Abstract
Background
The diagnosis of behavioral variant of frontotemporal dementia (bvFTD) relies primarily on clinical features and remains challenging. The specificity of the recently revised criteria can be disappointing, justifying development of new clinical tools.
Objective
We produced a behavioral inventory named DAPHNE. This scale (adapted from Rascovsky's criteria) explores six domains: disinhibition, apathy, perseverations, hyperorality, personal neglect and loss of empathy. It is composed of ten items (five answer categories). The aim was (1) to assess the validity and reliability of DAPHNE and (2) to evaluate its contribution in differentiating patients.
Methods
Two scores were computed: DAPHNE-6 (screening) from the six domains and DAPHNE-40 (diagnosis) from the ten items. Reliability and reproducibility were assessed. External validity was studied with the Frontal Behavioral Inventory (FBI) and the Frontotemporal Behavioral Scale (FBS). Finally, the diagnostic performance of DAPHNE was compared to revised criteria, FBI and FBS.
Results
DAPHNE was administered to the caregivers of 89 patients, 36 with bvFTD, 22 with Alzheimer's disease, 15 with progressive supranuclear palsy and 16 with bipolar disorder. Reliability and reproducibility were excellent, as was external validity. DAPHNE-6 allowed bvFTD diagnosis (score ≥4) with a sensitivity of 92%, while DAPHNE-40 (score ≥15) had a specificity of 92%.
Conclusion
We demonstrate excellent psychometric features for DAPHNE. This quick tool could help for both diagnosing and screening bvFTD.
Key Words: Frontotemporal dementia, Behavioral disorders, Alzheimer's disease, Bipolar disorder, Progressive supranuclear palsy, Scale
Introduction
Different clinical variants of frontotemporal dementia (FTD) have been delineated, the most common of which is behavioral variant of FTD (bvFTD). Patients with bvFTD exhibit early decline in social behavior and personal conduct [1,2,3]. In the absence of definitive biomarkers, the diagnosis of bvFTD relies primarily on clinical features and remains challenging as bvFTD patients are frequently underdiagnosed or misdiagnosed, with psychiatric disorders for example.
Recently an international consortium developed revised guidelines for the diagnosis of bvFTD [4]. The validation process retrospectively reviewed clinical records and compared the sensitivity of proposed and earlier criteria in a multisite sample of patients with pathologically verified frontotemporal lobar degeneration. Among these new criteria developed by Rascovsky et al. [4], five out of the six clinically discriminating features concern psycho-behavioral disturbances. Unfortunately, as stated by the authors themselves, these criteria were not tested in neurological or psychiatric comparison groups to assess specificity. Thus, the use of these criteria might be sensitive but not specific enough to really help in differentiating non-bvFTD patients with quite similar clinical presentations.
In clinical practice, early-onset Alzheimer's disease (AD) is often difficult to distinguish from bvFTD [5]. Firstly, memory testing sometimes has limited value to differentiate AD from FTD [6], and secondly, behavioral disorders may be more frequent in younger AD patients than in older ones [7]. Patients with frontal damage, such as progressive supranuclear palsy (PSP), can also be difficult to differentiate from bvFTD patients at an early stage when behavior disorders are prominent [8]. Another major differential diagnosis is bipolar disorder (BP) [9].
To improve clinical diagnostic accuracy, numerous scales have been proposed, mostly focusing on the assessment of the behavioral symptoms in bvFTD patients. These scales are either not specific, based on outdated consensus criteria and/or too time-consuming to be routinely performed. For instance, the Frontal Behavioral Inventory (FBI), proposed by Kertesz et al. in 1997 [10], was based on the Lund and Manchester criteria [11]. Furthermore, using these scales ‘at the patient's bedside’ for routine evaluation takes too much time in clinical practice, with the scoring system often much too complicated for both patients and caregivers.
In order to better and more readily identify the behavioral symptoms in FTD, we have developed a new and original scale named DAPHNE. The primary aim of this prospective multicenter study was to test the validity and reliability of this new scale and then to investigate whether it could be helpful in diagnosing bvFTD and especially in differentiating patients with or without bvFTD.
If behavioral disturbances are essential to differentiate between bvFTD and non-bvFTD patients, the neuropsychological evaluation seem also important [12]. Thus, the secondary purpose of the present study was to investigate, among different groups of patients with or without bvFTD, the possible relationship between this new behavioral scale, DAPHNE, and other measures such as traditional cognitive efficiency and social cognition tests, as well as caregiver's burden.
Patients and Methods
Study Design
This was a prospective multicenter cohort study. Patients and caregivers were enrolled from five French expert memory centers. All these centers have extensive experience in neurodegenerative diseases, especially in FTD. Psychiatric patients (and caregivers) were also referred from the psychiatric department of Nantes University Hospital. The patients and their caregivers were seen for evaluation, at the investigation center, on day 0, then at 6 months and finally 1 year after enrollment.
Ethical Considerations
The study protocol was approved by the Committee on Ethics and Human Research (Comité de Protection des Personnes Ouest VI), in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). All patients and caregivers gave written informed consent.
Subjects and Inclusion Criteria
For inclusion in the study, the following criteria had to be met: (1) the patient had a caregiver and both had accepted to participate to the study, and (2) the patient was referred to one reference memory center (3) where the diagnostic was either bvFTD, AD, PSP or BP. Inclusion of healthy subjects did not seem relevant since the aim was to investigate a new behavioral disturbance scale among different groups of ill patients.
The following clinical features were required. To avoid inclusion of patients with too advanced disease, a Mini-Mental State Examination (MMSE) score >18 was necessary [13]. For FTD patients, the revised Rascovsky criteria had to be met for possible bvFTD. AD patients had to fulfill the modified McKhann et al.'s criteria [14] while PSP patients had to fulfill the criteria for possible or probable PSP [15]. Psychiatrists referred BP patients. The DSM-IV criteria for BP were checked. Acute mood symptoms were not allowed.
