Abstract
Curcumin, a yellow pigment and principal polyphenolic Curcuminoid obtained from the turmeric rhizome Curcuma longa, is commonly used as a food-coloring agent. Studies suggest that curcumin has a wide range of beneficial properties e.g., anti-inflammatory, anti-oxidant, anti-cancer, anti-proliferative, anti-fungal and anti-microbial. These pleiotropic activities prompted several research groups to elucidate the role of curcumin in Helicobacter pylori (H. pylori) infection. This is the first review with this heading where we discussed regarding the role of curcumin as an anti-H. pylori agent along with its potential in other gastrointestinal diseases. Based on several in vitro, early cell culture, animal research and few pre-clinical trials, curcumin projected as a potential therapeutic candidate against H. pylori mediated gastric pathogenesis. This review sheds light on the anti-H. pylori effects of curcumin in different models with meticulous emphasis on its anti-oxidant, anti-inflammatory and anti-carcinogenic effects as well as some critical signaling and effecter molecules. Remarkably, non-toxic molecule curcumin fulfills the characteristics for an ideal chemopreventive agent against H. pylori mediated gastric carcinogenesis but the foremost challenge is to obtain the optimum therapeutic levels of curcumin, due to its low solubility and poor bioavailability. Further, we have discussed about the possibilities for improving its efficacy and bioavailability. Lastly, we concluded with the anticipation that in near future curcumin may be used to develop a therapeutic drug against H. pylori mediated gastric ailments through improved formulation or delivery systems, facilitating its enhanced absorption and cellular uptake.
Keywords: Curcumin, Helicobacter pylori, Duodenal ulcer, Gastric cancer, Anti-oxidant, Anti-inflammatory, Nuclear factor-κB
Core tip: Curcumin, a yellow polyphenolic pigment, used as a food-coloring agent has wide range of beneficial properties (e.g., anti-inflammatory, anti-oxidant, anti-cancer, anti-proliferative, anti-fungal and anti-microbial). Several research groups have elucidated the role of curcumin in Helicobacter pylori (H. pylori) infection. This is the first review where we have discussed the role of curcumin as an anti-H. pylori agent along with its potential in other gastrointestinal diseases. Based on several in vitro, early cell culture, animal research and few pre-clinical trials, curcumin projected as a potential therapeutic candidate against H. pylori mediated gastric pathogenesis with a challenge to improve its low solubility and poor bioavailability.
INTRODUCTION
Helicobacter pylori (H. pylori) is a Gram negative, microaerophilic, helical rod that colonizes in human gastric mucous layer. Although 50% of world population carries H. pylori, most infections are asymptomatic and 10%-15% of the H. pylori infected individuals develop chronic inflammation leading to atrophic gastritis, peptic ulcer as well as gastric adenocarcinoma[1]. Although infection occurs worldwide, there are significant variations in the prevalence of infection both within and between countries. In general, the overall prevalence of H. pylori infection in developed countries is lower than that in developing countries due to improved medical care, personal hygiene, sanitation or living conditions[2-4]. H. pylori infection is very common in India, where millions of adults are at risk for developing gastric inflammation, ulcers and carcinoma. World health organization has classified H. pylori as a group I carcinogen with significant risk of gastric cancer[5]. So, in case of infection, eradication of H. pylori is the most effective treatment for H. pylori-associated diseases. Eradication of H. pylori infection by treatment with “Triple therapy” (TT), includes two anti-microbial agents (clarithromycin and amoxicillin or metronidazole) and a proton pump inhibitor, is recommended by several groups[6]. However, such multiple therapy regimens have not been very successful in clinical practice due to their misuse and side effects.
Increasing difficulties in the conventional triple-therapy due to antimicrobial resistance, incomplete cure, undesirable side effects, noncompliance among the patients, cost of the antibiotic regimens, and few other factors promote a critical need to develop new non-antibiotic antibacterial agents against H. pylori-infection that are highly effective, safe, have specific cellular targets and of course low cost[7,8]. Moreover, several studies using extracts of traditional medicinal plants from several parts of the world have reviewed the in vitro susceptibility of H. pylori demonstrating the prospect of finding a cure from natural sources[9].
Plant derived compounds have shown their potential as therapeutic and chemopreventive agents for many chronic illness such as cardiovascular, neurodegenerative and neoplastic diseases. Dietary components present in spices, nuts, vegetables and fruits have demonstrated significant potential to suppress carcinogenesis in in vitro, pre-clinical and clinical cancer models[10]. Polyphenols with effective anti-oxidant and anti-inflammatory properties can modulate important signaling molecules are of enormous pharmacological interest. Catechins in green tea, curcumin in turmeric, lycopene in tomatoes, resveratrol in grapes and red wine, quercetin in apple and onions to name a few have showed considerable anti-carcinogenesis potential in several organs (e.g., skin, liver, breast, lung, and prostate)[10]. Several reports have suggested curcumin’s anti-H. pylori effect. In this review, we have discussed the potential role of curcumin as an anti-H. pylori molecule both in vitro and in vivo along with its future perspective in view of pharmacological importance.
CURCUMIN: THE INDIAN SOLID GOLD DERIVED FROM TURMERIC PLANT
Curcumin is a key polyphenolic yellow pigment found in turmeric root. Turmeric is a rhizomatous plant (Curcuma longa Linn), a perennial member of Zingiberaceae family mostly grown in India and Southeast Asia[11]. Turmeric is commonly used as a spices/food coloring agent in India. Except its use in skin care product and as a dye in textile industry, turmeric have a wide variety of use in Ayurvedic medicine for centuries[12]. As a traditional medicine, turmeric has also been broadly used to treat a diversity of disorders including rheumatism, sprains, runny nose, skin diseases, wounds, diarrhea, dyspepsia, intermittent fevers, hepatic disorders, intestinal worms, biliousness, urinary discharges, Blood Sugar, inflammation, constipation, leukoderma, amenorrhoea and colic inflammation[13].
Alcoholic extracts of turmeric mainly contain three curcuminoids viz. curcumin (curcumin I or diferuloylmethane), curcumin II (desmethoxycurcumin) and bisdesmethoxycurcumin (curcumin III) (Figure 1). Although C. longa is the main source of curumin, it has also been reported from C. aromatica, C. phaecaulis, C. zedoaria, C. xanthorrhiza, C. mangga amongst more than 120 Curcuma plants identified so far[14]. Commercially available purified compound is also a mixture of curcumin I (approximately 77%), curcumin II (approximately 18%) and curcumin III (approximately 5%).
Figure 1.
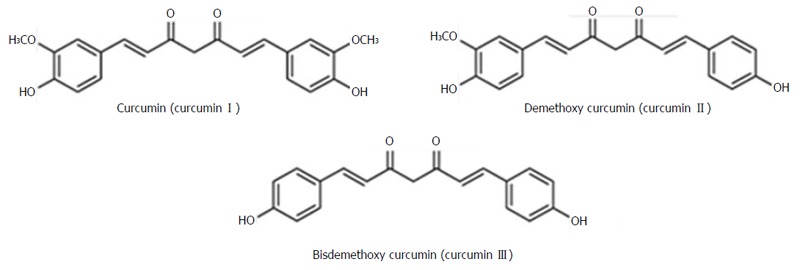
Structure of curcuminoids: Curcumin I, II and III. Adapted from Park et al[75]. New perspectives of curcumin in cancer prevention.
Curcumin (diferuloylmethane), a poly-phenolic molecule, exists as a keto-enol tautomer with the enol isomer most likely the more stable in both solid and liquid state[15]. It is intensely yellow at acidic pH and red at basic pH. This lipophilic and sparingly water soluble molecule have two aromatic rings connected by two unsaturated carbonyl groups. The molecule is stabilized by hydrogen bond with the central hydroxyl group. This is thought to be an important functional site responsible for the property of free radical scavenger and the array of other biological activity[10,16]. It was a topic of long discussion whether the amount of curcumin in turmeric or curry powder is sufficient to show its biological activity. A study by Tayyem et al[17], estimated the amount of curcumin in a variety turmeric and curry powder by high performance liquid chromatography technique. They have found that pure turmeric contents the highest concentration of curcumin (3.14% w/w) and different commercial curry powder samples have relatively very small amount of curcumin[17].
