Abstract
Background
Oxidative stress and disturbances in serotonergic and dopaminergic neurotransmission may play a role in the pathophysiology of delirium.
Aims
In this study, we investigated levels of amino acids, amino acid ratios and levels of homovanillic acid (HVA) as indicators for oxidative stress and disturbances in neurotransmission.
Methods
Plasma levels of amino acids, amino acid ratios and HVA were determined in acutely ill patients aged ≥65 years admitted to the wards of Internal Medicine and Geriatrics of the Erasmus University Medical Center and the ward of Geriatrics of the Havenziekenhuis, Rotterdam, The Netherlands. Differences in the biochemical parameters between patients with and without delirium were investigated by analysis of variance in models adjusted for age, gender and comorbidities.
Results
Of the 86 patients included, 23 had delirium. In adjusted models, higher mean phenylalanine/tyrosine ratios (1.34 vs. 1.14, p = 0.028), lower mean tryptophan/large neutral amino acids ratios (4.90 vs. 6.12, p = 0.021) and lower mean arginine levels (34.8 vs. 45.2 µmol/l, p = 0.022) were found in patients with delirium when compared to those without. No differences were found in HVA levels between patients with and without delirium.
Conclusion
The findings of this study suggest disturbed serotonergic neurotransmission and an increased status of oxidative stress in patients with delirium.
Key Words: Delirium, Oxidative stress, Tetrahydrobiopterin, Amino acids, Arginine, Tryptophan, Serotonin, Homovanillic acid
Introduction
Delirium is a common and severe complication among elderly patients and is associated with increased morbidity and mortality, prolonged hospital stay, increased risk of post-discharge institutionalization and dementia [1,2]. The pathophysiology of delirium is still largely hypothetical. Identifying accurate biomarkers for delirium may shed light on the pathophysiology and may help to improve delirium recognition and care.
Oxidative stress and disturbances in serotonergic and dopaminergic neurotransmission might all be involved in the pathophysiology of delirium and probably act together [3]. Within the central nervous system, tetrahydrobiopterin (BH4) functions as an essential cofactor in enzymatic reactions responsible for the production of serotonin and dopamine. In addition, BH4 is a cofactor for nitric oxide synthase (NOS) that catalyzes the production of nitric oxide (NO) and citrulline from arginine [4]. If BH4 becomes limited, this could impair serotonin and dopamine synthesis. Besides, when BH4 is partially deficient, some cellular sources of NOS may generate superoxide (O2∙-) instead of NO and citrulline [4,5]. In patients with delirium, BH4 status has only been investigated after elective cardiac surgery [6].
In order to assess BH4 status, we measured amino acid levels and subsequently calculated the phenylalanine/tyrosine (Phe/Tyr) ratio. This ratio is an indicator for the BH4 status as it reflects the activity of the enzyme Phe hydroxylase, an enzyme that uses BH4 as a cofactor [4,7]. An elevated ratio is suggestive for decreased BH4 availability. Furthermore, we determined the ratios of tryptophan (Trp), Phe and Tyr to the other large neutral amino acids (LNAAs). Trp is the precursor of serotonin, while Phe and Tyr are the precursors of dopamine. The LNAAs (Trp, Phe, Tyr, valine, isoleucine and leucine) compete with each other for transport across the blood-brain barrier. Therefore, a decreased Trp/LNAAs ratio is suggestive for a decline in the amount of Trp that enters the brain and consequently for reduced synthesis of serotonin [8]. Moreover, we measured plasma levels of the dopamine metabolite homovanillic acid (HVA), approximately 30% of which is estimated to originate from dopamine neurons in the central nervous system and which is therefore thought to be a reliable indicator for central dopamine activity [9]. Finally, we measured plasma levels of arginine and citrulline to investigate the production of NO by NOS.
The aim of the study was to investigate BH4 status, potential disturbances in serotonergic and dopaminergic neurotransmission and the production of NO in patients with and without delirium.
