predominant expression,10 they might similarly provide specific anti-AF effects. In contrast to IKur, K2P current is up-regulated in cAF,10 potentially enhancing anti-AF efficacy of K2P-channel inhibition, although no in vivo data are available. Stretch-related channels are also a potential target,95 although their ubiquity and complex nature present a challenge to therapeutic targeting.
Figure 5.
Schematic overview of pharmacological anti-AF therapies and their targets. (A) Antiarrhythmic drugs targeting ion channels/transporters involved in atrial repolarization and Ca2+ handling. (B) Compounds targeting Ca2+-dependent signalling pathways involved in electrical remodelling, including Ca2+/calmodulin-dependent protein kinase-II (CaMKII) and calcineurin-A (CnA)-mediated signalling cascades, as well as calpain-dependent protein degradation. Clinically approved antiarrhythmic drugs are shown in green, compounds evaluated in clinical trials are shown in orange, with abandoned compounds indicated with †. Experimental compounds with anti-AF properties in animal studies in vivo or human atrial samples in vitro are shown in blue and purple, respectively, whereas compounds with anti-arrhythmic properties in animal samples are shown in grey.
Very limited data are available from human studies on these developing targets. The only IKur inhibitor tested in man was MK-0448, which did not prolong atrial ERPs in healthy volunteers.96 However, it has been known for over 15 years that IKur block is likely to prolong APD only in remodelled tissue and at rapid rates,97 so these negative data are not surprising and do not exclude benefit from IKur inhibition. Studies need to be performed looking for AF prevention or termination, and/or ERP effects at rapid atrial rates or in tachycardia-remodelled atria. Similar considerations apply to IK,ACh blockade. Constitutive IK,ACh is negligible in normal human atrium, but becomes significant with AF-related remodelling.98 Recent work showed that IK,ACh blockers did not affect atrial-ERP in atrial-flutter patients who had been in sinus rhythm for >3 months.91 Given the rapid reversal of electrical remodelling (within 48 h of tachycardia termination), the lack of effect is not surprising and does not exclude possible value of IK,ACh blockade.
4.1.2. Ca2+-handling targets
Ca2+-handling abnormalities have been proposed as promising antiarrhythmic targets.15,94 SCaEs and their arrhythmogenic consequences could be targeted through RyR2-stabilizing drugs or NCX inhibitors. For both targets, several experimental compounds have shown promise in vitro or in animal models, but successful clinical application still awaits (Figure 5A). The differences in molecular mechanisms underlying SCaEs between pAF and cAF suggest the need for AF-type-tailored therapy. For example, cardiac CaMKII inhibition might be a useful strategy for cAF patients, but appears less suitable for pAF.15,18,19 Carvedilol and several derivatives suppress arrhythmogenic Ca2+ release.99 The only clinical data available are from a small but well-designed trial in post-operative AF, in which triggered activity is believed to play a prominent role and did not show superiority of carvedilol over a comparator β-blocker metoprolol.100 Modulation of SERCA2a activity might be used to limit SR Ca2+ overload in pAF patients.15
The persistent RyR2 Ca2+ leak in AF patients might also initiate Ca2+-dependent pathways contributing to the remodelling processes that promote AF progression (Figure 5B).31 The Ca2+-dependent protease calpain is activated in AF patients and likely contributes to the ICa,L reduction and PKCα-dysregulation/constitutive IK,ACh activation that underlie AF-related APD shortening.101 Thus, calpain inhibitors might help to reduce AF-related electrical remodelling. Activation of NFAT signalling down-regulates the IK1-inhibitory microRNA-26, contributing to AF stabilization due to increased IK1,102 and inhibition of the Ca2+-dependent calcineurin/NFAT-system is another option to target pro-arrhythmic Ca2+-dependent remodelling. A number of microRNAs (miRs) have been shown to play a role in experimental AF. A detailed discussion is outside the scope of the present paper—the interested reader is referred to a recent detailed review.103
4.1.3. Atrial metabolism
AF activates the atria very rapidly, enhancing their metabolic demands and increasing atrial oxygen extraction and lactate production.104 Metabolomic studies point to disruption of atrial metabolic chains in AF and AF-predisposing pathologies.105,106 Metabolic dysfunction might thus contribute to AF promotion and be a therapeutic target. AMP-dependent protein kinase (AMPK) is a metabolic stress sensor/adaptor activated by AF or AF-like pacing paradigms.107,108 AMPK activation limits the electrophysiological/contractile dysfunction caused by metabolic dysfunction and might prevent AF progression.107 On the other hand, AMPK activation can also increase cardiomyocyte lipid uptake and decrease glucose uptake.108,109 AMPK suppression via upstream-kinase knockout causes spontaneous AF in mice.110 Metformin is an AMPK activator,111 so it should be possible to test the effects of AMPK activation on AF in both animal and clinical models. Epidemiological data suggest that metformin therapy might indeed reduce AF risk.112
4.1.4. Autonomic-tone manipulation
A variety of approaches are being developed to modulate the ANS, given its important role in determining AF occurrence.34 Paradoxically, low-level vagal stimulation suppresses autonomic outflow and AF occurrence in dogs.113 A non-invasive approach using transcutaneous nerve stimulation on the tragus to modulate cardiac neural tone reduced AF-duration, increased AF cycle length, and suppressed inflammatory cytokines in anaesthetized patients.114 A controlled trial (NCT02548754) is testing the value of transcutaneous electrical vagus nerve stimulation in pAF patients.