At inclusion, all patients underwent structural magnetic resonance imaging and 99mTc- ECD brain single-photon emission computed tomography. For bvFTD patients the findings had to be consistent with the diagnosis. Thus, at inclusion all bvFTD patients met Rascovsky's criteria for probable bvFTD.
Cerebrospinal fluid biomarkers had to be in accordance with the diagnosis for AD patients (Aβ 1-42 <500 ng/l, T-tau >350 ng/l and P-tau181P >50 ng/l) and were also required for FTD patients when an AD diagnosis could not be ruled out based solely on clinical or radiologic and scintigraphic features.
At enrollment the patients were allocated to one out of four groups: bvFTD, AD, PSP or BP. Since the initial diagnosis could sometimes be challenging, the reference physician was asked to confirm the diagnosis at the end of the follow-up (minimum 1 year).
DAPHNE, a New Behavioral Disturbance Scale
In early 2012, a national consortium of French-speaking experts was selected. A first draft scale was produced (C.B.B. and C.T.A.). Ten experts participated in a modified Delphi process in order to validate proposals for each item of this initial scale. These clinicians are all working in French university hospitals. The experts were asked to evaluate proposals for each item (with a score from 0 to 4) and, if necessary, to comment on it. Their responses were gathered and analyzed. If the proposal item did not have the same score at 80%, it was modified according to the experts' comments. Three rounds were necessary to produce a six-domain ten-item scale, named DAPHNE for disinhibition, apathy, perseverations, hyperorality, personal neglect and loss of empathy.
Domains were selected from the core diagnostic features of Rascovsky's criteria. The ‘personal neglect’ domain was added in accordance with Kertesz et al.'s FBI [10], Lebert et al.'s Frontotemporal Behavioral Scale (FBS) [16] and following our experience. The DAPHNE scale was designed as a series of semi-structured propositions to be asked of caregivers. It was composed of items with five possible answer categories. The process determined that one item was sufficient to characterize the following deficits: apathy, loss of empathy, perseverations and personal neglect. However, disinhibition and hyperorality required several items for accurate assessment. In our experience, it is often difficult for caregivers to evaluate the degree of severity, so we proposed a scoring system similar to the Clinical Dementia Rating (CDR) scale instead of scoring with only a severity score [17]. The scoring on a five-point scale (none, very mild, mild, moderate and severe) for each item is dependent on the severity and/or frequency, gauged by an increase in behavioral troubles. We proposed progressive graduation for each item with description and examples.
At the end of 2012, the Delphi process had produced the final DAPHNE scale. The English version is presented in table 1 while the French version can be found as a supplementary online file (online supplementary table S1; for all online supplementary material, see www.karger.com/doi/10.1159/000440859).
Table 1.
DAPHNE scale
| Normal (0) | Very mild (1) | Mild (2) | Moderate (3) | Severe (4) | |
|---|---|---|---|---|---|
| Disinhibition | |||||
| Loss of social convenience | no trouble | subject makes unpleasant, hurtful comments to family members; subject seeks out contact with strangers | subject makes unpleasant, hurtful comments to strangers | subject is unable to participate in any social activity because of inappropriate social behavior (impatience, etc.) | subject interrupts strangers’ activities, behaves inappropriately and disturbs public order (obscene words, urination, etc.) |
| Inappropriate joviality | no trouble | subject is jovial and laughs unreasonably but in appropriate situations and can stop when asked to | subject is jovial and laughs unreasonably in appropriate situations but cannot stop when asked to | subject is jovial in embarrassing situations (talks to strangers, etc.) | subject is jovial and says unacceptable words (jokes, sneers) in inappropriate situations (at funerals, with young children, etc.) |
| Unrestrained spending habits | no trouble | subject buys a lot by mail order or repeatedly buys the same low-value things, but can listen to reason | subject buys a lot by mail order or repeatedly buys the same low-value things, but cannot listen to reason | subject buys lots of useless things, buys expensive objects and does not understand that they are excessive and inappropriate | subject is indebted because of lots of expensive purchases or gambling (card games, casino, etc.) |
| Sexual disinhibition | no trouble | subject makes inappropriate sexual comments or jokes, but can stop if asked to | subject makes inappropriate and uncontrolled sexual comments or jokes, which he/she then acts on | subject makes inappropriate and uncontrolled sexual comments or jokes, which he/she then acts on; subject is indecent (undresses in inappropriate places, etc.) | subject displays unwanted and inappropriate sexual behavior (public masturbation, sexual touching of a minor, sexual attraction to animals, etc.) |
| Apathy | |||||
| Loss of initiative, social interest | no trouble | subject can take part in usual activities, but must be encouraged to do anything outside of the ordinary | subject can take part in usual activities, but does not complete them; subject can restart an activity, but only with stimulation | subject interrupts activities and does not restart them, even with stimulation; subject does not want to do usual activities | subject has no interest; does not do anything despite stimulation, stays in his/her seat or in bed all day |
| Loss of empathy | |||||
| Emotional blunting, indifference | no trouble | subject complains about loss of emotion towards relatives | subject shows little interest in stories from relatives or in emotionally current matters; subject has difficulty expressing feelings | subject is indifferent to relatives, does not care about them, and is not concerned when people speak about him/her | subject is unable to express or decipher any emotion, can have inappropriate emotional responses |
| Perseverations | |||||
| Fixed ideas, stereotypical behavior | no trouble | subject collects usual objects or has trouble getting rid of things or has routine activities | subject collects unusual objects or does not throw anything away, has ritualized activities or has obsessions (hours, etc.), but this is consistent with social life | subject collects lots of objects or has difficulty sitting still, has obsessional rituals that interfere with social life | subject has continuous rituals (grinding of teeth, rubbing of body, grasping of objects, repetition of words or sentences); subject does not stand still |
| Hyperorality | |||||
| Eating disorders, new preference for sweets | no trouble | subject has a new preference for sweets | subject has new or bizarre food preferences but can listen to reason | subject eats or drinks excessively and cannot listen to reason (padlock on cupboard, etc.) | subject eats and drinks everything within reach, including in other people's plates or glasses, or eats inedible substances |
| Bulimia, gluttony | no trouble | subject eats much more, has put on weight | subject eats gluttonously, voraciously, without getting dirty | subject eats quickly and gets dirty, takes big pieces, risking choking | subject eats with hands, uncleanly, does not cut his food, keeps food in mouth; subject has put on a lot of weight |
| Neglect | |||||
| Personal neglect | no trouble | subject looks less neat | subject must be stimulated to wash or change clothes | subject can wash or change clothes only when threatened or tricked | subject has very poor hygiene (dirty fingernails, dirty hair, dirty clothes, etc.) |
DAPHNE-6 (screening) is computed from the six synthetic binary domains. For a given domain, score 1 point if at least one symptom is present, regardless of the number of items present in the domain and irrespective of the severity. The maximum score is six.
