Abstract
Cystic fibrosis (CF) results in inflammation, malabsorption of fats and other nutrients, and obstruction in the gastrointestinal (GI) tract, yet the mechanisms linking these disease manifestations to microbiome composition remain largely unexplored. Here we used metagenomic analysis to systematically characterize fecal microbiomes of children with and without CF, demonstrating marked CF-associated taxonomic dysbiosis and functional imbalance. We further showed that these taxonomic and functional shifts were especially pronounced in young children with CF and diminished with age. Importantly, the resulting dysbiotic microbiomes had significantly altered capacities for lipid metabolism, including decreased capacity for overall fatty acid biosynthesis and increased capacity for degrading anti-inflammatory short-chain fatty acids. Notably, these functional differences correlated with fecal measures of fat malabsorption and inflammation. Combined, these results suggest that enteric fat abundance selects for pro-inflammatory GI microbiota in young children with CF, offering novel strategies for improving the health of children with CF-associated fat malabsorption.
People with the genetic disease cystic fibrosis (CF) have dysfunction in multiple organs with epithelial-lined lumens. Among these individuals, disease of the respiratory and gastrointestinal (GI) tracts are the most important determinants of quality and length of life1. The manifestations of CF GI disease begin early in life and include maldigestion and malabsorption of specific nutrients, particularly proteins, fats, and fat-soluble vitamins. This malabsorption leads not only to malnutrition, but also to intestinal obstruction and altered transit times of luminal contents through the GI tract2. Therefore, the CF GI tract has an abnormal physicochemical environment that could select for different GI microbiota.
In support of this hypothesis, we recently identified a fecal dysbiosis among children with CF characterized by a strikingly high abundance of Escherichia coli that was most marked in samples from younger subjects. We further showed that the magnitude of this E. coli dysbiosis correlated with a fecal measure of inflammation (calprotectin) as well as with fecal fat content3. However, a more systematic profiling of the CF microbiome could elucidate additional relationships between dysbiosis, intestinal inflammation, and luminal fat content.
In this study, we therefore set out to comprehensively analyze metagenomic data from this fecal sample collection, to fully characterize the taxonomic and functional composition of these metagenomes, and to leverage the longitudinal nature of the sample collection in order to better define CF-associated taxonomic dysbioses and functional imbalances, their temporal dynamics, and their links to inflammation and fat content. We hypothesized that taxonomic dysbiosis in the fecal microbiota of children with CF would lead to imbalances in the fecal abundance of microbial genes involved in the metabolism of fat or products related to host inflammation. Our results support a model of CF GI dysbiosis and dysfunction in which the malabsorption of dietary fat selects for a pro-inflammatory enteral microbiome.
Methods
Subjects and samples
The samples and source subjects for this analysis were collected as part of a prior study comparing the fecal microbiota of children aged <3 years old with and without CF. This study was approved by the Seattle Children’s Hospital Institutional Review Board, all procedures were carried out in accordance with the approved guidelines, and informed consent was obtained for all subjects. Subject inclusion/exclusion criteria, as well as demographic information, have been previously described3. The current analysis included not only the specimens described in our prior publication3, but additional specimens collected from the same subjects after our earlier analysis has begun. The final sample set (after removing low-coverage samples as described below) comprised 104 fecal samples from 14 children with CF (sampled between the ages of 15 days to 5 years) and 12 children without CF (sampled between the ages of 55 days to 3.5 years), and with between 2–5 samples per subject collected over an approximately one year period. In several analyses described here, we additionally binned samples by age group (e.g., first, second, or third year of life) to further control for potential age-related differences in the microbiome. Detailed information regarding the included specimens and source patients are provided in Supplementary Table S1.
Fecal fat and calprotectin analyses
Fecal fat content was measured by the acid steatocrit method, performed as previously described for each sample in the collection3. Calprotectin–a product of neutrophils – was used as a fecal measure of inflammation and was defined using an FDA-approved enzyme-linked immunosorbent assay as previously described3.
