Abstract
Pulmonary fibrosis affects millions worldwide and, even though there has been a significant investment in understanding the processes involved in wound healing and maladaptive repair, a complete understanding of the mechanisms responsible for lung fibrogenesis eludes us, and interventions capable of reversing or halting disease progression are not available. Pulmonary fibrosis is characterized by the excessive expression and uncontrolled deposition of extracellular matrix (ECM) proteins resulting in erosion of the tissue structure. Initially considered an ‘end-stage’ process elicited after injury, these events are now considered pathogenic and are believed to contribute to the course of the disease. By interacting with integrins capable of signal transduction and by influencing tissue mechanics, ECM proteins modulate processes ranging from cell adhesion and migration to differentiation and growth factor expression. In doing so, ECM proteins help orchestrate complex developmental processes and maintain tissue homeostasis. However, poorly controlled deposition of ECM proteins promotes inflammation, fibroproliferation, and aberrant differentiation of cells, and has been implicated in the pathogenesis of pulmonary fibrosis, atherosclerosis and cancer. Considering their vital functions, ECM proteins are the target of investigation, and oxidation–reduction (redox) reactions have emerged as important regulators of the ECM. Oxidative stress invariably accompanies lung disease and promotes ECM expression directly or through the overproduction of pro-fibrotic growth factors, while affecting integrin binding and activation. In vitro and in vivo investigations point to redox reactions as targets for intervention in pulmonary fibrosis and related disorders, but studies in humans have been disappointing probably due to the narrow impact of the interventions tested, and our poor understanding of the factors that regulate these complex reactions. This review is not meant to provide a comprehensive review of this field, but rather to highlight what has been learned and to raise interest in this area in need of much attention.
Keywords: Redox, Oxidative stress, Pulmonary fibrosis, Extracellular matrix, Integrins
Graphical abstract
1. Introduction
Lung fibrosis is characterized by, among other things, the effacement of the original architecture of the lung due to excessive expression and deposition of the extracellular matrix (ECM) [1]. In normal lungs, this acellular substance is a complex admixture of glycoproteins, collagens, and polysaccharides neatly assembled so as to maintain tissue integrity and to separate epidermal and mesenchymal cell layers in tissues [2]. In injured lungs, however, inflammation, oxidative stress, and other events drive the expression and turnover of ECM proteins. In most cases, this process is regulated and is inhibited once the injuring agent is eliminated. Yet, on occasion, this process remains activated leading to thickening of the interstitium followed by permanent obliterations of the alveolar spaces and loss of lung function [3] (Fig. 1). These events underlie fibrosing lung disorders affecting millions worldwide.
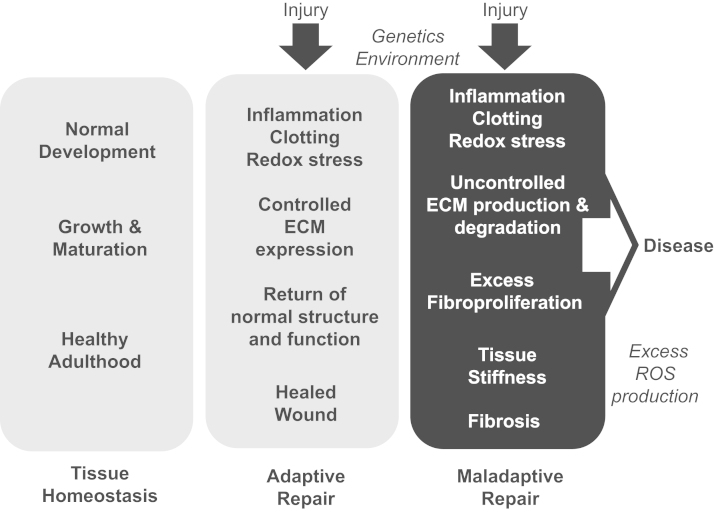
Fig. 1.
Development, tissue homeostasis, and response to injury are dependent on ECM expression and deposition. ECM expression and turnover are tightly controlled during organ development and during adulthood. Tissue injury triggers inflammation, clotting, redox stress, and regulated expression and degradation of the ECM. In general, elimination of the injurious agents is followed by ‘turning off’ this wound healing response resulting in inhibition of ECM expression and, ultimately, a return to the original tissue structure and function (Adaptive Repair). However, on occasion, injury triggers an exuberant response characterized by uncontrolled ECM expression and turnover leading to increased stiffness of the tissue and eradication of the original tissue architecture leading to loss of function (Maladaptive Repair). These events are greatly influenced by genetics and environmental exposures. Uncontrolled generation of reactive oxidant species (ROS) is thought to contribute to maladaptive repair, in part, by promoting aberrant ECM expression and fibroproliferation.
Cells differ in their capacity for producing, secreting, and assembling ECM, and its composition differs amongst organs and between organ compartments. The ECM was initially considered to be an inert substance providing scaffold for the adhesion of cells and for their organization into complex organs. In the early 1980s, however, a better appreciation of the true role of the ECM began to emerge with the discovery of a family of cell surface adhesion receptors termed integrins [4]. Integrin activation by ligand binding to ECM proteins triggers diverse intracellular signals capable of influencing gene expression [5]. This early work laid the foundation for our current understanding that cell functions are greatly influenced by the composition of their surrounding ECM and by the repertoire of matrix-binding integrins expressed on their surface. Moreover, ECM proteins are the main contributors to tissue stiffness, which also influences cell behavior [6].
It is well documented that ECM proteins play roles in regulating important cell functions such as adhesion, migration, and differentiation as well as in complex processes like tissue morphogenesis and wound healing [7], [8]. However, the exact roles ECM proteins play in cellular and tissue homeostasis in human health and disease remain incompletely elucidated mainly because in vitro models fail to reflect the complex nature of in vivo ECM, because genetically-engineered animals with knockout mutations of genes coding for ECM and matrix-binding integrin receptors are often embryonic lethal [9], and because of the complex, robust and insoluble nature of assembled ECM which makes their study arduous even in the most experienced hands [10]. However, these challenges are being partially overcome by the emergence of new experimental models (e.g., organ decellularization) and novel approaches to genetic targeting of proteins [11]. Together, these technologies have helped generate data about the lung ‘matrisome’, thereby adding to the overwhelming literature available in support of the role of ECM proteins in embryogenesis and tissue homeostasis after birth, as well as in the development of disorders ranging from atherosclerosis and kidney disease to rheumatoid arthritis and cancer [8], [12], [13].
In lung, ECM proteins have been implicated in lung branching morphogenesis, vasculogenesis, and alveolar maturation during development, as well as in tissue repair after injury [14], [15]. However, ECM proteins have also implicated in pathologic processes leading to acute and chronic pulmonary disorders such as asthma, acute lung injury, and idiopathic pulmonary fibrosis (IPF) [16], [17], [18]. Considering the above, and the fact that essentially all pulmonary disorders are associated with alterations in the expression, deposition and turnover of ECM proteins, it is no surprise that understanding the factors that regulate ECM-dependent events in lung has remained a focus of attention for over two decades.
Oxidants and redox reactions have been found to influence ECM expression and turnover, and these appear to be important physiological processes relevant to health, but also to diseased states such as lung fibrogenesis [19]. Patients with fibrosing lung disorders manifest evidence of oxidative stress [20], [21], [22], [23], which triggers intracellular signals that stimulate fibroproliferation and the expression of pro-fibrotic factors [24], while interventions targeting the oxidant-anti-oxidant balance ameliorate progression of fibrosis in animal models of lung injury [25], [26]. Together, these observations strongly implicate oxidative stress in the pathogenesis of fibrosing lung disorders, and even though its role as a target for intervention in lung fibrosis remains controversial [27], the exploration of redox as an important modulator of ECM production, modification, function, and recognition by cells is justified. Excellent reviews have been published addressing aspects of this area of investigation [28], [29], [30]. Thus, we will focus on the impact of redox reactions on the lung ECM considering that this organ is exposed to higher levels of oxygen than other tissues. A description of the lung ECM and its functions in lung development and in injury and repair will be followed by a discussion of redox reactions considered to influence these events. Finally, information will be provided that provide strong evidence supporting the concept that ECM protein expression, turnover and recognition are redox-dependent events.
2. Role of lung ECM
The importance of ECM proteins in multicellular organisms is highlighted by their function as a multi-dimensional structural support for tissues, and their ability to bind growth factors, activate signaling cell surface receptors, and regulate cell proliferation and differentiation, among other processes [31], [32]. Together with soluble factors, these functions enable ECM proteins to orchestrate tissue morphogenesis, differentiation, and homeostasis [7], [8], processes critical for the adequate development and repair of all major organs, including the lung. In the embryonic lung, branching morphogenesis, alveolar septation, and terminal differentiation of the various cells of the lung are all dependent on proper signals derived from the ECM [7], [14]. The maintenance of cell polarity and the regulation of cell functions by the epithelium are also highly dependent on the ECM, including its three-dimensional and topographic cues [33].
ECM deposition, remodeling, and resorption are dynamic processes that are precisely controlled during normal lung development, homeostasis, and tissue repair. Since the primary function of the adult lung is gas exchange, the specialized structure and composition of the pulmonary ECM is designed to facilitate this function. In the upper airways, the ECM is specialized for structural support in order to prevent airway collapse. However, in the lower airways, the ECM forms a specialized basement membrane layer made from many different ECM proteins such as chondroitin sulfate proteoglycans, heparan sulfate proteoglycans, entactin, laminins [34], [35], fibronectins [36], collagens [37] and glycosaminoglycans [38]. These ECM components affect bronchial epithelial cell attachment [39] and migration [40] as well as the differentiation of cells lining the alveolus. The latter is best depicted by the observation that isolated type II airway epithelial cells quickly lose their specialized phenotype if not cultured on the proper ECM. In contrast, when cultured on three-dimensional collagen gel matrices, these cells proliferate and form branching structures that contain lumens lined by both flattened and cuboidal epithelium [41], while expressing surfactant-associated proteins SP-A, SP-B, and SP-C [42], [43]. Claudin-dependent cell–cell interactions responsible for lung epithelial cell monolayer formation and control of permeability are also affected by the ECM [44]. Lung development is clearly influenced by ECM proteins and redox reactions, but how these events relate to each other remains unclear [160].
In contrast to the uninjured developing and adult lung, the diseased lung shows exaggerated and uncontrolled expression, deposition and turnover of collagens and other ECM proteins leading to, at the very least, qualitative alterations in the composition of the ECM, and at worse, disruption of organ architecture with loss of tissue function [1], [3]. Remodeling is triggered early after tissue injury, and could be dissociated from (and therefore controlled by processes other than) inflammation [45]. Recent studies have further emphasized the role of ECM proteins in lung injury and repair. For example, normal fibroblasts cultured atop human fibrotic lungs after decellularization manifest changes in phenotype characterized by increased expression of myofibroblast markers [46]. Moreover, animals deficient in a splicing variant of fibronectin termed fibronectin EDA, a matrix glycoprotein highly expressed after injury and implicated in tissue repair, show protection against fibrosis in the bleomycin model [47].
