Abstract
AIM: To determine the safety profile of new hepatitis C virus (HCV) treatments in liver transplant (LT) recipients with recurrent HCV infection.
METHODS: Forty-two patients were identified with recurrent HCV infection that underwent LT at least 12 mo prior to initiating treatment with a Sofosbuvir-based regimen during December 2013-June 2014. Cases were patients who experienced hepatic decompensation and/or serious adverse events (SAE) during or within one month of completing treatment. Controls had no evidence of hepatic decompensation and/or SAE. HIV-infected patients were excluded. Cumulative incidence of decompensation/SAE was calculated using the Kaplan Meier method. Exact logistic regression analysis was used to identify factors associated with the composite outcome.
RESULTS: Median age of the 42 patients was 60 years [Interquartile Range (IQR): 56-65 years], 33% (14/42) were female, 21% (9/42) were Hispanic, and 9% (4/42) were Black. The median time from transplant to treatment initiation was 5.4 years (IQR: 2.1-8.8 years). Thirteen patients experienced one or more episodes of hepatic decompensation and/or SAE. Anemia requiring transfusion, the most common event, occurred in 62% (8/13) patients, while 54% (7/13) decompensated. The cumulative incidence of hepatic decompensation/SAE was 31% (95%CI: 16%-41%). Risk factors for decompensation/SAE included lower pre-treatment hemoglobin (OR = 0.61 per g/dL, 95%CI: 0.40-0.88, P < 0.01), estimated glomerular filtration rate (OR = 0.95 per mL/min per 1.73 m2, 95%CI: 0.90-0.99, P = 0.01), and higher baseline serum total bilirubin (OR = 2.43 per mg/dL, 95%CI: 1.17-8.65, P < 0.01). The sustained virological response rate for the cohort of 42 patients was 45%, while it was 31% for cases.
CONCLUSION: Sofosbuvir/ribavirin will continue to be used in the post-transplant population, including those with HCV genotypes 2 and 3. Management of anemia remains an important clinical challenge.
Keywords: Hepatitis C virus, Sofosbuvir, Ribavirin, Anemia, Hepatic decompensation, Serious adverse event, Liver transplant
Core tip: Direct acting antivirals have changed the landscape of managing hepatitis C virus (HCV) infection, but there is limited data on the full safety profile of these drugs. We studied a group of liver transplant recipients with recurrent HCV who had hepatic decompensation and/or serious adverse events while on treatment with sofosbuvir-based regimens. We found that cases had lower pre-treatment hemoglobin, estimated glomerular filtration rate, and higher pre-treatment serum total bilirubin levels compared to controls. Anemia was the most common event and 62% of cases required blood transfusion. Similar to registration trials, sofosbuvir was generally well-tolerated, while ribavirin-induced anemia remains a challenge.
INTRODUCTION
Hepatitis C virus (HCV) is a leading cause of cirrhosis and hepatocellular carcinoma, and thus a primary indication for liver transplantation (LT) in the United States[1,2]. HCV infection recurs in virtually all patients who have a detectable HCV viral load at the time of LT[1-3]. Recurrent HCV infection is an important cause of graft failure and excess mortality[2,3]. Up to 30% of patients with recurrent HCV develop graft cirrhosis within 5 years[1,2,4], and 5% experience fibrosing cholestatic hepatitis, an aggressive and rapidly-progressive liver disease[2,4,5]. Successful antiviral treatment leading to a sustained virological response (SVR) may decelerate the rate of fibrosis, positively impacting patient and graft survival[1,4,6-9].
HCV treatment options are evolving rapidly. Pegylated-interferon (PEG) plus ribavirin (RBV) was the standard of care for over ten years, but it has low efficacy and is poorly tolerated due to side effects[10]. Triple therapy with the protease inhibitors telaprevir and boceprevir combined with PEG/RBV was more efficacious than dual therapy, with SVR rates ranging from 50%-67%[11-13], but these regimens had severe drug-drug interactions with calcineurin inhibitors (immunosuppressive agents commonly used in post-LT patients) due to an interaction with CYP3A enzymes[14]. Complications included severe anemia, infections, and acute renal insufficiency, limiting its use[14-16].
In December 2013, sofosbuvir (SOF), an inhibitor of the HCV NS5B polymerase, was approved by the United States Food and Drug Administration for use in combination with RBV, with or without PEG, for the treatment of chronic HCV infection in genotypes 1-4[17,18]. SOF has pangenotypic activity and a high barrier to resistance[19]. In a multicenter study, SOF/RBV was given for 24 wk to 40 LT patients with compensated liver disease[20]; 28 (70%) achieved SVR12. The safety profile was favorable compared to PEG-containing regimens. Most adverse events leading to drug discontinuation were attributed to RBV[19]. There was no evidence of drug-drug interactions between SOF and commonly used calcineurin inhibitors (tacrolimus and cyclosporine), and there were no deaths or episodes of graft rejection. Despite these favorable outcomes, there is limited real-world data on the safety profile and efficacy of SOF-based regimens for the treatment of recurrent HCV infection after LT.
The goals of this study were to characterize and determine the incidence and clinical significance of hepatic decompensation and/or serious adverse events (SAE) in LT recipients with recurrent HCV infection on SOF-containing regimens. This information will help clinicians identify patients at high risk of adverse outcomes who may benefit from more intensive monitoring during treatment. Although a new combination of SOF and ledipasvir (LDV), a NS5A inhibitor, was recently approved for patients with genotype 1 HCV[21-23], SOF/RBV will continue to be used for patients with other genotypes, particularly in those regions where newer direct-acting antivirals (DAA) may not be readily available.
MATERIALS AND METHODS
Study design and patients
In this observational cohort study, all LT recipients ≥ 18 years of age initiating HCV treatment with SOF at the Mount Sinai Medical Center from December 2013 to June 30, 2014 were identified by their providers. Patients who experienced a SAE and/or a hepatic decompensation episode during treatment or up to one month following the end of treatment (EOT) were chosen for the study. All patients were treated with SOF/RBV ± PEG and received at least one dose of SOF. Most patients were started on 400 mg of SOF and weight-based RBV, with doses ranging from 200 mg to 400 mg daily or twice per day. PEG was added to the regimens of those patients who had HCV recurrence after treatment post-LT. In response to decreased hemoglobin concentrations, doses of RBV were decreased at the discretion of providers. HIV-positive patients and patients who underwent LT within one year of treatment initiation were excluded. Data collection included demographics, comorbid conditions, baseline and on-treatment laboratory values including HCV RNA levels, stage of liver disease, and description of decompensation and/or SAE. Cirrhosis was defined by any of the following: liver biopsy consistent with stage 4 fibrosis; liver biopsy with stage 3 fibrosis plus one of the following: platelet count < 140000, esophageal varices identified on upper endoscopy, imaging study with evidence of cirrhosis and/or portal hypertension, history of ascites, or FibroSure test equivalent to stage 4. In the absence of a liver biopsy, any two of the following indicated cirrhosis: platelet count < 140000, esophageal varices identified on upper endoscopy, imaging study with evidence of cirrhosis and/or portal hypertension, history of ascites, or FibroSure test equivalent to stage 4. FIB-4 score was used to estimate the degree of liver fibrosis. This was calculated as [age (years) × AST (U/L)/[Platelet count (109/L) × ALT (U/L)1/2] with a value ≥ 3.25 signifying F3-F4 fibrosis. Glomerular filtration rates were estimated using the CKD-EPI formula. The study was conducted in accordance with the Helsinki accord and had IRB approval (GCO # 10-0032).