Metagenomic sequencing
Sample processing and DNA extraction were performed as previously described3. Briefly, sequencing data for this study resulted from Illumina HiSeq-2000 sequencing using the Nextera platform. The Human Microbiome Project (HMP) protocol was used for processing reads4,5. Specifically, BMTagger was used to remove human reads. Duplicates were removed using the HMP documented protocol. Runs for which a pair failed were not duplicate-filtered. Reads were quality-trimmed using HMP scripts, modified to work with single-end runs. Reads shorter than 60 bases after quality trimming were removed. Four samples that had less than 10 M reads after filtering human reads were also removed from the analysis. This process resulted in a total of 104 samples with an average of 58 million reads per sample.
Taxonomic profiling and analysis
The taxonomic composition of each sample was defined using metagenomic phylogenetic analysis (MetaPhlAn6; version 1.7.3). To examine variation in taxonomic profiles across samples, a principal component analysis (PCA) was performed. Differentially abundant taxa in CF versus non-CF samples were identified using the Wilcoxon rank-sum test with false discovery rate (FDR) <0.1. To examine the role of E. coli in CF vs. non-CF samples, we performed several analyses using both the original taxonomic profiles and taxonomic profiles in which E. coli was excluded and the abundances of the remaining species were renormalized within each sample. In addition, we confirmed that our results held when excluding samples that were taken from children who had been administered antibiotics in the prior 60 days, and again when excluding samples from children who were breastfed at sampling.
Functional annotation of metagenomic reads
To determine the presence and relative abundance of genes in each metagenomic sample, reads were mapped to the Kyoto Encyclopedia of Genes and Genomes (KEGG). Specifically, each sequencing read was aligned to a peptide database containing the peptide sequences from all annotated KEGG organisms (KEGG7; v. 67.0, July 15th, 2013 weekly release) using mBLASTx with standard parameters and accepting all matches with an E-value < 1 (in accordance with HMP protocol4,5). Each read was then annotated according to the KEGG Orthology groups (KOs) associated with the identified alignments using the ‘top gene’ approach that was previously described and carefully validated8. Notably, while significantly fewer KOs were identified in CF versus non-CF samples on average (rank-sum p < 0.03), when adjusted for the number of reads per sample (which were on average lower in CF samples), this difference became non-significant.
To streamline the analysis, we analyzed the data at the level of KEGG functional pathways and modules by summing the relative abundances of all KOs associated with each pathway or module. Pathways and modules were further filtered to verify that downstream analysis considers only bacterial pathways/modules. Specifically, a pathway (module) was included in our analysis only if at least 1% of bacterial genomes in KEGG contained at least 1 KO from that pathway (module), and if these bacterial genomes contained at least 5% (20%) of the KOs in the pathway (module) on average. Using this criterion resulted in a list of 146 and 409 bacterial pathways and modules, respectively.
Comparative statistical analysis of functional profiles
For each pathway/module, CF samples were compared to non-CF samples using the Wilcoxon rank-sum test including a multiple comparisons correction using a 5% false discovery rate (FDR) threshold for both pathways and modules. Considering the relatively small number of samples available, several analyses were done by comparing all CF samples to all non-CF samples, ignoring age and subject identity. In additional analyses, samples were further binned by age group, to better control for subject age. For each pathway/module, the median number of non-zero KOs (i.e., KOs identified) across all samples was calculated, and only pathways and modules with more than 20 or 5 non-zero KOs, respectively, were considered. PCA was used to explore variation in functional profiles across samples. To further examine the patterns observed in this PCA, we additionally applied MUSiCC, a novel marker genes-based normalization scheme for accurate profiling of gene abundances in the microbiome9. In addition, we confirmed that our results held when excluding samples that were taken from children who had been administered antibiotics prior to sampling, both within 30 and 60 days, and when excluding samples from children who were breastfed at the time of sampling. We further confirmed that our main findings held when restricting our dataset to include only a single sample from each subject (from the first year of life), to verify that our results were not biased by the presence of multiple samples per individual.