Methods
Study Design and Participants
The present study was performed within the Delirium In The Old (DITO) study in which mean levels of neopterin, interleukin-6 and insulin-like growth factor-1 were compared between patients with and without delirium [10]. In the DITO study, a cross-sectional study, we included patients who were admitted to the wards of Internal Medicine and Geriatrics of the Erasmus University Medical Center and the ward of Geriatrics of the Havenziekenhuis, Rotterdam, The Netherlands. All acutely admitted patients aged ≥65 years were eligible to participate. Exclusion criteria were a diagnosis of Lewy body dementia, Parkinson's disease, neuroleptic malignant syndrome, tardive dyskinesia, ongoing treatment with antipsychotics or other psychiatric medications except haloperidol and benzodiazepines, aphasia, insufficient understanding of the Dutch language and a Mini-Mental State Examination (MMSE) score <10 points out of 30. We excluded patients with a MMSE <10 because it can be quite difficult to distinguish between features of severe dementia and delirium at admission as well as to measure improvement of cognitive function in this group.
Written informed consent was obtained from all participants. In case of delirium or cognitive impairment at the time of admission, informed consent was obtained from a representative of the patient. The Medical Ethics Committee of the Erasmus University Medical Center approved the study protocol.
Procedures
All participants were observed daily by the nursing and medical staff and by members of the research team until discharge. To screen for a change in behavior, the 13-item Delirium Observation Screening scale was used during the first 5 days of admission [11]. The diagnosis of delirium was made by a geriatrician, according to the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) [12], and was based on the psychiatric examination of the patient, the medical and nursing records, including the Delirium Observation Screening scale scores, and information given by the patient's closest relative. When the diagnosis of delirium was doubtful, the case was discussed with the geriatric consultation team to gain consensus.
Demographic and clinical data were collected at admission. Age, gender and living situation before admission were documented. Cognitive functioning was assessed in absence of delirium using the MMSE [13]. When it was impossible to score the MMSE during admission because the patient was too ill, cognitive function was discussed with a clinician or assessed with information from the available medical records. When the clinical opinion was that the patient would have a MMSE score ≥10, the patient was not excluded from the study. Severity of comorbidities was scored using the Charlson Comorbidity Index. This index encompasses 19 medical conditions, including dementia, and each condition is weighted with a score of 1-6 by severity [14]. Physical functionality was assessed using the 6-item Katz Activities of Daily Living scale and the Barthel index [15,16]. Instrumental functionality was assessed using the 7-item Older Americans Resource Scale for Instrumental Activities of Daily Living [15]. Frailty was measured with the Identification of Seniors at Risk questionnaire [17]. Blood samples of all patients were collected within 48 h after admission. When a patient developed delirium during the hospital stay, new blood samples were collected within 24 h after the onset of the delirium and were used instead of the first blood samples for the statistical analyses.
Biochemical Measurements
Non-fasting blood was collected preferably between 8 and 10 a.m. in an 8-ml tube containing ethylene diamine tetraacetic acid. After blood sampling, the tubes were stored at room temperature to prevent changes in the transfer of amino acids between plasma and blood cells [18]. Within 3 h, the blood was centrifuged for 20 min at 2,650 g and 20°C. The obtained plasma was stored at −80°C until analysis.
Plasma amino acid levels were determined by high-performance liquid chromatography with automated pre-column derivatization with ortho-phthalaldehyde as previously described [18]. Plasma HVA levels were determined by reversed-phase high-performance liquid chromatography and electrochemical detection, as previously described for the determination of serotonin [19].
Statistical Analysis
Medians and interquartile ranges were determined for continuous participant characteristics and proportions for categorical characteristics. Biochemical parameters with a skewed distribution were logarithmically transformed (all amino acids, amino acid ratios and HVA). Univariate one-way analysis of variance was used to investigate the association between mean levels of amino acids, amino acid ratios and HVA (dependent variables) and the presence of delirium. Models were adjusted for age, gender and the Charlson Comorbidity Index. Additional analyses were performed for all amino acids, amino acid ratios and HVA after also adding MMSE score to the models. A two-tailed p <0.05 was defined as statistically significant.