In addition to the above, ECM proteins have been found to affect immune cell activation and cytokine expression, serve as a reservoir for growth factors, and influence autophagy [48], [49]. More recently, attention has been given to the role of ECM proteins in controlling tissue stiffness and how this impacts cellular behavior. The stiffness of the lung increases with enhanced deposition of collagens during tissue fibrosis, and this mechanical stimulus is sufficient to exert changes in cell function that further drive fibrogenesis [6], [50].
3. Redox reactions
Reduction and oxidation reactions (also termed redox reactions) have profound effects on biological processes. The oxidation reactions that have received the most attention with respect to human health are those arising from the interaction of an oxygen metabolite with biological molecules. Much has been learned over the last several decades about the conditions that lead to the formation of reactive oxygen metabolites and the association of disease processes with levels of specific oxidation products.
Successive one-electron reductions of molecular oxygen (O2) yield the superoxide anion radical (O2•−), the non-radical species hydrogen peroxide (H2O2), the extremely reactive hydroxyl radical (•OH), and, finally, water (H2O). The most abundant source of superoxide is leakage of incompletely-reduced oxygen from the mitochondrial electron transport chain [51]. Superoxide can spontaneously dismutate to produce O2 and H2O2, but this reaction is greatly accelerated by superoxide dismutases (SODs). The H2O2 formed from these (and other) enzymes is more stable than superoxide, and, because it is uncharged, it is able to diffuse through biological membranes. Enzymes such as catalase, glutathione peroxidases, and peroxiredoxins catalyze the 2-electron reduction of H2O2 to H2O, but there are no enzymes that catalyze its 1-electron reduction to •OH. Rather, the hydroxylradical is formed non-enzymatically in the presence of unbound transition metals. Once formed, •OH is so reactive that it can remove an electron from the first molecule it encounters. Clearly, such indiscriminant reactivity would disrupt normal cellular functions. Fortunately, free transition metals are kept at extremely low levels under normal conditions.
An observable consequence of augmented production of reactive oxygen metabolites is an increase in the number of oxidative modifications to cellular and extracellular constituents such as proteins, DNA, and lipids. Stable (irreversible) modifications make good biomarkers because they accumulate in cells or tissues experiencing increased rates of oxidant production and, therefore, provide a measure of the overall oxidant burden. Oxidation products of lipids (e.g., isoprostanes), proteins (e.g., diTyrosine), DNA (e.g., 8-oxo-deoxyguanosine) and sugars (e.g., advanced glycation end products, AGE) have all been correlated with specific pathological processes [52], [53], [54], [55], [56]. In addition, these biomarkers can sometimes have signaling roles. For example, isoprostanes can activate the thrombane X receptor [52], [57] and AGE can bind to receptors of AGE (RAGE) [58]. Irreversible oxidative modifications of macromolecules also serve as signals for degradation or repair. For example, tyrosine cross-links (diTyrosine) mark proteins for turnover [59], and oxidized nucleotides trigger DNA repair [60].
Oxidant production is not always accidental or harmful. For example, superoxide and H2O2 are produced as signaling molecules in response to growth factor stimulation and are important in immunity [60], [61]. A major source of oxidants used for signaling purposes is the NADPH oxidase (Nox) family of enzymes. These enzymes catalyze the transfer of electrons from NADPH to O2 to produce superoxide or H2O2. The founding member of this family, Nox2, was discovered as a key component of the respiratory burst that enables macrophages and other cells of the innate immune system to convert oxygen to cytotoxic agents to be used in host defense [62]. Other family members have been identified with different tissue expression levels and subcellular distributions whose main functions appear to be related to signaling rather than host defense. All cell types found in the lung express at least one isoform of Nox3 [63].
Reversibility is a major characteristic of oxidative modifications that are used for signaling purposes. Targets that are irreversibly modified accumulate and need to be replaced if they serve a vital cellular process. Reversible modifications, on the other hand, provide a way to temporarily alter the function of a target. The sulfur atom associated with the amino acid cysteine, for example, can exist in multiple stable redox states. Most intracellular cysteines are found in the reduced or thiol form (SH), but can be oxidized to a sulfenic acid (SOH) or a disulfide (SS). These are equivalent 2-electron oxidations, and can be reduced back to the thiol form using 2 electrons from NADPH. The target cysteines can be the free amino acid, in the tripeptide glutathione (GSH), or found as one of the amino acids comprising all cellular proteins [64]. Different enzymes catalyze the transfer of electrons from NADPH depending on which of these forms of cysteine has been oxidized. These events may affect the oxidation of thiol disulfide couples. Redox couples are comprised of 2 chemical species that are interconvertible via exchange of electrons. Cysteine (Cys) and its disulfide form, cystine (CySS) constitute a redox couple, as does GSH and its disulfide (GSSG). Under conditions where the rate of oxidation is increased, the proportion of the couple in the oxidized form will increase and its redox potential will change. The redox potential of the Cys/CySS or GSH/GSSG redox couples is best described by the Nernst equation [64] because it takes into account the concentrations of the components as well as the stoichiometry of thiol–disulfide exchange reactions such as these (2Cys↔CySS+2e−+2H+). Thus, the Cys concentration is squared in the equilibrium expression. While redox couples are not at equilibrium in biological systems, the Eh value from the Nernst equation nonetheless provides the most comprehensive numerical representation of the redox potential under a given set of conditions. For this reason, Eh values are often referred to as expressing the redox state rather than the redox potential to emphasize that these values are derived from steady state concentrations instead of equilibrium concentrations. Eh values allow comparisons between different redox couples. For example, the Eh value for the Cys/CySS redox couple in human plasma (Eh Cys/CySS) is normally about −80 mV, whereas the Eh GSH/GSSG for human plasma is about −137 mV [65]. Clearly, these 2 redox couples are not in equilibrium with each other in human plasma, suggesting that they are independently regulated.
The Cys/CySS redox couple is the predominant thiol–disulfide couple in plasma. This is in contrast with intracellular spaces, where GSH/GSSG predominates. Plasma Eh Cys/CySS becomes more oxidizing (less negative) with age, cigarette smoking, type 2 diabetes and cardiovascular disease [66]. Nutrition also affects plasma Eh Cys/CySS [67]. These events have dramatic effects on cells. For example, culturing Caco-2 cells in media with an oxidizing Eh Cys/CySS stimulates their proliferation through activation of the epidermal growth factor receptor in a metalloproteinase-dependent manner [68], [69]. The effect could be blocked by adding a cell membrane-impermeable thiol-reactive maleimide, suggesting that the effect was dependent on extracellular thiols. In another example, endothelial cells cultured in media with oxidized Eh Cys/CySS showed increased H2O2 production and expression of adhesion molecules in concert with a decrease in extracellular membrane protein thiol content [70]. Interestingly, cells grown in culture will condition the medium in which they are growing such that the redox state of Cys/CySS stabilizes at −80 mV, the same value as seen in human plasma [71], [72]. Thus, the redox state of a specific redox couple reflects the summation of effects on rates of oxidation and rates of reduction, and this redox state can be sensed by cells leading to changes in cell behavior.
Data have emerged implicating oxidative stress as a pathogenic factor in tissue fibrosis. In humans with fibrosing lung disorders, deficiencies in anti-oxidant defenses have long been identified. For example, investigators studied 16 IPF and 15 healthy non-smoking subjects, and found that sputum glutathione (GSH) was decreased more than four-fold in IPF patients [20]. Plasma GSH levels were also lower in IPF. Interestingly, in IPF patients, there was a borderline correlation of sputum GSH levels with disease progression and lung function impairment in IPF patients. Others studied the metabolism of GSH and superoxide anion production of whole blood in 14 IPF patients and 12 healthy subjects [23]. They found that the total amount of GSH in the blood of IPF patients did not differ from the controls, but the amount of GSH in the oxidized disulfide (GSSG) and the ratio of GSSG to total GSH in blood significantly increased in IPF patients. Also, the production and generation of superoxide anions by blood were greater in IPF than in normal subjects; this correlated with the GSSG/GSH ratio. Yet others studied the alveolar lining fluid of newly diagnosed IPF patients and reported significantly lower levels of total GSH when compared to sarcoid patients and controls [22]. In this study, GSH levels increased after treatment. This underlying redox state can be enhanced by oxygen radicals produced by inflammatory cells [73] and may help maintain the pro-fibrotic phenotype of lung fibroblasts [74]. Together, these and other observations point to redox reactions as an important pathogenic mechanism in lung fibrosis. However, simple interventions (e.g., N-acetylcysteine) designed solely to improve GSH levels in lung have proven ineffective in some populations suggesting that redox reactions in lung fibrosis are complex and influenced by many factors including genetics and, therefore, will require further investigation.
4. Redox – dependent ECM expression
Redox reactions can stimulate ECM expression via at least three mechanisms of action: 1) activation of redox-signaling cascades in response to pro-fibrotic growth factors, 2) activation of transcription factors and/or epigenetic events that drive ECM gene transcription, and 3) oxidation of the redox potential of thiol disulfide couples.
4.1. Pro-fibrotic effects of transforming growth factor-beta (TGFβ) are dependent on redox signaling cascades
Of the many growth factors capable of stimulating ECM production, TGFβ is considered the ‘master switch’ for lung fibrosis; it promotes ECM expression directly through activation of TGFβ receptors and upregulation of signals involving the Smads and mitogen activated protein kinase pathways [75]. TGFβ also promotes the expression of other pro-fibrotic growth factors such as connective tissue growth factor (CTGF), which can amplify the fibrotic response [76]. It also acts via matrix metalloproteinases (MMPs) and the upregulation of matrix-binding integrins, which can, in turn, activate TGFβ [77], [78]. Through these activities, TGFβ promotes the proliferation of fibroblasts, considered effector cells in tissue fibrosis, as well as their production of matrix glycoproteins like fibronectins, fibrillar collagens (e.g., collagens type I and III), and proteoglycans, among other ECM proteins.
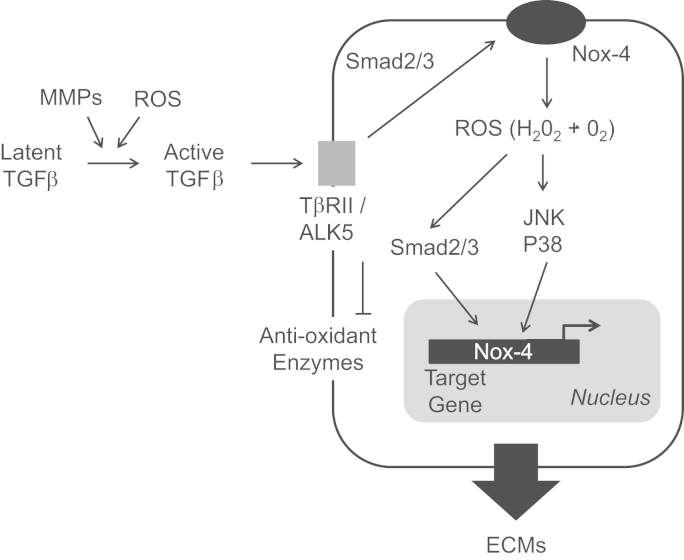
Interestingly, TGFβ represents the best example of redox-dependent growth factor-induced ECM expression since many of its pro-fibrotic effects are dependent on Nox-4 and the generation of reactive oxygen species (Fig. 2). Treatment with siRNA against NOX4 suppressed TGFβ-induced expression of fibronectin, collagen I, α-smooth muscle actin, and connective tissue growth factor in cardiac fibroblasts [79]. Similarly, chemical inhibition of Nox-4 suppressed ECM production and epithelial–mesenchymal transition in renal tubular epithelial cells [80], [81]. Furthermore, TGFβ stimulation of collagen synthesis and myofibroblast transdifferentiation is inhibited by a scavenger of superoxide and H2O2, and by a dominant negative form of Nox 4 [82]. Similar events have been noted in fetal lung mesenchymal cells [83], pulmonary fibroblasts [84], kidney fibroblasts [85] and liver stellate cells [86]. There also appears to be a reciprocal interaction between TGFβ and Nox-4 since TGFβ upregulates Nox-4 expression [87], [88], [89], [90] and this appears to be downstream of Smad 2/3 activation [91].
