Abstract
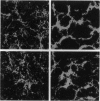
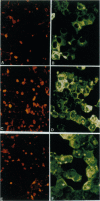
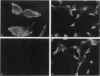
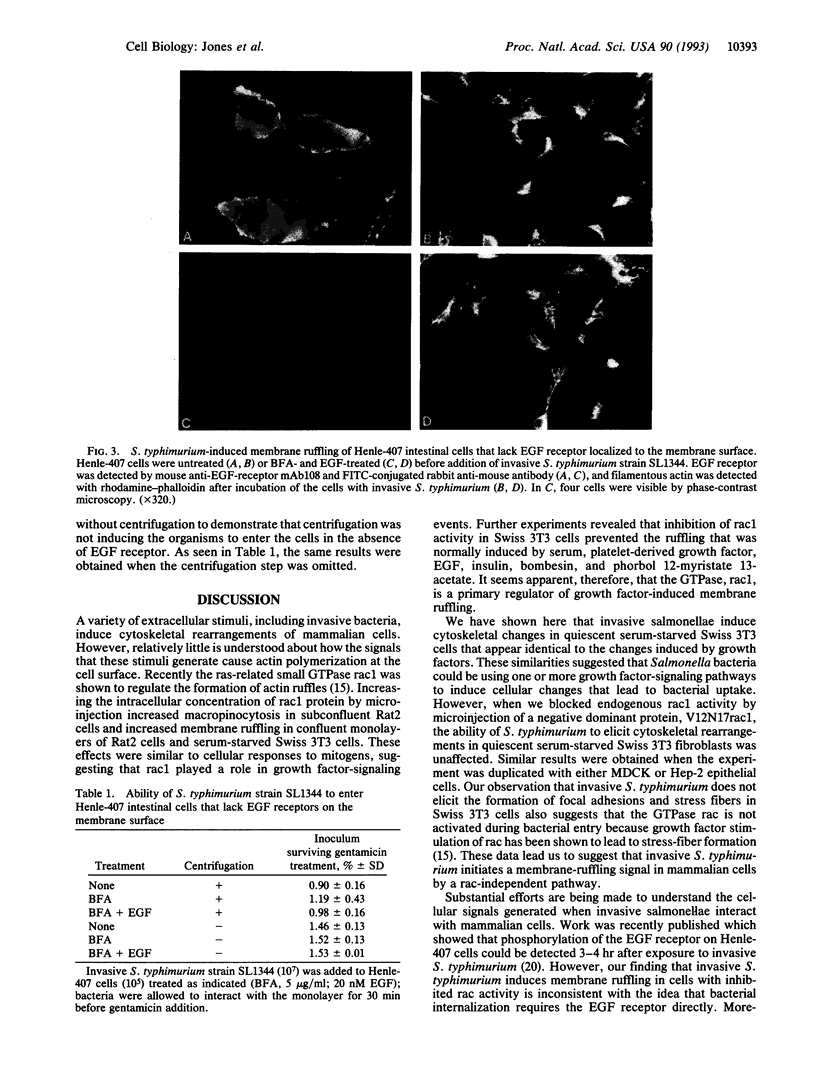
Invasive Salmonella typhimurium induces dramatic actin rearrangements on the membrane surface of mammalian cells as part of its entry mechanism. These changes, which are best characterized as membranous ruffles, closely resemble the membrane changes that occur when a growth factor binds to its receptor. Recently, inhibition of the function of the small GTPases rac and rho in quiescent serum-starved fibroblasts was demonstrated to abolish growth factor-mediated ruffling and stress-fiber formation, respectively. In addition, actin changes induced by the oncogene ras were also shown to be regulated by rac and rho. Because Salmonella-induced actin rearrangements resemble those caused by growth factors, we investigated whether ras, rho, or rac regulates the membrane ruffling elicited by S. typhimurium. Surprisingly, inhibition of the functions of these GTPases had no effect on the ability of invasive S. typhimurium to induce membrane ruffles on a variety of tissue culture cells including Madin-Darby canine kidney cells, Swiss 3T3 fibroblasts, and Hep-2 cells. These results led us to examine the interactions of S. typhimurium with Henle-407 intestinal cells, which lack epidermal growth factor receptor on their membrane surface. We found no difference in the ability of invasive S. typhimurium to induce membrane ruffling and to enter Henle-407 cells with or without the epidermal growth factor receptor on the membrane surface. We, therefore, conclude that invasive S. typhimurium induces membrane ruffling and its own internalization by a rac-independent, growth factor-receptor-independent signaling pathway.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellot F., Moolenaar W., Kris R., Mirakhur B., Verlaan I., Ullrich A., Schlessinger J., Felder S. High-affinity epidermal growth factor binding is specifically reduced by a monoclonal antibody, and appears necessary for early responses. J Cell Biol. 1990 Feb;110(2):491–502. doi: 10.1083/jcb.110.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devreotes P. N., Zigmond S. H. Chemotaxis in eukaryotic cells: a focus on leukocytes and Dictyostelium. Annu Rev Cell Biol. 1988;4:649–686. doi: 10.1146/annurev.cb.04.110188.003245. [DOI] [PubMed] [Google Scholar]
- Felder S., Miller K., Moehren G., Ullrich A., Schlessinger J., Hopkins C. R. Kinase activity controls the sorting of the epidermal growth factor receptor within the multivesicular body. Cell. 1990 May 18;61(4):623–634. doi: 10.1016/0092-8674(90)90474-s. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Gumbiner B., Falkow S. Penetration of Salmonella through a polarized Madin-Darby canine kidney epithelial cell monolayer. J Cell Biol. 1988 Jul;107(1):221–230. doi: 10.1083/jcb.107.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis C. L., Starnbach M. N., Falkow S. Morphological and cytoskeletal changes in epithelial cells occur immediately upon interaction with Salmonella typhimurium grown under low-oxygen conditions. Mol Microbiol. 1992 Nov;6(21):3077–3087. doi: 10.1111/j.1365-2958.1992.tb01765.x. [DOI] [PubMed] [Google Scholar]