Statistical Package for the Social Sciences, version 21.0 (SPSS Inc., Chicago, Ill., USA) was used to perform the statistical analyses. GraphPad Prism 5.01 for Windows (GraphPad Software, San Diego, Calif., USA) was used to draw all graphs.
Results
Participant Characteristics
Table 1 presents the baseline characteristics of the 86 participants who were included in the study. Of the 23 patients diagnosed with delirium, 21 were admitted to hospital with delirium and 2 developed delirium during admission.
Table 1.
Characteristics of the study participants
| No delirium (n = 63) | Delirium (n = 23) | |
|---|---|---|
| Male gender | 47.6% | 43.5% |
| Age, years | 81.0 (75.0–85.0) | 87.0 (84.0–88.0) |
| MMSE scorea | 25.5 (22.0–28.0)b | 20.0 (18.0–25.0)c |
| Living situation | ||
| Home | 47.6% | 26.1% |
| Home care | 31.7% | 30.4% |
| Residential home | 7.9% | 17.4% |
| Nursing home | 3.2% | 13.0% |
| Missing data | 9.5% | 13.0% |
| Katz Activities of Daily Living scored | 0.0 (0.0–3.0) | 2.0 (1.0–11.0) |
| OARS-IADL scoree | 5.0 (0.0–10.0) | 9.5 (3.5–14.0) |
| Barthel indexf | 18.0 (13.0–20.0) | 16.0 (9.5–19.0) |
| Identification of Seniors at Risk scoreg | 4.0 (2.0–6.0) | 6.0 (4.8–7.0) |
| Charlson Comorbidity Indexh | 1.0 (1.0–2.0) | 2.0 (1.0–3.0) |
Values are expressed as medians (interquartile ranges) or percentages.
OARS-IADL = Older Americans Resource Scale for Instrumental Activities of Daily Living.
Range 0 (severe cognitive impairment) to 30 (no cognitive impairment).
Three values missing.
Four values missing.
Range 0 (no disability) to 12 (severe disability).
Range 0 (no disability) to 14 (severe disability).
Range 0 (severe disability) to 20 (no disability).
Scores ≥2 indicate a high risk of functional decline.
Range 0–37 (severe burden of comorbidities).
Analyses of Biochemical Parameters
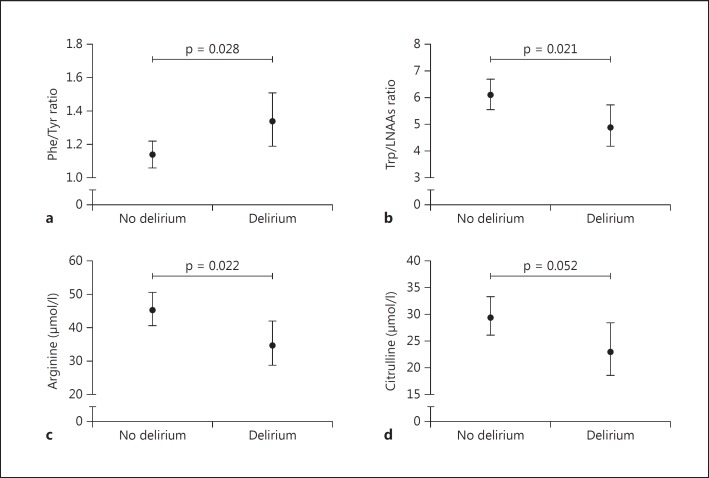
The mean levels and corresponding 95% confidence intervals (CIs) of the investigated biochemical parameters in patients with and without delirium are presented in tables 2 and 3. In adjusted models, mean levels of arginine were significantly lower in patients with delirium (34.8 µmol/l, 95% CI 28.8-42.0) than in those without (45.2 µmol/l, 95% CI 40.6-50.5) (p = 0.022) (fig. 1). Concerning the amino acid ratios, mean Phe/Tyr ratios were significantly higher in patients with delirium (1.34, 95% CI 1.19-1.51) than in patients without delirium (1.14, 95% CI 1.06-1.22) (p = 0.028) (fig. 1). In addition, mean Trp/LNAAs ratios were significantly lower in patients with delirium (4.90, 95% CI 4.19-5.74) than in those without (6.12, 95% CI 5.58-6.71) (p = 0.021) (fig. 1). No associations between the other amino acids and ratios and delirium were found, although citrulline (fig. 1) and Trp levels were at the border of significance lower in patients with delirium than in those without (p = 0.052 and p = 0.067, respectively).