4.1.5. Multiple ion-channel targets
Based on experience with amiodarone, drugs blocking multiple ion channels might have superior efficacy and/or safety profiles. While combination drug therapy for AF was first reported with hydroquinidine/reserpine in 1966115 and the first channel-blocking drug combination therapy for AF was reported in 1978,116 very little basic research has been done to define a rational basis for multichannel blocker-based AF therapy. Following up on experimental results,117 the HARMONY trial tested the value of combining reduced doses of the rapidly unblocking INa inhibitor ranolazine and the amiodarone-derivative dronedarone in AF. The results showed combination therapy to be synergistically effective and well tolerated.118 Recent in silico work indicates that Na+-channel-blocking properties for AF-selective action can be optimized for AF selectivity,119 and that AF selectivity can be improved by adding K+-channel blockade.120
5. Challenges for the translation of basic mechanisms to therapeutic innovation
5.1. Limitations of models
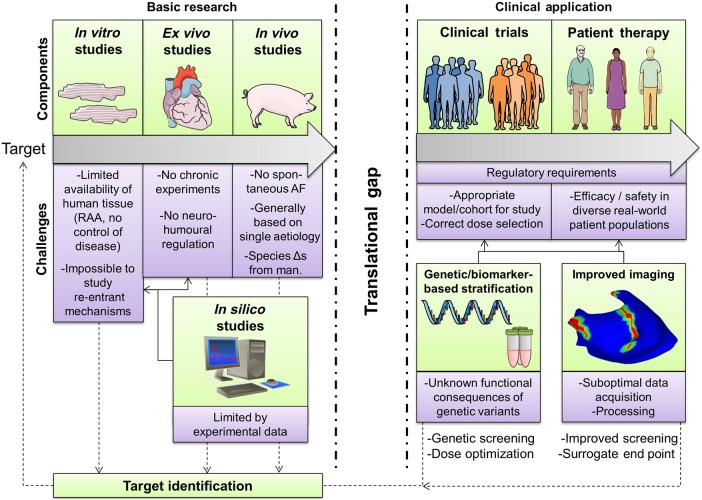
Each experimental AF model has advantages and drawbacks, as well as relevance to specific aspects of clinical AF, which have to be considered in translating basic research observations.26 Each type of basic research study (e.g. in vitro, ex vivo, in vivo, in silico) has its own limitations (Figure 6). Animal models allow control over co-morbidities and experimental conditions, both in vivo and during subsequent tissue and/or cell isolation, enabling detailed multi-scale investigation of specific AF-promoting mechanisms.26 Genetic manipulation allows the assessment of the roles of specific molecular species/proteins. Because of ethical considerations regarding patient studies, animal models represent a natural starting point for the evaluation of novel pharmacological and technological interventions. However, animal models have several drawbacks. The time course of substrate development and AF progression is much shorter than in patients and generally mono-factorial. Spontaneous AF initiation is rare: AF initiation by programmed electrical stimulation is usually needed to assess the AF substrate.6,26 Another gap is the paucity of animal models of pAF, with the limited information about pAF mechanisms available to date mainly based on experiments using tissue from pAF patients.19 Human tissue samples capture the full complexity of the pathophysiological mechanisms underlying AF in humans, but are limited by issues of tissue procurement, limited intraoperative access to sites other than the right-atrial appendage, and intrinsic clinical variability (age, co-morbidities, heart disease, drug therapy, etc.). Common applications of human samples include the validation of mechanisms identified in animal models, and the comparison of specific ion channels or proteins between patients with vs. without AF (or AF-promoting disease) to identify potential therapeutic targets. An evolving technology that may prove useful for preclinical investigations relevant to the study of human AF mechanisms and intervention involves the use of induced pluripotential stem-cell (iPSC) technology.121 In addition to generating APs with human ion-current composition for cellular studies, iPSCs can be engineered to produce tissue-like cellular arrays with coupling and conduction properties that are a step closer to in situ human heart conditions.122 However, electrophysiological and Ca2+-handling properties of iPSC-derived cardiomyocytes do not yet fully recapitulate those of the adult atrial cardiomyocyte. Computational modelling can integrate experimental findings from animal models and/or human studies into a conceptual framework. Computer models allow perfect control over parameters and complete observability, making them particularly suitable to investigate the relative contribution of specific molecular alterations to the overall cellular phenotype.123 However, computer models are limited by the experimental data on which they are based, do not capture inter-subject or cell-to-cell differences, and cannot simulate long-term remodelling processes or detailed macromolecular changes.123 Furthermore, the incorporation of detailed (sub)cellular models of the atrial cardiomyocyte in multi-cellular simulations of virtual tissue remains technically challenging. Careful use of a combination of AF models is thus needed to define basic mechanisms amenable to clinical translation.
Figure 6.
Elements involved in the translation from novel potential antiarrhythmic targets to clinical application. Several types of basic research studies (in vitro, ex vivo, in vivo) play critical roles in target identification and optimization, but each individually have several limitations/challenges (purple boxes), resulting in a significant translational gap before clinical application. Both clinical trials and patient therapy present additional challenges and are complicated by regulatory requirements. New/improved methodologies including in silico research, genetic/biomarker-based patient stratification and improved imaging may help to overcome some of these challenges, but also require further development. RAA, right atrial appendage.
5.2. Clinical considerations
Selection of the appropriate clinical model is crucial. The complexity of the AF population is a challenge, and the different forms/subtypes of AF need to be respected. It is likely a waste of time and money (and also potentially misleading) to test drugs expected to suppress re-entry, but not automaticity (like K+-channel blockers), in patients with frequent pAF episodes likely to be due to abnormal/triggered automaticity.79 On the other hand, such patients might be ideal to assess drugs suppressing SCaEs/triggered activity. Drugs designed to prevent atrial remodelling need to be used in a population at high risk of such remodelling, before remodelling is advanced and irreversible. Issues of appropriate dosing and population variability need to be considered. For example, women have more complications and less benefit from AF therapy, and a higher AF-related morbidity and mortality,124 indicating gender-specific mechanisms that need consideration.
The rapid development of medical genetics and imaging methods offer new opportunities. With time, improved genetic tools may refine identification of the correct target population and determination/adjustment of drug doses. Genetic variants at the 4q25 locus have been associated with improved symptom control with antiarrhythmic drugs, longer recurrence-free survival after AF ablation, and lower AF recurrence rates after electrical cardioversion.125,126 Moreover, individuals carrying certain 4q25 variants may respond better to Class I than to Class III antiarrhythmic drugs,125 highlighting opportunities for tailored therapy,126 which is currently under evaluation in a randomized, genotype-directed sequential cross-over study with flecainide and sotalol.126 However, the pathophysiological mechanisms modulated by these variants remain largely unknown and the results have not always been reproducible. For instance, genetic variants did not predict AF ablation success in an Asian population.127 Other parameters, based on imaging, ECG complexity, etc., have also been suggested.128 Finally, advances in the monitoring of AF (e.g. using implantable loop recorders) may help to perform trials with more detailed endpoints than just ‘AF recurrence’.126 Refinements in imaging may allow for better candidate screening and surrogate endpoints.
5.3. Practical issues
Regulatory requirements on drug development are extremely strict, contributing to development costs of $5 billion (4.4 billion Euro) for each new drug.129 The bar is particularly high for antiarrhythmic agents, for which mortality trials are generally demanded, greatly enhancing cost and complexity. All arrhythmia specialists are aware of the rising predominance of non-pharmacological vs. pharmacological therapies. Without in any way detracting from the advantages of non-pharmacological therapies, it is also true that the regulatory demands are generally less stringent for non-pharmacological therapies than pharmacological, both on the efficacy and safety sides. New antiarrhythmic compounds are often quickly abandoned with the slightest indication that they fail to meet expectations, in a ‘fail early fail cheaply’ approach.130 Thus, agents that are beneficial to specific types or subpopulations of AF are easily discarded. Since it is unlikely that there will be a single ‘magic bullet’ to treat all forms of AF, it will be critical to establish the subset of AF patients most likely to benefit from a given therapy based on mechanistic insights during preclinical studies and design clinical trials accordingly. AF is an enormous clinical problem and the available therapies are quite limited; regulatory requirements may have to be reconsidered to permit the effective development of new alternatives.