DAPHNE-40 (diagnosis) is computed as the sum of the boxes of the ten items. The maximum score is 40.
Procedures
Behavioral Scales. In order to assess the validity of DAPHNE, the FBI and the FBS were used. We previously demonstrated that FBI is a very useful scale in bvFTD, especially compared to the Neuropsychiatric Inventory [18]. The FBI constructed by Kertesz et al. [10] in 1997 is a 24-item questionnaire that targets behaviors or personality changes specific to bvFTD. The practical (sensitive) cut-off for the diagnosis of bvFTD was set at 27 out of 72 [10,19]. The FBS is a short scale appreciated for its specificity [16]. It indicates the presence of disturbances such as self-control disorder, physical neglect, mood disorder and lower general interest. The maximum score is 4, and a score of 3, with mild-to-moderate dementia (MMSE >18), is in favor of bvFTD.
Complete Neuropsychological Assessment. In addition to behavioral scales, all patients underwent a neuropsychological examination. Overall efficiency was evaluated using the MMSE [20]. Executive function was assessed by the Frontal Assessment Battery [21], the Trail Making Test [22], the modified Wisconsin Card Sorting Test [23], verbal fluency [24] and the Zoo Map Test [25]. Visuoconstructional praxis was assessed by copy of Rey figure [26], and memory was evaluated by Free and Cued Selective Reminding Test [27] as well as recall of Rey figure. DO 80 was performed for language examination [28]. All groups also underwent a set of tests assessing social and affective/emotional processes: Ekman pictures [29], Reading Mind in the Eyes [30] and Faux Pas Recognition Test [31] adapted by Boutantin et al. [32]. We also performed Frontotemporal Lobar Dementia - Clinical Dementia Rating (FTLD-CDR) for each patient. The classical CDR is a widely used scale in AD therapeutic trials [17]. Knopman et al. [33] added two additional domains: ‘language’ and ‘behavior, comportment and personality’ in order to adapt the CDR to FTD. Both the caregiver and the patient were interviewed to complete the FTLD-CDR.
Burden Inventory. Finally, the caregivers were asked to complete a burden inventory. We previously showed that burden was high for caregivers of those with bvFTD and that this score correlated with behavioral disturbances [34]. Caregiver burden was evaluated using the 22-item Zarit Burden Inventory (ZBI) [35], derived from a 29-item preliminary version [36]. The scale is made up of 22 items evaluating disease impact on caregiver's quality of life, psychological suffering, financial difficulties, shame, guilt and difficulties in social and familial relationships.
Statistical Analysis
Number of Patients. The number of patients was calculated to be sufficient to validate the new scale. Thus, preliminary calculation found that 45 bvFTD patients were needed while 15 patients were necessary for each of the three ‘control’ groups. According to the relative rarity of the disease, it was assumed that 1 year would be necessary to enroll that number of patients in the six investigation centers.
DAPHNE Validation. Two scores were computed. (1) DAPHNE-6 (screening) was computed from six synthetic binary domains. For a given domain, we scored one point if at least one symptom was present, regardless of the number of items present in the domain and irrespective of the severity. The maximum score is six. (2) DAPHNE-40 (diagnosis) was computed as the sum of the boxes of the ten items. The maximum score is 40. The validity of the DAPHNE scores (DAPHNE-6 and DAPHNE-40) was assessed using known groups' validity (scores of the bvFTD patients were assumed to be greater than scores of the other groups) and concurrent validity (scores of DAPHNE were assumed to be positively correlated using Spearman's correlation coefficient with FBS and FBI, but the hypothesis of a correlation with FTLD-CDR and ZBI was also tested). The reliability of the DAPHNE scores (DAPHNE-6 and DAPHNE-40) was assessed using Cronbach's alpha (internal consistency): a correct internal consistency was considered if the Cronbach's alpha coefficient was >0.7. The reproducibility of the DAPHNE scores (DAPHNE-6 and DAPHNE-40) was assessed using intra-class correlation coefficient (ICC): an excellent reproducibility was considered if ICC was >0.8 and a good reproducibility was considered if ICC was >0.6. The discriminating validity of the scores was assessed using receiver operating characteristics curves using each of the scales (DAPHNE-6, DAPHNE-40, FBI and FBS). For each scale, sensitivity and specificity as well as positive likelihood ratio were computed using optimal thresholds (for DAPHNE) or validated thresholds for FBI and FBS (≥27 for FBI and ≥3 for FBS).
General Statistics. Continuous outcomes were described using means and standard deviations. Binary outcomes were described using observed proportions. The four groups of patients (bvFTD, AD, PSP and BP) were compared using ANOVA. Initially the four groups were distinguished, and then the three control groups (AD, PSP and BP) were formed and bvFTD patients were compared to this set.
Results
Characteristics of Patients and Caregivers
Eighty-nine patients were prospectively enrolled between March 2013 and March 2014. The distribution was as follow: bvFTD (n = 36), AD (n = 22), PSP (n = 15) and BP patients (n = 16). The demographic characteristics of patients and caregivers, as well as the disease main features at inclusion, are summarized in table 2.
Table 2.