Table 2.
Mean levels of amino acids
| No delirium (n = 63) | Delirium (n = 23) | p value | |
|---|---|---|---|
| Glutamic acid, μmol/l | 46.2 (40.9–52.2) | 41.3 (33.5–50.9) | 0.368 |
| Serine, µmol/l | 84.5 (78.7–90.8) | 81.3 (71.9–91.8) | 0.596 |
| Glycine, μmol/l | 194.5 (179.5–210.9) | 187.1 (162.6–214.8) | 0.633 |
| Citrulline, μmol/l | 29.4 (26.1–33.3) | 23.0 (18.6–28.4) | 0.052 |
| Arginine, μmol/l | 45.2 (40.6–50.5) | 34.8 (28.8–42.0) | 0.022 |
| Alanine, μmol/1 | 329.6 (297.1–364.8) | 337.3 (283.1–402.7) | 0.814 |
| Taurine, μmol/1 | 41.3 (37.4–45.5) | 38.5 (32.6–45.6) | 0.496 |
| Tyr, µmol/1 | 58.6 (54.0–63.7) | 55.6 (48.2–64.1) | 0.529 |
| Valine, µmol/1 | 212.8 (198.2–228.0) | 214.8 (190.5–241.5) | 0.898 |
| Methionine, μmol/1 | 22.4 (20.8–24.3) | 23.3 (20.4–26.5) | 0.651 |
| Trp, µmol/1 | 32.1 (28.8–35.7) | 26.2 (21.8–31.5) | 0.067 |
| Phe, μmol/1 | 66.7 (62.2–71.4) | 74.5 (66.2–83.8) | 0.122 |
| Isoleucine, µmol/1 | 59.6 (55.0–64.6) | 57.7 (50.2–66.2) | 0.690 |
| Leucine, μmol/1 | 120.5 (111.4–130.6) | 123.3 (107.6–141.3) | 0.783 |
| Ornithine, μmol/1 | 78.0 (71.0–85.5) | 67.6 (57.5–79.4) | 0.146 |
Values are expressed as means (95% CIs) and are the back-transformed log10 values. Models are adjusted for age, gender and Charlson Comorbidity Index.
Table 3.
Mean levels of amino acid ratios and HVA
| No delirium (n = 63) | Delirium (n = 23) | p value | |
|---|---|---|---|
| Phe/Tyr ratio | 1.14 (1.06–1.22) | 1.34 (1.19–1.51) | 0.028 |
| Trp/LNAAs ratio (×100) | 6.12 (5.58–6.71) | 4.90 (4.19–5.74) | 0.021 |
| Tyr/LNAAs ratio (×100) | 11.8 (11.0–12.7) | 11.0 (9.7–12.4) | 0.342 |
| Phe/LNAAs ratio (×100) | 13.6 (12.7–14.7) | 15.3 (13.6–17.3) | 0.122 |
| HVA, nmol/l | 93.3 (79.4–109.4)a | 123.0 (93.3–162.6)a | 0.098 |
Values are expressed as means (95% CIs) and are the back-transformed log10 values. Models are adjusted for age, gender and Charlson Comorbidity Index.
One value missing.
Fig. 1.
Mean levels and corresponding 95% CIs of the Phe/Tyr ratio (a), the Trp/LNAAs ratio (b), arginine (c) and citrulline (d) in patients with and without delirium.
HVA data were missing for 2 patients (delirium, n = 1 [not enough plasma]; no delirium, n = 1 [measurement failed]). Mean HVA levels were not statistically significantly different between patients with delirium (123.0 nmol/l, 95% CI 93.3-162.6) and patients without delirium (93.3 nmol/l, 95% CI 79.4-109.4) (p = 0.098).