6. Conclusions
AF research and therapeutic development have been marked by complex mutual interactions between clinical and basic research. The insights developed from experimental work have contributed greatly to presently available AF therapies. Recent advances in basic research and technology development, as well as direct application to clinical contexts and models, promise to catalyze exciting future advances in AF management.
Conflict of interest: V.A. received scholarship funding from St Jude Medical, Biotronik, Boston, Medtronic and Sorin. X.H.T.W. is a founding partner of Elex Biotech, a startup company developing antiarrhythmic drugs. The other authors have no disclosures.
Funding
European Network for Translational Research in Atrial Fibrillation (EUTRAF, No. 261057 to D.D.), DZHK (German Center for Cardiovascular Research to D.D.); Canadian Institutes of Health Research (6957 and 44365 to S.N.) and Heart and Stroke Foundation of Canada (S.N.); Fédération française de Cardiologie (V.A.); National Institutes of Health (HL089598, HL091947, HL117641, HL129570 to X.H.T.W.), and American Heart Association (13EIA-14560061 to X.H.T.W.).
References
- 1.Nattel S. New ideas about atrial fibrillation 50 years on. Nature 2002;415:219–226. [DOI] [PubMed] [Google Scholar]
- 2.Nishida K, Nattel S. Atrial fibrillation compendium: historical context and detailed translational perspective on an important clinical problem. Circ Res 2014;114:1447–1452. [DOI] [PubMed] [Google Scholar]
- 3.Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res 2014;114:1453–1468. [DOI] [PubMed] [Google Scholar]
- 4.Dobrev D, Nattel S. New antiarrhythmic drugs for treatment of atrial fibrillation. Lancet 2010;375:1212–1223. [DOI] [PubMed] [Google Scholar]
- 5.Heijman J, Voigt N, Dobrev D. New directions in antiarrhythmic drug therapy for atrial fibrillation. Future Cardiol 2013;9:71–88. [DOI] [PubMed] [Google Scholar]
- 6.Heijman J, Voigt N, Nattel S, Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res 2014;114:1483–1499. [DOI] [PubMed] [Google Scholar]
- 7.Camm AJ, Al-Khatib SM, Calkins H, Halperin JL, Kirchhof P, Lip GY, Nattel S, Ruskin J, Banerjee A, Blendea D, Guasch E, Needleman M, Savelieva I, Viles-Gonzalez J, Williams ES. A proposal for new clinical concepts in the management of atrial fibrillation. Am Heart J 2012;164:292–302 e291. [DOI] [PubMed] [Google Scholar]
- 8.Schram G, Pourrier M, Melnyk P, Nattel S. Differential distribution of cardiac ion channel expression as a basis for regional specialization in electrical function. Circ Res 2002;90:939–950. [DOI] [PubMed] [Google Scholar]
- 9.Bartos DC, Grandi E, Ripplinger CM. Ion channels in the heart. Compr Physiol 2015;5:1423–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt C, Wiedmann F, Voigt N, Zhou XB, Heijman J, Lang S, Albert V, Kallenberger S, Ruhparwar A, Szabo G, Kallenbach K, Karck M, Borggrefe M, Biliczki P, Ehrlich JR, Baczko I, Lugenbiel P, Schweizer PA, Donner BC, Katus HA, Dobrev D, Thomas D. Upregulation of K2P3.1 K+ current causes action potential shortening in patients with chronic atrial fibrillation. Circulation 2015;132:82–92. [DOI] [PubMed] [Google Scholar]
- 11.Glasscock E, Voigt N, McCauley MD, Sun Q, Li N, Chiang DY, Zhou XB, Molina CE, Thomas D, Schmidt C, Skapura DG, Noebels JL, Dobrev D, Wehrens XH. Expression and function of Kv1.1 potassium channels in human atria from patients with atrial fibrillation. Basic Res Cardiol 2015;110:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qi XY, Diness JG, Brundel BJ, Zhou XB, Naud P, Wu CT, Huang H, Harada M, Aflaki M, Dobrev D, Grunnet M, Nattel S. Role of small-conductance calcium-activated potassium channels in atrial electrophysiology and fibrillation in the dog. Circulation 2014;129:430–440. [DOI] [PubMed] [Google Scholar]
- 13.Simard C, Hof T, Keddache Z, Launay P, Guinamard R. The TRPM4 non-selective cation channel contributes to the mammalian atrial action potential. J Mol Cell Cardiol 2013;59:11–19. [DOI] [PubMed] [Google Scholar]
- 14.Ravens U. Mechano-electric feedback and arrhythmias. Prog Biophys Mol Biol 2003;82:255–266. [DOI] [PubMed] [Google Scholar]
- 15.Heijman J, Voigt N, Ghezelbash S, Schirmer I, Dobrev D. Calcium handling abnormalities as a target for atrial fibrillation therapeutics: how close to clinical implementation? J Cardiovasc Pharmacol 2015;66:515–522. [DOI] [PubMed] [Google Scholar]
- 16.Comtois P, Kneller J, Nattel S. Of circles and spirals: bridging the gap between the leading circle and spiral wave concepts of cardiac reentry. Europace 2005;7(Suppl. 2):10–20. [DOI] [PubMed] [Google Scholar]
- 17.Aguilar M, Nattel S. The pioneering work of George Mines on cardiac arrhythmias: groundbreaking ideas that remain influential in contemporary cardiac electrophysiology. J Physiol 2015; doi:10.1113/JP270506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, Wehrens XH, Dobrev D. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation 2012;125:2059–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voigt N, Heijman J, Wang Q, Chiang DY, Li N, Karck M, Wehrens XH, Nattel S, Dobrev D. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation 2014;129:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiang DY, Kongchan N, Beavers DL, Alsina KM, Voigt N, Neilson JR, Jakob H, Martin JF, Dobrev D, Wehrens XH, Li N. Loss of microRNA-106b-25 cluster promotes atrial fibrillation by enhancing ryanodine receptor type-2 expression and calcium release. Circ Arrhythm Electrophysiol 2014;7:1214–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christ T, Rozmaritsa N, Engel A, Berk E, Knaut M, Metzner K, Canteras M, Ravens U, Kaumann A. Arrhythmias, elicited by catecholamines and serotonin, vanish in human chronic atrial fibrillation. Proc Natl Acad Sci USA 2014;111:11193–11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greiser M, Kerfant BG, Williams GS, Voigt N, Harks E, Dibb KM, Giese A, Meszaros J, Verheule S, Ravens U, Allessie MA, Gammie JS, van der Velden J, Lederer WJ, Dobrev D, Schotten U. Tachycardia-induced silencing of subcellular Ca2+ signaling in atrial myocytes. J Clin Invest 2014;124:4759–4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King JH, Wickramarachchi C, Kua K, Du Y, Jeevaratnam K, Matthews HR, Grace AA, Huang CL, Fraser JA. Loss of Nav1.5 expression and function in murine atria containing the RyR2-P2328S gain-of-function mutation. Cardiovasc Res 2013;99:751–759. [DOI] [PubMed] [Google Scholar]
