Abstract
Relapse rates are high amongst cases of Anorexia Nervosa (AN) suggesting that some alterations induced by AN may remain after weight restoration.
Objective
To study the consequences of AN without confounds of environmental variability, a rodent model of activity based anorexia (ABA) can be employed. We hypothesized that exposure to ABA during adolescence may have long-term consequences in taste function, cognition, and anxiety-like behavior after weight restoration.
Methods
To test this hypothesis we exposed adolescent female rats to ABA (1.5 hrs food access, combined with voluntary running wheel access) and compared their behavior to that of control rats after weight restoration was achieved. The rats were tested for learning /memory, anxiety, food preference and taste in a set of behavioral tests performed during the light period.
Results
Our data show that ABA exposure leads to reduced performance during the novel object recognition task, a test for contextual learning, without altering performance in the novel place recognition task or the Barnes maze, both tasks that test spatial learning. Furthermore, we do not observe alterations in unconditioned lick responses to sucrose nor quinine (described by humans as “sweet” and “bitter” respectively). Nor did we find alterations in anxiety-like behavior during an elevated plus maze or an open field test. Finally, preference for a diet high in fat was not altered.
Discussion
Overall our data suggest that ABA exposure during adolescence impairs contextual learning in adulthood without altering spatial leaning, taste, anxiety, or fat preference.
Introduction
Anorexia Nervosa (AN) is a disease characterized by food restriction, low body weight and fear of gaining weight (1). AN has one of the highest mortality rates of all psychiatric disorders (2) and is most prevalent in adolescent women (3). AN is difficult to treat and long term success rates range from 30–50% (4). With inpatient treatment weight restoration can be achieved. However, a large percentage of patients relapse within a year of termination of treatment (5). This suggests that either some facets of the disorder are not being sufficiently dealt with during treatment, or that AN has long term consequences that facilitate relapse. Therefore the aim of the current study is to evaluate whether exposure to AN has long lasting effects on parameters that may play an important role in relapse like taste, food preference, anxiety and cognition. To investigate this we used a rodent model for AN. The activity-based anorexia (ABA) model was developed to mimic consequences of AN in rats by combining food restriction and access to a running wheel. Similar to AN patients, rats exposed to ABA have low body weights, display hypophagia even when food is available and are hyperactive (6).
A change in taste may be a reason for AN patients to continue eating a diet low in fats and sugars even after recovery. However, Goldzak-Kunning et al. reported that intensity and hedonic ratings to a panel of gustatory stimuli did not differ in AN patients compared to controls (7). Changes in taste processing have been reported in currently-ill AN patients (8). Additionally, a functional MRI study suggested that recovered AN patients have reduced responses in the insula to orally applied sweet tastants (8, 9), though there were no differences in pleasantness ratings for the sweet taste, suggesting that the attenuated insula response does not lead to a shift in sweet taste preference (9). To date the effects of ABA exposure on taste responsivity in the rodent ABA model are not clear. Liang et al. showed that rats recovered from a single bout of ABA had faster acquisition of a conditioned taste aversion (CTA) and slower CTA extinction (10). One may argue that if ABA exposure alters taste responsivity, one may expect a stronger association between the tastant and the aversive stimuli, which could lead to faster CTA acquisition. To further investigate the effects of ABA exposure on the consummatory and appetitive components of taste-guided ingestive behavior, we tested ABA exposed rats in a brief-access taste test. This procedure involves measuring lick responses to a concentration range of a specific tastant across brief trials. The animal’s approach to the spout to initiate a trial reflects the appetitive component, whereas the licking response reflects the consummatory response.
Vulnerability to relapse may also be mediated by alterations in anxiety and fear learning in AN patients. Prior research suggests that AN patients frequently have co-morbid anxiety disorders (11), have higher trait anxiety levels (12), and increased food associated anxiety (13). Additionally, high trait anxiety was a negative predictor for recovery success (14). In addition to these studies in humans, Kinzig and Hargrave showed that adult rats that were recovered from two bouts of ABA during adolescence displayed increased anxiety-like behavior in the elevated plus maze (EPM) test and the open field test (15). Results from a study in which performance in an EPM during ABA in mice was measured suggested that food restriction, independent of running wheel access increased open arm exploration and may thus be considered anxiolytic. However, increases in running wheel activity during ABA (as compared to baseline) was positively correlated with the anxiety level displayed in the EPM, suggesting that heightened hyperactivity predicted increased anxiety (16). These studies suggest that increased anxiety may be a consequence of ABA. On the other hand, heightened anxiety may also be a predisposing factor for ABA. Gelegen et al. showed that mouse strains characterized by higher anxiety levels developed more hyperactivity during ABA (17). In contrast, trait anxiety as measured by open field performance prior to ABA did not predict weight loss or hyperactivity during ABA, suggesting that heightened trait anxiety may not be a predisposing factor for performance during ABA in rats. In the current study we examine anxiety in the EPM and open field test in weight restored rats exposed to a single bout of ABA.
Furthermore, ABA-induced alterations in learning and memory may contribute to relapse susceptibility. There have been some reports on long lasting effects on cognitive function in weight restored AN patients. Danner and colleagues reported impaired set-shifting and poor decision making in both currently ill and weight restored AN patients (18). Others report lower IQ scores in currently ill patients compared to weight restored patients (19), suggesting that impairments in IQ normalize when weight is restored. To our knowledge, effects of ABA exposure on cognitive function in a rodent model have not been reported. Therefore in the current study we investigated the effect of ABA on performance in three learning and memory related behavioral tasks: the Barnes maze, focused of spatial learning, the novel object recognition (NOR) test, a measure of contextual learning, and the novel place preference (NPR) task that combines both spatial and contextual learning.
In sum, to date there is limited information on the behavioral consequences of exposure to ABA after weight-restoration. Specifically, the question remains whether there are alterations in taste, food preference, or learning/memory as a result of ABA exposure. In the current study we therefore tested weight restored adult rats exposed to a single bout of ABA during adolescence using a battery of behavioral tests aimed at measuring taste, anxiety, food preference and learning/memory performance.
Materials and Methods
Experiment 1
Subjects
Thirty-two female adolescent (30 days old) Sprague-Dawley rats (Harlan) weighing 93.9 ± 1.0 g upon arrival were individually housed in polycarbonate cages with corn cob bedding in a room where humidity, temperature and a 12 h – 12 h light-dark cycle (lights on 6am) were automatically controlled. Rats had ad libitum access to chow (2018 Teklad, Harlan, Frederick, MD) and water, excepted where noted. Animals were provided a 7-day acclimation period to the lab environment upon arrival. All procedures were approved by the Institutional Animal Care and Use Committee at Johns Hopkins University.