Case: Cases were defined as liver transplant recipients with new-onset hepatic decompensation, signified by new or increased jaundice (total bilirubin ≥ 4.0 mg/dL), ascites, encephalopathy, variceal bleeding, spontaneous bacterial peritonitis, sepsis, or another SAE (FDA definition) while on HCV treatment or during the first month after EOT. Those with LT within one year prior to initiating SOF-containing regimens were excluded from the cohort.
Control: Controls were those who received LT at least one year prior to initiating treatment with SOF-containing regimens and did not experience a hepatic decompensation and/or SAE during treatment or up to one month following EOT.
Statistical analysis
Statistical analysis was performed by an experienced biostatistician (Kian Bichoupan) at the Icahn School of Medicine at Mount Sinai. The incidence of hepatic decompensation and SAE was determined using the Kaplan-Meier method. While all events experienced by the Cases were included in the analysis to identify factors associated with decompensation/SAE, only the time to the first event was used to calculate the incidence. Baseline descriptive variables were shown as the median with the interquartile range. Student t-test and Man-Whitney U test were used to compare categorical and continuous variables of Cases and Controls. Exact logistic regression analysis was used to identify factors associated with hepatic decompensation and SAE. Variables with P-values of less than 0.05 were used in the final model.
RESULTS
Among 57 patients who initiated treatment with a SOF-containing regimen during the study period, 12 were excluded because LT occurred during the prior 12 mo and 3 were excluded due to HIV co-infection. The remaining 42 patients were included in the study: 38 (90%) were on SOF/RBV and four (10%) were on SOF/PEG/RBV. The cumulative incidence of liver decompensation and/or SAE, which occurred in 13 patients, was 31% (95%CI: 2%-44%) (Figure 1). Details of the 13 cases, including pre-treatment laboratory values, years since LT, description of major events, and overall outcome are presented in Table 1. Episodes often involved multiple complications. Cases had an average of 2.5 separate episodes of hepatic decompensation/SAE during the course of treatment. The average time from the initiation of treatment to the first event of hepatic decompensation/SAE was 4.9 wk (SD: 3.6 wk, range: 2-15 wk).
Figure 1.
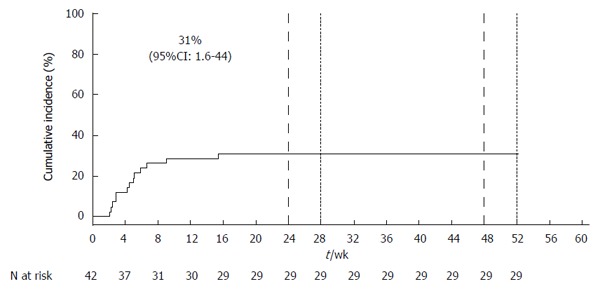
Cumulative incidence of hepatic decompensation and/or serious adverse events using the Kaplan-Meier method. The dashed line indicates the time at which 24-wk and 48-wk treatment regimens were completed. The dotted line indicates the 4-wk post end of treatment surveillance period.
Table 1.
Details of 13 cases with hepatic decompensation/serious adverse events
| Case | Rx | Genotype | Age | Sex | Years since transplant |
Baseline1 |
1st event wk | First episode | Later episodes | Overall outcome | SVR 12 (Y/N) | ||||
| Hemoglobin (g/dL) | Platelets (× 103/μL) | Albumin (g/dL) | Total bilirubin (mg/dL) | ALT (U/L) | |||||||||||
| 1 | SOF/RBV | 13 | 77 | F | 5.0 | 9.3 | 78 | 2.9 | 0.6 | 33 | 15.4 | Hospitalized for SBP | Hospitalized for SBP, ascites, anemia; jaundice, hyperkalemia | Hospitalized for fall soon after EOT, developed ICH, eventually died in hospital | Died |
| 24 wk2 | |||||||||||||||
| 2 | SOF/RBV | 2 | 70 | M | 17.0 | 7.9 | 13 | 3.9 | 4.7 | 15 | 4.3 | Hospitalized for PSE | None | Transferred to Hospice, died | Died |
| 24 wk | |||||||||||||||
| 3 | SOF/RBV | 13 | 37 | F | 1.2 | 8.9 | 143 | 2.9 | 16.9 | 39 | 2.9 | Hospitalized for failure to thrive, jaundice, anemia requiring transfusion | Hospitalized for jaundice, anemia requiring transfusion, blurred vision | Completed 48 wk of treatment, never undetectable | N |
| 48 wk | |||||||||||||||
| 4 | SOF/RBV | 3 | 49 | M | 1.9 | 12.4 | 138 | 4.4 | 0.7 | 43 | 2.0 | Anemia requiring transfusion | Anemia requiring transfusion | Completed treatment | Y |
| 24 wk | |||||||||||||||
| 5 | SOF/RBV | 13 | 67 | M | 13.2 | 11.8 | 118 | 3.1 | 2.1 | 72 | 2.9 | Anemia requiring transfusion | Worsening ascites requiring increased dose of diuretics | Hemoglobin level and ascites improved after intervention; completed treatment but relapsed | N |
| 24 wk | |||||||||||||||
| 6 | SOF/RBV | 12 | 56 | F | 1.5 | 13.0 | 152 | 5.0 | 0.7 | 44 | 2.9 | Anemia requiring transfusion | None | Hemoglobin improved after transfusion; completed treatment | Y |
| 24 wk | |||||||||||||||
| 7 | SOF/RBV | 12 | 70 | F | 7.3 | 12.0 | 127 | 3.6 | 0.4 | 60 | 4.4 | Symptomatic anemia, no transfusion, treatment discontinued | None | Viral load detectable 4 mo after treatment discontinuation | N |
| 24 wk | |||||||||||||||
| 8 | SOF/PEG/RBV | 2 | 65 | F | 6.0 | 12.7 | 85 | 3.6 | 0.3 | 38 | 6.6 | Anemia requiring transfusion | Anemia requiring multiple transfusions | Completed treatment | Y |
| 24 wk3 | |||||||||||||||
| 9 | SOF/RBV | 12 | 60 | M | 9.1 | 11.3 | 94 | 3.6 | 0.8 | 199 | 5.0 | Anemia requiring transfusion | Hospitalized for hyperglycemia, anemia requiring transfusion; treatment discontinued | Detectable viral load 7 wk after treatment discontinued | N |
| 24 wk | |||||||||||||||
| 10 | SOF/RBV | 4 | 64 | F | 7.5 | 9.6 | 98 | 2.7 | 5.5 | 24 | 2.4 | Anemia requiring transfusion | Hospitalized for SBP, anemia requiring transfusion, treatment discontinued | Hemoglobin improved after transfusions; viral load undetectable at treatment discontinuation; became detectable 1 mo later | N |
| 24 wk | |||||||||||||||
| 11 | SOF/RBV | 12 | 49 | M | 2.2 | 15.2 | 113 | 3.9 | 0.6 | 74 | 5.9 | Admitted for acute cholangitis after presenting with fever, jaundice, diarrhea | None | Resolution of presenting symptoms with antibiotics, biliary stent placement; completed treatment, relapsed | N |