- 24.Liu MB, de Lange E, Garfinkel A, Weiss JN, Qu Z. Delayed afterdepolarizations generate both triggers and a vulnerable substrate promoting reentry in cardiac tissue. Heart Rhythm 2015;12:2115–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pandit SV, Berenfeld O, Anumonwo JM, Zaritski RM, Kneller J, Nattel S, Jalife J. Ionic determinants of functional reentry in a 2-D model of human atrial cells during simulated chronic atrial fibrillation. Biophys J 2005;88:3806–3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishida K, Michael G, Dobrev D, Nattel S. Animal models for atrial fibrillation: clinical insights and scientific opportunities. Europace 2010;12:160–172. [DOI] [PubMed] [Google Scholar]
- 27.Kato T, Iwasaki YK, Nattel S. Connexins and atrial fibrillation: filling in the gaps. Circulation 2012;125:203–206. [DOI] [PubMed] [Google Scholar]
- 28.Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol 2008;1:62–73. [DOI] [PubMed] [Google Scholar]
- 29.Dzeshka MS, Lip GY, Snezhitskiy V, Shantsila E. Cardiac fibrosis in patients with atrial fibrillation: mechanisms and clinical implications. J Am Coll Cardiol 2015;66:943–959. [DOI] [PubMed] [Google Scholar]
- 30.Burstein B, Libby E, Calderone A, Nattel S. Differential behaviors of atrial versus ventricular fibroblasts: a potential role for platelet-derived growth factor in atrial-ventricular remodeling differences. Circulation 2008;117:1630–1641. [DOI] [PubMed] [Google Scholar]
- 31.Li N, Chiang DY, Wang S, Wang Q, Sun L, Voigt N, Respress JL, Ather S, Skapura DG, Jordan VK, Horrigan FT, Schmitz W, Muller FU, Valderrabano M, Nattel S, Dobrev D, Wehrens XH. Ryanodine receptor-mediated calcium leak drives progressive development of an atrial fibrillation substrate in a transgenic mouse model. Circulation 2014;129:1276–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harada M, Luo X, Qi XY, Tadevosyan A, Maguy A, Ordog B, Ledoux J, Kato T, Naud P, Voigt N, Shi Y, Kamiya K, Murohara T, Kodama I, Tardif JC, Schotten U, Van Wagoner DR, Dobrev D, Nattel S. Transient receptor potential canonical-3 channel-dependent fibroblast regulation in atrial fibrillation. Circulation 2012;126:2051–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalifa J, Zaitsev A, Warren M, Berenfeld O, Nattel S, Jalife J. High atrial pressure-induced stretch during atrial fibrillation reveals high frequency periodic sources in the pulmonary vein region of the sheep heart (Abstract). Pacing Clin Electrophysiol 2003;26:950. [Google Scholar]
- 34.Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res 2014;114:1500–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nattel S, Hadjis T, Talajic M. The treatment of atrial fibrillation. An evaluation of drug therapy, electrical modalities and therapeutic considerations. Drugs 1994;48:345–371. [DOI] [PubMed] [Google Scholar]
- 36.Anderson JL, Stewart JR, Perry BA, Van Hamersveld DD, Johnson TA, Conard GJ, Chang SF, Kvam DC, Pitt B. Oral flecainide acetate for the treatment of ventricular arrhythmias. N Engl J Med 1981;305:473–477. [DOI] [PubMed] [Google Scholar]
- 37.Banitt EH, Bronn WR, Coyne WE, Schmid JR. Antiarrhythmics. 2. Synthesis and antiarrhythmic activity of N-(piperidylalkyl)trifluoroethoxybenzamides. J Med Chem 1977;20:821–826. [DOI] [PubMed] [Google Scholar]
- 38.Campbell TJ, Vaughan Williams EM. Voltage- and time-dependent depression of maximum rate of depolarisation of guinea-pig ventricular action potentials by two new antiarrhythmic drugs, flecainide and lorcainide. Cardiovasc Res 1983;17:251–258. [DOI] [PubMed] [Google Scholar]
- 39.Goy JJ, Maendly R, Grbic M, Finci L, Sigwart U. Cardioversion with flecainide in patients with atrial fibrillation of recent onset. Eur J Clin Pharmacol 1985;27:737–738. [DOI] [PubMed] [Google Scholar]
- 40.Wang Z, Page P, Nattel S. Mechanism of flecainide's antiarrhythmic action in experimental atrial fibrillation. Circ Res 1992;71:271–287. [DOI] [PubMed] [Google Scholar]
- 41.Rensma PL, Allessie MA, Lammers WJ, Bonke FI, Schalij MJ. Length of excitation wave and susceptibility to reentrant atrial arrhythmias in normal conscious dogs. Circ Res 1988;62:395–410. [DOI] [PubMed] [Google Scholar]
- 42.Kneller J, Kalifa J, Zou R, Zaitsev AV, Warren M, Berenfeld O, Vigmond EJ, Leon LJ, Nattel S, Jalife J. Mechanisms of atrial fibrillation termination by pure sodium channel blockade in an ionically-realistic mathematical model. Circ Res 2005;96:e35–e47. [DOI] [PubMed] [Google Scholar]
- 43.Hondeghem LM, Katzung BG. Time- and voltage-dependent interactions of antiarrhythmic drugs with cardiac sodium channels. Biochim Biophys Acta 1977;472:373–398. [DOI] [PubMed] [Google Scholar]
- 44.Starmer CF, Grant AO, Strauss HC. Mechanisms of use-dependent block of sodium channels in excitable membranes by local anesthetics. Biophys J 1984;46:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nattel S, De Blasio E, Wang W-Q, Beatch GN. RSD1235: a novel antiarrhythmic agent with a unique electrophysiological profile that terminates AF in dogs. Eur Heart J 2001;22(Suppl):448 (Abstract).11237538 [Google Scholar]