Experimental set-up
Animals were assigned to one of four groups: ABA, SED, RW and BWM. The sedentary control animals (SED) (n=12) were provided ad libitum access to chow and water throughout the entire experiment. Prior to the introduction of the running wheel, groups were matched by body weight and food intake. Rats in the activity-based anorexia group (ABA) (n=12) and running wheel group (RW) (n=12) had 16 days access to a Nalgene running wheel (radius 13.5 cm) (Minimitter, Bend, OR). Running wheel activity was recorded by Vitalview software (Minimitter, Bend, OR). Animals in the running wheel group (RW) were provided ad libitum access to chow and water. After 10 days habituation to the running wheel, animals in the ABA group were restricted to 1.5 hrs of food access at the start of the dark period for 6 days or until they lost 25% of their baseline body weight (day 0). When 25% body weight loss was achieved the running wheel of the rat was blocked, and food was provided ad lib. The period of 1.5 hrs food access was chosen because pilot experiments showed that this was the most severe restriction that leads to a clean ABA phenotype in female rats of this age. Animals in the body weight-matched group (BWM) (n=12) had no access to a running wheel but were restricted in the amount of food given in an attempt to decrease body weights to a level comparable to that of the ABA group (the amount of food given is shown in Figure 1B). After 25% body weight loss was achieved or sixteen days of running wheel access, running wheels in the ABA and RW groups were locked and all groups were presented ad libitum access to chow and water for the remainder of the experiment.
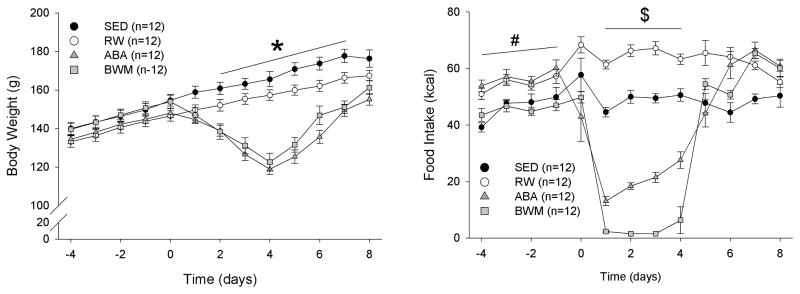
Figure 1.
Body weight. (A) Mean ± SE body weights and (B) mean ± SE food intake for sedentary (SED), running-wheel (RW), activity-based anorexia (ABA) and body weight matched (BWM) groups. * indicates a significant difference between SED and RW vs. ABA and BWM groups p≤0.05. # indicates a significant difference between SED and BWM vs. RW and ABA groups p≤0.05. $ indicates a significant difference among SED, RW, ABA and BWM groups p≤0.05.
Five days after food access was resumed and when body weights were back to baseline behavioral testing was started. First, anxiety-like behavior of the rats were tested in an elevated plus maze (day 10) and an open field test (day 12). Next, learning and memory were assessed using a NOR test (days 13–14), and a Barnes maze test (days 21–25). A brief access taste test was performed to assess taste responsivity (days 28–56). Finally, a diet preference test was performed to assess preference for a high fat diet (days 66–71). The experimental design is summarized in supplemental figure 1. All behavioral tests were performed during the light phase in a dim-light room (± 60 lux). Data are expressed as average ± the standard error of the mean (SE). Differences in food intake, body weight, running wheel activity, were compared across groups with repeated-measured ANOVAs, followed by Bonferroni post-hoc analysis. For all statistical analysis a confidence interval of 95% was used. For the figures we only display food intake and body weight until day 4 of ABA, because that was when the first rats reached 25% body weight loss, and thus was returned on ad lib diet access.
Elevated Plus Maze test
The rats were tested in an EPM in the middle of the light period (day 10). The EPM apparatus consisted of two open arms (45 × 10 cm) and two closed arms (45 × 10 × 50 cm) connected by a center platform (10 × 10 cm) made of opaque black Plexiglas (Harvard Apparatus). The arms of the plus maze were elevated 70 cm above the floor. The behavior of the animals was recorded with an overhead video camera. Animals were placed in the center of the EPM facing an open arm and allowed to explore the plus maze for 5 minutes. Time spent in each arm was scored using Hindsight behavioral scoring software. The middle point of the rat excluding the tail was used as the reference point to determine the position of the rat. The apparatus was cleaned with 70% EtOH between each animal. For each experimental group the mean ± SE duration spent in the open arm was calculated. Group differences were statistically tested with a ANOVA, followed by bonferonni post hoc analysis.
Open Field test
Two days after the EPM test the rats were tested in the open field (OF). All testing was performed during the light period. During this test the animals were placed in a Plexiglas box (60 × 60 × 60 cm) for a period of 10 minutes. The behavior of the animals was recorded with an overhead video camera. A circle with a 15 cm radius was indicated in the center of the test box floor and the time the animal spent in this circle, the “inner zone”, was scored using the Hindsight behavioral scoring software. Additionally, time spent exploring, immobile, and grooming was scored. The test-box was cleaned with 70% EtOH between each animal. For each experimental group the mean ± SE duration spent in the inner zone, and the time spent on each behavior was calculated. Group differences were statistically tested with an ANOVA, followed by bonferonni post hoc analysis.
Novel Object Recognition test
For the novel object recognition test (NOR), the same test-box used in the OF test was used. The rats had been habituated to the test-box during the OF test and therefore no additional habituation session to the test-box was included. On the first day of the NOR test, two objects different in color, shape and size (made with Duplo-Lego blocks, Lego, USA) were placed in opposite corners of the test-box with the center of each object 20 cm from the corner of the box. The rats were placed in a corner of the box without an object and allowed to explore the objects for 5 minutes. Hereafter rats were returned to its home cage. This first test trial will be referred to as “acquisition”. On the second day of testing, one of the objects in the test box was replaced by a distinctly different novel object. Rats were tested 24 hrs after their acquisition trial and were allowed to explore the familiar and novel objects for 5 minutes. This second trial will be referred to as “recollection”. The behavior of the animals was recorded with an overhead video camera. The time spent exploring the area, exploring the familiar and exploring the novel object was scored using Hindsight behavioral scoring software. The test-box and objects were cleaned with 70% EtOH between each rat. For each experimental group the mean ± SE duration spent interacting with the novel and the familiar objects were calculated. Group differences were statistically tested with an ANOVA, followed by bonferonni post hoc analysis. Additionally to test whether the rats spent more time interacting with the novel object than would be expected a chi-squared analysis, using 50% as predicted value was performed.
Barnes Maze test
The rats were tested in a Barnes maze starting from day 21. All testing was performed in the middle of the light period. The Barnes maze consisted of a dark grey PVC circular platform (radius 61 cm) with 18 holes (radius 4.75 cm) around the perimeter of the platform, equally spaced out 20° from each other. The platform was elevated 70 cm from the floor. An escape box (38.7 × 12.1 × 14.2 cm) was placed under one of the holes. Three neutral visual cues and one aversive cue (bright light) were placed around the edge of the platform.
At the start of each test session the rats were placed under a starting box in the center of the platform, the starting box was removed and the latency to locate and enter the escape box was measured. If a rat did not reach the escape box within 3 minutes, the rat was gently guided to the escape box. After the rat entered the escape box, the hole was covered and the animal remained in the escape box for 30 seconds before being returned to its home cage. Each animal underwent 2 test sessions per day ~3 hours apart for 5 consecutive days (10 test sessions in total). Between each animal, the test apparatus was thoroughly cleaned with 70% EtOH. For each experimental group, for each separate test trial the mean ± SE duration to reach the escape hole was calculated. Group differences were statistically tested with repeated measures ANOVA with group as between subject factor, and test trial as within subject factor. This was followed with planned comparison post hoc analysis to assess group differences on a specific day.