| 24 wk | |||||||||||||||
| 12 | SOF/RBV | 3 | 56 | M | 1.1 | 10.7 | 65 | 3.6 | 1.1 | 94 | 9.0 | Hospitalized for partial SBO | Hospitalized for hyperkalemia | SBO resolved with conservative medical management | Y |
| 24 wk | |||||||||||||||
| 13 | SOF/RBV | 13 | 68 | M | 1.7 | 10.7 | 196 | 1.9 | 2.2 | 24 | 5.0 | Hospitalized, PSE, UTI | Hospitalized for recurrent PSE due to UTI | Completed treatment, +EOT response, relapsed 4 wk post-EOT | N |
| 24 wk | |||||||||||||||
Baseline: indicates the most recent laboratory values prior to treatment initiation;
SOF/RBV: Sofosbuvir/Ribavirin;
SOF/PEG/RBV: Sofosbuvir/PEG-interferon/Ribavirin. Laboratory Reference Range: Hemoglobin (g/dL), Ref: 13.9-16.3; Platelets (× 103/μL), Ref: 150-450; Albumin (g/dL), Ref: 3.5-4.9; Total Bilirubin (mg/dL), Ref: 0.1-1.2; ALT (U/L), Ref: 1-53. ALT: Alanine aminotransferase; SVR: Sustained virological response; SBP: Spontaneous bacterial peritonitis; EOT: End of treatment; ICH: Intracranial hemorrhage; PSE: Portosystemic encephalopathy; SBO: Small bowel obstruction; UTI: Urinary tract infection.
The most common SAEs were hospitalization (8 patients) and blood transfusion for symptomatic anemia (8 patients). Other SAEs that occurred in more than 1 patient were failure to thrive, hyperkalemia, hyperglycemia, and partial small bowel obstruction. Two of the Cases died. One of these patients was hospitalized shortly before the end of treatment and eventually completed treatment but died as a consequence of complications from an intracranial hemorrhage. The second patient was hospitalized for portosystemic encephalopathy about 1 mo after starting treatment and was eventually transitioned to palliative care and hospice.
Hepatic decompensation events included new or worsening jaundice (3 cases), portosystemic encephalopathy (2 cases), sepsis (4 cases), spontaneous bacterial peritonitis (2 cases), urosepsis (1 Case), acute cholangitis (1 case), and worsening ascites requiring increased dose of diuretics (1 case). Of the 13 cases, 10 (77%) completed treatment and 4 (31%) had SVR12. Of the 29 Controls, 15 (52%) achieved SVR12. In the cohort of 42 patients, SVR12 was achieved in 19 (45%).
Table 2 shows the characteristics of Cases and Controls. Cases had lower body weight (P = 0.04), baseline hemoglobin (P = 0.01) and estimated glomerular filtration rate (eGFR) (P = 0.03) than Controls. There were no significant differences in age, sex, or years since transplant between Cases and Controls. There were also no significant differences in comorbid conditions (hypertension, diabetes, hepatocellular carcinoma), FIB-4 scores, treatment regimen, genotype, HCV viral load, or markers of liver impairment [platelets, aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, total bilirubin, and INR].
Table 2.
Baseline characteristics of cases and controls
| Categorical: n (%) | P value1 | |||
|
Continuous: median (IQR) | ||||
| Total | Case (n = 13) | Controls (n = 29) | ||
| Demographics and clinical characteristics | ||||
| Age (yr) | 60 (56-65) | 64 (56-68) | 60 (57-64) | 0.97 |
| Gender, female | 14 (33) | 6 (46) | 8 (28) | 0.15 |
| Race, black | 4 (9) | 1 (7) | 3 (10) | 1.00 |
| Ethnicity, Hispanic | 9 (21) | 1 (7) | 8 (28) | 0.23 |
| Weight (lbs) | 163 (146-182) | 155 (122-168) | 170 (147-193) | 0.04 |
| BMI (kg/m2) | 26.4 (22.6-28.7) | 25.6 (21.6-27.8) | 26.7 (24.6-29.0) | 0.12 |
| Years since Transplant | 5.4 (2.1–8.8) | 4.98 (1.7-7.5) | 5.6 (2.7-8.8) | 0.52 |
| Comorbidities | ||||
| Hepatocellular Carcinoma | 16 (38) | 5 (38) | 11 (38) | 1.00 |
| Diabetes | 21 (50) | 6 (46) | 15 (52) | 1.00 |
| Hypertension | 27 (64) | 9 (69) | 18 (62) | 0.74 |
| Depression | 5 (12) | 1 (8) | 4 (14) | 1.00 |
| Liver disease severity | ||||
| Cirrhosis | 8 (19) | 3 (23) | 5 (17) | 0.69 |
| FIB-4 | 4.81 (3.00-7.02) | 6.57 (3.12-8.94) | 4.49 (2.78-6.71) | 0.24 |
| FIB-4, ≥ 3.25 | 28 (67) | 9 (69) | 19 (65) | 1.00 |
| Treatment naïve | 9 (21) | 3 (23) | 6 (21) | 1.00 |
| Treatment Regimen | ||||
| SOF/RBV | 38 (90) | 12 (92) | 26 (90) | 1.00 |
| SOF/PEG/RBV | 4 (10) | 1 (8) | 3 (10) | 1.00 |
| Genotype | ||||
| 1 | 31 (74) | 8 (62) | 23 (79) | 0.27 |
| 2 | 3 (7) | 2 (15) | 1 (3) | 0.22 |
| 3 | 5 (12) | 2 (15) | 3 (10) | 0.64 |
| 4 | 2 (5) | 1 (8) | 1 (3) | 0.53 |
| Labs | ||||
| Hemoglobin (g/dL) (Ref: 13.9-16.3 g/dL) | 12.7 (10.8-14.2) | 11.3 (9.6-12.4) | 12.9 (12.2-14.5) | 0.01 |
| Platelets (× 103/μL) (Ref: 150-450 × 103/μL) | 117 (82-148) | 113 (85-138) | 121 (81-150) | 0.21 |
| HCV viral load (log10) (IU/mL) (Ref: 15-108 IU/mL) | 6.53 (6.27-6.72) | 6.59 (6.07-6.70) | 6.53 (6.32-6.76) | 0.81 |
| Serum creatinine (mg/dL) (Ref: 0.70-1.40 mg/dL) | 1.44 (1.10-1.72) | 1.60 (1.33-1.86) | 1.23 (1.04-1.60) | 0.06 |
| eGFR (mL/min per 1.73 m2 Albumin, g/dL) (Ref: 3.5-4.9 g/dL) | 49 (41-67) | 42 (32-49) | 56 (44-70) | 0.01 |
| 3.8 (3.5-4.1) | 3.6 (2.9-3.9) | 3.8 (3.6-4.1) | 0.23 | |
| ALT (U/L) (Ref: 1-53 U/L) | 59 (39-82) | 43 (33-72) | 65 (42-89) | 0.15 |
| AST (U/L) (Ref: 1-50 U/L) | 62 (49-92) | 61 (53-77) | 64 (41-93) | 0.95 |
| INR | 1.0 (1.0-1.2) | 1.0 (0.9-1.3) | 1.0 (1.0-1.1) | 0.852 |
| Total bilirubin (mg/dL) (Ref: 0.1-1.2 mg/dL) | 0.7 (0.5-1.1) | 0.8 (0.6-2.2) | 0.7 (0.5-1.0) | 0.10 |
| Alpha fetoprotein (ng/mL) (Ref: 0.0-9.0 ng/mL) | 5.0 (3.1-9.2) | 5.0 (3.5-12.1) | 5.0 (3.0-8.2) | 0.40 |
Student t test unless noted otherwise;
Mann-Whitney. BMI: Body mass index; SOF: Sofosbuvir; RBV: Ribavirin; PEG: Pegylated interferon; eGFR: Estimated glomerular filtration rate; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; INR: International normalized ratio.