- 46.Roy D, Rowe BH, Stiell IG, Coutu B, Ip JH, Phaneuf D, Lee J, Vidaillet H, Dickinson G, Grant S, Ezrin AM, Beatch GN, CRAFT Investigators. A randomized, controlled trial of RSD1235, a novel anti-arrhythmic agent, in the treatment of recent onset atrial fibrillation. J Am Coll Cardiol 2004;44:2355–2361. [DOI] [PubMed] [Google Scholar]
- 47.Comtois P, Sakabe M, Vigmond EJ, Munoz M, Texier A, Shiroshita-Takeshita A, Nattel S. Mechanisms of atrial fibrillation termination by rapidly unbinding Na+ channel blockers: insights from mathematical models and experimental correlates. Am J Physiol Heart Circ Physiol 2008;295:H1489–H1504. [DOI] [PubMed] [Google Scholar]
- 48.Aguilar M, Nattel S. The past, present and potential future of sodium channel block as an atrial fibrillation suppressing strategy. J Cardiovasc Pharmacol 2015;66:432–440. [DOI] [PubMed] [Google Scholar]
- 49.Singh BN, Vaughan Williams EM. A third class of anti-arrhythmic action. Effects on atrial and ventricular intracellular potentials, and other pharmacological actions on cardiac muscle, of MJ 1999 and AH 3474. Br J Pharmacol 1970;39:675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prakash R, Parmley WW, Allen HN, Matloff JM. Effect of Sotalol on clinical arrhythmias. Am J Cardiol 1972;29:397–400. [DOI] [PubMed] [Google Scholar]
- 51.Singh BN, Vaughan Williams EM. The effect of amiodarone, a new anti-anginal drug, on cardiac muscle. Br J Pharmacol 1970;39:657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hester JB, Gibson JK, Cimini MG, Emmert DE, Locker PK, Perricone SC, Skaletzky LL, Sykes JK, West BE. N-[(omega-amino-1-hydroxyalkyl)phenyl]methanesulfonamide derivatives with class III antiarrhythmic activity. J Med Chem 1991;34:308–315. [DOI] [PubMed] [Google Scholar]
- 53.Nattel S. Experimental evidence for proarrhythmic mechanisms of antiarrhythmic drugs. Cardiovasc Res 1998;37:567–577. [DOI] [PubMed] [Google Scholar]
- 54.Wang ZG, Pelletier LC, Talajic M, Nattel S. Effects of flecainide and quinidine on human atrial action potentials. Role of rate-dependence and comparison with guinea pig, rabbit, and dog tissues. Circulation 1990;82:274–283. [DOI] [PubMed] [Google Scholar]
- 55.Rosenbaum MB, Chiale PA, Halpern MS, Nau GJ, Przybylski J, Levi RJ, Lazzari JO, Elizari MV. Clinical efficacy of amiodarone as an antiarrhythmic agent. Am J Cardiol 1976;38:934–944. [DOI] [PubMed] [Google Scholar]
- 56.Mason JW, Hondeghem LM, Katzung BG. Amiodarone blocks inactivated cardiac sodium channels. Pflugers Arch 1983;396:79–81. [DOI] [PubMed] [Google Scholar]
- 57.Nattel S, Talajic M, Quantz M, DeRoode M. Frequency-dependent effects of amiodarone on atrioventricular nodal function and slow-channel action potentials: evidence for calcium channel-blocking activity. Circulation 1987;76:442–449. [DOI] [PubMed] [Google Scholar]
- 58.Nattel S, Talajic M. Recent advances in understanding the pharmacology of amiodarone. Drugs 1988;36:121–131. [DOI] [PubMed] [Google Scholar]
- 59.Roy D, Talajic M, Dorian P, Connolly S, Eisenberg MJ, Green M, Kus T, Lambert J, Dubuc M, Gagne P, Nattel S, Thibault B. Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators. N Engl J Med 2000;342:913–920. [DOI] [PubMed] [Google Scholar]
- 60.Ehrlich JR, Nattel S. Novel approaches for pharmacological management of atrial fibrillation. Drugs 2009;69:757–774. [DOI] [PubMed] [Google Scholar]
- 61.Hohnloser SH, Crijns HJ, van Eickels M, Gaudin C, Page RL, Torp-Pedersen C, Connolly SJ; ATHENA Investigators. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med 2009;360:668–678. [DOI] [PubMed] [Google Scholar]
- 62.Køber L, Torp-Pedersen C, McMurray JJ, Gøtzsche O, Lévy S, Crijns H, Amlie J, Carlsen J; Dronedarone Study Group. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med 2008;358:2678–2687. [DOI] [PubMed] [Google Scholar]
- 63.Connolly SJ, Camm AJ, Halperin JL, Joyner C, Alings M, Amerena J, Atar D, Avezum Á, Blomström P, Borggrefe M, Budaj A, Chen SA, Ching CK, Commerford P, Dans A, Davy JM, Delacrétaz E, Di Pasquale G, Diaz R, Dorian P, Flaker G, Golitsyn S, Gonzalez-Hermosillo A, Granger CB, Heidbüchel H, Kautzner J, Kim JS, Lanas F, Lewis BS, Merino JL, Morillo C, Murin J, Narasimhan C, Paolasso E, Parkhomenko A, Peters NS, Sim KH, Stiles MK, Tanomsup S, Toivonen L, Tomcsányi J, Torp-Pedersen C, Tse HF, Vardas P, Vinereanu D, Xavier D, Zhu J, Zhu JR, Baret-Cormel L, Weinling E, Staiger C, Yusuf S, Chrolavicius S, Afzal R, Hohnloser SH; PALLAS Investigators. Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med 2011;365:2268–2276. [DOI] [PubMed] [Google Scholar]
- 64.Nattel S. Dronedarone in atrial fibrillation—Jekyll and Hyde? N Engl J Med 2011;365:2321–2322. [DOI] [PubMed] [Google Scholar]
- 65.Nattel S, Carlsson L. Innovative approaches to anti-arrhythmic drug therapy. Nat Rev Drug Discov 2006;5:1034–1049. [DOI] [PubMed] [Google Scholar]
- 66.Nattel S, Bourne G, Talajic M. Insights into mechanisms of antiarrhythmic drug action from experimental models of atrial fibrillation. J Cardiovasc Electrophysiol 1997;8:469–480. [DOI] [PubMed] [Google Scholar]
- 67.Nattel S, Waters D. What is an antiarrhythmic drug? From clinical trials to fundamental concepts. Am J Cardiol 1990;66:96–99. [DOI] [PubMed] [Google Scholar]
- 68.Sicilian Gambit Group. The search for novel antiarrhythmic strategies. Sicilian Gambit. Eur Heart J 1998;19:1178–1196. [DOI] [PubMed] [Google Scholar]
- 69.Savelieva I, Kakouros N, Kourliouros A, Camm AJ. Upstream therapies for management of atrial fibrillation: review of clinical evidence and implications for European Society of Cardiology guidelines. Part II: secondary prevention. Europace 2011;13:610–625. [DOI] [PubMed] [Google Scholar]