Curcumin has been the subject of hundreds of published paper in more than last three decades which includes it’s anti-oxidant, anti-inflammatory, anti-carcinogenic and anti-microbial properties[18]. Knowing these properties of curcumin is the pre-requisite to better understand its anti-H. pylori effect. Moreover chemotherapeutic[19], chemo-preventive[19], hepato-protective, nephro-protective, thrombosis suppressing, hypoglycemic and anti-rheumatic effects of curcumin are also well established[18].
Anti-oxidant property
Besides its important nature of donating hydrogen ions, curcuminoids also have a number of moieties (phenolic hydroxyl group, methoxy group and 1,3 β-diketone) with a potential to neutralize reactive oxygen intermediates. Curcuminoids can undergo nucleophilic addition and imparts its anti-oxidant property by a various, complex mechanism. These free radical scavenging and anti-oxidant activity counteract with reactive oxygen and nitrogen species and thereby protects cells from oxidative damage. In alkaline pH, curcumin is less stable but its stability markedly increases in acidic pH. These explain why curcumin is stable within the gastrointestinal tract (pH 1 to 6)[15,20].
Oxidative stress and oxidative damage play important role in pathogenesis of many diseases, e.g., cardiovascular diseases, diabetes, neurodegenerative diseases and cancer etc. Curcuminoids have been shown to have the potential to scavenge free radical and attenuate free radical mediated lipid peroxidation in a number of experimental systems[21]. Besides its direct anti-oxidant property, curcumin may function indirectly as anti-oxidant by inhibiting the activity of inflammatory enzymes.
Anti-inflammatory property
Curcumin is known for its remarkable anti-inflammatory effect. Its anti-inflammatory property is comparable with the recognized steroidal and non-steroidal anti-inflammatory drugs, e.g., Indomethacin and Phenylbutazone which have dangerous side effects. For example, curcumin considerably inhibits carrageenan-induced paw edema in mice, acute lung injury of rats by cyclophosphamide and other inflammatory diseases (arthritis, ulcerative colitis or pancreatitis) in different animal models[12,22]. It’s anti-inflammatory mechanism is attributed through inhibition of inducible nitric oxide synthase (iNOS), Cycloxygenase-2 (COX-2), inhibition in production of pro-inflammatory cytokines [IL-1, IL-6, IL-8, IL-12, interferon γ (IFN-γ), tumor necrosis factor-α (TNF-α) etc.], monocyte chemoattractant protein (MCP), migration inhibitory protein (MIP) and down regulation of mitogen activated and Janus kinases[11]. Menon and Sudheer[23] have nicely reviewed the anti-inflammatory property of curcumin. Several reports have indicated that curcumin not only inhibits prostaglandins it also inhibits lipoxigenase (LOX) activity. Inhibition of pro-inflammatory cytokines, COX-2 and iNOS are mainly mediated via suppression of transcription factor nuclear factor κB (NF-κB) and activating protein (AP-1). Down regulation of intercellular signaling proteins (e.g., protein kinase C) is another way in which curcumin inhibits production of pro-inflammatory cytokines[11]. This molecular mechanism finally inhibits cell proliferation, cell invasion and apoptosis causing suppression of carcinogenesis.
Anti-carcinogenic property
Curcumin is well known for its anti-carcinogenesis activity and plays important role in all stages of cancer. Inflammation and carcinogenesis are broadly interlinked. Inhibition of I-κB kinase is required to inhibit potent inflammatory transcription factor NF-κB which is also involved in progression of carcinogenesis. Curcumin has the potential to decrease matrix metalo-protease activity which is thought to be a prognostic marker of neoplasm[24]. In in vitro studies, where bioavailability is not an issue curcumin plays its role like a master manipulator. It inhibits important transcription factors and thereby decreases production of pro-inflammatory cytokines which leads to reduced inflammation and cell survival. Curcumin also effectively prevented its anti-cancer activity in animal models of oral, esophageal, stomach, duodenal and colon cancer[13]. Plenty of articles are available for anti-cancer activity of curcumin. in vitro, pre-clinical and clinical studies on anti-carcinogenic property of curcumin was thoroughly reviewed by Sharma et al[19].
Anti-microbial property
Curcumin can suppress the growth of a variety of parasite, bacteria and pathogenic fungi. It shows anti-microbial effect against Helicobacter pylori, Bacillus subtilis, Plasmodium falciparum etc[25]. In a study with chick infected with caecal parasite Eimera maxima showed that 1% dietary turmeric resulted in a decrease intestinal lesions and improved weight gain. Topically applied curcumin demonstrated its potential anti-microbial effect against either dermatophytes, pathogenic molds, or yeast in guinea pigs. Curcumin also possesses a pro-microbial effect against Leishmania and some species of Plasmodium[26].
WHY CURCUMIN SHOULD HAVE ANTI H. PYLORI EFFECT?
H. pylori upon adhering to the gastric epithelial cells, they inject their virulence factors by Type-IV secretion system. Those virulence factors then activate different signaling molecules to initiate a pro-inflammatory response. H. pylori induced generation of pro-oxidants and pro-inflammatory cytokines then causes apoptotic cell death which leads to gastric mucosal damage. In some cases this inflammation can also stimulate carcinogenesis which resulted into gastric carcinoma.
In search for a plant derived anti-H. pylori molecule one should obviously search for a compound which have anti-oxidant, anti-inflammatory, anti-carcinogenic and pro-apoptotic properties. The Indian solid gold “Curcumin” have all these properties and that’s why it is a fairly studied compound in H. pylori infection in last two decades. Literature suggests that curcumin is a highly pleiotropic molecule and able to interact with numerous molecular targets associated with inflammation[11]. Curcumin was found to have an astonishing power in anti-inflammatory response. The natural anti-inflammatory activity of curcumin is at par with steroidal drugs and non-steroidal drugs (e.g., indomethacin and phenylbutazone) which have harmful side effects. Its anti-inflammatory property mediated through the inhibition of COX-2, LOX, iNOS and production of cytokines such as IFN-γ and TNF-α and activation of transcription factors like NF-κB, and AP-1. Therefore, agents that interfere signaling pathways involved COX-2 transcription should also reduce inflammation and subsequently tumorigenesis. Further study suggests that arachidonic acid metabolites derived from LOX pathways plays a significant role in signal transduction related to growth. This implies that intervention of these pathways should be useful for arresting cancer progression. Curcumin also showed strong anti-oxidant and anti-cancer properties through regulating the expression of genes that require the activation of activator protein (AP1) and NF-κB. Suppression of TNF by curcumin led to inhibition of NF-κB and cell proliferation, as was the case when TNF secretion was neutralized using anti-TNF antibody[23]. As inflammation is closely linked to tumor promotion, curcumin with its potent anti-inflammatory property is anticipated to exert chemopreventive effects on carcinogenesis. Anti-inflammatory mechanisms implicated in the anti-carcinogenic potential of curcumin includes: (1) inhibition of NF-κB and COX-2 (increased COX-2 level is associated with many types of cancer); (2) inhibition of arachidonic acid metabolism via lipoxygenase and scavenging of free radicals generated in this pathway; (3) decreased expression of inflammatory cytokines e.g., IL-1β, IL-6, and TNF-α, resulting in inhibition cancer cells growth; and (4) down regulation of enzymes for instance protein kinase C that mediate inflammation and tumor-cell proliferation[11].
The anti-inflammatory property of curcumin hinders carcinogenesis by inhibiting it’s all three stages e.g., initiation, promotion and progression, as documented by animal research. During initiation and promotion curcumin shows many of its activities. It starts modulating transcription factors controlling phaseI and II detoxification of carcinogens; scavenges free radicals and thereby free radical mediated transcription factors, down regulates pro-inflammatory cytokines and arachidonic metabolism (via COX and LOX pathways). At promotion and progression stages curcumin induces apoptosis via suppression of NF-κB and AP-1 transcription factors and thereby decreases the frequency and size of many types of tumors[11].