BWA-based analysis of the impact of E. coli on fatty acid biosynthesis pathway
To verify that the observed depletion of the fatty acid biosynthesis pathway is not solely the result of increased E. coli abundance in CF samples, we re-mapped the reads from all samples to a set of genome clusters using BWA10, following the method described in Greenblum et al.11. We analyzed the relative abundance of the fatty acid biosynthesis pathway using this alternative mapping method, confirming that it reproduced results obtained with the original BLASTx-based mapping (specifically, the depletion of this pathway in CF samples; P = 2.9e–05, Wilcoxon rank-sum test). We then removed all reads that mapped to the E. coli genome cluster and applied the same analysis, confirming that the fatty acid biosynthesis pathway was still significantly depleted in CF samples (P = 0.039).
Creation of butyrate and propionate catabolism modules
To create functional modules representing butyrate catabolism and non-catabolism, a list of 8 enzymes from the butyrate metabolism pathway found to be associated with butyrate catabolism12,13 was compiled based on a detailed literature survey. The 14 KOs corresponding to these 8 enzymes were defined as the “butyrate catabolism” module, while the other 50 KOs representing enzymes in the butyrate metabolism pathway were defined as the “butyrate non-catabolism” module. Removing bcd (K00248) or both bcd and atoA-atoD (K01034, K01035), for which literature support was weaker, from the “butyrate catabolism” module did not qualitatively change the results reported below. We similarly partitioned the propionate metabolism pathway into propionate catabolism (19 KOs) and propionate non-catabolism (43 KOs) modules14,15.
Results
Taxonomic analysis of pediatric CF fecal samples reveals taxonomic dysbiosis that diminishes with age
In a previous cross-sectional microbiota analysis, we showed that E. coli was markedly more abundant in fecal samples from young children with CF than in those without CF3. This prior study focused on this striking E. coli dysbiosis, but did not characterize in detail how these differences related to the age of the source subjects, how these differences impacted the rest of the microbiota, and how community-wide dysbiosis links to E. coli abundance. To address these questions, here we obtained Illumina shotgun sequence reads from an expanded sample set, including a total of 104 fecal specimens collected over a period averaging approximately 12 months from 26 children (14 with CF and 12 without CF), comprising 52 CF and 52 non-CF samples, and characterized their microbiota using metagenomic phylogenetic analysis (MetaPhlAn6). We found that CF samples had a high relative abundance of Proteobacteria (including but not limited to E. coli) and Actinobacteria and low abundance of Firmicutes, Bacteroidetes and Verrucomicrobia compared to samples from children without CF (Fig. 1a; see also Supplementary Fig. S1). For this sample set, CF fecal microbiota also exhibited significantly lower α-diversity (Shannon index) than did non-CF microbiota (p < 0.0063, two-sided t-test). Importantly, however, this difference was no longer significant after excluding E. coli (p = 0.094; Methods), suggesting that the expansion of E. coli in CF fecal communities is not accompanied by a significant difference in the microbial diversity of the rest of the microbiota.
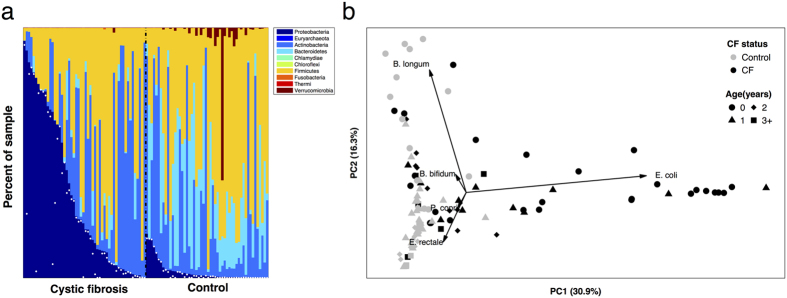
Figure 1.