Univariable exact logistic regression analysis of factors associated with hepatic decompensation/SAE
Exact logistic regression analysis was used to identify factors associated with both hepatic decompensation and SAE (Table 3). Univariable analysis revealed that lower hemoglobin (OR = 0.61 per g/dL, P < 0.01) and eGFR (OR = 0.95, P = 0.01) and higher serum total bilirubin (OR = 2.43 per mg/dL, P < 0.01) were factors potentially associated with both hepatic decompensation and SAE. Other markers of liver disease severity and hepatic impairment, including FIB-4 score, transaminase levels, albumin, and INR, were not associated with decompensation and SAE. Multivariable analysis was not performed due to the small size of the study group.
Table 3.
Unadjusted logistic regression analysis of factors associated with hepatic decompensation/serious adverse events
|
Unadjusted |
|||
| OR | 95%CI | P value | |
| Demographics and clinical characteristics | |||
| Age (yr) | 1.00 | 0.92-1.09 | 0.97 |
| Gender, female | 2.13 | 0.47-9.72 | 0.40 |
| Race, black | 0.73 | 0.01-9.98 | 1.00 |
| Ethnicity, Hispanic | 0.22 | 0.01-2.07 | 0.30 |
| Weight (lbs) | 0.98 | 0.95-1.00 | 0.06 |
| BMI (kg/m2) | 0.87 | 0.73-1.04 | 0.13 |
| Years since transplant | 0.96 | 0.83-1.10 | 0.52 |
| Comorbidities | |||
| Hepatocellular carcinoma | 1.02 | 0.21-4.78 | 1.00 |
| Diabetes | 0.80 | 0.17-3.58 | 1.00 |
| Hypertension | 1.40 | 0.29-7.76 | 0.91 |
| Depression | 0.54 | 0.01-6.05 | 1.00 |
| Liver disease severity | |||
| Cirrhosis | 1.42 | 0.19-9.49 | 0.96 |
| FIB-4 | 1.07 | 0.95-1.20 | 0.29 |
| FIB-4, ≥ 3.25 | 1.17 | 0.25-6.70 | 1.00 |
| Treatment naïve | 1.14 | 0.15-6.77 | 1.00 |
| Treatment Regimen | |||
| SOF/RBV | REF | REF | REF |
| SOF/PEG/RBV | 0.86 | 0.11-3.19 | 1.00 |
| Genotype | |||
| 1 | REF | REF | REF |
| 2 | 6.25 | 0.26-477.66 | 0.39 |
| 3 | 1.90 | 0.13-18.36 | 0.86 |
| 4 | 2.75 | 0.03-228.51 | 0.95 |
| Labs | |||
| Hemoglobin (g/dL) | 0.61 | 0.40-0.88 | < 0.01 |
| (Ref: 13.9-16.3 g/dL) | |||
| Platelets (× 103/μL) | 0.99 | 0.98-1.00 | 0.26 |
| (Ref: 150-450 × 103/μL) | |||
| HCV viral load [log10 (IU/mL)] | 0.90 | 0.38-2.44 | 0.82 |
| (Ref: 15-108 IU/mL) | |||
| Serum creatinine (mg/dL) | 2.27 | 0.88-8.82 | 0.09 |
| (Ref: 0.70-1.40 mg/dL) | |||
| eGFR (mL/min per 1.73 m2) | 0.95 | 0.90-0.99 | 0.01 |
| Albumin (g/dL) | 0.45 | 0.13-1.32 | 0.15 |
| (Ref: 3.5-4.9 g/dL) | |||
| ALT (U/L) | 0.99 | 0.97-1.00 | 0.19 |
| (Ref: 1-53 U/L) | |||
| AST (U/L) | 1.00 | 0.99-1.00 | 0.54 |
| (Ref: 1-50 U/L) | |||
| INR | 1.27 | 1.12-1.14 | 0.22 |
| Total bilirubin (mg/dL) | 2.43 | 1.17-8.65 | < 0.01 |
| (Ref: 0.1-1.2 mg/dL) | |||
| Alpha fetoprotein (ng/mL) | 0.25 | 0.99-1.11 | 0.15 |
| (Ref: 0.0-9.0 ng/mL) | |||
BMI: Body mass index; SOF: Sofosbuvir; RBV: Ribavirin; PEG: Pegylated interferon; eGFR: Estimated glomerular filtration rate; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; INR: International normalized ratio.
Sensitivity analysis
Sensitivity analyses were done to identify variables associated with either hepatic decompensation or SAE, rather than the composite endpoint. Factors associated with SAE (Table 4) included lower baseline weight (P = 0.02), hemoglobin (P = 0.02), and estimated glomerular filtration rate (P = 0.03), and higher total bilirubin (P = 0.02). Factors associated with hepatic decompensation (Table 5) included lower baseline hemoglobin (P = 0.02), estimated glomerular filtration rate (P = 0.02), albumin (P < 0.01), and ALT (P = 0.03), and higher INR (P < 0.01).
Table 4.