Fig. 2.
ROS-dependent TGFβ activation and function. TGFβ can be produced by cells or liberated from ECM reservoirs during tissue remodeling. Its latent form is converted into active TGFβ through the actions of MMPs or ROS. Once activated, TGFβ interacts with cell surface receptors leading to the activation of Nox-4 and the generation of ROS. In turn, ROS promotes target gene expression through effects on protein kinases and transcription factors.
These studies strongly suggest that Nox-4 modulates many of the pro-fibrotic actions of TGFβ in vitro. Studies in animal models of lung fibrosis suggest that these mechanisms of action are also relevant in vivo. For example, bleomycin-induced fibrosis was suppressed with tracheal administration of Nox-4 siRNA [92]. Similar observations were made in Nox-4 knockout mice [93], whereas knockdown of Nox-2 was not as effective [94].
TGFβ not only promotes oxidative stress, but it also decreases the expression of several anti-oxidant enzymes. For example, TGFβ inhibits the mRNA expression and activity of glutathione peroxidase 1 and catalase in hamster pancreatic cell lines [95], and suppresses the expression of superoxide dismutase-1 (SOD1) and -2 (SOD2) and catalase in cultured rat hepatocytes and in airway smooth muscle cells [96], [97]. Interestingly, oxidation of latency-associated protein or MMPs can facilitate the activation of latent TGFβ [98], [99], providing a means for continued TGFβ activity under conditions of oxidative stress. Thus, the actions of TGFβ, the pro-fibrotic growth factor most implicated in tissue fibrosis, appear to be tightly linked to redox reactions.
4.2. Redox-dependent ECM expression via activation of transcription factors and/or epigenetic events
Redox reactions are known to affect gene transcription through effects on transcription factor activation or via epigenetic events. For example, redox reactions are well-known to influence fos and jun DNA binding [100]. A member of this family, Activator Protein-1 (AP-1), a transcription factor needed for the expression of alpha2(I) collagen and fibronectin in response to TGFβ [101], [102]. MMPs are also affected by these mechanisms. For example, age-dependent increases in MMP-1 are dependent on the generation of reactive oxidant species, which appears to occur through regulation of MMP-1 gene transcription as well as chromatin remodeling affecting gene activation [103]. H2O2 inactivates histone deacetylase-2 (HDAC-2), thereby maintaining the MMP-1 promoter accessible to transcription factors such as c-Fos, c-Jun, and Ets-1. These and other transcriptions factors may also be activated by redox-sensitive pathways including MAPK activation [104], [105]. Thus, ECM expression and MMP production appear dependent on redox reactions affecting transcription factor activation and epigenetic changes. This is intriguing considering emerging data implicating aberrant control of gene expression through epigenetic mechanisms in the setting of aging and IPF [106].
4.3. Redox potential – mediated ECM expression
Aging, malnutrition, alcohol abuse, cigarette smoking, and cancer, among other disorders, are associated with oxidation of thiol disulfide couples [66], [67]. Although how this process promotes ECM expression in lung remains incompletely elucidated, several observations have provided important new insight into this area. Fibroblast cell lines (NIH 3T3 fibroblasts) and primary murine lung fibroblasts cultured in media with oxidized Eh Cys/CySS (−41 mV) show increased proliferation as well as increased expression of fibronectin when compared to cells cultured in normal (−81 mV) and reduced (−105 mV) Eh Cys/CySS [24]. These events are associated with increased expression of α-smooth muscle actin suggesting concomitant myofibroblast transdifferentiation. Interestingly, the expression of these pro-fibrotic markers was inhibited by anti-TGFβ antibodies as well as chemical blockers of TGFβ receptor function suggesting a critical role for TGFβ in mediating redox potential – mediated ECM expression [24].
Oxidation of the extracellular redox potential also stimulates the expression of integrins and other cell adhesion molecules including ICAM-1, VCAM-1, P-selectin, and E-selectin in vitro [107]. However, few in vivo studies are available regarding the role of redox potential of thiol disulfide couples in lung fibrosis. Of relevance, mice exposed to bleomycin, a well-known model of lung fibrosis, develop oxidation of the Eh Cys/CySS and Eh GSH/GSSG [108]. Furthermore, oxidation of Eh Cys/CySS correlates with increased pro-inflammatory cytokine levels in these animals [109]. Finally, preliminary studies suggest that patients with IPF also manifest oxidation of Eh Cys/CySS (personal observations).
5. Redox-dependent ECM modification
Redox reactions can promote ECM modifications, which may alter their interactions with each other, with cells, and with growth factors. In general, ECM proteins can be modified by enzymatic digestion or by oxidation. Of the cell-derived proteinases capable of ECM degradation, MMPs are some of the best studied. MMPs are highly expressed in inflammatory conditions and have been implicated in many pulmonary disorders ranging from asthma and other obstructive airways disorders to acute lung injury and idiopathic pulmonary fibrosis [110]. Most MMPs are released from cells as ‘pro-enzymes’, which are activated by proteolysis of a cysteine-zinc prodomain, called a ‘cysteine switch’ [110]. Once activated, these proteinases can act on many ECM substrates including collagens I, II, III, V, VII, X, gelatin, elastin, fibronectin, proteoglycans, and basement membrane components such as collagen IV and laminins. MMPs can also target other MMPs as well as soluble growth factors including latent TGFβ and pro-TNFα [110], [111], thereby affecting their function. The activity of MMPs is regulated by tissue inhibitors of MMPs (TIMPs) in a 1:1 stoichiometric fashion; thus, their activity is dependent on the relative concentration of MMPs and TIMPs [112]
Oxidation is another important mechanism of ECM modification (Fig. 3). Most oxidants generated within cells cannot diffuse out into the extracellular space and, consequently, have limited effect on the ECM [113]. However, oxidants formed extracellularly may gain access to the ECM and modify its components to the point of altering their impact on cells. This is particularly relevant in inflammatory disorders, which are characterized by the influx of activated neutrophils, macrophages, and eosinophils, among other immune cells. These cells assemble Nox complexes on their membranes and produce superoxide radicals, which are released extracellularly and can be catalyzed into hydrogen peroxide by extracellular superoxide dismutases, thereby generating more oxidants. Activated neutrophils, monocytes and macrophages can also release myeloperoxidase, an enzyme that binds to ECM proteins and localizes damage to specific sites. Furthermore, the resting Fe3+ form of myeloperoxidase reacts with hydrogen peroxide and generates more oxidants [114], [115], [116]. Eosinophils produce eosinophil peroxidase with similar capabilities. Chloramines, bromamines and reactive aldehydes can cross membranes as well and promote further generation of oxidant radicals [117].
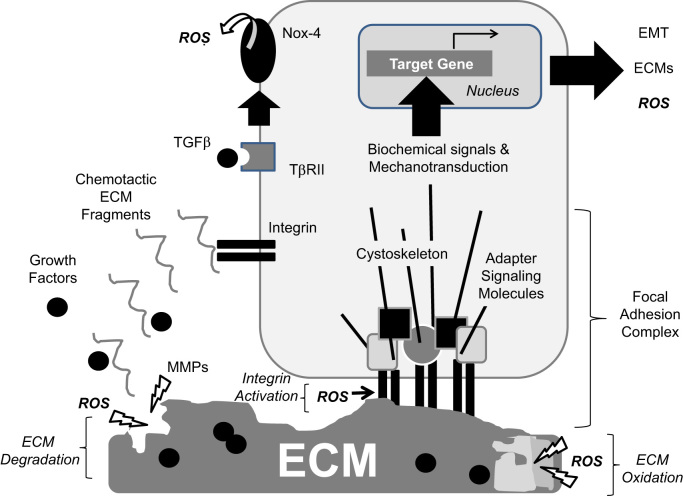
Fig. 3.
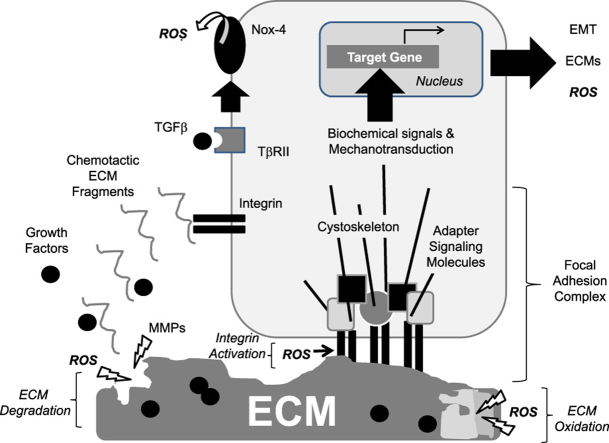
ROS effects on ECM-integrin interactions and signaling. Cells interact with ECM proteins via integrins that cluster at the cell surface in focal adhesion complexes containing signaling adapter molecules and cytoskeletal structures. Activation of integrins results in biochemical signals that influence differential gene expression. By integrating the extracellular insoluble ECM with the intracellular cytoskeleton, integrins transmit mechanical signals (mechanotransduction) that also influence gene expression. Ultimately, cellular responses to ECM proteins are dependent on the composition and stiffness of the ECM, which can be altered by MMP- or ROS-mediated degradation or by ROS-mediated oxidation. Integrin activation by ECM can be affected by ROS through effects on cysteines contained within the α and β subunits of integrins leading to conformational changes in the receptors. Tissue remodeling after injury resulting from the activity of MMPs and ROS can liberate growth factors from their ECM reservoir. One growth factor with pro-fibrotic activity is TGFβ, which can interact with surface receptors leading to Nox-4 activation (and locations to focal adhesion complexes) and further generation of ROS.
The production of radicals through the aforementioned reactions can lead to carbohydrate oxidation on glycosaminoglycans yielding α-hydroxyalkyl radicals capable of catalyzing reactions with nearby C–OH and C–OR bonds [118]. This can result in glycosidic bond cleavage and the formation of peroxyl radicals, which can trigger further chain reactions. These and related events result in the oxidation of collagens, elastin, fibronectin, laminin and glycosaminoglycans [119]. Glycosaminoglycans are particularly susceptible since peroxynitrite can modify their core protein and heparan sulfate chains as has been document for perlecan, a basement membrane-specific heparin sulfate proteoglycan [120]. Polyanionic molecules like heparan sulfate proteoglycans can bind cationic proteins and transition metals, thereby serving as substrates for metal-catalyzed redox events.