- 70.Savelieva I, Kakouros N, Kourliouros A, Camm AJ. Upstream therapies for management of atrial fibrillation: review of clinical evidence and implications for European Society of Cardiology guidelines. Part I: primary prevention. Europace 2011;13:308–328. [DOI] [PubMed] [Google Scholar]
- 71.Abed HS, Samuel CS, Lau DH, Kelly DJ, Royce SG, Alasady M, Mahajan R, Kuklik P, Zhang Y, Brooks AG, Nelson AJ, Worthley SG, Abhayaratna WP, Kalman JM, Wittert GA, Sanders P. Obesity results in progressive atrial structural and electrical remodeling: implications for atrial fibrillation. Heart Rhythm 2013;10:90–100. [DOI] [PubMed] [Google Scholar]
- 72.Iwasaki YK, Kato T, Xiong F, Shi YF, Naud P, Maguy A, Mizuno K, Tardif JC, Comtois P, Nattel S. Atrial fibrillation promotion with long-term repetitive obstructive sleep apnea in a rat model. J Am Coll Cardiol 2014;64:2013–2023. [DOI] [PubMed] [Google Scholar]
- 73.Abed HS, Wittert GA, Leong DP, Shirazi MG, Bahrami B, Middeldorp ME, Lorimer MF, Lau DH, Antic NA, Brooks AG, Abhayaratna WP, Kalman JM, Sanders P. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA 2013;310:2050–2060. [DOI] [PubMed] [Google Scholar]
- 74.Naruse Y, Tada H, Satoh M, Yanagihara M, Tsuneoka H, Hirata Y, Ito Y, Kuroki K, Machino T, Yamasaki H, Igarashi M, Sekiguchi Y, Sato A, Aonuma K. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart Rhythm 2013;10:331–337. [DOI] [PubMed] [Google Scholar]
- 75.Benjamin EJ, Chen PS, Bild DE, Mascette AM, Albert CM, Alonso A, Calkins H, Connolly SJ, Curtis AB, Darbar D, Ellinor PT, Go AS, Goldschlager NF, Heckbert SR, Jalife J, Kerr CR, Levy D, Lloyd-Jones DM, Massie BM, Nattel S, Olgin JE, Packer DL, Po SS, Tsang TS, Van Wagoner DR, Waldo AL, Wyse DG. Prevention of atrial fibrillation: report from a national heart, lung, and blood institute workshop. Circulation 2009;119:606–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nishida K, Datino T, Macle L, Nattel S. Atrial fibrillation ablation: translating basic mechanistic insights to the patient. J Am Coll Cardiol 2014;64:823–831. [DOI] [PubMed] [Google Scholar]
- 77.Cox JL, Schuessler RB, D'Agostino HJ Jr, Stone CM, Chang BC, Cain ME, Corr PB, Boineau JP. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg 1991;101:569–583. [PubMed] [Google Scholar]
- 78.Jais P, Haissaguerre M, Shah DC, Chouairi S, Gencel L, Hocini M, Clementy J. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation 1997;95:572–576. [DOI] [PubMed] [Google Scholar]
- 79.Nattel S. Paroxysmal atrial fibrillation and pulmonary veins: relationships between clinical forms and automatic versus re-entrant mechanisms. Can J Cardiol 2013;29:1147–1149. [DOI] [PubMed] [Google Scholar]
- 80.Arentz T, Macle L, Kalusche D, Hocini M, Jais P, Shah D, Haissaguerre M. “Dormant” pulmonary vein conduction revealed by adenosine after ostial radiofrequency catheter ablation. J Cardiovasc Electrophysiol 2004;15:1041–1047. [DOI] [PubMed] [Google Scholar]
- 81.Datino T, Macle L, Qi XY, Maguy A, Comtois P, Chartier D, Guerra PG, Arenal A, Fernandez-Aviles F, Nattel S. Mechanisms by which adenosine restores conduction in dormant canine pulmonary veins. Circulation 2010;121:963–972. [DOI] [PubMed] [Google Scholar]
- 82.Macle L, Khairy P, Weerasooriya R, Novak P, Verma A, Willems S, Arentz T, Deisenhofer I, Veenhuyzen G, Scavée C, Jaïs P, Puererfellner H, Levesque S, Andrade JG, Rivard L, Guerra PG, Dubuc M, Thibault B, Talajic M, Roy D, Nattel S; ADVICE trial investigators. Adenosine-guided pulmonary vein isolation for the treatment of paroxysmal atrial fibrillation: an international, multicentre, randomised superiority trial. Lancet 2015;386:672–679. [DOI] [PubMed] [Google Scholar]
- 83.Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, Macle L, Morillo CA, Haverkamp W, Weerasooriya R, Albenque JP, Nardi S, Menardi E, Novak P, Sanders P; STAR AF II Investigators. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–1822. [DOI] [PubMed] [Google Scholar]
- 84.Krinskii VI. Excitation propagation in nonhomogenous medium (actions analogous to heart fibrillation). Biofizika 1966;11:676–683. [PubMed] [Google Scholar]
- 85.Sarmast F, Kolli A, Zaitsev A, Parisian K, Dhamoon AS, Guha PK, Warren M, Anumonwo JM, Taffet SM, Berenfeld O, Jalife J. Cholinergic atrial fibrillation: IK,ACh gradients determine unequal left/right atrial frequencies and rotor dynamics. Cardiovasc Res 2003;59:863–873. [DOI] [PubMed] [Google Scholar]
- 86.Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel WJ, Miller JM. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol 2012;60:628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cuculich PS, Wang Y, Lindsay BD, Faddis MN, Schuessler RB, Damiano RJ Jr, Li L, Rudy Y. Noninvasive characterization of epicardial activation in humans with diverse atrial fibrillation patterns. Circulation 2010;122:1364–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Haissaguerre M, Hocini M, Denis A, Shah AJ, Komatsu Y, Yamashita S, Daly M, Amraoui S, Zellerhoff S, Picat MQ, Quotb A, Jesel L, Lim H, Ploux S, Bordachar P, Attuel G, Meillet V, Ritter P, Derval N, Sacher F, Bernus O, Cochet H, Jais P, Dubois R. Driver domains in persistent atrial fibrillation. Circulation 2014;130:530–538. [DOI] [PubMed] [Google Scholar]
- 89.Eckstein J, Zeemering S, Linz D, Maesen B, Verheule S, van Hunnik A, Crijns H, Allessie MA, Schotten U. Transmural conduction is the predominant mechanism of breakthrough during atrial fibrillation: evidence from simultaneous endo-epicardial high-density activation mapping. Circ Arrhythm Electrophysiol 2013;6:334–341. [DOI] [PubMed] [Google Scholar]