Characteristics of patients and caregivers at inclusion
| bvFTD (n = 36) | AD (n = 22) | PSP (n = 15) | BP (n = 16) | All controls1 (n = 53) | p value2 (4 groups/2 groups) | |
|---|---|---|---|---|---|---|
| Age, years | 66.0 ± 8.3 | 65.4 ± 7.3 | 69.8± 5.3 | 61.0 ± 7.6 | 65.3 ± 7.6 | 0.010/0.51 |
| Female sex | 42% (n = 15) | 45% (n = 10) | 40% (n = 6) | 69% (n = 11) | 51% (n = 27) | 0.30/0.52 |
| Disease duration, years | 4.5 ± 2.7 | 3.8 ± 1.3 | 3.0 ± 1.4 | 19.6 ± 15.6 | 8.4 ± 11.3 | 0.0001/0.30 |
| MMS/30 | 23.6 ± 3.6 | 23.5 ± 2.5 | 24.2 ± 3.3 | 26.2 ± 2.7 | 24.5 ± 3.0 | 0.033/0.18 |
| Caregiver age, years | 59.1 ± 13.5 | 64.2 ± 6.6 | 69.2 ± 6.8 | 54.5 ± 15.6 | 62.8 ± 11.6 | 0.0032/0.19 |
Corresponds to the sum of the three control groups (AD, PSP and BP patients).
The first comparison was performed between the four groups of patients (bvFTD, AD, PSP and BP) while the second analysis compared bvFTD patients to all controls (AD, PSP and BP patients as a whole). p values <0.05 were considered significant and are written in bold.
Validation of the DAPHNE Scale
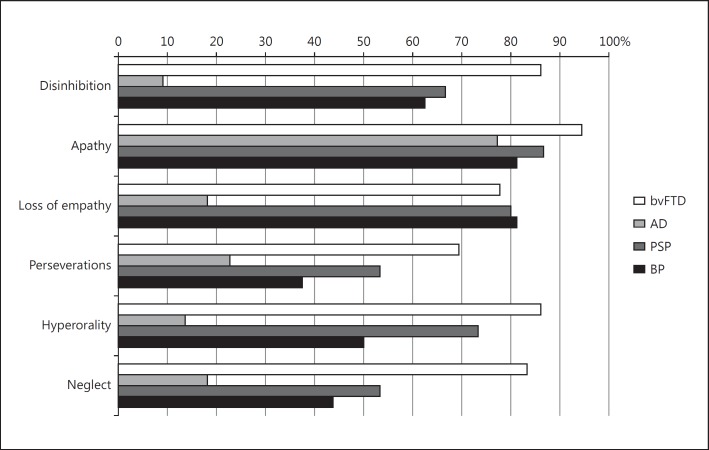
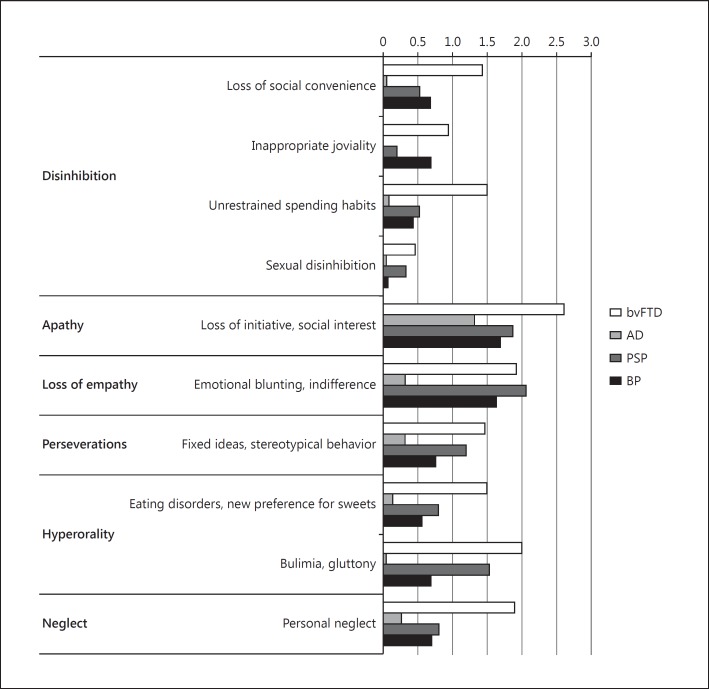
Internal Validity. The score obtained respectively with DAPHNE-6 (screening) and DAPHNE-40 (diagnosis) is detailed for each group in table 3. Results by domains and items are presented in figures 1 and 2, respectively. As expected, patients with bvFTD obtained significantly higher behavioral scores on DAPHNE than AD patients. Moreover, the mean scores of bvFTD patients on DAPHNE-6 and DAPHNE-40 were significantly greater compared to all other groups. The ICC for reproducibility was 0.94 for DAPHNE-40 and 0.84 for DAPHNE-6.
Table 3.
Diagnostic accuracy of behavioral scales to differentiate bvFTD (area under curve, sensitivity, specificity)
| bvFTD (n = 36) | AD (n = 22) | PSP (n = 15) | BP (n = 16) | All controls1 (n = 53) | p value2 (4 groups/2 groups) | |
|---|---|---|---|---|---|---|
| DAPHNE-6 | 5.0 ± 1.1 | 1.6 ± 1.5 | 4.1 ± 1.3 | 3.6 ± 1.5 | 2.9 ± 1.8 | 0.0001/0.0001 |
| DAPHNE-40 | 15.7 ± 6.0 | 2.6 ± 2.6 | 10.2 ± 5.3 | 7.6 ± 5.1 | 6.2 ± 5.3 | 0.0001/0.0001 |
Scores are presented as mean ± SD.
Corresponds to the sum of the three control groups (AD, PSP and BP patients).
The first comparison was performed between the four groups of patients (bvFTD, AD, PSP and BP) while the second analysis compared bvFTD patients to all controls (AD, PSP and BP patients as a whole). p values <0.05 were considered significant and are written in bold.
Fig. 1.