Unadjusted sensitivity analysis of factors associated with serious adverse events
| OR | 95%CI | P value | |
| Demographics and clinical characteristics | |||
| Age, yr | 0.99 | 0.91-1.08 | 0.79 |
| Gender, female | 2.73 | 0.54-18.42 | 0.28 |
| Race, black | 0.44 | < 0.01-2.76 | 0.49 |
| Ethnicity, Hispanic | 0.26 | < 0.01-2.37 | 0.39 |
| Weight, lbs | 0.97 | 0.94-0.99 | 0.02 |
| BMI, kg/m2 | 0.85 | 0.69-1.02 | 0.08 |
| Years since transplant | 0.98 | 0.85-1.12 | 0.75 |
| Comorbidities | |||
| Hepatocellular carcinoma | 1.21 | 0.24-6.04 | 1.00 |
| Diabetes | 1.01 | 0.22-4.74 | 1.00 |
| Hypertension | 1.15 | 0.23-6.45 | 1.00 |
| Depression | 0.6 | 0.01-7.16 | 1.00 |
| Liver disease severity | |||
| Cirrhosis | 0.8 | 0.07-5.68 | 1.00 |
| FIB-4 | 1.08 | 0.94-1.22 | 0.28 |
| FIB-4, ≥ 3.25 | 0.99 | 0.20-5.55 | 1.00 |
| Treatment naïve | 1.33 | 0.18-8.56 | 1.00 |
| Treatment Regimen | |||
| SOF/RBV | REF | REF | REF |
| SOF/PEG/RBV | 0.91 | 0.12-3.46 | 1.00 |
| Genotype | |||
| 1 | REF | REF | REF |
| 2 | 8.47 | 0.30-744.15 | 0.31 |
| 3 | 2.27 | 0.16-31.69 | 0.72 |
| 4 | 3.03 | 0.04-256.92 | 0.90 |
| Labs | |||
| Hemoglobin (g/dL) | 0.63 | 0.36-0.95 | 0.02 |
| (Ref: 13.9-16.3 g/dL) | |||
| Platelets (× 103/μL) | 0.99 | 0.98-1.00 | 0.14 |
| (Ref: 150-450 × 103/μL) | |||
| HCV viral load [log10 (IU/mL)] | 1.03 | 0.41-2.60 | 0.95 |
| (Ref: 15-108 IU/mL) | |||
| Serum creatinine (mg/dL) | 2.02 | 0.81-5.91 | 0.14 |
| (Ref: 0.70-1.40 mg/dL) | |||
| eGFR (mL/min per 1.73 m2) | 0.95 | 0.91-0.99 | 0.03 |
| Albumin (g/dL) | 0.76 | 0.26-2.31 | 0.63 |
| (Ref: 3.5-4.9 g/dL) | |||
| ALT (U/L) | 0.99 | 0.98-1.00 | 0.43 |
| (Ref: 1-53 U/L) | |||
| AST (U/L) | 0.99 | 0.99-1.01 | 0.78 |
| (Ref: 1-50 U/L) | |||
| INR | 2.93 | 0.35-29.0 | 0.36 |
| Total bilirubin (mg/dL) | 1.94 | 1.05-4.69 | 0.02 |
| (Ref: 0.1-1.2 mg/dL) | |||
| Alpha fetoprotein (ng/mL) | 1.04 | 0.99-1.12 | 0.16 |
| (Ref: 0.0-9.0 ng/mL) |
BMI: Body mass index; SOF: Sofosbuvir; RBV: Ribavirin; PEG: Pegylated interferon; eGFR: Estimated glomerular filtration rate; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; INR: International normalized ratio.
Table 5.
Unadjusted sensitivity analysis for factors associated with hepatic decompensation
| OR | 95%CI | P value | |
| Demographics and clinical characteristics | |||
| Age (yr) | 1.03 | 0.92-1.16 | 0.67 |
| Gender, female | 2.23 | 0.26-19.54 | 0.63 |
| Race, black | 2.2 | 0.04-35.75 | 0.94 |
| Ethnicity, Hispanic | 0.39 | < 0.01-2.30 | 0.41 |
| Weight (lbs) | 0.99 | 0.96-1.02 | 0.37 |
| BMI (kg/m2) | 0.94 | 0.75-1.16 | 0.61 |
| Years since Transplant | 0.96 | 0.79-1.15 | 0.63 |
| Comorbidities | |||
| Hepatocellular Carcinoma | 0.28 | 0.01-2.94 | 0.49 |
| Diabetes | 2.21 | 0.28-27.69 | 0.66 |
| Hypertension | 3.22 | 0.30-173.80 | 0.57 |
| Depression | 0.86 | < 0.01-5.35 | 0.90 |
| Liver disease severity | |||
| Cirrhosis | 5.41 | 0.61-56.98 | 0.14 |
| FIB-4 | 1.11 | 0.94-1.31 | 0.24 |
| FIB-4, ≥ 3.25 | 2.81 | 0.26-151.24 | 0.67 |
| Treatment naïve | 4.85 | 0.50-58.79 | 0.21 |
| Treatment Regimen | |||
| SOF/RBV | REF | REF | REF |
| SOF/PEG/RBV | < 0.01 | < 0.01-> 99.99 | 0.97 |
| Genotype | |||
| 1 | REF | REF | REF |
| 2 | 4.67 | 0.32-68.03 | 0.26 |
| 3 | 2.33 | 0.19-28.25 | 0.51 |
| 4 | 9.33 | 0.46-190.63 | 0.15 |
| Labs | |||
| Hemoglobin (g/dL) | 0.17 | 0.04-0.75 | 0.02 |
| (Ref: 13.9-16.3 g/dL) | |||
| Platelets (× 103/μL) | 0.99 | 0.97-1.01 | 0.29 |
| (Ref: 150-450 × 103/μL) | |||
| HCV viral load [log10 (IU/mL)] | 0.79 | 0.29-2.18 | 0.65 |
| (Ref: 15-108 IU/mL) | |||
| Serum creatinine (mg/dL) | 3.12 | 0.99-9.77 | 0.05 |
| (Ref: 0.70-1.40 mg/dL) | |||
| eGFR (mL/min per 1.73 m2) | 0.92 | 0.86-0.99 | 0.02 |
| Albumin (g/dL) | 0.13 | 0.02-0.58 | < 0.01 |
| (Ref: 3.5-4.9 g/dL) | |||
| ALT (U/L) | 0.96 | 0.90-0.99 | 0.03 |
| (Ref: 1-53 U/L) | |||
| AST (U/L) | 0.99 | 0.98-1.01 | 0.70 |
| (Ref: 1-50 U/L) | |||
| INR | 1.59 | 1.09-2.97 | < 0.01 |
| Total bilirubin (mg/dL) | 5.65 | 0.99-32.34 | 0.05 |
| (Ref: 0.1-1.2 mg/dL) | |||
| Alpha fetoprotein (ng/mL) | 1.03 | 0.98-1.08 | 0.27 |
| (Ref: 0.0-9.0 ng/mL) |
BMI: Body mass index; SOF: Sofosbuvir; RBV: Ribavirin; PEG: Pegylated interferon; eGFR: Estimated glomerular filtration rate; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; INR: International normalized ratio.