- 90.Hansen BJ, Zhao J, Csepe TA, Moore BT, Li N, Jayne LA, Kalyanasundaram A, Lim P, Bratasz A, Powell KA, Simonetti OP, Higgins RS, Kilic A, Mohler PJ, Janssen PM, Weiss R, Hummel JD, Fedorov VV. Atrial fibrillation driven by micro-anatomic intramural re-entry revealed by simultaneous sub-epicardial and sub-endocardial optical mapping in explanted human hearts. Eur Heart J 2015;36:2390–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation 2011;124:2264–2274. [DOI] [PubMed] [Google Scholar]
- 92.Lemola K, Chartier D, Yeh YH, Dubuc M, Cartier R, Armour A, Ting M, Sakabe M, Shiroshita-Takeshita A, Comtois P, Nattel S. Pulmonary vein region ablation in experimental vagal atrial fibrillation: role of pulmonary veins versus autonomic ganglia. Circulation 2008;117:470–477. [DOI] [PubMed] [Google Scholar]
- 93.Katritsis DG, Giazitzoglou E, Zografos T, Pokushalov E, Po SS, Camm AJ. Rapid pulmonary vein isolation combined with autonomic ganglia modification: a randomized study. Heart Rhythm 2011;8:672–678. [DOI] [PubMed] [Google Scholar]
- 94.Dobrev D, Carlsson L, Nattel S. Novel molecular targets for atrial fibrillation therapy. Nat Rev Drug Discov 2012;11:275–291. [DOI] [PubMed] [Google Scholar]
- 95.Bode F, Sachs F, Franz MR. Tarantula peptide inhibits atrial fibrillation. Nature 2001;409:35–36. [DOI] [PubMed] [Google Scholar]
- 96.Pavri BB, Greenberg HE, Kraft WK, Lazarus N, Lynch JJ, Salata JJ, Bilodeau MT, Regan CP, Stump G, Fan L, Mehta A, Wagner JA, Gutstein DE, Bloomfield D. MK-0448, a specific Kv1.5 inhibitor: safety, pharmacokinetics, and pharmacodynamic electrophysiology in experimental animal models and humans. Circ Arrhythm Electrophysiol 2012;5:1193–1201. [DOI] [PubMed] [Google Scholar]
- 97.Courtemanche M, Ramirez RJ, Nattel S. Ionic targets for drug therapy and atrial fibrillation-induced electrical remodeling: insights from a mathematical model. Cardiovasc Res 1999;42:477–489. [DOI] [PubMed] [Google Scholar]
- 98.Dobrev D, Friedrich A, Voigt N, Jost N, Wettwer E, Christ T, Knaut M, Ravens U. The G protein-gated potassium current IK,ACh is constitutively active in patients with chronic atrial fibrillation. Circulation 2005;112:3697–3706. [DOI] [PubMed] [Google Scholar]
- 99.Zhou Q, Xiao J, Jiang D, Wang R, Vembaiyan K, Wang A, Smith CD, Xie C, Chen W, Zhang J, Tian X, Jones PP, Zhong X, Guo A, Chen H, Zhang L, Zhu W, Yang D, Li X, Chen J, Gillis AM, Duff HJ, Cheng H, Feldman AM, Song LS, Fill M, Back TG, Chen SR. Carvedilol and its new analogs suppress arrhythmogenic store overload-induced Ca2+ release. Nat Med 2011;17:1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jalalian R, Ghafari R, Ghazanfari P. Comparing the therapeutic effects of carvedilol and metoprolol on prevention of atrial fibrillation after coronary artery bypass surgery, a double-blind study. Int Cardiovasc Res J 2014;8:111–115. [PMC free article] [PubMed] [Google Scholar]
- 101.Brundel BJ, Ausma J, van Gelder IC, Van der Want JJ, van Gilst WH, Crijns HJ, Henning RH. Activation of proteolysis by calpains and structural changes in human paroxysmal and persistent atrial fibrillation. Cardiovasc Res 2002;54:380–389. [DOI] [PubMed] [Google Scholar]
- 102.Luo X, Pan Z, Shan H, Xiao J, Sun X, Wang N, Lin H, Xiao L, Maguy A, Qi XY, Li Y, Gao X, Dong D, Zhang Y, Bai Y, Ai J, Sun L, Lu H, Luo XY, Wang Z, Lu Y, Yang B, Nattel S. MicroRNA-26 governs profibrillatory inward-rectifier potassium current changes in atrial fibrillation. J Clin Invest 2013;123:1939–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Luo X, Yang B, Nattel S. MicroRNAs and atrial fibrillation: mechanisms and translational potential. Nat Rev Cardiol 2015;12:80–90. [DOI] [PubMed] [Google Scholar]
- 104.van Bragt KA, Nasrallah HM, Kuiper M, Luiken JJ, Schotten U, Verheule S. Atrial supply-demand balance in healthy adult pigs: coronary blood flow, oxygen extraction, and lactate production during acute atrial fibrillation. Cardiovasc Res 2014;101:9–19. [DOI] [PubMed] [Google Scholar]
- 105.Mayr M, Yusuf S, Weir G, Chung YL, Mayr U, Yin X, Ladroue C, Madhu B, Roberts N, De Souza A, Fredericks S, Stubbs M, Griffiths JR, Jahangiri M, Xu Q, Camm AJ. Combined metabolomic and proteomic analysis of human atrial fibrillation. J Am Coll Cardiol 2008;51:585–594. [DOI] [PubMed] [Google Scholar]
- 106.De Souza AI, Cardin S, Wait R, Chung YL, Vijayakumar M, Maguy A, Camm AJ, Nattel S. Proteomic and metabolomic analysis of atrial profibrillatory remodelling in congestive heart failure. J Mol Cell Cardiol 2010;49:851–863. [DOI] [PubMed] [Google Scholar]
- 107.Harada M, Tadevosyan A, Qi X, Xiao J, Liu T, Voigt N, Karck M, Kamler M, Kodama I, Murohara T, Dobrev D, Nattel S. Atrial fibrillation activates AMP-dependent protein kinase and its regulation of cellular calcium handling: potential role in metabolic adaptation and prevention of progression. J Am Coll Cardiol 2015;66:47–58. [DOI] [PubMed] [Google Scholar]
- 108.Lenski M, Schleider G, Kohlhaas M, Adrian L, Adam O, Tian Q, Kaestner L, Lipp P, Lehrke M, Maack C, Bohm M, Laufs U. Arrhythmia causes lipid accumulation and reduced glucose uptake. Basic Res Cardiol 2015;110:40. [DOI] [PubMed] [Google Scholar]
- 109.Heijman J, Dobrev D. Irregular rhythm and atrial metabolism are key for the evolution of proarrhythmic atrial remodeling in atrial fibrillation. Basic Res Cardiol 2015;110:41. [DOI] [PubMed] [Google Scholar]