Irving et al[15] adequately described the signaling pathways which are modulated by curcumin to initiate apoptosis. Some properties of curcumin that helps to recognize it’s anti-H. pylori effect are pro-apoptotic, prevention of angiogenesis, proliferation and metastasis. Caspase activation or p53 signalling ultimately induces apoptosis. Curcumin a potent stimulator of caspase-3, can also increase the activation of caspase-7, 8 and releases cytochrome-C[27]. It increases p21 expression in response to DNA damage and induces p53 driven apoptosis[28,29]. Curcumin has direct anti-angiogenic activity in vitro and in vivo[30] by inhibiting growth factors, growth factor receptors or by modification of inflammatory mediators associated with neovascularisation. Curcumin suppresses cell proliferation in colorectal cancer by inhibiting TNF-induced NF-κB-dependent gene products (COX-2, cyclin- D, c-myc)[31]. It also inhibits NF-κB along with other important proteins e.g., matrix metalloproteinase 9 (MMP-9), vascular endothelial growth factor (VEGF) and intra-cellular adhesion molecule 1 (ICAM-1) which are associated with proliferation, adhesion and metastasis[15].
Several studies have demonstrated that H. pylori-infection induces the secretion of MMPs from a range of gastric cells in vivo as well as in cultured cells, which in turn contribute to the pathogenesis of gastric ulcer and gastric cancer. Docking analysis has confirmed that curcumin derivatives have the potential to interact with MMPs[32]. So, it is assumed that curcumin should have immense beneficial effect in H. pylori induced gastric pathogenesis.
CURCUMIN IN GASTROINTESTINAL DISEASES
Due to easy administration through oral route or relatively fair bioavailablity, curcumin has widely been studied in different gastrointestinal aliments. Its anti-inflammatory property was studied from microbial gastric infection, inflammatory disease and several gastrointestinal cancers[11,33]. Curcumin has been proved to be effective in many gastrointestinal diseases, e.g., irritable bowel syndrome (IBS), dyspepsia, gastric ulcer, pancreatitis[34], ulcerative colitis and gastrointestinal cancers.
It is established that low grade inflammation is responsible for symptoms, e.g., bloating, abdominal pain, flatulence, irregular bowl movement in IBS. In a pilot study with IBS patients, standard curcumin extract reduced the symptoms in 60% patients[11]. Prucksunand et al[35] in a phase II clinical trial have evaluated the effectiveness or healing property of turmeric (Curcuma longa) in patients with dyspepsia and peptic ulcer. Patients who have no detectable ulceration but have symptoms e.g., erosions, gastritis, and dyspepsia were treated with turmeric (600 mg curcumin five times daily before meals). Curcumin treatment up to 2 wk improves the symptoms. Patients with endoscopically diagnosed ulceration were treated up to 12 wk and a gradual improvement was observed depending upon the severity in almost 76% cases[35].
Curcumin is also found to be effective in decreasing inflammatory mediators in ethanol induced or non-ethanol (cerulein) induced Rat pancreatitis model. In 1,4,6-trinitrobenzene sulphonic acid (TNBS) induced mice colitis model, curcumin was found to decrease mucosal injury. Pre-treatment of curcumin in mice before TNBS induced colitis resulted in to a significant inhibition of diarrhea, better colonic architecture, significantly reduced neutrophil infiltration and lipid peroxidation in colonic tissue[34].
Inflammation in directly linked with tumorigenesis. Curcumin causes inhibition of NF-κB pathway and thereby reduces inflammation. Other molecules which thought to be responsible in gastric tumorigenesis are rho effectors rhotekin (RTKN) and NF-κB are also inhibited by curcumin. p21-activated kinase 1 (PAK1) and different cell cycle regulators which can cause rapid proliferation are also found to be reduced with curcumin treatment in different gastric carcinoma cell line[33]. Curcumin and its analogues were well studied with Intestinal cancers both in animal models as well as in cell lines. Treatment of HCT-116 colon tumor-bearing mice with curcumin resulted in reduced tumor[36]. In case of pancreatic cancer, curcumin was found to be a good alternate anti-tumor agent alone or in combination with other drugs. Treatment with polymeric nanoparticle-encapsulated curcumin blocked tumor growth and metastasis in xenograft models of pancreatic cancer and liposomal curcumin diminish the tumor growth in cultured pancreatic cancer cells as well as subcutaneous xenografts[33]. A number of in vitro and in vivo studies with different cancers whether it is gastrointestinal or other system indicates curcumin can modulate multiple cellular signalling molecules and thereby proved it’s immense therapeutic value[37].
CURCUMIN AND ANTI-H. PYLORI EFFECT
From long before it is anticipated that curcumin which is a potent anti-inflammatory and anti-carcinogenic molecule should have anti-H. pylori effect (as the reasons discussed above). First report on direct anti-H. pylori effect came in the year of 2002[38]. Subsequently, ex vivo, in vivo and even clinical trials have been completed to establish curcumin as a potential therapeutic candidate against H. pylori infections (Table 1 and Figure 2)[7,8,38-47].
Table 1.
Anti-Helicobacter pylori effect of curcumin
| Year of report | Model of the study | Concentration/doses used | Observations and conclusion | Ref. |
| 1997 | In vitro co-culture | 20 μmol/L | Demonstrates H. pylori mediated inflammation or IL-8 secretion requires NF-κB activation. Anti-oxidant curcumin can inhibit NF-κB activation and thereby IL-8 secretion. NF-κB activation requires a secreted H. pylori product, which is not secreted by strains mutated in picB/cagE (putative transport protein) | [39] |
| 2002 | In vitro | 0.78-100 μg/mL | Methanol extract of turmeric rhizome and curcumin, both have the MIC within 6.25-50 μg/mL and more effective against cag (+ve) strains | [38] |
| 2004 | In vitro co-culture | Up to 80 μmol/L | H. pylori-induced NF-κB activation and subsequent IL-8 induction and mitogenic response (cell scattering) are inhibited by curcumin. It can inhibit IκBα degradation, activity of IκB kinases (IKKα and β) and NF-κB DNA-binding but cannot suppress mitogen-activated protein kinases (P38MAPK or ERK1/2) | [40] |
| 2006 | In vitro | 16 μg/mL | Curcumin inhibited H. pylori shikimate dehydrogenase with an IC50 values of 15.4 μmol/L. Curcumin also found to inhibit the growth of H. pylori with MIC at 16 μg/mL | [41] |
| 2007 | Pre-clinical trial with 25 H. pylori infected patients | Curcumin 30 mg b.i.d. for 7 d | Found to be not effective in eradicating H. pylori infection. However, despite the presence of bacterium, a significant improvement of dyspeptic symptoms and reduction of serologic signs of gastric inflammation were observed after 2 mo completion of treatment | [42] |
| 2009 | In vitro and in vivo mice model | 5-50 μg/mL and 25 mg/kg BW (once daily for 7 d) respectively | Potential In vitro anti-H. pylori effect of curcumin re-established, which was irrespective of the genetic makeup of the strains used. Although due to less bioavailability, curcumin’s minimal inhibitory concentration In vitro is high (most strains are within 10-30 μg/mL range), but still showed its efficiency in eradicating H. pylori from infected mice along with restoration of H. pylori induced gastric damage. This study also showed that curcumin-mediated inhibition of H. pylori growth possibly not associated on shikimate pathway | [7] |
| 2009 | In vitro co-culture | 5 and 10 μmol/L | Non-bactericidal concentration of curcumin failed to inhibit adhesion of H. pylori to epithelial cells but down-regulated H. pylori-induced activation induced cytidine deaminase (AICD, an enzyme which may cause tumorigenesis) expression possibly via inhibition of NF-κB pathway | [43] |
| 2010 | Pre-clinical trial with 36 H. pylori infected patients | 700 mg orally three times a day for 4 wk | H. pylori eradication rate with curcumin (5.9%) was very poor against conventional triple therapy (78.9%). Triple therapy significantly decreases IL-8 expression but no inhibition was observed in curcumin group | [44] |
| 2010 | In vivo rat model | 200 or 600 mg/kg BW | Curcumin supplementation exhibits its anti-inflammatory effect by decreasing macromolecular leakage through the suppression of NF-κB p65 expression in gastric epithelium. Curcumin reduces the H. pylori induced gastric inflammation in rat model | [45] |
| 2011 | In vitro co-culture and in vivo mice model | 60 μmol/L and 25 mg/kg or 50 mg/kg BW (once daily for 7 d) respectively | Elevated levels of MMP-3 and -9 in cultured cells or gastric tissues of mice due to H. pylori infections are inhibited by curcumin. Curcumin is more efficient in re-stabilizing the distorted balance between MMPs and TIMPs than triple therapy, suggests curcumin’s immense therapeutic potential | [8] |
| 2014 | Bioinformatics tools/software based analysis | Mucoadhesive microspheres of curcumin | Purpose was to develop and characterize mucoadhesive microspheres of curcumin for treatment of H. pylori mediated gastric aliments. Drug release was found to be slower than free curcumin. Prolonged stomach residence of this molecule is expected to completely eradicate H. pylori infection with other anti-microbials | [46] |
| 2015 | In vivo mice model | 500 mg/kg three times a week, for 6 and 18 wk | Curcumin treatment exhibited a significant anti-inflammatory role in H. pylori-infected gastric mucosa. Inflammatory mediators (cytokines, chemokines, toll-like receptors and MyD88) which are up regulated with H. pylori infection, has been decreased with curcumin. No sign of inflammation was observed in curcumin treated group | [47] |
MMPs: Matrix metalloproteinases; H. pylori: Helicobacter pylori; NF-κB: Nuclear factor-κB; BW: Body weight.