DISCUSSION
This observational cohort study was performed to investigate the safety profile of SOF-containing regimens in liver transplant recipients. There have been limited real-world studies that have described the safety profile of SOF in the LT population. Our goals were to determine the nature, incidence, and risk factors for hepatic decompensation/SAE in order to allow healthcare providers to identify high-risk patients when evaluating treatment options in post-LT patients with recurrence of HCV infection.
Of the 42 patients that were eligible for this study, 13 patients had a liver decompensation and/or a SAE. The cumulative incidence for hepatic decompensation/SAE was 31%. It is important to note that hepatic decompensation and/or SAE did not necessarily affect treatment outcome. Nine of the 13 patients (77%) who had a decompensation/SAE completed the treatment regimen, of which four (31%) achieved SVR, while 15 (52%) of the Controls achieved SVR. Of the remaining five cases that completed treatment, three relapsed, one failed treatment (viral load remained detectable despite 48 wk of treatment), and one patient was hospitalized immediately following the EOT and died during that hospitalization. Of the four cases that did not complete therapy, one Case died and three relapsed (became HCV viral load detectable) weeks after treatment discontinuation. None of the Cases experienced fibrosing cholestatic hepatitis.
Our analysis revealed that lower hemoglobin and estimated glomerular filtration rate and higher serum total bilirubin were potential factors associated with the composite outcome. Anemia is a well-known adverse effect of RBV use. Both RBV and SOF are metabolized in the liver and excreted through the kidneys, therefore if estimated glomerular filtration rate is impaired, intravascular concentrations of SOF and RBV metabolites are elevated, leading to potential complications including RBV-induced hemolytic anemia. Given that all LT patients take a form of immunosuppression that frequently impairs renal function, intense monitoring and dose adjustments are needed for medications that are renally excreted. Elevated bilirubin is a marker of liver impairment used in estimating the severity of liver disease, which supports the finding that advanced liver disease generally leads to more complications. While understanding the natural course of chronic HCV infection, we acknowledge that many of the decompensation/SAE events may not be treatment-related. Particularly, the study design is not optimized to infer causality of treatment given the lack of a true control group, but we feel that describing the events experienced by this specific population while on newer direct acting antivirals (DAA) will educate providers and allow them to closely monitor these high-risk patients while carefully individualizing treatment regimens. As observed in other studies, SOF was generally well tolerated and most of the SAEs observed in our study were from RBV use, while hepatic decompensation was likely due to the natural progression of HCV in the allograft. These findings are similar to a compassionate use program that administered SOF and RBV with or without PEG for 12 or 24 wk to patients with severe recurrent hepatitis or cirrhosis. In this program, SVR was 59%, while most SAEs were due to hepatic decompensation that was not thought to be treatment-related[24].
In October 2014, the fixed-dose combination of ledipasvir/sofosbuvir (LDV/SOF) was approved by the Food and Drug Administration to be used in combination with or without RBV for HCV genotypes 1 and 4[21-23]. The current standard of care for recurrent HCV infection in genotypes 1 and 4 in the allograft is LDV/SOF with or without weight-based RBV for 12 or 24 wk[25] in both treatment-naïve and treatment-experienced patients. In a recent study, LDV/SOF plus weight-based RBV was given to patients with advanced liver disease for 12 or 24 wk, including those with HCV recurrence post-LT. SVR for the post-LT group ranged from 60%-75% in those with severe hepatic impairment to 96%-98% in those without cirrhosis or those with compensated cirrhosis[26]. In December 2014, the all-oral regimen of ombitasvir, paritaprevir, ritonavir, and dasabuvir was approved for use in genotype 1 chronic HCV infection. In a phase 2 study, 34 liver transplant recipients with recurrent genotype 1 HCV infection were given a 24 wk regimen of fixed dose ombitasvir-paritaprevir-ritonavir with dasabuvir plus low-dose RBV[27]. Thirty-three patients (97%) achieved SVR12. There were no episodes of graft rejection or interaction with calcineurin inhibitors, and the rate of SAEs was 6%[27].
For HCV genotype 2 or 3 infection in the allograft, the standard of care remains SOF/RBV for 24 wk in non-LT and post-LT patients, compensated and decompensated cirrhotics, and treatment-naïve and treatment experienced patients[28]; therefore, RBV will continue to be used extensively. Even with the approval of LDV/SOF, the combination of SOF/RBV will continue to be used internationally across all genotypes, particularly in areas where LDV/SOF may not be accessible. It is imperative that patients that are pre-disposed to complications secondary to RBV be closely monitored. These patients should have appropriate dose reductions to neutralize the effects of RBV. SOF may also be used as salvage therapy with simeprevir (SMV) without RBV in those patients who relapsed with newer NS5A inhibitors. A recent study presented by Hezode et al[29] evaluated the effectiveness of retreatment with 12 wk of SOF/SMV in patients infected with HCV genotypes 1 and 4 who relapsed after treatment with daclatasvir-based regimens. Of those retreated with SMV/SOF, 13/15 (87%) achieved SVR 12.
This observational study has significant strengths and some limitations. The strengths of this study include the real-world setting and a cohort from a single referral center. The real-world setting allows us to report experiences with newer HCV regimens in clinical practice. Registration trials often include a specific patient population that may exclude patients with more advanced liver disease, and real-world experiences can shed new light on adverse effects that may not have been seen in clinical trials. The low response rate in this series resulted from the fact that most patients were infected with HCV genotype 1. This is important given the fact that SOF/RBV will continue to be prescribed to patients with HCV genotypes 2 and 3, despite the introduction of newer DAAs. Although we were not able to perform a multivariable analysis, our study serves as a platform to further study factors associated with decompensation and/or SAE in patients treated with these drugs.
There were several limitations. Several patients included in the cohort had episodes of hepatic decompensation within 12 mo prior to initiating treatment, making it difficult to infer causality for on-treatment decompensation/SAE, as one could argue that on-treatment episodes could be related to the natural progression of liver disease. Another limitation is the lack of a true matched control group that did not receive HCV treatment, which would be optimal in a study design created to investigate treatment-related complications; however, the patients who are not treated differ in comparison to those who are treated, particularly in liver disease severity, resulting in selection bias. We were limited to 42 patients in the cohort. Consequently, multivariable analysis could not be performed. We found that lower pre-treatment hemoglobin and eGFR and higher total bilirubin may be factors associated with decompensation/SAE. However, a larger sample size is needed to confirm these findings.