- 110.Kim GE, Ross JL, Xie C, Su KN, Zaha VG, Wu X, Palmeri M, Ashraf M, Akar JG, Russell KS, Akar FG, Young LH. LKB1 deletion causes early changes in atrial channel expression and electrophysiology prior to atrial fibrillation. Cardiovasc Res 2015;108:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001;108:1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chang SH, Wu LS, Chiou MJ, Liu JR, Yu KH, Kuo CF, Wen MS, Chen WJ, Yeh YH, See LC. Association of metformin with lower atrial fibrillation risk among patients with type 2 diabetes mellitus: a population-based dynamic cohort and in vitro studies. Cardiovasc Diabetol 2014;13:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shen MJ, Shinohara T, Park HW, Frick K, Ice DS, Choi EK, Han S, Maruyama M, Sharma R, Shen C, Fishbein MC, Chen LS, Lopshire JC, Zipes DP, Lin SF, Chen PS. Continuous low-level vagus nerve stimulation reduces stellate ganglion nerve activity and paroxysmal atrial tachyarrhythmias in ambulatory canines. Circulation 2011;123:2204–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stavrakis S, Humphrey MB, Scherlag BJ, Hu Y, Jackman WM, Nakagawa H, Lockwood D, Lazzara R, Po SS. Low-level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J Am Coll Cardiol 2015;65:867–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lampugnani P. The therapy of atrial fibrillation with the combination of hydroquinidine and reserpine. Clin Ter 1966;36:491–525. [PubMed] [Google Scholar]
- 116.de Mendonca JV. Verapamil associated with quinidine in the treatment of chronic atrial fibrillation. Arq Bras Cardiol 1978;31(Suppl. 1):87–93. [PubMed] [Google Scholar]
- 117.Burashnikov A, Sicouri S, Di Diego JM, Belardinelli L, Antzelevitch C. Synergistic effect of the combination of ranolazine and dronedarone to suppress atrial fibrillation. J Am Coll Cardiol 2010;56:1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Reiffel JA, Camm AJ, Belardinelli L, Zeng D, Karwatowska-Prokopczuk E, Olmsted A, Zareba W, Rosero S, Kowey P. The HARMONY Trial: combined ranolazine and dronedarone in the management of paroxysmal atrial fibrillation: mechanistic and therapeutic synergism. Circ Arrhythm Electrophysiol 2015;8:1048–1056. [DOI] [PubMed] [Google Scholar]
- 119.Aguilar-Shardonofsky M, Vigmond EJ, Nattel S, Comtois P. In silico optimization of atrial fibrillation-selective sodium channel blocker pharmacodynamics. Biophys J 2012;102:951–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Aguilar M, Xiong F, Qi XY, Comtois P, Nattel S. Potassium channel blockade enhances atrial fibrillation-selective antiarrhythmic effects of optimized state-dependent sodium channel blockade. Circulation 2015;132:2203–2211. [DOI] [PubMed] [Google Scholar]
- 121.Paci M, Hyttinen J, Aalto-Setala K, Severi S. Computational models of ventricular- and atrial-like human induced pluripotent stem cell derived cardiomyocytes. Ann Biomed Eng 2013;41:2334–2348. [DOI] [PubMed] [Google Scholar]
- 122.Zhao Y, Feric NT, Thavandiran N, Nunes SS, Radisic M. The role of tissue engineering and biomaterials in cardiac regenerative medicine. Can J Cardiol 2014;30:1307–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Heijman J, Erfanian Abdoust P, Voigt N, Nattel S, Dobrev D. Computational models of atrial cellular electrophysiology and calcium handling, and their role in atrial fibrillation. J Physiol 2015; doi:10.1113/JP271404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Michelena HI, Powell BD, Brady PA, Friedman PA, Ezekowitz MD. Gender in atrial fibrillation: Ten years later. Gend Med 2010;7:206–217. [DOI] [PubMed] [Google Scholar]
- 125.Parvez B, Vaglio J, Rowan S, Muhammad R, Kucera G, Stubblefield T, Carter S, Roden D, Darbar D. Symptomatic response to antiarrhythmic drug therapy is modulated by a common single nucleotide polymorphism in atrial fibrillation. J Am Coll Cardiol 2012;60:539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Darbar D. The role of pharmacogenetics in atrial fibrillation therapeutics – is personalized therapy in sight? J Cardiovasc Pharmacol 2016;67:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Choi EK, Park JH, Lee JY, Nam CM, Hwang MK, Uhm JS, Joung B, Ko YG, Lee MH, Lubitz SA, Ellinor PT, Pak HN. Korean Atrial Fibrillation (AF) Network: Genetic Variants for AF Do Not Predict Ablation Success. J Am Heart Assoc 2015;4:e002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kirchhof P, Breithardt G, Aliot E, Al Khatib S, Apostolakis S, Auricchio A, Bailleul C, Bax J, Benninger G, Blomstrom-Lundqvist C, Boersma L, Boriani G, Brandes A, Brown H, Brueckmann M, Calkins H, Casadei B, Clemens A, Crijns H, Derwand R, Dobrev D, Ezekowitz M, Fetsch T, Gerth A, Gillis A, Gulizia M, Hack G, Haegeli L, Hatem S, Georg Hausler K, Heidbuchel H, Hernandez-Brichis J, Jais P, Kappenberger L, Kautzner J, Kim S, Kuck KH, Lane D, Leute A, Lewalter T, Meyer R, Mont L, Moses G, Mueller M, Munzel F, Nabauer M, Nielsen JC, Oeff M, Oto A, Pieske B, Pisters R, Potpara T, Rasmussen L, Ravens U, Reiffel J, Richard-Lordereau I, Schafer H, Schotten U, Stegink W, Stein K, Steinbeck G, Szumowski L, Tavazzi L, Themistoclakis S, Thomitzek K, Van Gelder IC, von Stritzky B, Vincent A, Werring D, Willems S, Lip GY, Camm AJ. Personalized management of atrial fibrillation: Proceedings from the fourth Atrial Fibrillation competence NETwork/European Heart Rhythm Association consensus conference. Europace 2013;15:1540–1556. [DOI] [PubMed] [Google Scholar]
- 129.Nattel S, Andrade J, Macle L, Rivard L, Dyrda K, Mondesert B, Khairy P. New directions in cardiac arrhythmia management: present challenges and future solutions. Can J Cardiol 2014;30:S420–S430. [DOI] [PubMed] [Google Scholar]
- 130.Jackson N, Atar D, Borentain M, Breithardt G, van Eickels M, Endres M, Fraass U, Friede T, Hannachi H, Janmohamed S, Kreuzer J, Landray M, Lautsch D, Le Floch C, Mol P, Naci H, Samani NJ, Svensson A, Thorstensen C, Tijssen J, Vandzhura V, Zalewski A, Kirchhof P. Improving clinical trials for cardiovascular diseases: a position paper from the Cardiovascular Round Table of the European Society of Cardiology. Eur Heart J 2016;37:747–754. [DOI] [PubMed] [Google Scholar]