In the models additionally adjusted for MMSE score, the association between arginine and delirium did not reach statistical significance (delirium: mean 36.0 µmol/l, 95% CI 28.7-45.1 vs. no delirium: mean 44.8 µmol/l, 95% CI 39.8-50.5, p = 0.107). Mean Phe/Tyr ratios remained borderline significantly higher in patients with delirium (1.34, 95% CI 1.16-1.55) than in those without (1.15, 95% CI 1.07-1.24) (p = 0.089) and mean Trp/LNAAs ratios remained borderline significantly lower in patients with delirium (5.00, 95% CI 4.15-6.01) compared to those without (6.15, 95% CI 5.58-6.78) (p = 0.062). Estimates for the other biochemical parameters remained statistically insignificant (data not shown).
Discussion
In the present study we found disturbed serotonergic neurotransmission and an increased status of oxidative stress in patients with delirium when compared to patients without delirium.
As far as we are aware, this is the first delirium study investigating BH4 status and levels of arginine and citrulline in acutely ill elderly hospitalized patients. In order to assess the BH4 status, we measured the Phe/Tyr ratio. In patients with delirium we found an increased ratio, suggesting a deficiency in the essential cofactor BH4 for the production of serotonin, dopamine and NO. Decreased BH4 availability has already been found in other neuropsychiatric disorders such as Alzheimer's disease [20], Parkinson's disease [20] and schizophrenia [21]. Our finding is not in agreement with the results of a previous delirium study which showed that levels of BH4 and Phe/Tyr ratios did not differ between patients with and without delirium [6]. However, that study included a relatively younger group of patients undergoing elective cardiac surgery.
In the present study, serotonergic neurotransmission was investigated with the Trp/LNAAs ratio and the Phe/Tyr ratio. We found that patients with delirium had a decreased Trp/LNAAs ratio, which might suggest reduced serotonin production in the central nervous system. This hypothesis is strengthened by the finding that patients with delirium had an elevated Phe/Tyr ratio, which might suggest deficiency in the essential cofactor BH4 in the production of serotonin. In previous studies, controversial results have been reported. Several studies found a reduced Trp/LNAAs ratio during delirium [6,8,22,23], whereas two studies reported no difference in this ratio between patients with and without delirium [24,25]. The study performed by Flacker and Lipsitz [24] included only patients with mild illnesses not requiring hospitalization. Therefore, the findings may not be generalizable to acutely ill patients who needed medical care in hospital. The study performed by van der Cammen et al. [25] included delirium patients with Alzheimer's disease. It might be possible that in those patients a disturbance in cholinergic neurotransmission played a more important role in the development of delirium than disturbances in other pathophysiological pathways [26].
Furthermore, we found no differences in Phe/LNAAs ratios, Tyr/LNAAs ratios and HVA levels between patients with and without delirium, suggesting that dopaminergic neurotransmission is not impaired during delirium. The finding that plasma HVA levels are not significantly increased in patients with delirium compared to patients without delirium is not in agreement with earlier results [6,25]. However, those studies were performed in patients with Alzheimer's disease [25] and patients undergoing cardiac surgery [6], and therefore the results may not be generalizable. Ramirez-Bermudez et al. [27] found that cerebrospinal fluid HVA levels correlated with psychotic symptoms of delirium (hallucinations and delusions) in neurological patients. It might also be possible that we did not find an association between the dopaminergic markers and the presence of delirium because we included patients both with and without psychotic features.