Result of the DAPHNE scale by domains for each group. For each domain the proportion was significantly different between the four groups except for apathy (Fisher's exact test; all p values <0.01). When bvFTD patients were compared to all the other patients (as a control group), the same difference was observed: the proportion was significantly higher for bvFTD patients whatever the domain except for apathy (Fisher's exact test; p = 0.04 for empathy; all other p values <0.01).
Fig. 2.
DAPHNE scale - mean item score for each group. The x axis (theoretical maximum value of 4) was censored, considering the highest mean observed. For each item, the score was significantly different between the four groups (Kruskal-Wallis test; all p values <0.01) except for sexual disinhibition. When bvFTD patients were compared to all the other patients (as a control group), the same difference was observed (Student's t test; all p values <0.01): the score was significantly higher for bvFTD patients whatever the item except for sexual disinhibition.
Concurrent Validity. The overall mean FBI and FBS scores were abnormal for the bvFTD group (30.8 ± 9.9 and 3.6 ± 0.5, respectively) and were both significantly greater when compared to all other groups. We showed a significant correlation (p < 0.05) between the FBI and DAPHNE (r = 0.80 and r = 0.89 for DAPHNE-6 and DAPHNE-40, respectively) and between the FBS and DAPHNE (r = 0.77 and 0.71 for DAPHNE-6 and DAPHNE-40, respectively) in all patients.
Is DAPHNE a Discriminating Tool for bvFTD Diagnosis? Three AD patients, 14 PSP patients and 14 BP patients fulfilled Rascovsky's criteria for possible bvFTD, having been assessed with three or more clinical criteria. This confirms a very low specificity of these criteria. On the other hand, a cut-off on DAPHNE-40 of 15/40 allowed us to distinguish all groups (score <15) from bvFTD (score ≥15), with a specificity of 92%. This scale can thus be considered as an efficient clinical tool to distinguish bvFTD patients from patients exhibiting a possible very close clinical presentation. A logistic regression (results not shown) indicated that neglect, inappropriate joviality and unrestrained spending habits were the best items used to discriminate bvFTD from the others groups. A cut-off on DAPHNE-6 of 4/6 allowed us to distinguish all groups (score <4) from bvFTD (score ≥4), with a sensitivity of 92%. DAPHNE can then also be used as a screening tool. When applied in this study population, the diagnostic features of the FBI and the FBS were as follows. For the FBI we found a sensitivity of 67% and a specificity of 91% when a cut-off value of 27 was used. For the FBS sensitivity was 97% and specificity 45% when a cut-off value of 3 was used. These results confirm the major interest of behavioral scales, in particular the DAPHNE scale, to differentiate bvFTD patients from AD, PSP or BP patients (table 4). Additional material (area under the curve, diagnostic accuracy for each group, etc.) can be found in online supplementary table S2.
Table 4.
Diagnostic accuracy of the revised criteria and behavioral scales to differentiate bvFTD
| Threshold | Sensitivity | Specificity | Positive likelihood ratio | |
|---|---|---|---|---|
| Rascovsky's clinical criteria | ≥3 | 100% | 41% | 1.7 |
| DAPHNE-6 | ≥4 | 92% | 57% | 2.1 |
| DAPHNE-40 | ≥15 | 56% | 92% | 7.0 |
| DAPHNE ‘combined’ | – | 92% | 92% | 11.5 |
| FBS | ≥3 | 97% | 45% | 1.7 |
| FBI | ≥27 | 67% | 91% | 7.4 |
The positive likelihood ratio is assumed to demonstrate the interest of a diagnostic tool when >5, and better >10. Thus, values >5 are written in bold.
Complete Neuropsychological Assessment
As presented in Patients and Methods, many neuropsychological tools were used to try to show differences between the four groups of patients. Unfortunately, none of these tests (memory, executive functions and social cognition) was significant between the bvFTD patients and the others, except for the Zoo Map Test. The detailed neuropsychological characteristics at baseline are presented in online supplementary table S3.
Significant correlations of DAPHNE were obtained with the Zoo Map Test and the Ekman test (the correlations between DAPHNE-6 and DAPHNE-40 with Zoo Map Test latency were −0.29 and −0.34, respectively, and the correlations between the DAPHNE-6 score and the DAPHNE-40 score with the Ekman test were −0.34 and −0.38, respectively). FTLD-CDR correlated significantly with DAPHNE-6 and DAPHNE-40 (0.63 and 0.70, respectively).
Burden Inventory
The ZBI score was significantly higher in bvFTD than in AD and PSP, but no statistical difference was observed with BP. We showed a significant correlation between the ZBI and DAPHNE (r = 0.56 and 0.62 for DAPHNE-6 and DAPHNE-40, respectively).
Discussion
Clinicians in memory centers need tools designed to help in the screening and diagnosing of neurodegenerative diseases. The findings from this study support the utility of DAPHNE for bvFTD screening and diagnosis. DAPHNE-6, with a sensitivity of 92%, permits screening to guide investigations in order to validate a diagnostic hypothesis. DAPHNE-40 is a clinical diagnostic tool which permits a differential diagnosis.
DAPHNE and the Revised Criteria of bvFTD
In the study population, specificity was very low for the revised criteria of bvFTD. This result was expected. As mentioned earlier, the domains chosen for DAPHNE were selected in accordance with Rascovsky's criteria, but also from pre-existing behavioral scales and our experience. In order to meet the criteria for possible bvFTD, three of the following six behavioral or cognitive symptoms had to be present: early behavioral disinhibition, early apathy or inertia, early loss of sympathy or empathy, early perseverative/stereotyped or compulsive behavior, hyperorality and dietary changes, and executive deficits in neuropsychological profile, with a sensitivity of 85% in a cohort of 176 pathology-confirmed bvFTD patients. In our study, bvFTD obviously fulfilled the revised criteria (sensitivity 100%), but most PSP and BP patients did so too (specificity 41%). In a recent work, Kobylecki et al. [37] also showed that 20 patients (32%) with PSP fulfilled the diagnostic criteria for possible bvFTD, with three or more clinical criteria. DAPHNE allows us to distinguish these populations from each other as demonstrated by a high positive likelihood ratio. First of all, we added personal neglect. In our study more than 80% of the bvFTD patients present with this symptom. Early personal neglect is a very frequent symptom in bvFTD, with 36% of patients responding to the criteria of Diogenes syndrome [38]. The FBI and the FBS included this symptom too. Our results support the finding that this sign is essential for a differential diagnosis of BP or PSP.