Figure 2.
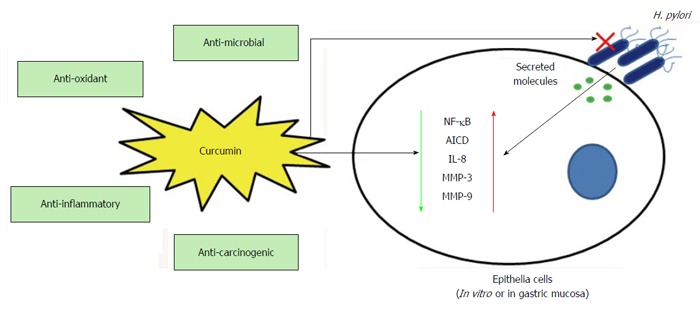
Role of curcumin in Helicobacter pylori infection. Important properties of curcumin which probably helps host cells in H. pylori infection are anti-oxidant, anti-inflammatory and anti-carcinogenic. Other than its direct anti-H. pylori effect (anti-microbial) curcumin inhibits H. pylori induced NF-κB, AICD, IL-8, MMP-3 and MMP-9 in host epithelial cells and thereby protects from inflammatory response (may sometime leads to host cell apoptosis). MMPs: Matrix metalloproteinases; IL: Interleukin; H. pylori: Helicobacter pylori; NF-κB: Nuclear factor-κB; AICD: Activation induced cytidine deaminase.
In vitro studies
Considering the strong association of H. pylori and gastric cancer the authors of the study by Mahady et al[38] hypothesized that curcumin may exerts its chemopreventive activity by directly inhibiting H. pylori. They have showed that both curcumin and methanolic extract of turmeric rhizome inhibited the growth of 19 different strains of H. pylori (including cag positive isolates). MIC50 and MIC90 of the turmeric rhizome extract were 12.5 μg/mL and 25 μg/mL respectively where as curcumin at a concentration of 12.5 μg/mL inhibited 100% growth of all strains with a MIC range from 6.25-12.5 μg/mL. Later, a study by our group with a large number of clinical isolates (n = 65) was also found to be in agreement with regard to MIC result. We have reported that MIC of curcumin ranged from 5 μg/mL to 50 μg/mL and the majority of the strains (81%) showed a MIC of either 10 μg/mL (23%) or 15 μg/mL (58%)[7]. To understand the reason of this wide range in sensitivity, we have examined sequence uniformity of aroE gene encoding sikimate dehydrogenase (SDH) of H. pylori which is involved in shikimate pathway known to play crucial role in developing non-toxic antimicrobial agents. Although curcumin is a potent inhibitor of SDH, we were unable to establish any correlation in between aroE sequence and MIC of curcumin towards H. pylori. These results clearly confirmed that curcumin acts as a potent growth inhibitor for Indian H. pylori strains irrespective of different genetic makeup and disease status[7].
In vitro co-culture and in vivo studies
The mouse model of H. pylori infection has been extensively used in investigations of host responses to H. pylori infection as well as in eradication studies. In our study, we have shown that curcumin treatment completely eradicated H. pylori from infected mouse stomach. This eradication by curcumin was irrespective of the bacterial genotype, which is independent of the presence of the cag PAI. Histological analysis clearly showed that curcumin is remarkably effective in repairing damaged tissue. Our microscopical observations revealed substantial damage in the mouse gastric tissues infected with H. pylori in comparison to the control. Intact epithelial layer and glandular cells with continuous gastric pits in control gastric mucosa have damaged by denudation of the surface epithelial layer in H. pylori an infected mouse gastric tissue which was almost restored to normal upon treatment with curcumin. Inflammation in the gastric pit cells of infected tissue was significantly reduced by curcumin treatment. Disruption of submucosal, muscularis mucosal layers and Glandular atrophy of infected mouse gastric tissues was also considerably recovered by curcumin treatment. Negligible inflammatory cells in the control tissue infiltered more in the submucosal region of H. pylori-infected mouse gastric tissues and was prevented to a significant degree in curcumin-treated mice[7]. Hence, these results pointed towards that high efficacy of curcumin in healing the overall damage caused by H. pylori infection. These observations not only indicate the therapeutic potential of curcumin against H. pylori infections but also highlight the anti-inflammatory effect of curcumin and also draw our attention to its potential as an alternative therapy against H. pylori infection[7].
Effect on MMPs
MMPs are a family of various zinc dependant endopeptidases that have wide substrate specificity and play a critical role in a variety of physiological processes including tissue inflammatory processes, remodeling, wound repair and organ development. Among them, gelatinases (MMP-2 and MMP-9) and stromelysin-1 (MMP-3) collectively cleave gelatins (types I and V), collagens (type IV, V, VII, IX and X), elastin, fibronectin, laminin and proteoglycan core proteins[8]. Apart from curcumin’s antioxidant, anti-inflammatory, anti-microbial and anti-carcinogenic properties, it has been shown to target a number of molecules like cytokines, growth factors, transcription factors and enzymes including MMPs, that are involved in the etiology of diverse diseases[32]. Several studies have demonstrated that H. pylori-infection induces the secretion of MMPs from a variety of gastric cells in vivo as well as in cultured cells, which in turn contributes to the pathogenesis of gastric ulcer and gastric cancer[8].
Since MMP-3 and -9 play a critical role in gastric ECM degradation during H. pylori induced pathogenesis, and are associated with various carcinomas including gastric cancer, attenuation of their increased secretion and synthesis is crucial for restoration of the gastric damage caused by H. pylori infection. In our recent study, we have tested the effect of curcumin on the activity of MMP-3 and -9 during protection against H. pylori infection in cultured cells and mice. We have also compared the efficiency of curcumin and conventional TT in stabilizing the balance between MMPs and its inhibitor[8]. It was the first report where we have confirmed that curcumin dose dependently suppressed the increased secretion of MMP-3 and MMP-9 in H. pylori infection. The more interesting part was that secreted MMP-3 and -9 were inhibited 90% by curcumin while 50% by TT and antibiotic alone. We demonstrate that curcumin, apart from eradication of H. pylori strains from infected mice, regulated the expressions and activities of MMP-3 and -9 in the gastric tissues. Furthermore, curcumin was highly effective in restoring the denudation of epithelial region, disruption in gastric mucosal layer and infiltration of inflammatory cells that occurred due to H. pylori infection in mouse gastric tissues. Curcumin is more effective than TT in restabilizing the altered balance between MMPs and TIMPs during protection against H. pylori-infection. This curcumin mediated down regulation of MMP-3 and -9 levels in H. pylori-infected mice and cultured cells suggest its immense therapeutic potential against H. pylori associated gastrointestinal diseases.
Effect on NF-κB signaling and inflammation
NF-κB plays an essential role in regulating human immune response. It is normally present as a cytosolic-inactive form, and upon stimulation by a huge varity of pathogenic agents (bacterial, viral or others), inflammatory cytokines, UV or γ-irradiation, NF-κB is activated. Those stimulants help phosphorylation and ubiquitination of the inhibitory subunit IκB, which leads to the release of NF-κB dimer. Released NF-κB dimer then rapidly translocates into the nucleus, where it activates transcription of target genes e.g., IL-1, IL-6, IL-8, TNF-α as well as other cytokines, IFNs, cell adhesion molecules and hemopoietic growth factors[39]. NF-κB is known to be inhibited by many anti-oxidants[48] and therefore, curcumin which have a strong anti-oxidant property can be considered as an inhibitor of this nuclear factor in H. pylori infection.