One can envision how these events may influence ECM regulation of cell adhesion and signaling, interactions with growth factors, epithelial and endothelial cell permeability, and other cellular processes, yet formal studies providing insight into these events are only fairly recent [121], [122]. Oxidative modifications of the protein core of perlecan, for example, influence the adhesion of endothelial cells [123]. Oxidation events can also destabilize interactions between ECM components and growth factors. For example, FGF2 binds to perlecan via its heparin sulfates, and modification of perlecan by oxidation may render FGF2 susceptible to proteolysis [124]. ECM oxidation may also lead to changes in the assembly and stability of the structure of fibrillary collagens. For example, collagen III is a homotrimer C-terminally cross-linked by an inter-chain of three disulfide bridges (also known as the cystine knot) with two adjacent cysteine residues on each of the three a chains, and these structures are important for the folding and stability of the molecule [125]. This might be an important mechanism involved in the unveiling of auto-antigens in collagen V, which have been implicated in the pathogenesis of IPF [126]. Oxidants may also result in the shedding of ECM components and/or the generation of ECM protein fragments with chemotactic activity. In vivo studies have shown that lung hyaluronan and heparan sulfates are cleaved by superoxides, while related redox mechanisms affect the distribution of syndecan-1 [127], [128]. Of course, not all oxidative modifications of ECM proteins are detrimental and some may have physiological roles in wound healing and other processes. For example, oxidative modifications of ECM proteins can lead to covalent cross-linkages which stabilize the supramolecular structure of the ECM [129].
Thus, ECM proteins can be modified by MMP-dependent cleavage or by oxidation. Interestingly, there is a mechanistic link between these two pathways since reactive oxidant species can cleave and activate MMPs. Oxidized glutathione can oxidize MMPs, thereby inducing their activation, while concomitantly repressing TIMP gene transcription. In normal myocardium, latent MMPs are activated during end-stage heart failure through oxidized glutathione [130].
6. Modulation of redox by ECM
Attention has recently turned to the ability of ECM proteins to modulate redox reactions. In SV40 MES 13 murine mesangial cells cultured on collagen type I, the cells produced more reactive oxidant species and less intracellular nitric oxide when compared to cells cultured on the basement membrane component collagen IV, thereby suggesting that the composition of the ECM can modulate the redox state in the kidney [131]. This is significant considering that diabetic nephropathy is associated with enhanced collagen I expression. Lung injury is also associated with increased expression of collagen I and other fibrillar collagens. In other work, the number of focal adhesions that attach cells to ECM proteins were found to correlate with age-related slowing down of wound healing in vitro in skin fibroblasts. This can be modulated by curcumin and appears dependent on modulation of redox reactions through the induction of the transcription factor Nrf-2 and hemeoxygenase-1 [132]. Nrf2 is a leucine zipper protein that regulates the expression of antioxidant proteins and is considered a protective mechanism in the pathogenesis of pulmonary fibrosis, although this requires formal proof [133]. Interestingly, hyaluronic acid upregulates the expression of Nrf2 in chondrocytes via Akt phosphorylation [134]. Finally, ECM regulated H2O2 consumption in endothelial cells via regulation of glutathione peroxidase activity [135].
The above studies suggest that there is interplay between redox reactions and the ECM with each modulating the other. Controlling these events to prevent, inhibit or, at least, ameliorate fibrogenesis in the lung will require a better understanding of these interactions.
7. Redox stress and integrins
Cells interact with the ECM via cell surface receptors called integrins, a family of cell–cell and cell–matrix binding transmembrane receptors capable of signal transduction. Integrins are among the most abundant cell surface receptors and are expressed in all cell types [136]. In mammals, the integrin receptor family consists of 18 alpha (α) and 8 beta (β) subunits that link non-covalently in at least 24 known combinations of αβ subunit heterodimers [4], [137]. Upon binding, integrins cluster at the cell surface into focal adhesion complexes where they are joined by several adapter and signaling molecules, thereby establishing a reversible signaling structure. Downstream signals include tyrosine kinase activation, calcium influx, pH changes, and induction of transcription factors, among others, ultimately leading to differential gene expression (Fig. 3). These events control fundamental cellular processes such as adhesion, migration, and differentiation, in addition to more coordinated tissue behaviors needed for adequate morphogenesis and wound healing.
High concentrations of reactive oxygen species have been found located to focal adhesion complexes where integrins cluster after ligand binding in fibroblasts and other cells [138]; these regions are considered ‘redox hotspots’. These hotspots reverse soon after cell–matrix contacts are established suggesting that reactive oxygen species production and concomitant protein oxidation is regulated in an ECM adhesion-dependent manner [139].
The ligand-binding activity of integrins can be affected by redox reactions as reported for the integrins α4β1 and α4β7, and this appears due to free cysteine thiol groups present in both α and β subunits that are targeted by reactive oxygen species [140]. These events affect leukocyte diapedesis and similar mechanisms affect platelet aggregation via oxidation of redox-sensitive thiol residues present in the integrin αΙΙβ/β3 [141]. In adherent cells, inhibition of matrix contacts triggers reactive oxygen species production and this is likely due to a decrease in the uptake of nutrients and the decrease in pyruvate dehydrogenase kinases, thereby decreasing ATP generation [142].
While the α subunits of integrins contain few cysteine residues, β subunits are rich in such cysteine residues [143]. Thus, thiol-related redox reactions can impact integrin conformation, activation and, therefore, regulate important functions. These events are important as highlighted by experiments showing that redox-dependent cleavage of the β2 subunit is required for integrin activation [144]. Hydrogen peroxide induces modifications in several cysteines present in the α7 subunit of the integrin α7β1, a laminin receptor, resulting in conformational changes leading to integrin activation [145]. Thus, redox modifications of cysteine residues within integrin α and β subunits result in conformational changes capable of promoting signal transduction. These redox-related changes modulate signals transmitted by the ECM, thereby having important implications for the cell–matrix interactions and their roles in tissues.
8. Implications
The above observations indicate that redox reactions modulate ECM production, modification, and turnover as well as their recognition and control of cell functions through integrin activation. Equally interesting are observations suggesting that ECM-integrin binding can modulate redox reactions, thereby unveiling an interplay between these processes. It is evident that these mechanisms are necessary for maintaining cell and tissue homeostasis and, therefore, their alteration is likely to play significant roles in diseased states. In particular, uncontrolled redox reactions are likely relevant in the pathogenesis of tissue fibrosis. It is for this reason that investigators have attempted to intervene in these events in disorders including IPF. One such intervention relates to the delivery of anti-oxidants with the intention of restoring the oxidant-antioxidant balance, an intervention shown to be feasible in several studies. For example, intravenous administration of N-acetylcysteine in subjects with IPF increased GSH levels in alveolar lining fluid [146]. In this work, the authors found that, at baseline, the levels of total GSH in IPF subjects were higher than in controls. This highlights the problems inherent with the reliance on measuring total GSH levels alone for determining antioxidant capacity in lung. The effects of oral N-acetylcysteine on lung GSH levels in IPF has been tested and these studies revealed a significant increase in GSH in bronchoalveolar lavage fluid [147]. When testing the effects of GSH aerosol in 10 patients with IPF, investigators showed increased GSH levels in the epithelial cell lining fluid as well as a decrease in spontaneous superoxide anion release by alveolar macrophages [148]. More recently, investigators tested the therapeutic effects of N-acetylcysteine in a cohort of patients with IPF; that study revealed a beneficial effect, which prompted the almost widespread use of N-acetylcysteine, in combination with other drugs, in the treatment of IPF [149]. However, a large, randomized, placebo-controlled study was halted early when an interim analysis revealed that IPF patients receiving a combination of prednisone, azathioprine, and NAC had an increased rate of death and hospitalizations [150]. Another component of that study was to study the effectiveness of NAC alone against placebo, and that arm of the study was allowed to continue. That study revealed that NAC alone did not prove beneficial. More recently, genetic studies suggested that NAC could be helpful in a subset of patients carrying a single nucleotide polymorphism in the TOLLIP gene, but this has not been tested prospectively [151]. The kinetics of N-acetylcysteine and its relatively narrow effect may have thwarted its impact in that particular trial. Pirfenidone is an exciting new drug for the treatment of IPF [152]. It has been suggested that it acts as an antioxidant, but the evidence for this is not very strong. In vitro assays showed that pirfenidone could scavenge hydroxyl radicals but not superoxide or hydrogen peroxide [152], [153]. Independent of this, the data implicating redox reactions in the pathogenesis of IPF are so strong that few are willing to dismiss the possibility that more effective interventions capable of affecting these reactions via distinct mechanisms of action may emerge in the not-to-distant future [154], [155].
In the meantime, others have begun to evaluate the possible detection of oxidative modified proteins in the plasma of human subjects as potential biomarkers of redox reactions in lung disease. In this regard, oxidative modifications of protein tyrosyl residues were found to be increased in the plasma of subjects with interstitial lung disease [156]. Biomarkers of oxidative stress may ultimately have prognostic implications with regards to disease course or responsiveness to treatment, but considering that oxidative stress is common in chronic respiratory disorders, they will not likely play a diagnostic role.
It must be acknowledged that, although this document focuses on the lung fibroblast, other studies point to the importance of epithelial and endothelial cells, among others, in pulmonary fibrosing disorders [157]. ROS production and the involvement of Nox-1 and Nox-4 in mediating fibrogenic effects through epithelial and endothelial cell death have been reported [158], [159]. In fact, blockade of epithelial cell apoptosis induced by bleomycin in Nox-4 deficient mice is protective [159].
In short, ECM proteins are considered important players in processes responsible for lung development, injury and repair, and abnormalities in their expression, turnover, and recognition represent potential targets for intervention in chronic fibrosing lung disorders. Considering that redox reactions play roles in each of these events, investigations into the mechanisms responsible for such interactions are likely to unveil novel insights into disease pathogenesis. This research is already unveiling molecules and pathways that could be targeted to reduce the burden of pulmonary fibrosing disorders (Table 1).
Table 1.
Examples of potential targets for intervention based on phenotype observed in the bleomycin model.
| Intervention | Bleomycin-induced phenotype | Ref. |
|---|---|---|
| EC-SOD knockdown | Increased fibrosis, inflammation, and oxidative protein fragmentation | [161] |
| Nox-4 knockdown | Decreased fibrosis, reduced accumulation of myofibroblasts | [159] |
| P47phox knockdown | Absence of collagen deposition, increased inflammation | [93] |
| Nrf knockdown | Increased fibrosis | [25] |
| Smad3knockdown | Decreased fibrosis | [162] |
| FN EDA knockdown⁎ | Decreased fibrosis | [47] |
| β6 integrin knockdown | Decreased fibrosis, increased inflammation | [163] |
⁎FN: fibronectin.
Acknowledgments
The author's research is funded by the National Institutes of Health (R01 AA019953 and U01 HL121807, JR) and the Department of Veterans Affairs (5I01 BX000216-02, JR).