Furthermore, we propose a quantification of behavioral symptoms. For example, the presence of apathy did not permit us to differentiate groups because this symptom is not specific and is well known to present therapeutic challenges in most forms of dementia. However, the quantification of apathy proposed in DAPHNE-40 seems to be a relevant factor when used to distinguish the diseases. A third point is that DAPHNE has such high specificity because it is a pure ‘behavioral’ scale and because it does not screen for the neuropsychological criterion of the revised criteria, which lacks specificity.
DAPHNE, FBI and FBS
In 1997, it was shown that a FBI score of ≥27 (maximum 72) was suggestive of bvFTD [10]. The results of that pilot study were confirmed in 2000 [19]. DAPHNE was constructed on the same dichotomy proposed by Kertesz et al. [10], with two main types of behavior: negative and positive. In our population, the cut-off >27 for the FBI corresponds to a sensitivity of 67% and a specificity of 96%. The psychometric features of DAPHNE are then better than the FBI when combined. The duration and the scoring system are also major advantages of DAPHNE. The FBI is composed of 24 items and DAPHNE of only 10, so the assessment with DAPHNE usually takes 5-10 min while that with the FBI takes 15-20 min. The propositions formulated by interviewers in DAPHNE lead to a more precise scoring. For the FBS, Lebert et al. [16] in their validation study proposed a cut-off of 3, with a sensitivity of 95% and a specificity of 91%. In our population, we obtained a sensitivity of 97% and a specificity of only 45% for FBS. This seems to indicate that the psychometric features of DAPHNE are more relevant (table 4). Indeed, DAPHNE is a compromise between a long and short scale, and the correlations with these two scales are very good.
DAPHNE and Neuropsychological Assessment
We observed in our population that neither executive assessment nor social cognition permits an easy distinction between bvFTD or psychiatric troubles or PSP. Finally, for executive functions only Zoo Map Test latency time appears very relevant in discriminating bvFTD. bvFTD patients rush to begin the test without adequate reflection, a tendency linked with behavioral trouble.
Concerning memory, it was established recently that memory testing has limited value in differentiating AD from FTD [39]. Our bvFTD group had memory deficit similar to amnesic syndrome of the AD type [40]. The relative preservation of memory, a criterion proposed by Rascovsky et al. [4], needs to be considered with caution.
Thus, our study confirms that traditional neuropsychological tests have a low diagnostic accuracy in bvFTD. In contrast, ‘theory of mind’ was assumed to be more relevant [41,42]. However, none of the tests, for the global score, permitted to distinguish bvFTD from the three other groups. Further specific analysis and discussion on these neuropsychological data will be presented elsewhere.
DAPHNE, FTLD-CDR and Burden
FTLD-CDR is a comprehensive measure of cognition and behavior, and Knopman et al. [33] showed in 107 subjects (including 36 bvFTD patients) that FTLD-CDR permits the measuring of disease progression. It is interesting to note that DAPHNE and FTLD-CDR are well correlated (DAPHNE-6 = 0.63, DAPHNE-40 = 0.70). In our ongoing study we are investigating the ability of DAPHNE to measure the progression of bvFTD.
It is now well known that caregiver burden is heavier in relatives of FTD patients than in relatives of AD patients [43], and that it is correlated with high scores on behavioral scales [34,44]. Our findings confirm these results. The good correlation between DAPHNE and ZBI is therefore clinically relevant.
Another scale, called Cambridge Behavioral Inventory, is an inventory that explores 13 domains (memory, orientation and care, daily activities, autonomy, mood, beliefs, behavioral disorders, disinhibition, diet, sleep, stereotyped behavior, motivation and introspection). It permits to distinguish a lot of degenerative diseases [45,46,47]. However, the score is only based on the frequency of the disorders and do not assess their intensity.
This study has potential limitations. The assessment with DAPHNE relies on caregiver reports, as do most behavioral scales. It is well known that the recognition of the disease, and more specifically the recognition of behavioral disorders, depends on several factors. The caregiver's characteristics such as young age, low level of education, depression and spending a lot of time with the person with cognitive impairment have been shown to be potentially associated with an overestimation of behavior problems [48]. For DAPHNE, this point would require further investigations. A second limitation is that diagnoses were not supported by neuropathological verification. However, it is worth noting that all patients with AD and FTD who had undergone lumbar puncture had cerebrospinal fluid biomarkers results in accordance with the diagnosis, and therefore could not be misclassified as amnesic FTD or frontal AD. Moreover, the clinicians who evaluated the patients are experts, with follow-up in memory clinics. Lastly, one could criticize the circularity of this study because of DAPHNE's structure, which is based on the revised criteria of bvFTD. Criteria are required for research, but they are often not sufficient for clinical practice. We demonstrated the low specificity of the revised criteria, while DAPHNE permits a better distinction between diseases. This scale does not replace the criteria, but it provides valuable help for the diagnosis of bvFTD in clinical practice. For the future, another approach will be to include patients and to investigate, blind to diagnosis, the ability of DAPHNE to identify the correct diagnosis, so that the study will be partially independent from the revised criteria.