Brief-Access Taste Procedure
Brief-access taste tests were conducted during the light cycle. Training and testing for the behavioral procedure were conducted in a lickometer (Davis MS-160, DiLog Instruments, Tallahassee FL) as previously described (20, 21)). The rat was placed in the testing chamber of the apparatus and presented with access to a single spout positioned approximately 5 mm behind a slot. The spout was connected to a glass container holding water or a taste solution. To minimize potential olfactory cues from the stimulus, a small fan was placed above the wall of the testing chamber to direct a current of air past the drinking spout. A trial was initiated when the rat licked the spout. At the end of each trial (10 s), the shutter closed. During each 8-s inter-trial interval the spout presentation was changed via a motorized block, after which the shutter reopened for the next trial. Concentrations were presented in randomized blocks without replacement. Animals were able to initiate as many trials as possible during each 30-min session.
During training in the lickometer and testing with water or quinine, the rats were placed on a water-restriction schedule. During behavioral training and testing with water and quinine, animals were placed on a ~23 h water restriction schedule in which water was available only during the 30-min sessions. On days 1 and 2 of behavioral training, rats were presented with a stationary spout of water for 30 minutes. Total number of licks and inter-lick-interval were measured. On day 3 of behavioral training, 7 tubes of water were presented one at a time in 10-s trials across 30-min sessions. Ad libitum access to water resumed in the home cages after the last session of a testing phase. For responses to sucrose, animals were tested without food or water restriction prior to testing (summarized in Supplemental Figure 1). Body weight was measured every day during testing and restriction conditions and did not fall below 90% of weight during ad libitum access to water. (Average weight loss after water restriction: 3.6% ± 0.7 g).
Taste stimuli
All solutions were prepared with distilled water and presented at room temperature. Six concentrations of sucrose (0.01, 0.03, 0.06, 0.1, 0.3, and 1.0 M; Sigma Aldrich, St. Louis MO) and six concentrations of quinine hydrochloride (0.01, 0.03, 0.1, 0.3, 1.0, 3.0 mM; Sigma Aldrich, St. Louis MO) were used.
Data analysis
Total licks and inter-lick interval (ILI) values to stationary water on day 2 were compared across the four groups using one-way ANOVAs. For ILI measures, values less than 50 ms were considered as double licks and ILI values larger than 250 ms were considered pauses between licking bursts (22). Thus only ILIs that were between 50 and 250 ms were included for analysis.
For a given animal during each stimulus type, the mean number of licks at each concentration was calculated by collapsing all trials across the three sessions. The mean number of licks to water was subtracted from the mean number of licks at each concentration, yielding a Licks Relative to Water value. This measure has been used in previous studies (21, 23–25) to produce concentration-response curves that are adjusted to a water baseline. The Licks Relative to Water value for each concentration was compared using analyses of variance (ANOVAs). Data for animals that did not initiate at least one trial per concentration were not included for Licks Relative to Water data analysis. The total number of trials initiated across the three sessions of each compound was compared for all animals. The statistical rejection criterion of 0.05 was used for all analyses.
Curves were fit to mean data for each animal and group by using the following logistic function:
where x = log10 stimulus concentration, a = asymptotic lick response adjusted for water, b = slope and c = log10 concentration at the inflection point.
Food Preference test
After the brief access taste test the rats had ad libitum access to water and chow for 10 days. Next, the animals were habituated to the novel 60% high fat diet (D12492, Research Diets, New Brunswick, NJ) by giving them a single pellet (~ 0.5 g) of the high fat diet for two consecutive days. Following habituation, rats were given ad libitum access to both their standard chow diet (2018 Teklad, Harlan, Frederick, MD) and the high fat diet (D12492, Research Diets, New Brunswick, NJ) for 5 consecutive days. Intake of both diets was monitored during this period to determine food preference (data were corrected for spillage). Average ± SE intake of both diets for all experimental groups were calculated. To calculate diet preference the total High fat diet intake was divided by the total intake (chow + HF diet) and multiplied by 100%. Group differences in dietary preference were assessed with a repeated measures ANOVA with group as between subject factor and day as within subject factor.
Experiment 2
Subjects
Sixteen female adolescent (27 days old) Sprague-Dawley rats (Harlan) weighing 63.5 ± 2.0 g upon arrival were housed and were provided a 10-day acclimation period to the lab environment upon arrival. Rats were hereafter habituated under the same conditions as described in experiment 1. At the start of the ABA regime, there were no significant differences in age, body weight or food intake between rats in experiment 1 and experiment 2.
Experimental Set-up
Upon arrival the rats were divided into 2 groups, a SED control group housed in standard tub cages, and an ABA group housed in Nalgene running wheel cages (radius 13.5 cm) (Minimitter, Bend, OR). Because the first experiment did not show deficiencies in the RW or BWM groups, we did not include these control groups in the current experiment to reduce the number of rats needed. Running wheel activity was recorded by Vitalview software (Minimitter, Bend, OR). Running wheels were locked for 3 days, and on postnatal day 30 the wheels were unlocked. After 10 days habituation to the running wheel, animals in the ABA group were restricted to 1.5 hrs of food access at the start of the dark period for 6 days or until they lost 25% of their baseline body weight (day 0). After ad libitum food access was returned, behavioral testing started. First, effects on learning and memory was assessed using novel object recognition and novel place recognition tests (day 12–15), followed by a Barnes maze test including a reversal learning trial (day 21–31).
Novel Object Recognition and Novel Place Recognition Tests
The same experimental procedure as described in Experiment 1 was used to conduct the NOR. Briefly, the rats were habituated to the test-box for 5 minutes on day 12. On day 13 the rats were tested in an acquisition trial. This was followed by a recollection trial on day 14.
To assess specificity of contextual learning a NPR test was performed (day 15), in which the two objects used in the “recollection” trial were used. However, during the NPR trial one of the objects was moved to another position in the test box. The “recollection” trial for the NOR was used as an acquisition trial for the NPR test. The rat was returned to the test box 24 hrs after their NOR “recollection” trial and was allowed to explore the objects in the familiar and novel positions for 5 minutes. This third trial will be referred to as “place recollection”.
The behavior of the animal was taped with an overhead video camera. The time spent exploring the area, exploring the object in the familiar place and exploring object in the novel place was scored using Hindsight software. The test-box and objects were cleaned with 70% EtOH between each rat.
For each experimental group the mean ± SE duration spent interacting with the novel object/place and the familiar objects were calculated. Group differences were statistically tested with an ANOVA, followed by bonferonni post hoc analysis. Additionally to test whether the rats spent more time interacting with the novel/novel place object than would be expected a chi-squared analysis, using 50% as predicted value was performed.