Münzenmaier et al[39] first reported the activation of NF-κB by H. pylori infection in gastric epithelial cells. They have demonstrated that ability to produce IL-8 by a variety of H. pylori strains correlates with the activation of NF-κB. IL-8 production requires NF-κB activation, and prevention by the anti-oxidant curcumin leads to suppression of cytokine production. Unlike many Gram-negative bacteria H. pylori does not use LPS to activate NF-κB, instead they use a secreted bacterial product[39].
Later, Foryst-Ludwig et al[40] described how curcumin blocks NF-κB and thus reduces gastric inflammatory response. Curcumin, a known inhibitor of AP-1, blocks the synthesis of H. pylori induced IL-8 production and inhibited the H. pylori-induced cell scattering in the gastric epithelial cells. Cucumin have the ability to reduce v-Src activity which might prevent Phosphorylation of cytotoxicity associated immunodominant antigen (CagA)[40]. The phenomenon of curcumin’s effect on inhibition of NF-κB and alleviating inflammatory response was also found to be effective in rat model of H. pylori infection[45]. Authors in that study have also described that supplementation of curcumin helped in suppression of macromolecular leakage. In a very recent study with mouse model also corroborate that curcumin treatment exerted a significant anti-inflammatory response in gastric mucosa. In treatment group, no sign of inflammation and decreased expression of different inflammatory mediators were demonstrated by histology[47]. H. pylori induced activation of signaling molecules e.g., mitogen-activated protein kinases (MAPK), extracellular signal-regulated kinases (ERK1/2) and p38 are not inhibited by curcumin[26] but inhibition potential of NF-κB and cell scattering justifies therapeutic properties of curcumin in H. pylori-associated stomach ailments[40].
Effect on activation induced cytidine deaminase
Activation induced cytidine deaminase (AICD) is an enzyme encoded by aicd gene in human which creates mutations in DNA by deamination of cytosine base by turning it into uracil. This also creates immune diversity by inducing somatic hypermutations and class-switch recombinations in human immunoglobulin genes. By somatic mutation in several host genome of nonlymphoid tissues like TP53 tumor suppressor gene it can cause tumorigenesis[43]. An unusual expression of AICD in H. pylori infected gastric epithelial cells is induced by NF-κB[49]. NF-κB-regulated gene products e.g., AICD, have found to be been closely associated with H. pylori-induced gastric carcinogenesis. Therefore, agents that inhibits NF-κB pathway might have potential in preventing H. pylori-related tumorogenesis[43]. Study by Zaidi et al[43] tested the effect of curcumin on the expression of AICD in H. pylori-infected gastric epithelial cells and the role of NF-κB. They have demonstrated, low concentration of curcumin down regulates H. pylori induced AICD expression possibly by inhibiting NF-κB pathway in MKN-45 cells. Consequently, this study also suggested that curcumin can serve as potentially alternative treatment option.
TREATMENT OUTCOME IN PATIENTS WITH H. PYLORI INFECTION
A number of in vitro (direct and with cell line infection) and in vivo (mouse and rat) studies (as described above) have encouraged some investigators to examine further in a group of patients with H. pylori induced gastric aliments. Di Mario et al[42] have carried out first human trial of curcumin based therapy in a group of 25 patients with functional dyspepsia and H. pylori infection. Patients were treated with curcumin, other anti-oxidants and proton pump inhibitor for 7 d. Two months after treatment completion, it was found that only 3 out of 25 patients were cured from H. pylori infection. In rest of the cases, despite the persistence of H. pylori, a significant improvement of dyspeptic symptoms and decrease in serological parameters of gastric inflammation were demonstrated. In another study, 36 clinically confirmed H. pylori infected chronic gastritis patients were randomly assigned in two groups to receive either OAM (Omeprazole, Amoxicillin and Metronidazole) or course of curcumin[44]. In that study, patients who were randomly assigned OAM group were significantly more efficient in eradication of H. pylori than curcumin (78.9% vs 5.9%). A significant decrease in IL-8 mRNA was observed in OAM group but no changes in other cytokines were found. On the other hand no decrease in cytokine production was found with curcumin treatment[44]. From the above discussion it is understandable that although curcumin was found highly effective in in vitro and animal study, some contradictory and less effective results were reported in patients with H. pylori infection. This low efficacy could be due to less solubility and bioavailability of curcumin in human. Further studies are needed to understand the toxicity and bioavailability of curcumin in greater details along with the search for appropriate steps to have more safe and bio-available drug.
Toxicity
It is always essential to carefully evaluate the toxicity of any compound in preclinical and early clinical studies. We can’t assume plant derived product which have been continuously in dietary use will be always harmless, as the doses may exceed or the formulation could differ. Early studies in rat model have demonstrated no significant toxicity at doses upto 5 g/kg body weight (BW) when given orally[50]. The safety issues have been lucidly elaborated in a review “Curcumin: The story so far” by Sharma et al[19]. One more organized pre-clinical study by prevention division of National Cancer Institute (NCI, US) did not find any side effects/toxicity in rat, dogs or monkeys using the doses upto 3.5 g/kg BW for 3 mo. Although, some infrequent adverse reactions (e.g., gastrointestinal upset or ulcerogenic response) reported were either spontaneously healed or not confirmed by subsequent studies. Some NCI reports confirmed this gastrointestinal disturbances in patients with different curcumin doses (0.45-3.6 g/d)[19]. Conversely, in another study where patients with advanced colorectal cancer were treated with curcumin upto a dose of 3.6 g daily for 4 mo and found to be well tolerated[51]. Moreover, three different phase I clinical trials showed that curcumin is extremely safe and well tolerated at very high doses (12 g/d)[18]. This pharmacological safety and potential efficacy have prompted investigators for further analysis.
Pharmacokinetics, bioavailability and problems
Even though the toxicity study of curcumin assures an immense possibility for treatment and prevention of a variety of diseases, the relatively low bioavailability of curcumin is the main difficulty in drug development. The bioavailability depends on absorption, metabolism, tissue distribution and excretion of that compound. Studies over the past few decades have established that curcumin undergoes poor absorption, fast metabolism and rapid clearance which severely reduced it’s bioavailability. Early study by Wahlstrom et al[50] showed that almost 75% of the dose (1 g/kg BW) was excreted in the faeces of rats and proved that curcumin is poorly absorbed in the gut.
A study of oral curcumin administration (400 mg) to rats demonstrated 60% absorption of curcumin, absence of any traces in heart blood and as a small amount (less than 5 μg/mL) was found in portal blood[52]. Same investigators, using tritiated curcumin showed majority of the oral dose being excreted in faeces, including one-third dose was excreted unchanged[53]. Pharmacokinetics of curcumin was also studied in mice either orally or intraperitonealy (ip) by Pan et al[54]. With oral administration of 1 g/kg of curcumin, low plasma level (0.22 μg/mL) was obtained at 1 h which was declined below the detection limit as soon as after 6 h. Although some studies have reported different results but all are in agreement that curcumin exhibits low bioavailability. In rodents it undergoes intestinal metabolism and excreted rapidly[18]. Result from pilot studies and phase I clinical trial confirmed first-pass and some degree of intestinal metabolism of curcumin, specially glucuronidation and sulphation, which explain curcumin’s low bioavailability[19]. The liver and intestinal mucosa were found to be the major organs responsible for metabolism of curcumin. Tissue distribution studies for curcumin showed its better accumulation in the intestine, liver, and colon and might be the most important cause why its most promising in vivo effects have been found in gastrointestinal diseases when compared with other organ systems[33]. H. pylori mediated gastrointestinal ailments can be treated with curcumin and the above phenomenon is the rationale behind its potential.
Effort to manage the bioavailability issue
Various classical techniques e.g., heat, pH, and complexations with metal ions, polymers or serum have been applied for enhancement of curcumin solubility and efficacy. It has been claimed that the solubility of curcumin can be increased by 12 fold by the use of heat[55]. Some novel approaches to overcome curcumin’s less bioavailability are adjuvants (which can block metabolic pathway of curcumin), effective delivery system e.g., phospholipid complexes, liposomes, micelles, and nanoparticles.