Conclusion
In summary, a behavioral inventory appears to be the best assessment method to help in the differential diagnosis of bvFTD compared to executive assessment or social cognition. DAPHNE, adapted to the revised criteria, with excellent psychometric features, has the advantage of an innovative scoring system on a five-point scale, swiftness and efficiency. In recent years, FTD has appeared to be a pathologically and genetically heterogeneous disorder. DAPHNE, as a specific behavioral inventory, could permit the identification of phenotypic signatures and provide valuable help when differential diagnosis is challenging.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Acknowledgements
Dr. Boutoleau-Bretonnière is funded by a grant from the Association France Alzheimer. The authors thank George Michael (Lyon), Didier Hannequin (Rouen), Olivier Godefroy (Amiens), Emilie Beaufils (Tours), Catherine Belin (Paris), Bernard Croisile (Lyon) and Pierre Jean Ousset (Toulouse) for their participation in the Delphi group.
References
- 1.Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 2.Diehl-Schmid J, Pohl C, Perneczky R, Forstl H, Kurz A. Behavioral disturbances in the course of frontotemporal dementia. Dement Geriatr Cogn Disord. 2006;22:352–357. doi: 10.1159/000095625. [DOI] [PubMed] [Google Scholar]
- 3.Pasquier F, Lebert F, Lavenu I, Guillaume B. The clinical picture of frontotemporal dementia: diagnosis and follow-up. Dement Geriatr Cogn Disord. 1999;10(suppl 1):10–14. doi: 10.1159/000051206. [DOI] [PubMed] [Google Scholar]
- 4.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frisoni GB, Rozzini L, Gozzetti A, Binetti G, Zanetti O, Bianchetti A, Trabucchi M, Cummings JL. Behavioral syndromes in Alzheimer's disease: description and correlates. Dement Geriatr Cogn Disord. 1999;10:130–138. doi: 10.1159/000017113. [DOI] [PubMed] [Google Scholar]
- 6.Hornberger M, Piguet O. Episodic memory in frontotemporal dementia: a critical review. Brain. 2012;135:678–692. doi: 10.1093/brain/aws011. [DOI] [PubMed] [Google Scholar]
- 7.Ducharme S, Dickerson BC. The neuropsychiatric examination of the young-onset dementias. Psychiatr Clin North Am. 2015;38:249–264. doi: 10.1016/j.psc.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Bak TH, Crawford LM, Berrios G, Hodges JR. Behavioural symptoms in progressive supranuclear palsy and frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2010;81:1057–1059. doi: 10.1136/jnnp.2008.157974. [DOI] [PubMed] [Google Scholar]
- 9.Woolley JD, Khan BK, Murthy NK, Miller BL, Rankin KP. The diagnostic challenge of psychiatric symptoms in neurodegenerative disease: rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease. J Clin Psychiatry. 2011;72:126–133. doi: 10.4088/JCP.10m06382oli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kertesz A, Davidson W, Fox H. Frontal Behavioral Inventory: diagnostic criteria for frontal lobe dementia. Can J Neurol Sci. 1997;24:29–36. doi: 10.1017/s0317167100021053. [DOI] [PubMed] [Google Scholar]
- 11.Brun A, Englund E, Gustafson L, Passant U, Mann DMA, Neary D. Clinical and neuropathological criteria for fronto-temporal dementia. J Neurol Neurosurg Psychiatry. 1994;57:416–418. doi: 10.1136/jnnp.57.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gregory CA. Frontal variant of frontotemporal dementia: a cross-sectional and longitudinal study of neuropsychiatric features. Psychol Med. 1999;29:1205–1217. doi: 10.1017/s0033291799008934. [DOI] [PubMed] [Google Scholar]
- 13.Chow TW, Hynan LS, Lipton AM. MMSE scores decline at a greater rate in frontotemporal degeneration than in AD. Dement Geriatr Cogn Disord. 2006;22:194–199. doi: 10.1159/000094870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Litvan I, Hauw JJ, Bartko JJ, Lantos PL, Daniel SE, Horoupian DS, McKee A, Dickson D, Bancher C, Tabaton M, Jellinger K, Anderson DW. Validity and reliability of the preliminary NINDS neuropathologic criteria for progressive supranuclear palsy and related disorders. J Neuropathol Exp Neurol. 1996;55:97–105. doi: 10.1097/00005072-199601000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Lebert F, Pasquier F, Souliez L, Petit H. Frontotemporal behavioral scale. Alzheimer Dis Assoc Disord. 1998;12:335–339. doi: 10.1097/00002093-199812000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 18.Boutoleau-Bretonnière C, Lebouvier T, Volteau C, Jaulin P, Lacomblez L, Damier P, Thomas-Anterion C, Vercelletto M. Prospective evaluation of behavioral scales in the behavioral variant of frontotemporal dementia. Dement Geriatr Cogn Disord. 2012;34:75–82. doi: 10.1159/000341784. [DOI] [PubMed] [Google Scholar]
- 19.Kertesz A, Nadkarni N, Davidson W, Thomas AW. The Frontal Behavioral Inventory in the differential diagnosis of frontotemporal dementia. J Int Neuropsychol Soc. 2000;6:460–468. doi: 10.1017/s1355617700644041. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state'. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55:1621–1626. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 22.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 23.Nelson HE. A modified card sorting test sensitive to frontal lobe defects. Cortex. 1976;12:313–324. doi: 10.1016/s0010-9452(76)80035-4. [DOI] [PubMed] [Google Scholar]
- 24.Cardebat D, Doyon B, Puel M, Goulet P, Joanette Y. Formal and semantic lexical evocation in normal subjects. Performance and dynamics of production as a function of sex, age and educational level. Acta Neurol Belg. 1990;90:207–217. [PubMed] [Google Scholar]