(a) The relative abundance of bacterial phyla among fecal samples from children with CF (left) and without CF (right). Samples are ordered for ease of display in decreasing abundance of Proteobacteria; relative abundance of E. coli is marked in each sample by a white dot. (b) A principal components analysis (PCA) of the taxonomic profile of each sample. The percent of variation explained by each of the first two components is noted on the axes and the top five loadings (scaled for ease of display) are illustrated. Evidently, the microbiota of CF fecal samples differ the most from non-CF samples at earlier source subject ages, driven largely by relative abundance of E. coli and Bifidobacterium longum.
Going beyond phylum-level, we further characterized the taxa that differed significantly in relative abundance between CF and non-CF samples (Supplementary Table S2; Methods). We found that although Firmicutes was relatively depleted as a phylum in CF samples (P < 0.01), one order of Firmicutes, Lactobacillales, was significantly enriched in CF samples (P < 0.001), including the known pathogens E. faecalis and E. faecium, which are known to frequently exhibit antibiotic resistance16,17. In addition, the Firmicutes genus Veillonella was significantly enriched in CF samples (P < 10−5), including the species V. parvula, which has been identified in the lungs of children with CF18. In contrast, the Firmicutes order Clostridiales (which includes many taxa that contribute to GI immune homeostasis and development19,20) was significantly depleted in CF samples (P < 10−4); one of the only two Clostridiales species that were enriched in CF samples was C. difficile, well-known for its pathogenic potential after infancy21. Importantly, since environmental factors such as antibiotic exposure and breastfeeding can impact an infant’s microbiome22,23,24,25, we confirmed that analyses yielded similar results when excluding all samples from children who were breastfed at the time of collection, or samples taken after antibiotic treatment up to 60 days, as well as when controlling for E. coli abundance (see Supplementary Tables S3–S5).
To further characterize how the phylogenetic differences observed above change with age, we performed a principal components analysis (PCA) of the obtained taxonomic profiles. As shown in the resulting PCA plot (Fig. 1b), microbiota from control samples were largely distinguished from those of CF samples by the first component, owing mostly to differences in E. coli abundance (evidenced by the PCA loadings). Notably, however, CF samples from younger children were more clearly separated from younger control samples, while samples from older children with CF seemed to converge towards samples from older controls. The second principal component, predominantly governed by the relative abundance of Bifidobacterium spp, further separated younger children from older children in both groups. Next, to specifically examine the role of E. coli in CF and non-CF microbiota composition over time, we performed a similar PCA analysis after excluding the abundance of E. coli from the microbiota of each sample (Fig. S2; see Methods). The resulting CF and non-CF samples were still largely separated on the PCA plot, mostly owing to the abundances of the Actinobacterium Bifidobacterium bifidum (which is generally higher in CF samples) and the Firmicute Eubacterium rectale (lower in CF samples). Indeed, a statistical analysis of the abundances of the various species in CF versus non-CF samples while controlling for E. coli abundance confirmed that Eubacterium rectale (as well as several other species) had significantly lower relative abundance in CF samples (Supplementary Table S3). Again, the magnitude of the differences between CF and non-CF samples tended to diminish with source subject age (Fig. 1 and Supplementary Fig. S2), indicating that the taxonomic dysbiosis was most marked in infancy and waned over time.
Functional metagenomic analysis shows differences in metabolic capacity between CF and non-CF pediatric fecal microbiota
To investigate potential functional differences between CF and non-CF fecal microbiota, shotgun metagenomic reads were mapped to the KEGG database to estimate the abundance of each KEGG orthology group (KO) in each sample (Methods). In total, we identified 13,840 KOs across the entire sample set, with an average of 5,725 KOs per sample. We then summed the abundances of all KOs associated with each microbial pathway (or module) to obtain a comprehensive functional profile of each sample (Methods).