Barnes Maze reversal learning
Acquisition trials to the Barnes maze test were conducted according to the procedures described in Experiment 1. Each animal underwent 2 acquisition trials per day for 4 consecutive days (8 test trials in total). Then on the 5th day, the rats were first given another acquisition trial (trial 9) followed by a probe trial. During this probe trial the escape box was removed. This session was recorded by an overhead video camera, and the time spent in each quadrant of the Barnes maze was scored. This trial was followed by 2 trials (trials 10 and 11) where the escape box was placed back in its original position and the rat was re-conditioned to the position of the escape box. Next, the position of the escape box relative to the spatial cues was altered to assess behavioral flexibility; the escape box was located at the opposite side of the maze compared to its position during acquisition. The rats were tested for 4 consecutive days (8 trials in total) with the escape box in the novel position. Between each animal, the test apparatus was thoroughly cleaned with 70% EtOH. For each experimental group, for each separate test trial the mean ± SE duration to reach the escape hole was calculated. Group differences were statistically tested with repeated measures ANOVA with group as between subject factor, and test trial as within subject factor. This was followed with planned comparison post hoc analysis to assess group differences on a specific day. To test whether the rats spent more time in the target quadrant than would be expected by chance during the probe trial a chi-squared analysis, using 25% as predicted value was performed.
Results
Experiment 1
Body weight
During habituation to the running wheels (days −10 through 0), repeated measures ANOVA revealed no main group (F(3,45)=0.941, p= 0.428) or time*group interaction (F(33,495)=1.023, p= 0.435) effects on body weight. During food restriction (days 0–6) a main effect of group (F(3,45)=18.761, p< 0.001) and significant time* group interaction effect (F(18,420)=85.555, p< 0.001) on body weight was shown. Post-hoc analysis showed that the body weights of rats in the BWM and ABA groups were significantly lower than that of rats in the SED and RW groups on days 2,3,4,5, and 6 (Figure 1A). There were no significant differences in body weight between BWM and ABA rats. Nor were there significant differences in body weight between SED and RW rats. After food restriction and running wheel access was stopped, repeated measures ANOVA showed a main group effect (F(3,45)=19.75, p< 0.001) and a time*group interaction effect (F(9,135)=8.36, p<0.001) for the first 4 days of recovery (days 7–10). Bonferroni post hoc analysis revealed lower body weight in BWM and ABA rats compared to SED and RW rats on days 7, 8, and 9 (p<0.05). From day 10, no significant group (F(3,45)=1.22, p= 0.311) or time*group interaction effects (F(87,1305)=1.03, p=0.400) on body weight were observed.
Food intake
During habituation to the running wheels, repeated measures ANOVA revealed a main group effect (F(3,45)=15.671, p< 0.001) but no time*group interaction effect (F(33,495)=0.784, p= 0.801) on food intake. Bonferroni post hoc analysis showed higher food intake in ABA and RW groups compared to SED and BWM groups during habituation. During the period of food restriction, repeated measures ANOVA showed a main group effect (F(3,45)=5.783, p= 0.003) but no time*group interaction effect on food intake (F(18,420)=0.616, p= 0.884). Post hoc analysis showed that food intake was significantly higher in RW rats compared to SED rats. Additionally, food intake was significantly lower in ABA rats compared to RW and SED rats, and food intake of the BWM group was significantly lower than ABA, RW and SED groups. During the first 4 days of recovery (ad libitum food access and RW blocked) a main group effect (F(3,45)=9.105, p< 0.001) was shown. Post hoc analysis revealed higher food intake in ABA, BWM and RW groups compared to the SED group (Figure 1B). There were no significant differences between ABA, BWM and RW rats. After day 10, no significant group (F(3,45)=1.11, p= 0.409) or time*group interaction effects (F(87,1305)=1.01, p=0.441) on food intake between the groups were observed.
Running wheel activity
During the baseline period, repeated measures ANOVA analysis did not show significant group (F(1,22)=0.291, p= 0.590) or time*group interaction (F(11.242)=0.463 p=0.924) effects. There was a main effect of time (F(11,242)=50.308, p< 0.001); all rats increased running distance throughout habituation. During food restriction a significant group effect was revealed (F(1,22)=6.304, p= 0.043). No time*group interaction effect was observed (F(5,110)= 1.634 p= 0.154). Post hoc analysis revealed that rats in the ABA group ran significantly more than rats in the RW group on days 1, 2, 3, 4 and 5 (Supplemental Table 2).
Elevated plus maze test
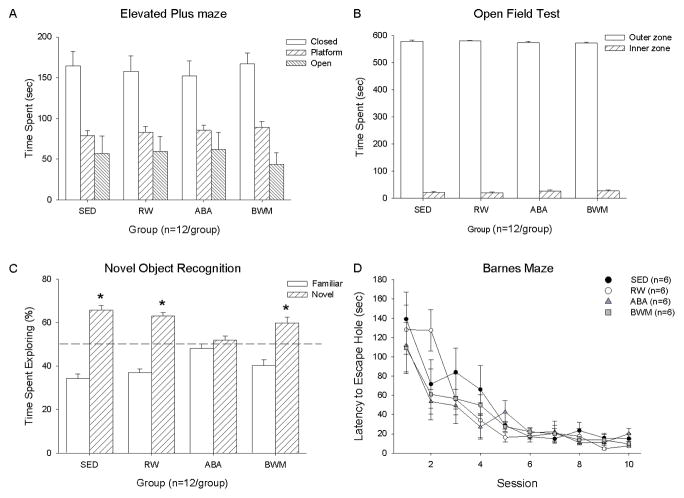
One-way ANOVA analyses revealed no group effects on time spent in the closed arm (F(3,43)=0.154 p=0.925), on the platform (F(3,43)=0.454 p=0.715) or in the open arm (F(3,43)=0.195 p=0.889) (Figure 2A). No significant group effects on the total number of arm entries were observed (F(3,43)=1.640 p=0.194).
Figure 2.
Anxiety and cognition test (A) time spent on the closed arms, platform and open arms during the elevated plus maze test, (B) time spent in the outer and inner zones during the open field test, (C) percentage time spent exploring the novel and the familiar objects during the recollection trial of the novel object recognition test, and (D) latency to enter the escape hole during the Barnes Maze test by sedentary (SED), running-wheel (RW), activity-based anorexia (ABA) and body weight matched (BWM) groups. * indicates a significant difference between time spent with the familiar and the novel object p≤0.05. Data are presented as mean ± SE.
Open field test
There were no group effects on the time spent in the inner zone (F(3,43)=1.037 p=0.386) or the number of entries into the inner zone (F(3,43)=0.171 p=0.912) during the open field test (Figure 2B). One-way ANOVAs furthermore revealed no group effects on time spent exploring the area (F(3,43)=2.081 p=0.116), time spent immobile (F(3,43)=1.524 p=0.219), or time spent grooming (F(3,43)= 0.162 p=0.920) (Supplemental Table 3).
Novel object recognition test
A one way ANOVA revealed no group effect on the time spent exploring an object during theacquisition phase (F(3,20)=0.943 p=0.438). During acquisition, chi-square testing revealed no difference in the time spent with object A versus object B (Chi-Square = 24.28412 df = 23 p =0.388). During the recollection trial no group difference in time spent exploring an object was observed (F(3,20)=0.903 p=0.413) (Supplemental Table 4). One-way ANOVA revealed a group effect on the percentage of time spent with the novel object (F(3,20)= 7.566 p=0.001). Post-hoc analysis showed that the ABA group spent a lower percentage of time with the novel object than the SED, RW or BWM groups. Chi-square analysis revealed that the SED (Chi-Square = 32.161 df = 5, p< 0.001), the RW (Chi-Square = 21.776 df = 5, p<0.001) and the BWM (Chi-Square = 16.065 df = 5, p=0.006) rats spent significantly more time with the novel object than expected by chance, whereas ABA rats did not spend more time with the novel object than expected by chance (Chi-Square = 1.189 df = 5, p=0.946) (Figure 2C).