References
- 1.Larsen B.T., Colby T.V. Update for pathologists on idiopathic interstitial pneumonias. Arch. Pathol. Lab. Med. 2012;136:1234–1241. doi: 10.5858/arpa.2012-0225-RA. [DOI] [PubMed] [Google Scholar]
- 2.Raghu G., Striker L.J., Hudson L.D., Striker G.E. Extracellular matrix in normal and fibrotic human lungs. Am. Rev. Respir. Dis. 1985;131:281e289. doi: 10.1164/arrd.1985.131.2.281. [DOI] [PubMed] [Google Scholar]
- 3.Kuhn C., 3rd, Boldt J., King T.E., Jr, Crouch E., Vartio T., McDonald J.A. An immunohistochemical study of architectural remodeling and connective tissue synthesis in pulmonary fibrosis. Am. Rev. Respir. Dis. 1989;140:1693e1703. doi: 10.1164/ajrccm/140.6.1693. [DOI] [PubMed] [Google Scholar]
- 4.Hynes R.O. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 5.Barczyk M., Carracedo S., Gullberg D. Integrins. Cell Tissue Res. 2009;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu F., Mih J.D., Shea B.S., Kho A.T., Sharif A.S., Tager A.M., Tschumperlin D.J. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J. Cell Biol. 2010;190:693–706. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bachman H., Nicosia J., Dysart M., Barker T.H. Utilizing fibronectin integrin-binding specificity to control cellular responses. Adv. Wound Care. 2015;4:501–5011. doi: 10.1089/wound.2014.0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swinehart I.T., Badylak S.F. Extracellular matrix bioscaffolds in tissue remodeling and morphogenesis. Dev. Dyn. 2016;245:351–360. doi: 10.1002/dvdy.24379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Georges-Labouesse E.N., George E.L., Rayburn H., Hynes R.O. Mesodermal development in mouse embryos mutant for fibronectin. Dev. Dyn. 1996;207:147–156. doi: 10.1002/(SICI)1097-0177(199610)207:2<145::AID-AJA3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 10.Theocharis A.D., Skandalis S.S., Gialeli C., Karamanos N.K. Extracellular matrix structure. Adv. Drug. Deliv. Rev. 2016;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Booth A.J., Hadley R., Cornett A.M., Dreffs A.A., Matthes S.A., Tsui J.L., Weiss K., Horowitz J.C., Fiore V.F., Barker T.H., Moore B.B., Martinez F.J., Niklason L.E., White E.S. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am. J. Respir. Crit. Care Med. 2012;186:866e876. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osidak M.S., Osidak E.O., Akhmanova M.A., Domogatsky S.P., Domogatskaya A.S. Fibrillar, fibril-associated and basement membrane collagens of the arterial wall: architecture, elasticity and remodeling under stress. Curr. Pharm. Des. 2015;21:1124–1133. doi: 10.2174/1381612820666141013122906. [DOI] [PubMed] [Google Scholar]
- 13.Zheng Y., Ritzenthaler J.D., Roman J., Han S. Nicotine stimulates human lung cancer cell growth by inducing fibronectin expression. Am. J. Respir. Cell Mol. Biol. 2007;37:681–690. doi: 10.1165/rcmb.2007-0051OC. [DOI] [PubMed] [Google Scholar]
- 14.Roman J., Little C.W., McDonald J.A. Potential role of RGD-binding integrins in mammalian lung branching morphogenesis. Development. 1991;112:551–558. doi: 10.1242/dev.112.2.551. [DOI] [PubMed] [Google Scholar]
- 15.Muro A.F., Chauhan A.K., Gajovic S., Iacononcig A., Porro F., Stanta G., Baralle F.E. Regulated splicing of the fibronectin EDA exon is essential for proper skin wound healing and normal lifespan. J. Cell Biol. 2003;162:149–160. doi: 10.1083/jcb.200212079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeffery P.K. Remodeling in asthma and chronic obstructive lung disease. Am. J. Respir. Crit. Care Med. 2001;164:S28–S38. doi: 10.1164/ajrccm.164.supplement_2.2106061. [DOI] [PubMed] [Google Scholar]
- 17.Martin C., Papazian L., Payan M.J., Saux P., Gouin F. Pulmonary fibrosis correlates with outcome in adult respiratory distress syndrome. A study in mechanically ventilated patients. Chest. 1995;107:196–200. doi: 10.1378/chest.107.1.196. [DOI] [PubMed] [Google Scholar]
- 18.Xia H., Diebold D., Nho R., Perlman D., Kleidon J., Kahm J., Avdulov S., Peterson M., Nerva J., Bitterman P., Henke C. Pathological integrin signaling enhances proliferation of primary lung fibroblasts from patients with idiopathic pulmonary fibrosis. J. Exp. Med. 2008;205:1659e1672. doi: 10.1084/jem.20080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacNee I., Rahman I. Oxidants/antioxidants in idiopathic pulmonary fibrosis. Thorax. 1995;50:553–558. doi: 10.1136/thx.50.suppl_1.s53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beeh K.M., Beier J., Haas I.C., Kornmann O., Micke P., Buhl R. Glutathione deficiency of the lower respiratory tract in patients with idiopathic pulmonary fibrosis. Eur. Respir. J. 2002;19:1119–1123. doi: 10.1183/09031936.02.00262402. [DOI] [PubMed] [Google Scholar]
- 21.Rahman I., Skwarska E., Henry M., Davis M., O’Connor C.M., FitzGerald M.X., Greening A., MacNee W. Systemic and pulmonary oxidative stress in idiopathic pulmonary fibrosis. Free Radic. Biol. Med. 1999;27:60–68. doi: 10.1016/s0891-5849(99)00035-0. [DOI] [PubMed] [Google Scholar]
- 22.Montaldo C., Cannas E., Ledda M., Rosetti L., Congui L., Atzori L. Bronchoalveolar glutathione and nitrite/nitrate in idiopathic pulmonary fibrosis and sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2002;19:54–58. [PubMed] [Google Scholar]
- 23.Teramoto S., Fukuchi Y., Uejima Y., Shu C.Y., Orimo H. Superoxide anion formation and glutathione metabolism of blood in patients with idiopathic pulmonary fibrosis. Biochem. Mol. Med. 1995;55:66–70. doi: 10.1006/bmme.1995.1033. [DOI] [PubMed] [Google Scholar]
- 24.Ramirez A., Ramadan B., Ritzenthaler J.D., Rivera H.N., Jones D.P., Roman J. Extracellular cysteine/cystine redox potential controls lung fibroblast proliferation and matrix expression through upregulation of transforming growth factor-b. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;293:L972–L981. doi: 10.1152/ajplung.00010.2007. [DOI] [PubMed] [Google Scholar]
- 25.Kikuchi N., Ishii Y., Morishima Y., Yageta Y., Haraguchi N., Itoh K., Yamamoto M., Hizawa N. Nrf2 protects against pulmonary fibrosis by regulating the lung oxidant level and Th1/Th2 balance. Respir. Res. 2010;11:31. doi: 10.1186/1465-9921-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinnula V.L., Myllarniemi M. Oxidant-antioxidant balance as a potential contributor to the progression of human pulmonary fibrosis. Antioxid. Redox Signal. 2008;10:727–738. doi: 10.1089/ars.2007.1942. [DOI] [PubMed] [Google Scholar]
- 27.Martinez F.J., De Andrade J.A., Anstrom K.J., King T.E., Jr, Raghu G. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis (Idiopathic pulmonary fibrosis clinical research network) N. Engl. J. Med. 2014;370:2093–2101. doi: 10.1056/NEJMoa1401739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chuang C.Y., Degendorfer G., Davies M.J. Oxidation and modification of extracellular matrix and its role in disease. Free Radic. Res. 2014;48:970–989. doi: 10.3109/10715762.2014.920087. [DOI] [PubMed] [Google Scholar]
- 29.Kliment C.R., Oury T.D. Oxidative stress, extracellular matrix targets, and idiopathic pulmonary fibrosis. Free Radic. Biol. Med. 2010;49:707–717. doi: 10.1016/j.freeradbiomed.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 30.Eble J.A., Figueiredo de Rezende F. Redox-relevant aspects of the extracellular matrix and its cellular contacts via integrins. Antioxid. Redox Signal. 2014;20:1977–1993. doi: 10.1089/ars.2013.5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hansen N.U., Genovese F., Leeming D.J., Karsdal M.A. The importance of extracellular matrix for cell function and in vivo likeness. Exp. Mol. Pathol. 2015;98:286–294. doi: 10.1016/j.yexmp.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Hynes R.O. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manninem A. Epithelial polarity – generating and integrating signals from the ECM with integrins. Exp. Cell Res. 2015;334:337–349. doi: 10.1016/j.yexcr.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Couchman J.R. Immunohistochemical localization of chrondroitin sulfate, chondroitin sulfate proteoglycan, heparan sulfate proteoglycan, entactin, and laminin in basement membranes of postnatal and adult rat lungs. Am. J. Respir. Cell Mol. Biol. 1993;8:245–251. doi: 10.1165/ajrcmb/8.3.245. [DOI] [PubMed] [Google Scholar]
- 35.Gil J., Martinez-Hernandez A. The connective tissue of the rat lung: electron immunohistochemical studies. J. Histothem. Cytochem. 1984;32:230–238. doi: 10.1177/32.2.6363520. [DOI] [PubMed] [Google Scholar]
- 36.Stenman S., Vaheri A. Distribution of a major connective tissue protein, fibronectin, in normal human tissues. J. Exp. Med. 1978;147:1054–1064. doi: 10.1084/jem.147.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madri J.A., Furthmayr H. Isolation and tissue localization of type ABZ collagen from normal lung parenchyma. Am. J. Pathol. 1979;94:323–332. [PMC free article] [PubMed] [Google Scholar]
- 38.Heine U.I., Munoz E.F., Flanders K.C., Roberts A.B., Sporn M.B. Colocalization of TGF-β1 and collagen I and III, fibronectin, and glycosaminoglycans during lung branching morphogenesis. Development. 1990;109:29–36. doi: 10.1242/dev.109.1.29. [DOI] [PubMed] [Google Scholar]
- 39.Rickard K.A., Shoji S., Spurzem J.R., Rennard S.I. Attachment characteristics of bovine bronchial epithelial cells to extracellular matrix components. Am. J. Respir. Cell Mol. Biol. 1991;4:440–448. doi: 10.1165/ajrcmb/4.5.440. [DOI] [PubMed] [Google Scholar]
- 40.Rickard K.A., Taylor S.I., Rennard S.I., Spurzem J.R. Migration of bovine bronchial epithelial cells to extracellular matrix components. Am. J. Respir. Cell Mol. Biol. 1993;8:63–68. doi: 10.1165/ajrcmb/8.1.63. [DOI] [PubMed] [Google Scholar]
- 41.Sugihara H.S., Toda S., Miyabara S., Fujiyama C., Yonemitsu N. Reconstruction of alveolus-like structure from alveolar type II epithelial cells in three-dimensional collagen gel matrix culture. Am. J. Pathol. 1993;142:783–792. [PMC free article] [PubMed] [Google Scholar]
- 42.Shannon J.M., Emrie P.A., Fisher J.H., Kuroki Y., Jennings S.D., Mason R.J. Effect of a reconstituted basement membrane on expression of surfactant apoproteins in cultured adult rat alveolar type II cells. Am. J. Respir. Cell Mol. Biol. 1990;2:183–192. doi: 10.1165/ajrcmb/2.2.183. [DOI] [PubMed] [Google Scholar]