Use of adjuvants
An adjuvant is a compound that enhances an existing medical regimen, as a pharmacological agent added to a drug to increase or aid its effect. Glucuronidation of curcumin is known to cease its effect. Metabolic conversation of curcumin can be inhibited by an adjuvant Piperine, a known inhibitor of hepatic and intestinal glucuronidation. Concomitant use of curcumin with piperine increased the bioavailability of curcumin by 154% in rats and 2000% in human volunteers[56]. Other adjuvants which showed synergistic effect when used in combination with curcumin are quercetin[57], genistein[58] and Eugenol/terpeneol[59].
Derivatives and analogues
Structure of curcumin plays crucial role in determining its biological activity. As a result, several attempts have been made using curcumin derivatives/analogue with a number of achievements. EF-24, a curcumin analogue, was reported to be a lead compound presenting increased anti-tumor activity both in vitro and in vivo in comparison to curcumin[60] and less toxic[61]. EF-24 showed its higher bioavailability than curcumin of 60% and 35% with oral and ip respectively[18]. A review[18] from a well known group, working with curcumin’s biochemical property, has lucidly explained different reports where investigators have studied different complexes of curcumin (e.g., copper, boron, manganese, vanadyl, indium and gallium complexes). Lead complexes may be tested for anti-H. pylori effect.
Phospholipid complexes, liposomes and micelles
Curcumin phospholipid complexes exhibited better bioavailability, pharmacokinetics and hepato-protective property than free curcumin as describes by several researchers[32]. In a study with rat model of oral administration, curcumin phospholipid complex showed 1.5-fold more half-life over free cucumin[62]. A threefold high aqueous solubility and better efficacy in rat model was reported by Maiti et al[63].
Some important delivery system e.g., liposomes and micelles are also effectively used to study effect of curcumin. Liposomes are known drug delivery system due to the property of carrying both hydrophilic and hydrophobic molecules. Li et al[64] demonstrated that liposomal curcumin inhibits the growth of human pancreatic carcinoma cells and tumor angiogenic properties. Several other studies have reported the efficacy of liposomal curcumin in colorectal cancer or lymphoma cells but a controlled in vivo study is essential to check the effect of its bioavailability. On the other hand, use of micelles increases the solubility of curcumin in a number of systems. Letchford et al[65], showed a stiff increase in curcumin solubility using a polymeric micelle. Micelles inhibited curcumin uptake by liver/spleen, and also increased the distribution of curcumin in the other organs (e.g., lung and brain)[66].
Use of nanoparticles
Recently use of nanoparticle has been come out as a possible solution of compounds with compromised bioavailability. Nanoparticle-based delivery systems will most likely be appropriate for very hydrophobic agents like curcumin evading the drawbacks of poor aqueous solubility. Nanocurcumin has found to have more anti-inflammatory property and gives more protection to oxidative stress and apoptosis[67-69]. Main aim for using nanocarcumin is to have more dispersed and available curcumin in aqueous solution[70,71]. Use of nanocurcumin in different cancers is now a matter of regular discussion due to its better efficacy than native curcumin[67,72]. Oral bioavailability has been increased by 9 fold with nano-delivery system than piperine adjuvant administration. PLGA (polylactic-co-glycolic acid) nano formulation of curcumin have shown increased bioavailability upto 22 fold in rat model than free curcumin[73]. Better efficacy and solubility was described in a number of cancer cells and pre-clinical studies[74] but yet to analyze in H. pylori infection.
CONCLUSION
In different model systems and pre-clinical trials, curcumin has been widely studied for the treatment of various cancers; amongst them gastrointestinal cancer is mostly highlighted[13,75]. Considering the strong association between gastric cancer and H. pylori infection, different investigators in the world have examined the effect of curcumin in last two decades. Although we have got some beneficial to more promising results, still the use of curcumin in this bacterial infection is not in practice. Curcumin showed its potential mostly in in vitro studies or in small animals[7,8,38,39]. Long-term in vivo or pre-clinical studies are either very less or not that much effective[42]. Low solubility, poor absorption and less bioavailability are probably the key reasons for this ineffectiveness. Since curcumin have efficient anti-H. pylori effect, cheap and easily available in developing countries like India, additional studies are necessary, with large number of subjects to establish curcumin as an easy therapeutic solution for a potentially complicated infections.
Mostly studied and capable promising curcumin complexes (e.g., EF-24, phospholipid complexes, nano-formulations, use of adjuvants, micelles or liposomes) should be examined with regards to anti-H. pylori effect in a stepwise manner from in vitro to in vivo studies up to pre-clinical trials. This well controlled and organized research will help to identify the appropriate derivative or analogue of curcumin which can be effectively used in H. pylori infection. Future studies will elucidate more critical signaling molecules involved in H. pylori infection and better analyze the role of curcumin. This understanding along with progress in personal genome based risk analysis may help us to select appropriate curcumin formulation for particular patient populations. We can hope that in near future curcumin, either alone or in combination with other anti-microbial, could be considered as a potential chemopreventive agent against H. pylori-induced gastric carcinogenesis.
ACKNOWLEDGMENTS
Sarkar A acknowledges Indian Council of Medical Research, Government of India for providing a postdoctoral fellowship.
Footnotes
Conflict-of-interest statement: All authors declare no conflicts of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 8, 2015
First decision: December 11, 2015
Article in press: January 18, 2016
P- Reviewer: Chmiela M, El-Bendary M, Handa O, Kim GH, Maldonado-Bernal C S- Editor: Yu J L- Editor: A E- Editor: Ma S
References
- 1.Chattopadhyay S, Patra R, Chatterjee R, De R, Alam J, Ramamurthy T, Chowdhury A, Nair GB, Berg DE, Mukhopadhyay AK. Distinct repeat motifs at the C-terminal region of CagA of Helicobacter pylori strains isolated from diseased patients and asymptomatic individuals in West Bengal, India. Gut Pathog. 2012;4:4. doi: 10.1186/1757-4749-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bardhan PK. Epidemiological features of Helicobacter pylori infection in developing countries. Clin Infect Dis. 1997;25:973–978. doi: 10.1086/516067. [DOI] [PubMed] [Google Scholar]
- 3.Graham S, Haughey B, Marshall J, Brasure J, Zielezny M, Freudenheim J, West D, Nolan J, Wilkinson G. Diet in the epidemiology of gastric cancer. Nutr Cancer. 1990;13:19–34. doi: 10.1080/01635589009514042. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell HM, Li YY, Hu PJ, Liu Q, Chen M, Du GG, Wang ZJ, Lee A, Hazell SL. Epidemiology of Helicobacter pylori in southern China: identification of early childhood as the critical period for acquisition. J Infect Dis. 1992;166:149–153. doi: 10.1093/infdis/166.1.149. [DOI] [PubMed] [Google Scholar]
- 5.Smoot DT, Elliott TB, Verspaget HW, Jones D, Allen CR, Vernon KG, Bremner T, Kidd LC, Kim KS, Groupman JD, et al. Influence of Helicobacter pylori on reactive oxygen-induced gastric epithelial cell injury. Carcinogenesis. 2000;21:2091–2095. doi: 10.1093/carcin/21.11.2091. [DOI] [PubMed] [Google Scholar]