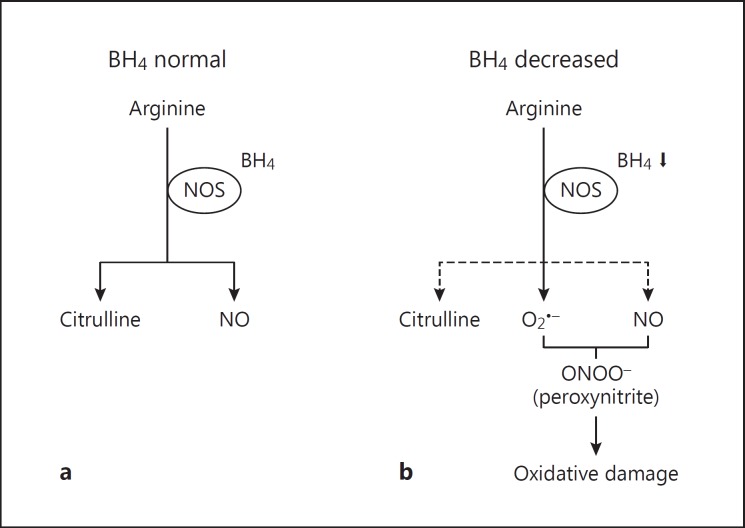
In our study, we also found reduced plasma arginine levels and borderline statistically significantly reduced citrulline levels in patients with delirium. Considering the cross-sectional study design, these findings could mean several things. First, it is possible that patients with delirium had a pre-existing arginine deficiency which might have resulted in a reduced production of citrulline by NOS (fig. 2a). Second, when BH4 is partially deficient, as our results do suggest, some cellular sources of NOS may generate O2∙- instead of citrulline and NO from arginine (fig. 2b) [4,5], leading to decreased levels of both arginine and citrulline. If this latter scenario is true for delirium, this would also suggest an increased status of oxidative stress, since it favors peroxynitrite formation (fig. 2b). If peroxynitrite is not scavenged by antioxidants, it may cause oxidative damage to cellular macromolecules [20,28], which has already been hypothesized to occur in Alzheimer's disease [29]. However, both amino acids have been investigated previously by Osse et al. [6] in patients with delirium after cardiac surgery, but they found no differences in arginine and citrulline levels between patients with and without delirium. Since they also reported no difference in BH4 status between patients with and without delirium, the results are probable not generalizable to our study.
Fig. 2.
Schematic presentation of the role of BH4 in the formation of citrulline, NO and O2∙- by NOS. a Situation in which there is sufficient supply of BH4 for NOS. b Situation in which there is insufficient supply of BH4. Some cellular sources of NOS will generate O2∙- instead of NO and citrulline. Generation of O2∙- and NO together will lead to the formation of peroxynitrite, which may cause oxidative damage.
Limitations and Strengths
This study has some limitations. First, our findings were obtained in a relatively small group of patients; therefore, confirmation in a larger population is recommended. Second, it might be speculated that the degree of the patients' cognitive functioning influenced the mean levels of the biochemical parameters [20]. In this study, we adjusted for the Charlson Comorbidity Index, which includes dementia, and our estimates remained statistically significant. However, for our additional analysis MMSE scores were not available for all patients; therefore, we can neither confirm nor deny that the presence of a comorbid cognitive disturbance, not diagnosed as dementia (yet), was a confounding factor. Third, the timing of blood sampling might be a factor of significance. It is possible that the levels of biochemical markers are dependent on delirium duration and severity or even fluctuate during the day in patients with delirium, just like delirium symptoms. In the present study, blood sampling and delirium occurred on the same day, but there is a possibility that the patients had no delirium symptoms at the moment of blood sampling. This might have influenced our results. Finally, some potential participants were not included in the study and this may have resulted in some selection bias; however, since this was random and occurred in both patients with and without delirium, we think that our results are only minimally influenced by this.
The present study has several strengths. First, the intensive monitoring of clinical symptoms of patients with delirium until discharge and the DSM-IV diagnosis by a geriatrician makes it less likely that we missed delirium or misdiagnosed symptoms. Second, we did not focus on one but on several possible pathways that might lead to delirium as it has been suggested that the pathophysiology is multifactorial.