- 43.Shannon J.M., Jennings S.D., Nielson L.D. Modulation of alveolar type II cell differentiation function in vitro. Am. J. Physiol. Lung Cell. Mol. Physiol. 1992;262:L427–L436. doi: 10.1152/ajplung.1992.262.4.L427. [DOI] [PubMed] [Google Scholar]
- 44.Koval M., Ward C., Findley M.K., Roser-Page S., Helms M.N., Roman J. Extracellular matrix influences alveolar epithelial claudin expression and barrier function. Am. J. Respir. Cell Mol. Biol. 2010;42:172–180. doi: 10.1165/rcmb.2008-0270OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.King T.E., Jr, Pardo A., Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949–1961. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 46.Parker M.W., Rossi D., Peterson M., Smith K., Sikstrom K., White E.S., Connett J.E., Henke C.A., Larsson O., Bitterman P.B. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J. Clin. Investig. 2014;124:1622–1635. doi: 10.1172/JCI71386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muro A.F., Moretti F.A., Moore B.B., Yan M., Atrasz R.G., Wilke C.A., Flagerty K.R., Martinez F.J., Tsui J.L., Sheppard D., Baralle F.E., Toews G.B., White E.S. An essential role for fibronectin extra type III domain a in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2008;177:638e645. doi: 10.1164/rccm.200708-1291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roman J., Ritzenthaler J.D., Fenton M.W., Roser S., Schyler W. Transcriptional regulation of the human interleukin-1β gene by fibronectin: role of protein kinase C and activator protein-1 (AP-1) Cytokine. 2000;12:1581–1596. doi: 10.1006/cyto.2000.0759. [DOI] [PubMed] [Google Scholar]
- 49.Neill T., Schaefer L., Iozzo R.V. Instructive roles of extracellular matrix on autophagy. Am. J. Pathol. 2014;184:2146–2153. doi: 10.1016/j.ajpath.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tschumperlin D.J. Matrix, mesenchyme and mechanotransduction. Ann. Am. Thorac. Soc. 2015;12(Suppl 1):S24–S29. doi: 10.1513/AnnalsATS.201407-320MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beckman K.B., Ames B.N. The free radical theory of aging matures. Physiol. Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 52.Morrow J.D. The isoprostanes – unique products of arachidonate peroxidation: their role as mediators of oxidant stress. Curr. Pharm. Des. 2006;12:895–902. doi: 10.2174/138161206776055985. [DOI] [PubMed] [Google Scholar]
- 53.Feeney M.B., Schoneich C. Tyrosine modifications in aging. Antioxid. Redox Signal. 2012;17:1571–1579. doi: 10.1089/ars.2012.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kadiiska M.B., Gladen B.C., Barid D.D., Germolec D., Graham L.B., Parker C.E. Biomarkers of oxidative stress study II: are oxidation of products of lipids, proteins, and DNA markers of CCI4 poisoning? Free Radic. Biol. Med. 2005;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 55.Vetter S.W. Glkycated serum albumin and AGE receptors. Adv. Clin. Chem. 2015;72:205–275. doi: 10.1016/bs.acc.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 56.Heinecke J.W. Tyrosyl radical production by myeoloperoxidase: a phagocyte pathway for lipid peroxidation and dityrosine cross-linking of proteins. Toxicology. 2002;177:11–22. doi: 10.1016/s0300-483x(02)00192-0. [DOI] [PubMed] [Google Scholar]
- 57.Bauer J., Ripperger A., Frantz S., Ergun S., Schwedhelm E., Benndorf R.A. Pathophysiology of isoprostanes in the cardiovascular system: implications of isoprostane-mediated thromboxane A2 receptor activation. Br. J. Pharmacol. 2014;171:3115–3131. doi: 10.1111/bph.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ott C., Jacobs K., Haucke E., Navarrete Santos A., Grune T., Simm A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;2:411–429. doi: 10.1016/j.redox.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giulivi C., Traaseth N.J., Davies K.J. Tyrosine oxidation products: analysis and biological relevance. Amino Acids. 2003;25:227–232. doi: 10.1007/s00726-003-0013-0. [DOI] [PubMed] [Google Scholar]
- 60.Sundarresan M., Yu Z.X., Ferrans V.J., Irani K., Finkel T. Requirement for generation of H202 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 61.Lee Z.W., Kweon S.M., Kim S.J., Kim J.H., Cheong C., Park Y.M. The essential role of H202 in the regulation of intracellular Ca2+ by epidermal growth factor in rat-2 fibroblasts. Cell. Signal. 2000;12:91–98. doi: 10.1016/s0898-6568(99)00069-8. [DOI] [PubMed] [Google Scholar]
- 62.El-Benna J., Dang P.M., Gougerot-Pocidalo M.A., Marie J.C., Braut-Boucher F. p47phox, the phagocyte NADPH oxidase/Nox2 organizer: structure, phosphorylation and implication in diseases. Exp. Mol. Med. 2009;41:217–225. doi: 10.3858/emm.2009.41.4.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grandvaux N., Mariani M., Fink K. Lung epithelial NOX/DUOX and respiratory virus infections. Clin. Sci. 2015;128:337–347. doi: 10.1042/CS20140321. [DOI] [PubMed] [Google Scholar]
- 64.Schafer F.Q., Buettner G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 65.Jones D.P., Carlson J.L., Mody V.C., Cai J., Lynn M.J., Sternberg P. Redox state of glutathione in human plasma. Free Radic. Biol. Med. 2000;28:625–635. doi: 10.1016/s0891-5849(99)00275-0. [DOI] [PubMed] [Google Scholar]
- 66.Jones D.P. Redefining oxidant stress. Antioxid. Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 67.Jones D.P., Park Y., Gletsu-Miller N., Liang Y., Yu T., Accardi C.J, Ziegler T.R. Dietary sulfur amino acid effects on fasting plasma cysteine/cystine redox potential in humans. Nutrition. 2011;27:199–205. doi: 10.1016/j.nut.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nkabyo Y.S., Go Y.M., Ziegler R.T., Jones D.P. Extracellular cysteine/cystine redox regulates the p44/p42 MAPK pathway by metalloproteinase-dependent epidermal growth factor receptor signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;289:G70–G78. doi: 10.1152/ajpgi.00280.2004. [DOI] [PubMed] [Google Scholar]
- 69.Jonas C.R., Ziegler T.R., Gu L.H., Jones D.P. Extracellular thiol/disulfide redox state affects proliferation rate in a human colon carcinoma (Caco2) cell line. Free Radic. Biol. Med. 2002;33:1499–1506. doi: 10.1016/s0891-5849(02)01081-x. [DOI] [PubMed] [Google Scholar]
- 70.Go Y.M., Jones D.P. Intracellular proatherogenic events and cell adhesion modulated by extracellular thiol/disulfide redox state. Circulation. 2005;111:2973–2980. doi: 10.1161/CIRCULATIONAHA.104.515155. [DOI] [PubMed] [Google Scholar]
- 71.Mannery Y.O., Ziegler T.R., Hao L., Shyntum Y., Jones D.P. Characterization of apical and basal thiol-disulfide redox regulation in human colonic epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G523–G530. doi: 10.1152/ajpgi.00359.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anderson C.L., Iyer S.S., Ziegler T.R., Jones D.P. Control of extracellular cysteine/cystine redox state by HT-29 cells is independent of cellular glutathione. Am. J. Physiol. – Regul. Integr. Comp. Physiol. 2007;293:R1069–R1075. doi: 10.1152/ajpregu.00195.2007. [DOI] [PubMed] [Google Scholar]
- 73.Strausz J., Muller-Quernheim J., Steppling H., Ferlinz R. Oxygen radical production by alveolar inflammatory cells in idiopathic pulmonary fibrosis. Am. Rev. Respir. Dis. 1990;141:124–128. doi: 10.1164/ajrccm/141.1.124. [DOI] [PubMed] [Google Scholar]
- 74.Bocchino M., Agneses S., Fagone E., Svegliati S., Grieco D., Vancheri C., Gabrielli A., Sanduzzi A., Avedimento E.V. Reactive oxygen species are required for maintenance and differentiation of primary lung fibroblasts in idiopathic pulmonary fibrosis. PLoS One. 2010;5:e14003. doi: 10.1371/journal.pone.0014003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fernandez I.E., Eickelberg O. The impact of TGF-b on lung fibrosis: from targeting to biomarkers. Proc. Am. Thorac. Soc. 2012;9:111–116. doi: 10.1513/pats.201203-023AW. [DOI] [PubMed] [Google Scholar]
- 76.Blom I.E., Goldschmeding R., Leask A. Gene regulation of connective tissue growth factor: new targets for antifibrotic therapy? Matrix Biol. 2002;21:473–482. doi: 10.1016/s0945-053x(02)00055-0. [DOI] [PubMed] [Google Scholar]
- 77.van der Kraan P.M., Blaney Davidson E.N., van den Berg W.B. A role for age-related changes in TGFbeta signaling in aberrant chondrocyte differentiation and osteoarthritis. Arthritis Res. Ther. 2010;12:201. doi: 10.1186/ar2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Travis M.A., Sheppard D. TGF-b activation and function in immunity. Annu. Rev. Immunol. 2014;32:51–82. doi: 10.1146/annurev-immunol-032713-120257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cucoranu I., Clempus R., Dikalova A., Phelan P.J., Ariyan S. NAD(P)H oxidase 4 mediates transforming growth factor beta 1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ. Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 80.Rhyu D.Y., Yang Y., Ha H., Lee G.T., Song J.S. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J. Am. Soc. Nephrol. 2005;16:667–675. doi: 10.1681/ASN.2004050425. [DOI] [PubMed] [Google Scholar]
- 81.Rhyu D.Y., Park J., Sharma H., Ha H. Role of reactive oxygen species in transforming growth factor beta1 induced extracellular matrix accumulation in renal tubular epithelial cells. Transplant. Proc. 2012;44:625–628. doi: 10.1016/j.transproceed.2011.12.054. [DOI] [PubMed] [Google Scholar]
- 82.Chan E.C., Peshavariya H.M., Liu G.S., Jiang F., Lim S.Y., Dusting G.J. Nox4 modulates collagen production stimulated by transforming growth factor beta1 in vivo and in vitro. Biochem. Biophys. Res. Commun. 2013;430:918–925. doi: 10.1016/j.bbrc.2012.11.138. [DOI] [PubMed] [Google Scholar]