- 6.Nahar S, Mukhopadhyay AK, Khan R, Ahmad MM, Datta S, Chattopadhyay S, Dhar SC, Sarker SA, Engstrand L, Berg DE, et al. Antimicrobial susceptibility of Helicobacter pylori strains isolated in Bangladesh. J Clin Microbiol. 2004;42:4856–4858. doi: 10.1128/JCM.42.10.4856-4858.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De R, Kundu P, Swarnakar S, Ramamurthy T, Chowdhury A, Nair GB, Mukhopadhyay AK. Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob Agents Chemother. 2009;53:1592–1597. doi: 10.1128/AAC.01242-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kundu P, De R, Pal I, Mukhopadhyay AK, Saha DR, Swarnakar S. Curcumin alleviates matrix metalloproteinase-3 and -9 activities during eradication of Helicobacter pylori infection in cultured cells and mice. PLoS One. 2011;6:e16306. doi: 10.1371/journal.pone.0016306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehrotra S, Jamwal R, Shyam R, Meena DK, Mishra K, Patra R, De R, Mukhopadhyay A, Srivastava AK, Nandi SP. Anti-Helicobacter pylori and antioxidant properties of Emblica officinalis pulp extract; a potential source for therapeutic use against gastric ulcer. J Med Plants Res. 2011;5:2577–2583. [Google Scholar]
- 10.Darvesh AS, Aggarwal BB, Bishayee A. Curcumin and liver cancer: a review. Curr Pharm Biotechnol. 2012;13:218–228. doi: 10.2174/138920112798868791. [DOI] [PubMed] [Google Scholar]
- 11.Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14:141–153. [PubMed] [Google Scholar]
- 12.Sareen R, Jain N, Pandit V. Curcumin: a boon to colonic diseases. Curr Drug Targets. 2013;14:1210–1218. doi: 10.2174/13894501113149990168. [DOI] [PubMed] [Google Scholar]
- 13.Kidd PM. Bioavailability and activity of phytosome complexes from botanical polyphenols: the silymarin, curcumin, green tea, and grape seed extracts. Altern Med Rev. 2009;14:226–246. [PubMed] [Google Scholar]
- 14.Basnet P, Skalko-Basnet N. Curcumin: an anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules. 2011;16:4567–4598. doi: 10.3390/molecules16064567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irving GR, Karmokar A, Berry DP, Brown K, Steward WP. Curcumin: the potential for efficacy in gastrointestinal diseases. Best Pract Res Clin Gastroenterol. 2011;25:519–534. doi: 10.1016/j.bpg.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Priyadarsini KI, Maity DK, Naik GH, Kumar MS, Unnikrishnan MK, Satav JG, Mohan H. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radic Biol Med. 2003;35:475–484. doi: 10.1016/s0891-5849(03)00325-3. [DOI] [PubMed] [Google Scholar]
- 17.Tayyem RF, Heath DD, Al-Delaimy WK, Rock CL. Curcumin content of turmeric and curry powders. Nutr Cancer. 2006;55:126–131. doi: 10.1207/s15327914nc5502_2. [DOI] [PubMed] [Google Scholar]
- 18.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 19.Sharma RA, Gescher AJ, Steward WP. Curcumin: the story so far. Eur J Cancer. 2005;41:1955–1968. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Weber WM, Hunsaker LA, Abcouwer SF, Deck LM, Vander Jagt DL. Anti-oxidant activities of curcumin and related enones. Bioorg Med Chem. 2005;13:3811–3820. doi: 10.1016/j.bmc.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 21.Ak T, Gülçin I. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact. 2008;174:27–37. doi: 10.1016/j.cbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol. 2007;595:105–125. doi: 10.1007/978-0-387-46401-5_3. [DOI] [PubMed] [Google Scholar]
- 24.Kumar D, Kumar M, Saravanan C, Singh SK. Curcumin: a potential candidate for matrix metalloproteinase inhibitors. Expert Opin Ther Targets. 2012;16:959–972. doi: 10.1517/14728222.2012.710603. [DOI] [PubMed] [Google Scholar]
- 25.Marathe SA, Sen M, Dasgupta I, Chakravortty D. Differential modulation of intracellular survival of cytosolic and vacuolar pathogens by curcumin. Antimicrob Agents Chemother. 2012;56:5555–5567. doi: 10.1128/AAC.00496-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akram M, Uddini S, Ahmed A, Usmanghani K, Hannan A, Mohiuddin E, Asif M. Curcuma longa and Curcumin: A Review article. Rom J Biol Plant Biol. 2010;55:65–70. [Google Scholar]
- 27.Anto RJ, Mukhopadhyay A, Denning K, Aggarwal BB. Curcumin (diferuloylmethane) induces apoptosis through activation of caspase-8, BID cleavage and cytochrome c release: its suppression by ectopic expression of Bcl-2 and Bcl-xl. Carcinogenesis. 2002;23:143–150. doi: 10.1093/carcin/23.1.143. [DOI] [PubMed] [Google Scholar]
- 28.Howells LM, Mitra A, Manson MM. Comparison of oxaliplatin- and curcumin-mediated antiproliferative effects in colorectal cell lines. Int J Cancer. 2007;121:175–183. doi: 10.1002/ijc.22645. [DOI] [PubMed] [Google Scholar]
- 29.Basile V, Ferrari E, Lazzari S, Belluti S, Pignedoli F, Imbriano C. Curcumin derivatives: molecular basis of their anti-cancer activity. Biochem Pharmacol. 2009;78:1305–1315. doi: 10.1016/j.bcp.2009.06.105. [DOI] [PubMed] [Google Scholar]
- 30.Arbiser JL, Klauber N, Rohan R, van Leeuwen R, Huang MT, Fisher C, Flynn E, Byers HR. Curcumin is an in vivo inhibitor of angiogenesis. Mol Med. 1998;4:376–383. [PMC free article] [PubMed] [Google Scholar]
- 31.Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol. 2006;69:195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- 32.Gupta SC, Prasad S, Kim JH, Patchva S, Webb LJ, Priyadarsini IK, Aggarwal BB. Multitargeting by curcumin as revealed by molecular interaction studies. Nat Prod Rep. 2011;28:1937–1955. doi: 10.1039/c1np00051a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajasekaran SA. Therapeutic potential of curcumin in gastrointestinal diseases. World J Gastrointest Pathophysiol. 2011;2:1–14. doi: 10.4291/wjgp.v2.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gukovsky I, Reyes CN, Vaquero EC, Gukovskaya AS, Pandol SJ. Curcumin ameliorates ethanol and nonethanol experimental pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2003;284:G85–G95. doi: 10.1152/ajpgi.00138.2002. [DOI] [PubMed] [Google Scholar]
- 35.Prucksunand C, Indrasukhsri B, Leethochawalit M, Hungspreugs K. Phase II clinical trial on effect of the long turmeric (Curcuma longa Linn) on healing of peptic ulcer. Southeast Asian J Trop Med Public Health. 2001;32:208–215. [PubMed] [Google Scholar]
- 36.Milacic V, Banerjee S, Landis-Piwowar KR, Sarkar FH, Majumdar AP, Dou QP. Curcumin inhibits the proteasome activity in human colon cancer cells in vitro and in vivo. Cancer Res. 2008;68:7283–7292. doi: 10.1158/0008-5472.CAN-07-6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett. 2008;267:133–164. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 38.Mahady GB, Pendland SL, Yun G, Lu ZZ. Turmeric (Curcuma longa) and curcumin inhibit the growth of Helicobacter pylori, a group 1 carcinogen. Anticancer Res. 2002;22:4179–4181. [PubMed] [Google Scholar]
- 39.Münzenmaier A, Lange C, Glocker E, Covacci A, Moran A, Bereswill S, Baeuerle PA, Kist M, Pahl HL. A secreted/shed product of Helicobacter pylori activates transcription factor nuclear factor-kappa B. J Immunol. 1997;159:6140–6147. [PubMed] [Google Scholar]
- 40.Foryst-Ludwig A, Neumann M, Schneider-Brachert W, Naumann M. Curcumin blocks NF-kappaB and the motogenic response in Helicobacter pylori-infected epithelial cells. Biochem Biophys Res Commun. 2004;316:1065–1072. doi: 10.1016/j.bbrc.2004.02.158. [DOI] [PubMed] [Google Scholar]
- 41.Han C, Wang L, Yu K, Chen L, Hu L, Chen K, Jiang H, Shen X. Biochemical characterization and inhibitor discovery of shikimate dehydrogenase from Helicobacter pylori. FEBS J. 2006;273:4682–4692. doi: 10.1111/j.1742-4658.2006.05469.x. [DOI] [PubMed] [Google Scholar]
- 42.Di Mario F, Cavallaro LG, Nouvenne A, Stefani N, Cavestro GM, Iori V, Maino M, Comparato G, Fanigliulo L, Morana E, et al. A curcumin-based 1-week triple therapy for eradication of Helicobacter pylori infection: something to learn from failure? Helicobacter. 2007;12:238–243. doi: 10.1111/j.1523-5378.2007.00497.x. [DOI] [PubMed] [Google Scholar]