Conclusion
In this study in older, acutely ill hospitalized patients, we found that patients with delirium had higher Phe/Tyr ratios, lower Trp/LNAAs ratios and lower levels of arginine and citrulline than patients without delirium. These findings might suggest that decreased BH4 availability, disturbed serotonergic neurotransmission and an increased status of oxidative stress may have played a role in the pathogenesis of delirium in our patient group. Since as far as we know this is the first delirium study investigating BH4 status and levels of arginine and citrulline in acutely ill elderly hospitalized patients, confirmation of our results in a larger, comparable population is recommended. Moreover, more research is needed to explore the potential differences in the pathophysiology of delirium in patients with and without cognitive disorders.
Disclosure Statement
The authors have no conflicts of interest to declare.
Acknowledgements
We thank all patients who participated in the study. We are also very grateful to Ans Voskuilen-Kooijman for her assistance in processing the blood samples and high-performance liquid chromatography analyses. This study was supported by a research grant of Fund NutsOhra (project number 0902-047).
References
- 1.Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304:443–451. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 2.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383:911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21:1190–1222. doi: 10.1016/j.jagp.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Werner ER, Blau N, Thony B. Tetrahydrobiopterin: biochemistry and pathophysiology. Biochem J. 2011;438:397–414. doi: 10.1042/BJ20110293. [DOI] [PubMed] [Google Scholar]
- 5.Werner ER, Gorren AC, Heller R, Werner-Felmayer G, Mayer B. Tetrahydrobiopterin and nitric oxide: mechanistic and pharmacological aspects. Exp Biol Med (Maywood) 2003;228:1291–1302. doi: 10.1177/153537020322801108. [DOI] [PubMed] [Google Scholar]
- 6.Osse RJ, Fekkes D, Tulen JH, Wierdsma AI, Bogers AJ, van der Mast RC, Hengeveld MW. High preoperative plasma neopterin predicts delirium after cardiac surgery in older adults. J Am Geriatr Soc. 2012;60:661–668. doi: 10.1111/j.1532-5415.2011.03885.x. [DOI] [PubMed] [Google Scholar]
- 7.van Gool AR, Fekkes D, Kruit WH, Mulder PG, ten Hagen TL, Bannink M, Maes M, Eggermont AM. Serum amino acids, biopterin and neopterin during long-term immunotherapy with interferon-alpha in high-risk melanoma patients. Psychiatry Res. 2003;119:125–132. doi: 10.1016/s0165-1781(03)00113-6. [DOI] [PubMed] [Google Scholar]
- 8.van der Mast RC, van den Broek WW, Fekkes D, Pepplinkhuizen L, Habbema JD. Is delirium after cardiac surgery related to plasma amino acids and physical condition? J Neuropsychiatry Clin Neurosci. 2000;12:57–63. doi: 10.1176/jnp.12.1.57. [DOI] [PubMed] [Google Scholar]
- 9.Amin F, Davidson M, Kahn RS, Schmeidler J, Stern R, Knott PJ, Apter S. Assessment of the central dopaminergic index of plasma HVA in schizophrenia. Schizophr Bull. 1995;21:53–66. doi: 10.1093/schbul/21.1.53. [DOI] [PubMed] [Google Scholar]
- 10.Egberts A, Wijnbeld EH, Fekkes D, van der Ploeg MA, Ziere G, Hooijkaas H, van der Cammen TJ, Mattace-Raso FU. Neopterin: a potential biomarker for delirium in elderly patients. Dement Geriatr Cogn Disord. 2015;39:116–124. doi: 10.1159/000366410. [DOI] [PubMed] [Google Scholar]
- 11.Schuurmans MJ, Shortridge-Baggett LM, Duursma SA. The Delirium Observation Screening scale: a screening instrument for delirium. Res Theory Nurs Pract. 2003;17:31–50. doi: 10.1891/rtnp.17.1.31.53169. [DOI] [PubMed] [Google Scholar]