- 83.Hecker L., Vittral R., Jones T., Jagirdar R., Luckhardt T.R. NAD(P)H oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat. Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Amara N., Govern D., Prost F., Muloway R., Crestani B., Boczkowski J. NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFb1-induced fibroblast differentiation into myofibroblasts. Thorax. 2010;65:733–738. doi: 10.1136/thx.2009.113456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bondi C.D., Manickam N., Lee D.Y., Block K., Gorin Y. NAD(P)H oxidase mediates TGFb1 induced activation of kidney myofibroblasts. J. Am. Soc. Nephrol. 2010;21:93–102. doi: 10.1681/ASN.2009020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sancho P., Mainez J., Crosas-Molist E., Roncero C., Fernandez-Rodriguez C.M. NADPH oxidase Nox4 mediates stellate cell activation and hepatocyte cell death during liver fibrosis development. PLoS One. 2012;7:e45285. doi: 10.1371/journal.pone.0045285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sturrock A., Cahill B., Norman K., Huecksteadt T.P., Hill K. Transforming growth factor beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am. J Physiol. Lung Cell. Mol. Physiol. 2006;290:L661–L673. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- 88.Michaeloudes C., Sukkar M.B., Khorasani N.M., Bhavsar P.K., Chung K.F. TGFβ regulates NOx4, MnSOD, and catalase expression, and IL-6 release in airway smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011;300:L295–L304. doi: 10.1152/ajplung.00134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boudreau H.E., Casterline B.W., Rada B., Korzeniowska A., Leto T.L. Nox4 involvement in TGF-beta and SMAD3-driven induction of the epithelial-to-mesenchymal transition and migration of breast epithelial cells. Free Radic. Biol. Med. 2012;53:1489–1499. doi: 10.1016/j.freeradbiomed.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carmona-Cuenca I., Roncero C., Sancho P., Caja I., Fausto N. Upregulation of the NADPH oxidase Nox4 by TGF-beta in hepatocytes is required for its pro-apoptotic activity. J. Hepatol. 2008;49:965–976. doi: 10.1016/j.jhep.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 91.Martin-Garrido A., Brown D.I., Lyle A.N., Dikalova A., Seidel-Rogol B. NADPH oxidase 4 mediates TGF-beta-induced smooth muscle alpha-actin via p38MAPK and serum response factor. Free Radic. Biol. Med. 2011;50:354–362. doi: 10.1016/j.freeradbiomed.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carnesecchi S., Deffert C., Donati Y., Basset O., Hinz B. A key role for NOX4 in epithelial cell death during development of lung fibrosis. Antioxid. Redox Signal. 2011;15:607–619. doi: 10.1089/ars.2010.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Manoury B., Nenan S., Leclerc O., Guenon I., Boichot E., Planquois J.M., Bertrand C.P., Lagente V. The absence of reactive oxygen species production protects mice against bleomycin-induced pulmonary fibrosis. Respir. Res. 2005;6:11. doi: 10.1186/1465-9921-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Richter K., Konzack A., Pihlajaniemi T., Heljasvaara R., Kietzmann T. Redox-fibrosis: Impact of TGFb1 on ROS generators, mediators, and functional consequences. Redox Biol. 2015;6:344–352. doi: 10.1016/j.redox.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Islam K.N., Kayamoki Y., Kaneto H., Suzuki K., Asah M., Fujii J., Taniguchi N. TGFb1 triggers oxidative modifications and enhances apoptosis in HIT cells through accumulation of reactive oxygen species by suppression of catalase and glutathione peroxidase. Free Radic. Biol. Med. 1997;22:1007–1017. doi: 10.1016/s0891-5849(96)00493-5. [DOI] [PubMed] [Google Scholar]
- 96.Kayanoki Y., Fujii J., Suzuki K., Kawata S., Matsuzawa Y., Taniguchi N. Suppression of antioxidative enzyme expression by transforming growth factor-beta in rat hepatocytes. J. Biol. Chem. 1994;269:15488–15492. [PubMed] [Google Scholar]
- 97.Michaeloudes C., Sukkar M.B., Khorasani N.M., Bhavsar P.K., Chung K.F. TGF-b regulates Nox4, MnSOD and catalase expression, and iL-6 release in airway smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011;300:L295–L304. doi: 10.1152/ajplung.00134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jiang F., Liu G.S., Dustin G.J., Chan E.C. NADPH oxidase-dependent redox signaling in TGFbeta mediated fibrotic responses. Redox Biol. 2014;2:267–272. doi: 10.1016/j.redox.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu R.M., Desai L.P. Reciprocal regulation of TGFbeta and reactive oxygen species: a perverse cycle for fibrosis. Redox Biol. 2015;6:565–577. doi: 10.1016/j.redox.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Abate C., Patel L., Rauscher F.J., 3rd, Curran T. Redox regulation of fos and jun DNA binding activity in vitro. Science. 1990;249:1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- 101.Chung K.Y., Agarwal A., Uitto J., Mauviel A. An AP-1 binding sequence is essential for regulation of the human alpha2(I) collagen (COL1A2) promoter activity by transforming growth factor-beta. J. Biol. Chem. 1996;271:3272–3278. doi: 10.1074/jbc.271.6.3272. [DOI] [PubMed] [Google Scholar]
- 102.Ignotz R.A., Endo T., Massague J. Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-beta. J. Biol. Chem. 1987;262:6443–6446. [PubMed] [Google Scholar]
- 103.Dasgupta J., Kar S., Liu R., Joseph J., Kalyanaraman B., Remington S.J., Chen C., Melendez J.A. Reactive oxygen species control senescence-associated matrix metalloproteinase-1 through c-Jun-N-terminal kinase. J. Cell. Physiol. 2010;225:52–62. doi: 10.1002/jcp.22193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Klotz L.O., Sanchez-Ramos C., Prieto-Arroyo I., Urbanek P., Steinbrenner H., Monsalve M. Redox regulation of FoxO transcription factors. Redox Biol. 2015;6:51–72. doi: 10.1016/j.redox.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Espinosa-Diez C., Miguel V., Mennerich D., Kietzmann T., Sanchez-Perez P., Cadenas S., Lamas S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015;6:183–197. doi: 10.1016/j.redox.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang I.V. Epigenomics of idiopathic pulmonary fibrosis. Epigenomics. 2012;4:195–203. doi: 10.2217/epi.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mastruzzo C., Crimi N., Vancheri C. Role of oxidative stress in pulmonary fibrosis. Monaldi Arch. Chest Dis. 2002;57:173–176. [PubMed] [Google Scholar]
- 108.Iyer S.S., Ramirez A.M., Ritzenthaler J.D., Torres-Gonzalez E., Roser-Page S., Mora A.L., Brigham K.L., Jones D.P., Roman J., Rojas M. Oxidation of extracellular cysteine/cystine redox state in bleomycin-induced lung fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;296:L37–L45. doi: 10.1152/ajplung.90401.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Iyer S.S., Accardi C.J., Ziegler T.R., Blanco R.A., Ritzenthaler J.D., Rojas M., Roman J., Jones D.P. Cysteine redox potential determines pro-inflammatory IL-1b levels. PLoS One. 2009;4:e5017. doi: 10.1371/journal.pone.0005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Houghton A.M. Matrix metalloproteinases in destructive lung disease. Matrix Biol. 2015;44–46:167–174. doi: 10.1016/j.matbio.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 111.Dancer R.C., Wood A.M., Thickett D.R. Metalloproteinases in idiopathic pulmonary fibrosis. Eur. Respir. J. 2011;38:1461–1467. doi: 10.1183/09031936.00024711. [DOI] [PubMed] [Google Scholar]
- 112.Arpino V., Brock M., Gill S.E. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015;44–66:247–254. doi: 10.1016/j.matbio.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 113.Kennett E.C., Chuang C.Y., Degendorfer G., Whitelock J.M., Davies M.J. Mechanisms and consequences of oxidative damage to extracellular matrix. Anal. Free Radic. Radic. Modif. Redox Signal. 2011;39:1279–1287. doi: 10.1042/BST0391279. [DOI] [PubMed] [Google Scholar]
- 114.Van der Veen B.S., de Winther M.P., Heeringa P. Myeloperoxidase: molecular mechanisms of action and their relevance to human health and disease. Antioxid. Redox Signal. 2009;11:2899–2937. doi: 10.1089/ars.2009.2538. [DOI] [PubMed] [Google Scholar]
- 115.Davies M.J., Hawkins C.L., Pattison D.I., Rees M.D. Mammalian heme peroxidases: from molecular mechanisms to health implications. Antioxid. Redox Signal. 2008;10:1199–1234. doi: 10.1089/ars.2007.1927. [DOI] [PubMed] [Google Scholar]
- 116.Davies M.J. Myeloperoxidase-derived oxidation: mechanisms of biological damage and its prevention. J. Clin. Biochem. Nutr. 2011;48:8–19. doi: 10.3164/jcbn.11-006FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thomas E.L., Grisham M.B., Jefferson M.M. Cytotoxicity of chloramines. Methods Enzymol. 1986;132:585–593. doi: 10.1016/s0076-6879(86)32043-3. [DOI] [PubMed] [Google Scholar]
- 118.Davies M.J., Gilbert B.C. Free radical reactions: fragmentation and rearrangements in aqueous solution. Adv. Detail. React. Mech. 1991;1:35–81. [Google Scholar]
- 119.Rees M.D., Kennett E.C., Whitelock J.M., Davies M.J. Oxidative damage to extracellular matrix and its role in human pathologies. Free Radic. Biol. Med. 2008;44:1973–2001. doi: 10.1016/j.freeradbiomed.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 120.Kennett E.C., Rees M.D., Malle E., Hammer A., Whitelock J.M., Davies M.J. Peroxynitrite modifies the structure and function of the extracellular matrix proteoglycan perlecan by reaction with both the protein core and the heparin sulfate chains. Free Radic. Biol. Med. 2010;49:282–293. doi: 10.1016/j.freeradbiomed.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Davies M.J. The oxidative environment and protein damage. Biochim. Biophys. Acta. 2005;1703:93–109. doi: 10.1016/j.bbapap.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 122.Hawkins C.L., Davies M.J. Generation and propagation of radical reactions on proteins. Biochim. Biophys. Acta. 2001;1504:196–219. doi: 10.1016/s0005-2728(00)00252-8. [DOI] [PubMed] [Google Scholar]
- 123.Whitelock J.M., Graham L.D., Melrose J., Murdoch A.D., Iozzo R., Underwood P.A. Human perlecan immunopurified from different endothelial cell sources has different adhesive properties for vascular cells. Matrix Biol. 1999;18:163–178. doi: 10.1016/s0945-053x(99)00014-1. [DOI] [PubMed] [Google Scholar]
- 124.Lord M.S., Chuang C.Y., Melrose J., Davies M.J., Iozzo R.V., Whitelock J.M. The role of vascular-derived perlecan in modulating cell adhesion, proliferation and growth factor signaling. Matrix Biol. 2014;35:112–122. doi: 10.1016/j.matbio.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Barth D., Kyrieleis O., Franck S., Renner C., Moroder C. The role of cystine knots in collagen folding and stability, Part II. conformational properties of (Pro-Hyp-Gly)n model trimers with N- and C- terminal collagen Type III cystine knots. Chemistry. 2003;9:3703–3714. doi: 10.1002/chem.200304918. [DOI] [PubMed] [Google Scholar]
- 126.Wilkes D.S., Chew T., Flagerty K.R., Frye S., Gibson K.F., Kaminski N., Klemsz M.J., Lange W., Noth I., Rothhaar K. Oral immunotherapy with type V collagen in idiopathic pulmonary fibrosis. Eur. Respir. J. 2015;45:1393–1402. doi: 10.1183/09031936.00105314. [DOI] [PubMed] [Google Scholar]