Barnes maze test
Repeated measures ANOVA revealed a significant time effect on the latency to reach the escape-box (F(9,180)= 27.529 p<0.001). The latency to reach the escape-box decreased over time. There were no group (F(3,20)= 0.609 p= 0.616) or time*group interaction (F(27,180)= 1.084 p= 0.362) effects found on the latency to the escape-box (Figure 2D).
Brief access taste test
There were no significant group differences in total licks (F(3,28)=0.672, p=0.576) or ILI values (F(3,28)=1.62, p=0.208) to water during 30-min access to a stationary spout or in the number of trials initiated to water (F(3,28)=0.488, p=0.693).
All groups increased licking to sucrose in a concentration-dependent manner. Two animals did not initiate a sufficient number of trials per sucrose concentration to be included in Licks Relative to Water data analysis. Two-way ANOVAs comparing Licks Relative to Water values between the four groups revealed no main effect of group (F(3,26)=2.892, p=0.054), a main effect of concentration (F(5,130)=254.552, p<0.001) and no significant interaction (F(15,130)=0.893, p=0.573). Comparing data from all animals, nor did the groups significantly differ in the total number of trials initiated during the sucrose sessions (F(3,28)=0.342, p=0.795) (Figure 3). Curves were fit to individual animal licking data accurately as reflected by the mean R2 value of 0.98±0.00 across animals. The groups did not significantly differ in parameter values representing asymptotic licking (a-parameter) (F(3,26)=0.992, p=0.412), representing slope (b-parameter) (F(3,26)=0.774, p=0.519), nor representing inflection point (c-parameter) (F(3,26)=2.386, p=0.092). One animal showed relatively flat licking responses across the concentration range with a sharp increase at the higher concentrations as reflected in its c-parameter value (Figure 3C).
Figure 3.
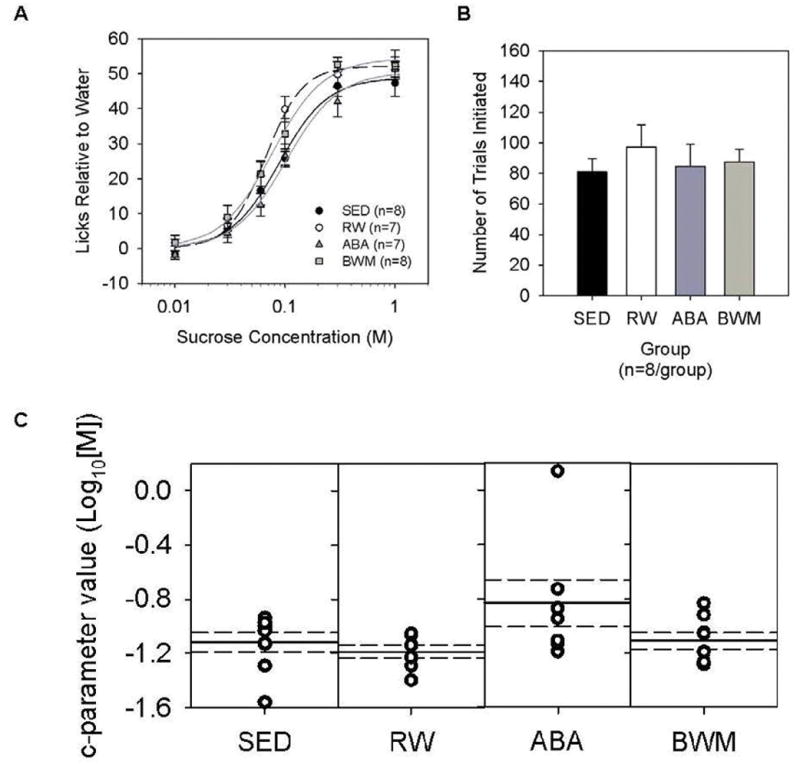
Brief access taste test – Sucrose. (A) Licks across a sucrose concentration series, and (B) Number of trials initiated across the sucrose sessions for sedentary (SED), running-wheel (RW), activity-based anorexia (ABA) and body weight matched (BWM) groups. Data are presented as mean ± SE. (C) c-parameter value distribution derived from sucrose sessions for individual rats (open circles), the group means (solid lines) and SE (dashed lines) for sedentary (SED), running-wheel (RW), activity-based anorexia (ABA) and body weight matched (BWM) groups.
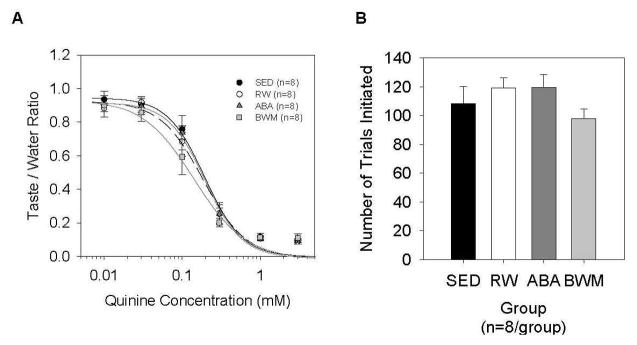
As concentration increased, all groups decreased licking to quinine. A two-way ANOVA revealed no main effect of group (F(3,28)=1.937, p=0.147), a main effect of concentration (F(5,140)=734.086, p<0.001) and no significant interaction (F(15,140)=1.342, p=0.185). Nor did the groups significantly differ in the number of trials initiated (F(3,28)=1.315, p=0.289) (Figure 4). For all the animals tested, curves were fit to individual licking data accurately as reflected by the mean R2 value of 0.98±0.01 across animals. The groups did not significantly differ in parameter values representing asymptotic licking (a-parameter) (F(3,28)=1.077, p=0.375), representing slope (b-parameter) (F(3,28)=0.363, p=0.552), nor representing inflection point (c-parameter) (F(3,28)=1.629, p=0.205).
Figure 4.
Brief access taste test – Quinine. (A) Licks across a quinine concentration series, and (B) Number of trials initiated across the quinine sessions for sedentary (SED), running-wheel (RW), activity-based anorexia (ABA) and body weight matched (BWM) groups. Data are presented as mean ± SE.