- Fujiwara T., Oda K., Yokota S., Takatsuki A., Ikehara Y. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J Biol Chem. 1988 Dec 5;263(34):18545–18552. [PubMed] [Google Scholar]
- Galán J. E., Ginocchio C., Costeas P. Molecular and functional characterization of the Salmonella invasion gene invA: homology of InvA to members of a new protein family. J Bacteriol. 1992 Jul;174(13):4338–4349. doi: 10.1128/jb.174.13.4338-4349.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán J. E., Pace J., Hayman M. J. Involvement of the epidermal growth factor receptor in the invasion of cultured mammalian cells by Salmonella typhimurium. Nature. 1992 Jun 18;357(6379):588–589. doi: 10.1038/357588a0. [DOI] [PubMed] [Google Scholar]
- Giannella R. A., Washington O., Gemski P., Formal S. B. Invasion of HeLa cells by Salmonella typhimurium: a model for study of invasiveness of Salmonella. J Infect Dis. 1973 Jul;128(1):69–75. doi: 10.1093/infdis/128.1.69. [DOI] [PubMed] [Google Scholar]
- Ginocchio C., Pace J., Galán J. E. Identification and molecular characterization of a Salmonella typhimurium gene involved in triggering the internalization of salmonellae into cultured epithelial cells. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5976–5980. doi: 10.1073/pnas.89.13.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Ras-related GTPases and the cytoskeleton. Mol Biol Cell. 1992 May;3(5):475–479. doi: 10.1091/mbc.3.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R., Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature. 1985 Sep 19;317(6034):262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- Jones B. D., Lee C. A., Falkow S. Invasion by Salmonella typhimurium is affected by the direction of flagellar rotation. Infect Immun. 1992 Jun;60(6):2475–2480. doi: 10.1128/iai.60.6.2475-2480.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Richardson L. A., Uhlman D. The invasion of HeLa cells by Salmonella typhimurium: reversible and irreversible bacterial attachment and the role of bacterial motility. J Gen Microbiol. 1981 Dec;127(2):351–360. doi: 10.1099/00221287-127-2-351. [DOI] [PubMed] [Google Scholar]
- Kihlström E., Nilsson L. Endocytosis of Salmonella typhimurium 395 MS and MR10 by HeLa cells. Acta Pathol Microbiol Scand B. 1977 Oct;85B(5):322–328. doi: 10.1111/j.1699-0463.1977.tb01982.x. [DOI] [PubMed] [Google Scholar]
- Misumi Y., Misumi Y., Miki K., Takatsuki A., Tamura G., Ikehara Y. Novel blockade by brefeldin A of intracellular transport of secretory proteins in cultured rat hepatocytes. J Biol Chem. 1986 Aug 25;261(24):11398–11403. [PubMed] [Google Scholar]
- Rosenshine I., Duronio V., Finlay B. B. Tyrosine protein kinase inhibitors block invasin-promoted bacterial uptake by epithelial cells. Infect Immun. 1992 Jun;60(6):2211–2217. doi: 10.1128/iai.60.6.2211-2217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon E. D. Cytokinesis in animal cells. Curr Opin Cell Biol. 1989 Jun;1(3):541–547. doi: 10.1016/0955-0674(89)90018-5. [DOI] [PubMed] [Google Scholar]
- Scheving L. A., Shiurba R. A., Nguyen T. D., Gray G. M. Epidermal growth factor receptor of the intestinal enterocyte. Localization to laterobasal but not brush border membrane. J Biol Chem. 1989 Jan 25;264(3):1735–1741. [PubMed] [Google Scholar]
- Takeuchi A. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am J Pathol. 1967 Jan;50(1):109–136. [PMC free article] [PubMed] [Google Scholar]