- 127.Kliment C.R., Tobolewski J.M., Manni M.L., Tan R.J., Enghild J., Oury T.D. Extracellular superoxide dismutase protects against matrix degradation of heparin sulfate in the lung. Antioxid. Redox Signal. 2008;10:261–268. doi: 10.1089/ars.2007.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gao F., Koenitzer J.R., Tobolewski J.M., Jiang D., Liang D., Ling J., Nobel P.W., Oury T.D. Extracellular superoxide dismutase inhibits inflammation by preventing oxidative fragmentation of hyaluronan. J. Biol. Chem. 2008;282:6058–6066. doi: 10.1074/jbc.M709273200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.van den Akker J., VanBavel E., van Geel R., Matlung H.L., Guvenic Tuna B., Janssen G.M.C., van Veelen P.A., Boelens W.C., De Mey J.G.R., Bakker E.N.T.P. The redox state of transglutaminase 2 controls arterial remodeling. PLoS One. 2011;6:e23067. doi: 10.1371/journal.pone.0023067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tyagi S.C., Kumar S., Borders S. Reduction-oxidation (redox) state regulation of extracellular matrix metalloproteinases and tissue inhibitors in cardiac normal and transformed fibroblast cells. J. Cell. Biochem. 1996;61:139–151. doi: 10.1002/(sici)1097-4644(19960401)61:1<139::aid-jcb15>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 131.Nakajima T., Hasegawa G., Kamiuchi K., Fukui M., Yamasaki M., Tominaga M., Asano M., Hosoda H., yoshikawa T., Nakamura N. Differential regulation of intracellular redox state by extracellular matrix proteins in glomerular mesangial cells: potential role in diabetic nephropathy. Redox Rep. 2006;11:223–230. doi: 10.1179/135100006X116736. [DOI] [PubMed] [Google Scholar]
- 132.Demirovic D., Rattan S.I. Curcurim induces stress response and hormetically modulates wound healing ability of human skin fibroblasts undergoing ageing in vitro. Biogerontology. 2011;12:437–444. doi: 10.1007/s10522-011-9326-7. [DOI] [PubMed] [Google Scholar]
- 133.Walters D.M., Cho H.Y., Kleeberger S.R. Oxidative stress and oxidants in the pathogenesis of pulmonary fibrosis: a potential role for Nrf2. Antioxid. Redox Signal. 2008;10:321–332. doi: 10.1089/ars.2007.1901. [DOI] [PubMed] [Google Scholar]
- 134.Onodera Y., Teramura T., Takehara T., Fukuda K. Hyaluronic acid regulates a key redox control factor Nrf2 via phosphorylation of Akt in bovine articular chondrocytes. FEBS Open Bio. 2015;5:476–484. doi: 10.1016/j.fob.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bagulho A., Vilas-Boas F., Pena A., Peneda C., Santos F.C., Jeronimo A., de Almeida R.F., Real C. The extracellular matrix modulates H202 degradation and redox signaling in endothelial cells. Redox Biol. 2015;6:454–460. doi: 10.1016/j.redox.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.De Franceschi N., Hamidi H., Alankol J., Sahgal P., Ivaska J. Integrin traffic – the update. J. Cell. Sci. 2015;128:839–852. doi: 10.1242/jcs.161653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bazan-Socha S., Bukiej A., Marcinkiewicz C., Musial J. Integrins in pulmonary inflammatory diseases. Curr. Pharm. Des. 2005;11:893–901. doi: 10.2174/1381612053381710. [DOI] [PubMed] [Google Scholar]
- 138.Ushio-Fukai M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid. Redox Signal. 2009;11:1289–1299. doi: 10.1089/ars.2008.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lin L.-J., Grimee J.M., Sun J., Lu S., Gai L., Cropek D.M., Wang Y. The antagonistic roles of PDGF and integrin avb3 in regulating ROS production at focal adhesions. Biomaterials. 2013;34:3807–3815. doi: 10.1016/j.biomaterials.2013.01.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chuang K.-P., Tsai W.-S., Wang Y.-J., Shieh C.-C. Superoxide activates very late antigen-4 on an eosinophil cell line and increases cellular binding to vascular cell adhesion molecule-1. Eur. J. Immunol. 2003;33:645–655. doi: 10.1002/eji.200323446. [DOI] [PubMed] [Google Scholar]
- 141.Mou Y., Ni H., Wilkins J.A. The selective inhibition of b1 and b7 integrin-mediated lymphocyte adhesion by bacitracin. J. Immunol. 1998;161:6323–6329. [PubMed] [Google Scholar]
- 142.Grassian A.R., Coloff J.L., Brugge J.S. Extracellular matrix regulation of metabolism and implications for tumorigenesis. Cold Spring Harb. Symp. Quant. Biol. 2011;76:313–324. doi: 10.1101/sqb.2011.76.010967. [DOI] [PubMed] [Google Scholar]
- 143.Eble J., Figueiredo de Rezende F. Redox-relevant aspects of the extracellular matrix and its cellular contacts via integrins. Antioxid. Redox Signal. 2014;20:1977–1993. doi: 10.1089/ars.2013.5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sun Q.-H., Liu C.-Y., Wang R., Paddock C., Newman P.J. Disruption of the long-range GPIIIa Cys5-Cys435 disulfide bond results in the production of constitutively active GPIIb/GPIIIa (aIIbB3) integrin complexes. Blood. 2002;100:2094–2101. doi: 10.1182/blood-2002-02-0418. [DOI] [PubMed] [Google Scholar]
- 145.de Rezende F.F., Matins Lima A., Niland S., Wittig I., Heide H., Schroder K., Eble J.A. Integrin a7b1 is a redox-regulated target of hydrogen peroxide in vascular smooth muscle cell adhesion. Free Radic. Biol. Med. 2012;53:521–531. doi: 10.1016/j.freeradbiomed.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 146.Meyer A., Buhl R., Kampf S., Magnussen H. Intravenous N-acetylcysteine and lung glutathione of patients with pulmonary fibrosis and normal lung. Am. J. Respir. Crit. Care Med. 1995;152:1055–1060. doi: 10.1164/ajrccm.152.3.7663783. [DOI] [PubMed] [Google Scholar]
- 147.Meyer A., Buhl R., Magnussen H. The effect of oral N-acetylcysteine on lung glutathione levels in idiopathic pulmonary fibrosis. Eur. Respir. J. 1994;7:431–436. doi: 10.1183/09031936.94.07030431. [DOI] [PubMed] [Google Scholar]
- 148.Borok Z., Buhl R., Grimes G.J., Bokser A.D., Hubbard R.C., Holroyd K.J., Roum J.H., Czerski D.B., Cantin A.M., Crystal R.G. Effect of glutathione aerosol on oxidant-antioxidant imbalance in idiopathic pulmonary fibrosis. Lancet. 1991;338:215–216. doi: 10.1016/0140-6736(91)90350-x. [DOI] [PubMed] [Google Scholar]
- 149.Demedts M., Behr J., Buhl R., Costabel U., Dekhuijzen R., Jansen H.M., MacNee W., Thomeer M., Wallaert B., Laurent F., Nicholson A.G., Verbeken E.K., Verschakelen J., Flower C.D., Capron F., Petruzzelli S., De Vuyst P., van den Bosch J.M., Rodriguez-Becerra E., Corvasce G., Lankhorst I., Sardina M., Montanari M., IFIGENIA Study Group High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl. J Med. 2005;353:2229–2242. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 150.Raghu G., Anstrom K.J., King T.E., Jr, Lasky J.A., Martinez F.J. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N. Engl. J. Med. 2012;366:1968–1977. doi: 10.1056/NEJMoa1113354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Oldham J.M., Ma S.F., Martinez F.J., Anstrom K.J., Raghu G., Schwartz D.A., Valenzi E., Witt L., Lee D.C., Vij R., Huang Y., Strek M.E., Noth I., IPFnet investigators TOLLIP, MUC5B, and the response to N-acetylcysteine among individuals with idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2015;192:1472–1482. doi: 10.1164/rccm.201505-1010OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Myllarniemi M., Kaarteenaho R. Pharmacological treatment of idiopathic pulmonary fibrosis – preclinical and clinical studies of pirfenidone, nintedanib, and N-acetylcysteine. Eur. Clin. Respir. J. 2015;2 doi: 10.3402/ecrj.v2.26385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Giri S.N., Leonard S., Shi X., Margolin S.B., Vallyathan V. Effects of pirfenidone on the generation of reactive oxygen species in vitro. J. Environ. Pathol. Toxicol. Oncol. 1999;18:169–177. [PubMed] [Google Scholar]
- 154.Misra H.P., Rabideau C. Pirfenidone inhibits NADPH-dependent microsomal lipid peroxidation and scavenges hydroxyl radicals. Mol. Cell. Biochem. 2000;204:19–126. doi: 10.1023/a:1007023532508. [DOI] [PubMed] [Google Scholar]
- 155.Jarman E.R., Khambata V.S., Cope C., Jones P., Roger J., Ye L.Y., Duggan N., Head D., Pearce A., Press N.J., Bellenie B., Sohal B., Jaral G. An inhibitor of NADPH oxidase-4 attenuates established pulmonary fibrosis in a rodent disease model. Am. J. Respir. Cell Mol. Biol. 2014;50:158–169. doi: 10.1165/rcmb.2013-0174OC. [DOI] [PubMed] [Google Scholar]
- 156.Pennathur S., Vivekanandan-Giri A., Locy M.L., Kulkarni T., Zhi D., Zeng L., Byun J., de Andrade Joao A., Thannickal V.J. Oxidative modifications of protein tyrosyl residues are increased in plasma of human subjects with interstitial lung disease. Am. J. Respir. Crit. Care Med. 2015 doi: 10.1164/rccm.201505-0992OC. Nov 17. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gabrielli A., Svegliati S., Moroncini G., Amico D. New insights into the role of oxidative stress in scleroderma fibrosis. Open Rheumatol. J. 2012;6:87–95. doi: 10.2174/1874312901206010087. Epub 2012 Jun 15. PMID: 22802906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Carnesecchi S., Deffert C., Pagano A., Garrido-Urbani S., Metrailler-Ruchonnet I., Schappi M., Donati Y., Matthay M.A., Krause K.H., Barazzone Argiroffo C. NADPH oxidase-1 plays a crucial role in hyperoxia-induced acute lung injury in mice. Am. J. Respir. Crit. Care Med. 2009;180:972–981. doi: 10.1164/rccm.200902-0296OC. [PubMed: 19661248] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Carnesecchi S., Deffert C., Donati Y., Basset O., Hinz B., Preynat-Seauve O., Guichard C., Arbiser J.L., Banfi B., Pache J.C., Barazzone-Argiroffo C., Krause K.H. A key role for NOX4 in epithelial cell death during development of lung fibrosis. Antioxid. Redox Signal. 2011;15:607–619. doi: 10.1089/ars.2010.3829. [PubMed: 21391892] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Land S.C., Wilson S.M. Redox regulation of lung development and perinatal lung epithelial function. Antioxid. Redox Signal. 2005;7:92–107. doi: 10.1089/ars.2005.7.92. [DOI] [PubMed] [Google Scholar]
- 161.Fattman C.L., Chang L.Y., Termin T.A., Petersen L., Enghild J.J., Oury T.D. Enhanced bleomycin-induced pulmonary damage in mice lacking extracellular superoxide dismutase. Free Radic. Biol. Med. 2003;35:763–771. doi: 10.1016/s0891-5849(03)00402-7. [DOI] [PubMed] [Google Scholar]
- 162.Zhao Jingsong. Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. Am. Physiol.– Lung Cell. Mol. Physiol. 2002;28(3):L585–L593. doi: 10.1152/ajplung.00151.2001. [DOI] [PubMed] [Google Scholar]
- 163.Munger J.S., Huang X., Kawakatsu H., Griffiths M.J., Dalton S.L., Wu J., Pittet J.F., Kaminski N., Garat C., Matthay M.A. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]