Food preference test
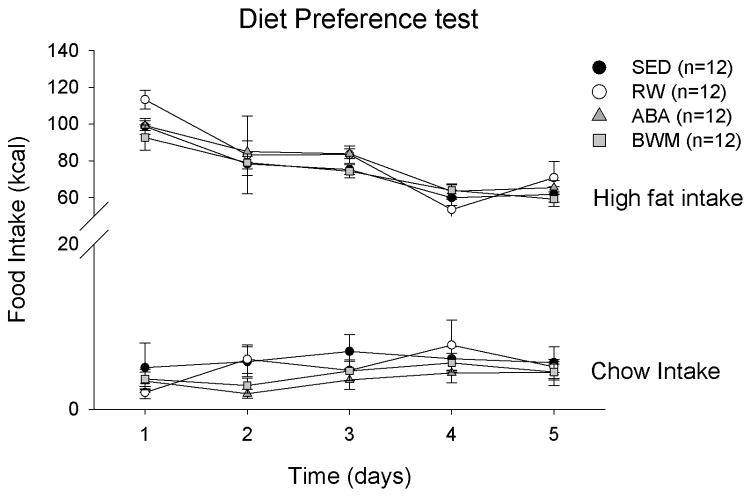
There were no group (F(3,41)= 1.487, p= 0.232) or group*time interaction (F(12,164)= 0.669, p= 0.778) effects on the total intake during the food preference test. Repeated measures ANOVA did reveal a time effect (F(4,164)= 72.815, p< 0.001) on the total intake. No time (F(4,164)= 2.309, p= 0.060), group (F(3,41)= 0.743, p= 0.532) or group*time interaction (F(12,164)= 1.007, p= 0.444) effects on standard chow intake were revealed. Repeated measures ANOVA showed that there was a significant time effect (F(4,164)= 82.633, p< 0.001) on high fat diet intake, and post hoc analysis revealed that over time high fat diet intake decreased significantly. There were no group (F(3,41)= 1.575, p= 0.209) or time*group interaction (F(12,164)= 0.802, p= 0.647) effects on high fat diet intake (Figure 5).
Figure 5.
Diet preference test. (A) Intake of the chow and the high fat diet for sedentary (SED), running-wheel (RW), activity-based anorexia (ABA) and body weight matched (BWM) groups. Data are presented as mean ± SE.
Experiment 2
Body weight
Prior to the introduction of the running wheel, groups were matched by body weight and food intake. During habituation to the running wheels (days −12 through 0), repeated measures ANOVA revealed no main group (F(1,17)=2.03, p= 0.178) or time*group interaction (F(10,170)=2.103, p= 0.194) effects on body weight. During food restriction (days 0–6), ABA rats had significantly lower body weights as indicated by main group (F(1,15) = 86.772, p<0.001) and time*group interaction (F(6,90)=71.931 p<0.001) effects. Body weights between ABA and SED rats remained significantly different (p<0.05) until day 11, after which no significant differences were observed (Supplemental Table 2A).
Food intake
Prior to the start of the food restriction there were no significant main effect of group (F(1,17)=1.283, p= 0.273) or a time*group interaction effect (F(9,153= 1.462, p=0.166) on food intake. During food restriction, ABA rats ate significantly less than the SED (Group: F(1,17)=51.01, p<0.001; time*group: F(6,90)=60.699, p=0.000). After the food restriction period there were no significant differences between the groups at any time point (F(1,17) =0.005, p=0.941) (Supplemental Table 2B).
Novel object recognition test
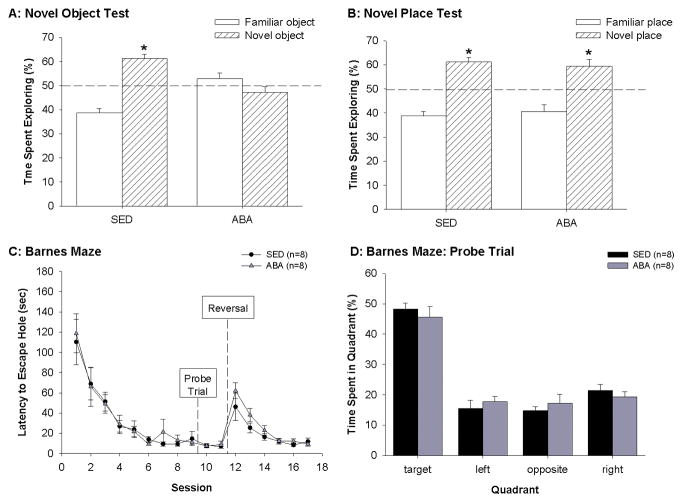
During the acquisition trial there were no significant differences between ABA and SED rats in the total time spent exploring an object (t(15)=0.902, df=8, p=0.382). The rats did not display a clear preference for either object (Chi-Square=7.228, df=8, p=0.370). During the novel object recollection trial, no statistical differences in total time spent exploring an object were observed (t(15)=1.618, p=0.126). However, ABA rats spent significantly less time exploring the novel object compared to SED rats (t(15)=−4.831, p<0.001). Chi-square analysis revealed that SED rats had a significant preference for the novel object (Chi-Square=27.230, df =8, p<0.001), whereas ABA rats did not display a clear preference (Chi-Square= 7.710, df =8, p = 0.358) (Figure 6A).
Figure 6.
Cognition tests. (A) percentage time spent exploring the novel and the familiar object during the recollection trial of the novel object recognition test. (B) percentage time spent exploring the novel and the familiar place object during the recollection trial of the novel place recognition test. (C) latency to enter the escape hole during the Barnes maze test, and (D) time spent exploring target, quadrats left to target (left), opposite of target (opposite) and right to target (right) of the Barnes maze during the probe trial of the Barnes maze test by sedentary (SED), and activity-based anorexia (ABA) groups. * indicates a significant difference between time spent with the familiar and the novel object p≤0.05.
Novel place recognition test
No significant differences in the time spent exploring an object were observed during the novel place recollection test (t(15)=−1.651, p= 0.118). There were also no group differences in the time spent exploring the novel place object (t(15)=−0.548, p=0.592). Chi-square analysis revealed that both SED (Chi-Square= 27.225, df=8, p < 0.001) and ABA (Chi-Square= 23.507, df=8, p = 0.001) rats preferred the novel place object over the familiar place object (Figure 6B).
Barnes maze
For the acquisition (trials 1–9), repeated measures ANOVA revealed a significant time effect on the latency to reach the escape-box (F(8,120)= 20.8640, p<0.001). The latency to reach the escape-box decreased over time. There were no group (F(1,15)= 0.0449 p= 0.834) or time*group interaction (F(8.120)= 0.144 p= 0.996) effects found on the latency to enter the escape-box. During the probe trial, both ABA and SED rats spent more time in the target quadrant than expected from chance (Chi-square = 369.2017, df = 16, p<0.001). There were no significant differences between the time spent in the target quadrant between ABA and SED rats (T(15)=−0.702, p=0.493) (Figure 6D). During the reversal trials there was a significant effect of time (F(5,75)= 19.581, p<0.001). There were however no group (F(1,15)= 2.662, p= 0.123), or time*group interaction effects (F(5,75)= 0.815, p=0.542) on reversal learning (Figure 6C).
Discussion
The aim of the current study was to evaluate whether ABA experience alters taste, diet preference and cognitive function in rats. Our studies showed that after weight restoration, rats in the ABA group did not differ in their responses during the brief access taste test compared to any of the control groups, suggesting that ABA experience does not have lasting effects on sweet or bitter taste responses. We did not find any effects of ABA exposure on performance in the anxiety-like behavior in the EPM or open field tests. We did, however, find impairments in NOR in weight restored ABA rats. These impairments were specific to the object recognition itself and not novel placement of a familiar object, suggesting that impairments were specific to contextual memory and not to spatial memory. This hypothesis was strengthened by the observation that control and ABA rats did not differ in Barnes maze performance, even when the location of the escape box was changed. We thus conclude that in weight restored ABA rats we do not observe impairments in spatial learning and cognitive flexibility, but that there may be impairments in contextual learning.