- 12.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington: American Psychiatric Association; 2000. text revision. [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state'. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.McDowell I, Newell C. Measuring Health: A Guide to Rating Scales and Questionnaires. New York: Oxford University Press; 1996. [Google Scholar]
- 16.Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 17.Dendukuri N, McCusker J, Belzile E. The Identification of Seniors at Risk screening tool: further evidence of concurrent and predictive validity. J Am Geriatr Soc. 2004;52:290–296. doi: 10.1111/j.1532-5415.2004.52073.x. [DOI] [PubMed] [Google Scholar]
- 18.Fekkes D. Automated analysis of primary amino acids in plasma by high-performance liquid chromatography. Methods Mol Biol. 2012;828:183–200. doi: 10.1007/978-1-61779-445-2_16. [DOI] [PubMed] [Google Scholar]
- 19.Fekkes D, Timmerman L, Pepplinkhuizen L. Effects of clomipramine on plasma amino acids and serotonergic parameters in panic disorder and depression. Eur Neuropsychopharmacol. 1997;7:235–239. doi: 10.1016/s0924-977x(97)00412-4. [DOI] [PubMed] [Google Scholar]
- 20.Foxton RH, Land JM, Heales SJ. Tetrahydrobiopterin availability in Parkinson's and Alzheimer's disease; potential pathogenic mechanisms. Neurochem Res. 2007;32:751–756. doi: 10.1007/s11064-006-9201-0. [DOI] [PubMed] [Google Scholar]
- 21.Richardson MA, Read LL, Taylor Clelland CL, Reilly MA, Chao HM, Guynn RW, Suckow RF, Clelland JD. Evidence for a tetrahydrobiopterin deficit in schizophrenia. Neuropsychobiology. 2005;52:190–201. doi: 10.1159/000089002. [DOI] [PubMed] [Google Scholar]
- 22.van der Mast RC, Fekkes D, Moleman P, Pepplinkhuizen L. Is postoperative delirium related to reduced plasma tryptophan? Lancet. 1991;338:851–852. doi: 10.1016/0140-6736(91)91504-n. [DOI] [PubMed] [Google Scholar]
- 23.Pandharipande PP, Morandi A, Adams JR, Girard TD, Thompson JL, Shintani AK, Ely EW. Plasma tryptophan and tyrosine levels are independent risk factors for delirium in critically ill patients. Intensive Care Med. 2009;35:1886–1892. doi: 10.1007/s00134-009-1573-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flacker JM, Lipsitz LA. Large neutral amino acid changes and delirium in febrile elderly medical patients. J Gerontol A Biol Sci Med Sci. 2000;55:B249–B252. doi: 10.1093/gerona/55.5.b249. discussion B253-B254. [DOI] [PubMed] [Google Scholar]
- 25.van der Cammen TJ, Tiemeier H, Engelhart MJ, Fekkes D. Abnormal neurotransmitter metabolite levels in Alzheimer patients with a delirium. Int J Geriatr Psychiatry. 2006;21:838–843. doi: 10.1002/gps.1569. [DOI] [PubMed] [Google Scholar]
- 26.Hshieh TT, Fong TG, Marcantonio ER, Inouye SK. Cholinergic deficiency hypothesis in delirium: a synthesis of current evidence. J Gerontol A Biol Sci Med Sci. 2008;63:764–772. doi: 10.1093/gerona/63.7.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramirez-Bermudez J, Ruiz-Chow A, Perez-Neri I, Soto-Hernandez JL, Flores-Hernandez R, Nente F, Montes S, Rios C. Cerebrospinal fluid homovanillic acid is correlated to psychotic features in neurological patients with delirium. Gen Hosp Psychiatry. 2008;30:337–343. doi: 10.1016/j.genhosppsych.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Reiter RJ, Tan DX, Burkhardt S. Reactive oxygen and nitrogen species and cellular and organismal decline: amelioration with melatonin. Mech Ageing Dev. 2002;123:1007–1019. doi: 10.1016/s0047-6374(01)00384-0. [DOI] [PubMed] [Google Scholar]
- 29.Smith MA, Richey Harris PL, Sayre LM, Beckman JS, Perry G. Widespread peroxynitrite-mediated damage in Alzheimer's disease. J Neurosci. 1997;17:2653–2657. doi: 10.1523/JNEUROSCI.17-08-02653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]