In summary, we found that lower pre-treatment hemoglobin level and eGFR and higher serum total bilirubin may be factors associated with both hepatic decompensation and/or SAE. Post-LT patients with recurrent HCV infection generally tolerate SOF well. Given that all post-LT patients are on immunosuppressant medications known to impair renal function and that all will likely receive RBV and/or SOF for any recurrence of HCV infection in the allograft, it is important that these patients are monitored closely for complications. Given that 19% of the cohort suffered from RBV-induced hemolytic anemia, management of anemia remains an important clinical challenge. Further studies with larger sample sizes need to be undertaken to confirm our findings and determine independent risk factors for decompensation/SAE.
ACKNOWLEDGMENTS
The authors would like to thank all of the patients and their providers who participated in this study, as well as all of the authors for their contributions to the final version of the manuscript.
COMMENTS
Background
Following liver transplantation of hepatitis C virus (HCV) infected patients, recurrent infection is nearly universal. More effective and less toxic regimens are now available. To improve their safe utilization, this study aimed to identify factors associated with hepatic decompensation and/or other serious adverse events in liver transplant recipients on Sofosbuvir-based regimens for recurrent HCV infection in a real-world setting.
Research frontiers
Interferon and ribavirin were the standard of care for the treatment of HCV for many years. With the advent of new direct acting antiviral (DAA) agents for HCV, there is a changing landscape in the management of HCV infection. Boceprevir and telaprevir, inhibitors of the HCV serine protease NS3/4A, were the first generation of DAAs approved by the United States Food and Drug Administration. Newer DAAs include sofosbuvir, a NS5B polymerase inhibitor, and simeprevir, a second phase NS3/4A protease inhibitor. These drugs may be used in combination with ribavirin and do not require the addition of interferon, and thus have less toxicity. By studying these drugs in a real-world setting, we can give providers more information on the safety profile. This in turn can influence individualization of HCV treatment regimens and closer monitoring in higher risk patients.
Innovations and breakthroughs
While sofosbuvir has shown remarkable efficacy, the full safety profile in a real-world setting is limited. Sofosbuvir in combination with ribavirin will continue to be used in HCV genotypes 2 and 3 in the post-LT population and in areas where 3rd generation DAAs are not available. Awareness of effects not seen in earlier trials may allow safer and more effective use of these medications.
Applications
This study identified low baseline hemoglobin and estimated glomerular filtration rate, and high serum total bilirubin as potential risk factors for hepatic decompensation and serious adverse events in post-LT patients on sofosbuvir-based regimens for recurrent HCV. Given that transplant patients are on immunosuppressive therapy that impairs renal function, careful dose adjustments and monitoring are necessary for those on medications that are renally metabolized. The high incidence of anemia requiring transfusion can be explained by an increased concentration of ribavirin metabolites due to decreased renal clearance.
Terminology
Hepatitis C is an infectious disease caused by the HCV, an infectious agent that primarily infects cells of the liver. Direct-acting antiviral drugs are medications that target specific areas of the HCV in order to prevent the virus from duplicating. Sustained virologic response is defined as the absence of detectable HCV RNA in blood 12 wk after the end of treatment. Hepatic decompensation is indicated by new or increased jaundice, ascites, encephalopathy, variceal bleeding, or sepsis. Serious adverse events, defined by the Food and Drug Administration, include those causing any death, hospitalization, significant or permanent disability, or an intervention to prevent permanent impairment or damage.
Peer-review
This manuscript “Hepatic decompensation/serious adverse events in post-liver transplantation recipients on sofosbuvir for recurrent HCV” is very interesting.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Icahn School of Medicine at Mount Sinai Institutional Review Board.
Informed consent statement: Study participants did not provide informed consent prior to study enrollment as the Icahn School of Medicine at Mount Sinai Institutional Review Board provided a waiver of authorization to release de-identified patient data for research purposes.
Conflict-of-interest statement: Kian Bichoupan is a paid consultant for Gilead Sciences and Janssen Pharmaceuticals, Inc. Dr. Andrea D. Branch is a paid consultant for Gilead Sciences and Janssen Pharmaceuticals, Inc. Dr. Douglas T. Dieterich serves as a paid lecturer, consultant and is a member on scientific advisory boards of companies which either develop or assess medicines used for the treatment of viral hepatitis. These companies include Gilead Sciences, Abbvie, Achillion, Bristol-Myers Squibb, Merck, and Janssen Pharmaceuticals, Inc. Thomas D. Schiano is a paid consultant for Salix, Merck, Gilead, BMS, Novartis, and Janssen. Alyson Harty is a paid consultant for Abbvie Pharmaceuticals, Gilead Sciences, and Janssen Pharmaceuticals, Inc. Michel Ng is a paid member of AbbVie’s Speakers bureau. Viktoriya Khaitova is a paid member of a scientific advisory board for AbbVie and Johnson & Johnson. Charissa Chang is a paid consultant for Gilead. Dr. Neal Patel, Lawrence Ku, Dr. Rachana Yalamanchili, Donald Gardenier, David Motamed, Dr. Nancy Bach, Dr. Meena Bansal, Dr. Priya Grewal, Dr. Ritu Agarwal, Dr. Lawrence Liu, Dr. Gene Im, Dr. Leona Kim-Schluger, Dr. Joseph Odin, Dr. Jawad Ahmad, Dr. Scott Friedman, and Dr. Ponni V. Perumalswmi do not have any disclosures.