Consistent with our data in rats exposed to ABA, studies in mice have shown that chronic food restriction (50%) impaired performance in a novel object recognition task (26). However, the mice in that study were food restricted during the cognitive testing, whereas the rats in our study were fully recovered from food restriction and body weight loss. In patients, impairments in full scale IQ have been reported in currently ill AN patients (19), however, these deficits disappeared with weight restoration. A recent study in adolescent AN patients showed no differences in full scale IQ, but impairments in the perceptual organization index (27). Whether or not these impairments persisted after weight restoration is not known. Studies investigating set-shifting typically report impairments in weight restored AN patients, although effect sizes seem variable in different subpopulations of patients (18, 28, 29). In the current study we did not specifically test set-shifting ability in weight restored ABA rats. Even though a set-shifting test has been designed for rodents (30), these procedures are lengthy, complex and use food cues which could be a major confounding factor with testing ABA rats. Additionally, though we did not directly assess set-shifting, we did include a related measure of behavioral flexibility using reversal learning in the Barnes maze. In this task we found no differences between control and ABA rats, suggesting that behavioral flexibility is not altered after recovery from ABA.
Together, the learning and memory data suggest that ABA might have a lasting effect on the perirhinal cortex which is known to play a crucial role in object recognition learning that seems independent of hippocampal function (31). Furthermore, ablation of the perirhinal cortex impairs novel object learning, without affecting novel place learning (32). Further investigation on the effects of ABA experience on perirhinal cortex structure and functioning is necessary to further evaluate cognitive impairments induced by ABA.
In contrast to previous reports by Kinzig and colleagues we did not find differences in the behavior in the elevated plus maze or the open field test between control and ABA rats (15). Our data suggest that a single exposure to ABA does not induce an anxiety-like phenotype in late adolescence. In Kinzig and Hargrave’s (15) study, the rats went through two cycles of ABA and were tested in adulthood, whereas our rats were tested during late adolescence and were only exposed to a single bout of ABA. This suggests that ABA-induced alterations in anxiety either manifest in later adulthood or only after repeated exposures to ABA. Additionally, anxiety has been suggested to be a predisposing factor for the development of ABA, Kinzig et al. used a different strain of rats (15), and baseline differences in anxiety-like behavior between the strains (33) may also have played a role. It is possible that having a genetic predisposition to anxiety without a clear anxiety-like phenotype under baseline conditions may facilitate anxiety development after ABA exposure in adulthood. Future, more detailed, studies addressing both the timing of anxiety development, as well as the environmental conditions needed to induce anxiety-like behavior after ABA exposure are required to fully understand the complex interaction between anxiety and weight loss during AN.
Finally, the current study shows that experience with ABA does not alter taste responsivity in rats. Previous studies by Liang and colleagues showed that rats exposed to ABA showed increased rates of CTA acquisition and decreased rates of CTA extinction (10). It is not clear whether ABA alters conditioning of any aversive stimulus, or whether the effects are specific to food related aversive cues. Our study, however, suggests that the effects on CTA are not due to a generalized anxiety phenotype or impaired taste function as we did not observe differences in elevated plus maze or brief access taste test performance. The results from the novel object recognition task suggest that experience with ABA may impair contextual learning. In contrast to their faster acquisition of CTA, however, object recognition was impaired. This may suggest that ABA specifically improves learning of aversive cues, whereas learning of neutral or positive cues may be impaired. In future research one may use imaging techniques, like fos mapping, to evaluate the effects of ABA exposure on activation of brain areas involved in aversive cue learning like the amygdala, parabrachial nucleus and thalamus, as well as areas involved in novel cue learning like the hippocampus and the perirhinal nucleus.
There are some limitations to the experimental design of the behavioral tests used in this study. First, all behavioral tests were performed during the light period. Since rodents are more active during the dark period, the amount of explorative behavior displayed by the animals may have been different when test were performed during the dark phase. Secondly, we exposed the rats to several behavioral test, and it is possible that exposure to multiple behavioral test may have influenced the results. Finally, the rats were exposed to 1,5 hr food access, other studies have used 1hr and 2 hr food access paradigm, which may impact the severity of food restriction. These limitations may explain some of the differences between this study and those described previously. And future studies may further address the role of the timing of test on the effects of ABA on the anxiety phenotype.
The data presented here suggest that with weight recovery after ABA experience there are no impairments in taste function or spatial learning. In contrast, perceptional memory, as measured in the NOR, was impaired in weight restored rats. To what extent these mild impairments in cognition may contribute to vulnerability to relapse merits further research. Insight into the origin of these cognitive impairments and understanding of the pathways involved may inform us about how to improve recovery success for AN patients by improving their cognitive functioning.
Supplementary Material
Acknowledgments
We would like to thank Leonard Marque, Shivany Aryal and Patricia Timi for their technical assistance in these studies. The studies in this manuscript were supported by grants from NIH (MH090585 to K.L. Tamashiro), The Klarmann Family foundation (to T.H. Moran).
Footnotes
Disclosures: No conflicts of interest, financial or otherwise, are declared by the authors.