- 25.Allain P, Roy A, Kefi Z, Etcharry-Bouyx F, Le Gall D. Fonctions exécutives et traumatisme crânien sévère: évaluation à l'aide de ‘behavioural assessment of the dysexecutive syndrome'. Rev Neuropsychol. 2004;14:285–323. [Google Scholar]
- 26.Osterrieth PA. Le test de copie d'une figure complexe: contribution à l'étude de la perception et de la mémoire. Arch Psychol. 1944;30:286–356. [Google Scholar]
- 27.Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology. 1988;38:900–903. doi: 10.1212/wnl.38.6.900. [DOI] [PubMed] [Google Scholar]
- 28.Deloche G, Hannequin D. DO 80: épreuve de dénomination orale d'images. Paris: ECPA; 1997. [Google Scholar]
- 29.Ekman P, Friesen WV. Facial Action Coding System: A Technique for the Measurement of Facial Movement. Palo Alto: Consulting Psychologists Press; 1978. [Google Scholar]
- 30.Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The ‘Reading the Mind in the Eyes’ test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. 2001;42:241–251. [PubMed] [Google Scholar]
- 31.Stone VE, Baron-Cohen S, Knight RT. Frontal lobe contributions to theory of mind. J Cogn Neurosci. 1998;10:640–656. doi: 10.1162/089892998562942. [DOI] [PubMed] [Google Scholar]
- 32.Boutantin J, Moroni C, Demeneix E, Marchand E, Lys H, Pasquier F, Delbeucq X. Normalisation du test des faux pas auprès d'une population adulte. Lille: Réunion de Printemps de la Société Française de Neuropsychologie de Langue Française; 2010. [Google Scholar]
- 33.Knopman DS, Kramer JH, Boeve BF, Caselli RJ, Graff-Radford NR, Mendez MF, Miller BL, Mercaldo N. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain. 2008;131:2957–2968. doi: 10.1093/brain/awn234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boutoleau-Bretonnière C, Vercelletto M, Volteau C, Renou P, Lamy E. Zarit Burden Inventory and activities of daily living in the behavioral variant of frontotemporal dementia. Dement Geriatr Cogn Disord. 2008;25:272–277. doi: 10.1159/000117394. [DOI] [PubMed] [Google Scholar]
- 35.Zarit SH, Orr NK, Zarit JM. The Hidden Victims of Alzheimer's Disease: Families under Stress. New York: New York University Press; 1985. [Google Scholar]
- 36.Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20:649–655. doi: 10.1093/geront/20.6.649. [DOI] [PubMed] [Google Scholar]
- 37.Kobylecki C, Jones M, Thompson JC, Richardson AM, Neary D, Mann DM, Snowden JS, Gerhard A. Cognitive-behavioural features of progressive supranuclear palsy syndrome overlap with frontotemporal dementia. J Neurol. 2015;262:916–922. doi: 10.1007/s00415-015-7657-z. [DOI] [PubMed] [Google Scholar]
- 38.Lebert F. Diogene syndrome, a clinical presentation of fronto-temporal dementia or not? Int J Geriatr Psychiatry. 2005;20:1203–1204. doi: 10.1002/gps.1430. [DOI] [PubMed] [Google Scholar]
- 39.Hornberger M, Piguet O, Graham AJ, Nestor PJ, Hodges JR. How preserved is episodic memory in behavioral variant frontotemporal dementia? Neurology. 2010;74:472–479. doi: 10.1212/WNL.0b013e3181cef85d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarazin M, Berr C, De Rotrou J, Fabrigoule C, Pasquier F, Legrain S, Michel B, Puel M, Volteau M, Touchon J, Verny M, Dubois B. Amnestic syndrome of the medial temporal type identifies prodromal AD: a longitudinal study. Neurology. 2007;69:1859–1867. doi: 10.1212/01.wnl.0000279336.36610.f7. [DOI] [PubMed] [Google Scholar]
- 41.Funkiewiez A, Bertoux M, de Souza LC, Levy R, Dubois B. The SEA (Social cognition and Emotional Assessment): a clinical neuropsychological tool for early diagnosis of frontal variant of frontotemporal lobar degeneration. Neuropsychology. 2012;26:81–90. doi: 10.1037/a0025318. [DOI] [PubMed] [Google Scholar]
- 42.Gregory C, Lough S, Stone V, Erzinclioglu S, Martin L, Baron-Cohen S, Hodges JR. Theory of mind in patients with frontal variant frontotemporal dementia and Alzheimer's disease: theoretical and practical implications. Brain. 2002;125:752–764. doi: 10.1093/brain/awf079. [DOI] [PubMed] [Google Scholar]
- 43.De Vugt ME, Riedijk SR, Aalten P, Tibben A, van Swieten JC, Verhey FR. Impact of behavioural problems on spousal caregivers: a comparison between Alzheimer's disease and frontotemporal dementia. Dement Geriatr Cogn Disord. 2006;22:35–41. doi: 10.1159/000093102. [DOI] [PubMed] [Google Scholar]
- 44.Riedijk SR, De Vugt ME, Duivenvoorden HJ, Niermeijer MF, Van Swieten JC, Verhey FR, Tibben A. Caregiver burden, health-related quality of life and coping in dementia caregivers: a comparison of frontotemporal dementia and Alzheimer's disease. Dement Geriatr Cogn Disord. 2006;22:405–412. doi: 10.1159/000095750. [DOI] [PubMed] [Google Scholar]
- 45.Bozeat S, Gregory CA, Ralph MA, Hodges JR. Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer's disease? J Neurol Neurosurg Psychiatry. 2000;69:178–186. doi: 10.1136/jnnp.69.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagahama Y, Okina T, Suzuki N, Matsuda M. The Cambridge Behavioral Inventory: validation and application in a memory clinic. J Geriatr Psychiatry Neurol. 2006;19:220–225. doi: 10.1177/0891988706286545. [DOI] [PubMed] [Google Scholar]
- 47.Wedderburn C, Wear H, Brown J, Mason SJ, Barker RA, Hodges J, Williams-Gray C. The utility of the Cambridge Behavioural Inventory in neurodegenerative disease. J Neurol Neurosurg Psychiatry. 2008;79:500–503. doi: 10.1136/jnnp.2007.122028. [DOI] [PubMed] [Google Scholar]
- 48.Sink KM, Covinsky KE, Barnes DE, Newcomer RJ, Yaffe K. Caregiver characteristics are associated with neuropsychiatric symptoms of dementia. J Am Geriatr Soc. 2006;54:796–803. doi: 10.1111/j.1532-5415.2006.00697.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data