Data sharing statement: Technical appendix, statistical code, and dataset available from the corresponding author at andrea.branch@mssm.edu. Consent was not obtained, but the presented data are anonymized and risk of identification is low.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 18, 2015
First decision: September 29, 2015
Article in press: November 19, 2015
P- Reviewer: Pan JJ S- Editor: Qi Y L- Editor: A E- Editor: Ma S
References
- 1.Crespo G, Mariño Z, Navasa M, Forns X. Viral hepatitis in liver transplantation. Gastroenterology. 2012;142:1373–1383.e1. doi: 10.1053/j.gastro.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucey MR. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology. 2002;122:889–896. doi: 10.1053/gast.2002.32418. [DOI] [PubMed] [Google Scholar]
- 3.Jeong SW, Choi Y, Kim JW. Management of viral hepatitis in liver transplant recipients. Clin Mol Hepatol. 2014;20:338–344. doi: 10.3350/cmh.2014.20.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gambato M, Lens S, Fernández-Carrillo C, Alfaro I, Forns X. Viral hepatitis and liver transplantation: pathogenesis, prevention and therapy of recurrent disease. Dig Dis. 2014;32:538–544. doi: 10.1159/000360831. [DOI] [PubMed] [Google Scholar]
- 5.Schluger LK, Sheiner PA, Thung SN, Lau JY, Min A, Wolf DC, Fiel I, Zhang D, Gerber MA, Miller CM, et al. Severe recurrent cholestatic hepatitis C following orthotopic liver transplantation. Hepatology. 1996;23:971–976. doi: 10.1002/hep.510230505. [DOI] [PubMed] [Google Scholar]
- 6.Berenguer M, Palau A, Aguilera V, Rayón JM, Juan FS, Prieto M. Clinical benefits of antiviral therapy in patients with recurrent hepatitis C following liver transplantation. Am J Transplant. 2008;8:679–687. doi: 10.1111/j.1600-6143.2007.02126.x. [DOI] [PubMed] [Google Scholar]
- 7.Abdelmalek MF, Firpi RJ, Soldevila-Pico C, Reed AI, Hemming AW, Liu C, Crawford JM, Davis GL, Nelson DR. Sustained viral response to interferon and ribavirin in liver transplant recipients with recurrent hepatitis C. Liver Transpl. 2004;10:199–207. doi: 10.1002/lt.20074. [DOI] [PubMed] [Google Scholar]
- 8.Bahra M, Neumann UP, Jacob D, Langrehr JM, Berg T, Neuhaus R, Neuhaus P. Fibrosis progression in hepatitis C positive liver recipients after sustained virologic response to antiviral combination therapy (interferon-ribavirin therapy) Transplantation. 2007;83:351–353. doi: 10.1097/01.tp.0000250575.92788.aa. [DOI] [PubMed] [Google Scholar]
- 9.Bizollon T, Ahmed SN, Radenne S, Chevallier M, Chevallier P, Parvaz P, Guichard S, Ducerf C, Baulieux J, Zoulim F, et al. Long term histological improvement and clearance of intrahepatic hepatitis C virus RNA following sustained response to interferon-ribavirin combination therapy in liver transplanted patients with hepatitis C virus recurrence. Gut. 2003;52:283–287. doi: 10.1136/gut.52.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berenguer M. Systematic review of the treatment of established recurrent hepatitis C with pegylated interferon in combination with ribavirin. J Hepatol. 2008;49:274–287. doi: 10.1016/j.jhep.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Coilly A, Roche B, Duclos-Vallée JC, Samuel D. Optimal therapy in hepatitis C virus liver transplant patients with direct acting antivirals. Liver Int. 2015;35 Suppl 1:44–50. doi: 10.1111/liv.12728. [DOI] [PubMed] [Google Scholar]
- 12.Fagiuoli S, Ravasio R, Lucà MG, Baldan A, Pecere S, Vitale A, Pasulo L. Management of hepatitis C infection before and after liver transplantation. World J Gastroenterol. 2015;21:4447–4456. doi: 10.3748/wjg.v21.i15.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verna EC, Saxena V, Burton JR, O’Leary JG, Dodge JL, Stravitz RT, Levitsky J, Trotter JF, Everson GT, Brown RS, et al. Telaprevir- and Boceprevir-based Triple Therapy for Hepatitis C in Liver Transplant Recipients With Advanced Recurrent Disease: A Multicenter Study. Transplantation. 2015;99:1644–1651. doi: 10.1097/TP.0000000000000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coilly A, Roche B, Dumortier J, Leroy V, Botta-Fridlund D, Radenne S, Pageaux GP, Si-Ahmed SN, Guillaud O, Antonini TM, et al. Safety and efficacy of protease inhibitors to treat hepatitis C after liver transplantation: a multicenter experience. J Hepatol. 2014;60:78–86. doi: 10.1016/j.jhep.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Londoño MC, Perelló C, Cabezas J, Cañete N, Lens S, Mariño Z, Gambato M, Rodríguez R, Menéndez S, Carrión JA, et al. The addition of a protease inhibitor increases the risk of infections in patients with hepatitis C-related cirrhosis. J Hepatol. 2015;62:311–316. doi: 10.1016/j.jhep.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 16.Tischer S, Fontana RJ. Drug-drug interactions with oral anti-HCV agents and idiosyncratic hepatotoxicity in the liver transplant setting. J Hepatol. 2014;60:872–884. doi: 10.1016/j.jhep.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 18.Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, Illeperuma A, Svarovskaia E, Brainard DM, Symonds WT, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370:1993–2001. doi: 10.1056/NEJMoa1316145. [DOI] [PubMed] [Google Scholar]
- 19.Koff RS. Review article: the efficacy and safety of sofosbuvir, a novel, oral nucleotide NS5B polymerase inhibitor, in the treatment of chronic hepatitis C virus infection. Aliment Pharmacol Ther. 2014;39:478–487. doi: 10.1111/apt.12601. [DOI] [PubMed] [Google Scholar]
- 20.Charlton M, Gane E, Manns MP, Brown RS, Curry MP, Kwo PY, Fontana RJ, Gilroy R, Teperman L, Muir AJ, et al. Sofosbuvir and ribavirin for treatment of compensated recurrent hepatitis C virus infection after liver transplantation. Gastroenterology. 2015;148:108–117. doi: 10.1053/j.gastro.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, Zarski JP, Agarwal K, Buggisch P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889–1898. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 22.Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, Shiffman ML, Schiff E, Ghalib R, Ryan M, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879–1888. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 23.Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, Nahass R, Ghalib R, Gitlin N, Herring R, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483–1493. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- 24.Forns X, Charlton M, Denning J, McHutchison JG, Symonds WT, Brainard D, Brandt-Sarif T, Chang P, Kivett V, Castells L, et al. Sofosbuvir compassionate use program for patients with severe recurrent hepatitis C after liver transplantation. Hepatology. 2015;61:1485–1494. doi: 10.1002/hep.27681. [DOI] [PubMed] [Google Scholar]
- 25.Reddy KR, Everson G, Flamm SL, denning J, Arterburn S, Brandt-Sarif T, Pang PS, McHutchison JG, Curry MP, Charlton M. Ledipasvir/Sofosbuvir with Ribavirin for the Treatment of HCV in Patients with Post Transpant Recurrence: Preliminary Results of a Prospective Multicenter Study. Hepatology. 2014;60:197A–200A. [Google Scholar]
- 26.Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS, Fried MW, Terrault NA, O’Leary JG, Vargas HE, et al. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients With Advanced Liver Disease. Gastroenterology. 2015;149:649–659. doi: 10.1053/j.gastro.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Kwo PY, Mantry PS, Coakley E, Te HS, Vargas HE, Brown R, Gordon F, Levitsky J, Terrault NA, Burton JR, et al. An interferon-free antiviral regimen for HCV after liver transplantation. N Engl J Med. 2014;371:2375–2382. doi: 10.1056/NEJMoa1408921. [DOI] [PubMed] [Google Scholar]
- 28.AASLD/IDSA/IAS-USA. Recommendations for testing, managing, and treating hepatitis C. Available from: http://www.hcvguidelines.org.
- 29.Hezode C, Chevaliez S, Scoazec G, Soulier A, Bouvier-Alias M, Ruiz I, Mallat A, Feray C, Pawlotsky JM. Retreatment with an interferon-free combination of simeprevir-sofosbuvir in patients who had previously failed on HCV NS5A inhibitor-based regimens. 13th European Resistance Workshop; 2015. [Google Scholar]