- 43.Zaidi SF, Yamamoto T, Refaat A, Ahmed K, Sakurai H, Saiki I, Kondo T, Usmanghani K, Kadowaki M, Sugiyama T. Modulation of activation-induced cytidine deaminase by curcumin in Helicobacter pylori-infected gastric epithelial cells. Helicobacter. 2009;14:588–595. doi: 10.1111/j.1523-5378.2009.00724.x. [DOI] [PubMed] [Google Scholar]
- 44.Koosirirat C, Linpisarn S, Changsom D, Chawansuntati K, Wipasa J. Investigation of the anti-inflammatory effect of Curcuma longa in Helicobacter pylori-infected patients. Int Immunopharmacol. 2010;10:815–818. doi: 10.1016/j.intimp.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 45.Sintara K, Thong-Ngam D, Patumraj S, Klaikeaw N, Chatsuwan T. Curcumin suppresses gastric NF-kappaB activation and macromolecular leakage in Helicobacter pylori-infected rats. World J Gastroenterol. 2010;16:4039–4046. doi: 10.3748/wjg.v16.i32.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ali MS, Pandit V, Jain M, Dhar KL. Mucoadhesive microparticulate drug delivery system of curcumin against Helicobacter pylori infection: Design, development and optimization. J Adv Pharm Technol Res. 2014;5:48–56. doi: 10.4103/2231-4040.126996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santos AM, Lopes T, Oleastro M, Gato IV, Floch P, Benejat L, Chaves P, Pereira T, Seixas E, Machado J, et al. Curcumin inhibits gastric inflammation induced by Helicobacter pylori infection in a mouse model. Nutrients. 2015;7:306–320. doi: 10.3390/nu7010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schreck R, Albermann K, Baeuerle PA. Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review) Free Radic Res Commun. 1992;17:221–237. doi: 10.3109/10715769209079515. [DOI] [PubMed] [Google Scholar]
- 49.Matsumoto Y, Marusawa H, Kinoshita K, Endo Y, Kou T, Morisawa T, Azuma T, Okazaki IM, Honjo T, Chiba T. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat Med. 2007;13:470–476. doi: 10.1038/nm1566. [DOI] [PubMed] [Google Scholar]
- 50.Wahlström B, Blennow G. A study on the fate of curcumin in the rat. Acta Pharmacol Toxicol (Copenh) 1978;43:86–92. doi: 10.1111/j.1600-0773.1978.tb02240.x. [DOI] [PubMed] [Google Scholar]
- 51.Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, Marczylo TH, Morgan B, Hemingway D, Plummer SM, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10:6847–6854. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 52.Ravindranath V, Chandrasekhara N. Absorption and tissue distribution of curcumin in rats. Toxicology. 1980;16:259–265. doi: 10.1016/0300-483x(80)90122-5. [DOI] [PubMed] [Google Scholar]
- 53.Ravindranath V, Chandrasekhara N. Metabolism of curcumin--studies with [3H]curcumin. Toxicology 1981- 1982;22:337–344. doi: 10.1016/0300-483x(81)90027-5. [DOI] [PubMed] [Google Scholar]
- 54.Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos. 1999;27:486–494. [PubMed] [Google Scholar]
- 55.Kurien BT, Scofield RH. Oral administration of heat-solubilized curcumin for potentially increasing curcumin bioavailability in experimental animals. Int J Cancer. 2009;125:1992–1993. doi: 10.1002/ijc.24547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 57.Cruz-Correa M, Shoskes DA, Sanchez P, Zhao R, Hylind LM, Wexner SD, Giardiello FM. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2006;4:1035–1038. doi: 10.1016/j.cgh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 58.Verma SP, Salamone E, Goldin B. Curcumin and genistein, plant natural products, show synergistic inhibitory effects on the growth of human breast cancer MCF-7 cells induced by estrogenic pesticides. Biochem Biophys Res Commun. 1997;233:692–696. doi: 10.1006/bbrc.1997.6527. [DOI] [PubMed] [Google Scholar]
- 59.Fang JY, Hung CF, Chiu HC, Wang JJ, Chan TF. Efficacy and irritancy of enhancers on the in-vitro and in-vivo percutaneous absorption of curcumin. J Pharm Pharmacol. 2003;55:593–601. doi: 10.1211/002235703765344496. [DOI] [PubMed] [Google Scholar]
- 60.Mosley CA, Liotta DC, Snyder JP. Highly active anticancer curcumin analogues. Adv Exp Med Biol. 2007;595:77–103. doi: 10.1007/978-0-387-46401-5_2. [DOI] [PubMed] [Google Scholar]
- 61.Ohori H, Yamakoshi H, Tomizawa M, Shibuya M, Kakudo Y, Takahashi A, Takahashi S, Kato S, Suzuki T, Ishioka C, et al. Synthesis and biological analysis of new curcumin analogues bearing an enhanced potential for the medicinal treatment of cancer. Mol Cancer Ther. 2006;5:2563–2571. doi: 10.1158/1535-7163.MCT-06-0174. [DOI] [PubMed] [Google Scholar]
- 62.Liu A, Lou H, Zhao L, Fan P. Validated LC/MS/MS assay for curcumin and tetrahydrocurcumin in rat plasma and application to pharmacokinetic study of phospholipid complex of curcumin. J Pharm Biomed Anal. 2006;40:720–727. doi: 10.1016/j.jpba.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 63.Maiti K, Mukherjee K, Gantait A, Saha BP, Mukherjee PK. Curcumin-phospholipid complex: Preparation, therapeutic evaluation and pharmacokinetic study in rats. Int J Pharm. 2007;330:155–163. doi: 10.1016/j.ijpharm.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 64.Li L, Braiteh FS, Kurzrock R. Liposome-encapsulated curcumin: in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer. 2005;104:1322–1331. doi: 10.1002/cncr.21300. [DOI] [PubMed] [Google Scholar]
- 65.Letchford K, Burt H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: micelles, nanospheres, nanocapsules and polymersomes. Eur J Pharm Biopharm. 2007;65:259–269. doi: 10.1016/j.ejpb.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 66.Song Z, Feng R, Sun M, Guo C, Gao Y, Li L, Zhai G. Curcumin-loaded PLGA-PEG-PLGA triblock copolymeric micelles: Preparation, pharmacokinetics and distribution in vivo. J Colloid Interface Sci. 2011;354:116–123. doi: 10.1016/j.jcis.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 67.Flora G, Gupta D, Tiwari A. Nanocurcumin: a promising therapeutic advancement over native curcumin. Crit Rev Ther Drug Carrier Syst. 2013;30:331–368. doi: 10.1615/critrevtherdrugcarriersyst.2013007236. [DOI] [PubMed] [Google Scholar]
- 68.Al-Rohaimi AH. Comparative anti-inflammatory potential of crystalline and amorphous nano curcumin in topical drug delivery. J Oleo Sci. 2015;64:27–40. doi: 10.5650/jos.ess14175. [DOI] [PubMed] [Google Scholar]
- 69.Nehra S, Bhardwaj V, Kalra N, Ganju L, Bansal A, Saxena S, Saraswat D. Nanocurcumin protects cardiomyoblasts H9c2 from hypoxia-induced hypertrophy and apoptosis by improving oxidative balance. J Physiol Biochem. 2015;71:239–251. doi: 10.1007/s13105-015-0405-0. [DOI] [PubMed] [Google Scholar]
- 70.Basniwal RK, Khosla R, Jain N. Improving the anticancer activity of curcumin using nanocurcumin dispersion in water. Nutr Cancer. 2014;66:1015–1022. doi: 10.1080/01635581.2014.936948. [DOI] [PubMed] [Google Scholar]
- 71.Shehzad A, Ul-Islam M, Wahid F, Lee YS. Multifunctional polymeric nanocurcumin for cancer therapy. J Nanosci Nanotechnol. 2014;14:803–814. doi: 10.1166/jnn.2014.9103. [DOI] [PubMed] [Google Scholar]
- 72.Terlikowska K, Witkowska A, Terlikowski S. Curcumin in chemoprevention of breast cancer. Postepy Hig Med Dosw (Online) 2014;68:571–578. doi: 10.5604/17322693.1102294. [DOI] [PubMed] [Google Scholar]
- 73.Tsai YM, Jan WC, Chien CF, Lee WC, Lin LC, Tsai TH. Optimised nano-formulation on the bioavailability of hydrophobic polyphenol, curcumin, in freely-moving rats. Food Chem. 2011;127:918–925. doi: 10.1016/j.foodchem.2011.01.059. [DOI] [PubMed] [Google Scholar]
- 74.Grynkiewicz G, Ślifirski P. Curcumin and curcuminoids in quest for medicinal status. Acta Biochim Pol. 2012;59:201–212. [PubMed] [Google Scholar]
- 75.Park W, Amin AR, Chen ZG, Shin DM. New perspectives of curcumin in cancer prevention. Cancer Prev Res (Phila) 2013;6:387–400. doi: 10.1158/1940-6207.CAPR-12-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]