References
- 1.Association AP. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 2.Birmingham CL, Su J, Hlynsky JA, Goldner EM, Gao M. The mortality rate from anorexia nervosa. The International Journal of Eating Disorders. 2005;38(2):143–6. doi: 10.1002/eat.20164. [DOI] [PubMed] [Google Scholar]
- 3.Hoek HW, van Hoeken D. Review of the prevalence and incidence of eating disorders. The International Journal of Eating Disorders. 2003;34(4):383–96. doi: 10.1002/eat.10222. [DOI] [PubMed] [Google Scholar]
- 4.Pike KM. Long-term course of anorexia nervosa: response, relapse, remission, and recovery. Clinical Psychology Review. 1998;18(4):447–75. doi: 10.1016/s0272-7358(98)00014-2. [DOI] [PubMed] [Google Scholar]
- 5.Eckert ED, Halmi KA, Marchi P, Grove W, Crosby R. Ten-year follow-up of anorexia nervosa: clinical course and outcome. Psychological Medicine. 1995;25(1):143–56. doi: 10.1017/s0033291700028166. [DOI] [PubMed] [Google Scholar]
- 6.Routtenberg A, Kuznesof AW. Self-starvation of rats living in activity wheels on a restricted feeding schedule. Journal of Comparative Physiolology Psycholology. 1967;64(3):414. doi: 10.1037/h0025205. [DOI] [PubMed] [Google Scholar]
- 7.Goldzak-Kunik G, Friedman R, Spitz M, Sandler L, Leshem M. Intact sensory function in anorexia nervosa. The American Journal of Clinical Nutrition. 2012;95(2):272–82. doi: 10.3945/ajcn.111.020131. [DOI] [PubMed] [Google Scholar]
- 8.Wagner A, Aizenstein H, Mazurkewicz L, Fudge J, Frank GK, Putnam K, et al. Altered insula response to taste stimuli in individuals recovered from restricting-type anorexia nervosa. Neuropsychopharmacology. 2008;33(3):513–23. doi: 10.1038/sj.npp.1301443. [DOI] [PubMed] [Google Scholar]
- 9.Oberndorfer TA, Frank GK, Simmons AN, Wagner A, McCurdy D, Fudge JL, et al. Altered insula response to sweet taste processing after recovery from anorexia and bulimia nervosa. The American Journal of Psychiatry. 2013;170(10):1143–51. doi: 10.1176/appi.ajp.2013.11111745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang NC, Bello NT, Moran TH. Experience with activity based anorexia enhances conditioned taste aversion learning in rats. Physiology & Behavior. 2011;102(1):51–7. doi: 10.1016/j.physbeh.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bulik CM, Sullivan PF, Fear JL, Joyce PR. Eating disorders and antecedent anxiety disorders: a controlled study. Acta Psychiatrica Scandinavica. 1997;96(2):101–7. doi: 10.1111/j.1600-0447.1997.tb09913.x. [DOI] [PubMed] [Google Scholar]
- 12.Klump KL, Strober M, Bulik CM, Thornton L, Johnson C, Devlin B, et al. Personality characteristics of women before and after recovery from an eating disorder. Psychological Medicine. 2004;34(8):1407–18. doi: 10.1017/s0033291704002442. [DOI] [PubMed] [Google Scholar]
- 13.Levinson CA, Byrne M. The fear of food measure: A novel measure for use in exposure therapy for eating disorders. The International Journal of Eating Disorders. 2014 doi: 10.1002/eat.22344. [DOI] [PubMed] [Google Scholar]
- 14.Zerwas S, Lund BC, Von Holle A, Thornton LM, Berrettini WH, Brandt H, et al. Factors associated with recovery from anorexia nervosa. Journal of Psychiatric Research. 2013;47(7):972–9. doi: 10.1016/j.jpsychires.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinzig KP, Hargrave SL. Adolescent activity-based anorexia increases anxiety-like behavior in adulthood. Physiology & Behavior. 2010;101(2):269–76. doi: 10.1016/j.physbeh.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wable GS, Min JY, Chen YW, Aoki C. Anxiety is correlated with running in adolescent female mice undergoing activity-based anorexia. Behavioral Neuroscience. 2015;129(2):170–82. doi: 10.1037/bne0000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelegen C, Collier DA, Campbell IC, Oppelaar H, van den Heuvel J, Adan RA, Kas MJ. Difference in susceptibility to activity-based anorexia in two inbred strains of mice. European Neuropsychopharmacology. 2007;17(3):199–205. doi: 10.1016/j.euroneuro.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Danner UN, Sanders N, Smeets PA, van Meer F, Adan RA, Hoek HW, van Elburg AA. Neuropsychological weaknesses in anorexia nervosa: set-shifting, central coherence, and decision making in currently ill and recovered women. The International Journal of Eating Disorders. 2012;45(5):685–94. doi: 10.1002/eat.22007. [DOI] [PubMed] [Google Scholar]
- 19.Koyama KI, Asakawa A, Nakahara T, Amitani H, Amitani M, Saito M, et al. Intelligence quotient and cognitive functions in severe restricting-type anorexia nervosa before and after weight gain. Nutrition. 2012;28(11–12):1132–6. doi: 10.1016/j.nut.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Glendinning JI, Gresack J, Spector AC. A high-throughput screening procedure for identifying mice with aberrant taste and oromotor function. Chemical Senses. 2002;27(5):461–74. doi: 10.1093/chemse/27.5.461. [DOI] [PubMed] [Google Scholar]
- 21.Smith JC. The history of the “Davis Rig”. Appetite. 2001;36(1):93–8. doi: 10.1006/appe.2000.0372. [DOI] [PubMed] [Google Scholar]
- 22.Davis JD, Smith GP. Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behavioral Neuroscience. 1992;106(1):217–28. [PubMed] [Google Scholar]
- 23.Jiang E, Blonde G, Garcea M, Spector AC. Greater superficial petrosal nerve transection in rats does not change unconditioned licking responses to putatively sweet taste stimuli. Chemical Senses. 2008;33(8):709–23. doi: 10.1093/chemse/bjn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Treesukosol Y, Blonde GD, Spector AC. T1R2 and T1R3 subunits are individually unnecessary for normal affective licking responses to Polycose: implications for saccharide taste receptors in mice. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2009;296(4):R855–65. doi: 10.1152/ajpregu.90869.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Treesukosol Y, Smith KR, Spector AC. Behavioral evidence for a glucose polymer taste receptor that is independent of the T1R2+3 heterodimer in a mouse model. Journal of Neuroscience. 2011;31(38):13527–34. doi: 10.1523/JNEUROSCI.2179-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carlini VP, Martini AC, Schioth HB, Ruiz RD, Fiol de Cuneo M, de Barioglio SR. Decreased memory for novel object recognition in chronically food-restricted mice is reversed by acute ghrelin administration. Neuroscience. 2008;153(4):929–34. doi: 10.1016/j.neuroscience.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Kjaersdam Telleus G, Jepsen JR, Bentz M, Christiansen E, Jensen SO, Fagerlund B, Thomsen PH. Cognitive profile of children and adolescents with anorexia nervosa. European Eating Disorders Review. 2015;23(1):34–42. doi: 10.1002/erv.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buhren K, Mainz V, Herpertz-Dahlmann B, Schafer K, Kahraman-Lanzerath B, Lente C, Konrad K. Cognitive flexibility in juvenile anorexia nervosa patients before and after weight recovery. Journal of Neural Transmission. 2012;119(9):1047–57. doi: 10.1007/s00702-012-0821-z. [DOI] [PubMed] [Google Scholar]
- 29.Roberts ME, Tchanturia K, Stahl D, Southgate L, Treasure J. A systematic review and meta-analysis of set-shifting ability in eating disorders. Psychological Medicine. 2007;37(8):1075–84. doi: 10.1017/S0033291707009877. [DOI] [PubMed] [Google Scholar]
- 30.Fox MT, Barense MD, Baxter MG. Perceptual attentional set-shifting is impaired in rats with neurotoxic lesions of posterior parietal cortex. Journal of Neuroscience. 2003;23(2):676–81. doi: 10.1523/JNEUROSCI.23-02-00676.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barker GR, Warburton EC. When is the hippocampus involved in recognition memory? Journal of Neuroscience. 2011;31(29):10721–31. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albasser MM, Davies M, Futter JE, Aggleton JP. Magnitude of the object recognition deficit associated with perirhinal cortex damage in rats: Effects of varying the lesion extent and the duration of the sample period. Behavioral Neuroscience. 2009;123(1):115–24. doi: 10.1037/a0013829. [DOI] [PubMed] [Google Scholar]
- 33.Turner KM, Burne TH. Comprehensive behavioural analysis of Long Evans and Sprague-Dawley rats reveals differential effects of housing conditions on tests relevant to neuropsychiatric disorders. PloS One. 2014;9(3):e93411. doi: 10.1371/journal.pone.0093411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.