A PCA of the resulting pathway-level abundance profiles demonstrated a clear distinction between samples from young children with CF (≤1 year) and those without CF (Fig. 2). Importantly, however, those distinctions were diminished in samples from older children, mirroring the pattern observed in the taxonomic profile (Fig. 1b). In addition, as observed for taxonomy, functional composition among samples from children with CF tended to differ between younger and older subjects much more than did those from children without CF. Using a novel normalization method that aims to correct potential biases that stem from using relative, rather than absolute, abundances (Methods) produced similar patterns and further highlighted the differences in functional capacity between CF and non-CF fecal microbiota (Fig. S3). These findings suggest that the GI microbiome in young children with CF have functional capacities that differ markedly from those without CF, but that this effect diminishes with age.
Figure 2. Principal component analysis (PCA) using pathway-level relative abundances of CF (black symbols) versus non-CF (gray symbols) samples.
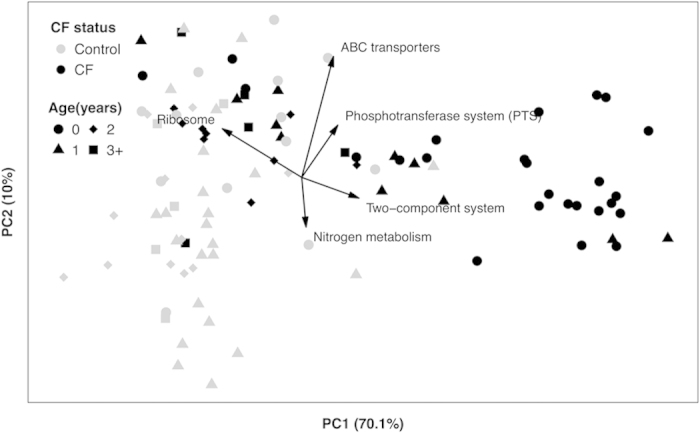
The percent of variation explained by each of the first two components is noted on the axes. The top five loadings (scaled for ease of display) are also illustrated.
Pediatric CF fecal microbiomes have altered capacities for fatty acid metabolism
We next used statistical analysis to identify significant differences in the relative abundance of specific bacterial functional pathways or modules that potentially underlie the separation observed in the PCA results above (Figs 2 and S3; Methods). We identified 17 pathways and 25 modules that were enriched in CF samples, and 36 pathways and 65 modules that were depleted in CF samples, relative to non-CF samples (p < 0.05, corrected for multiple comparisons; see Methods and Supplementary Tables S6–S9). Inspection of these functional differences revealed that multiple pathways and modules for fatty acid metabolism were differentially abundant in CF. Significantly, the KEGG fatty acid degradation pathway was enriched in CF, whereas the fatty acid biosynthesis pathway, as well as two fatty acid biosynthesis modules, were depleted in CF (Fig. S4 and Table 1). This decreased capacity of the CF fecal microbiota for fatty acid synthesis but an increased ability to metabolize fats overall might be expected if fatty acid availability was an important selective force for microbiota in the CF lumen. Examining the relative abundance of these pathways within subjects as a function of age again demonstrated that these differences in metabolic capacity between the CF and non-CF samples generally diminished with time (Fig. S4).
Table 1. Fatty acid metabolism functional differences between CF and non-CF fecal samples.
| Functional assignmenta | #non-zero KOsb | Shiftc | P valued |
|---|---|---|---|
| ko00650: Butyrate metabolism | 60 | E | 3.83e–07 |
| ko00640: Propionate metabolism | 53 | E | 1.01e–06 |
| ko00071: Fatty acid degradation | 28 | E | 5.70e–04 |
| ko00061: Fatty acid biosynthesis | 22 | D | 2.43e–08 |
| M00083: Fatty acid biosynthesis, elongation | 12 | D | 5.34e–10 |
| M00082: Fatty acid biosynthesis, initiation | 11 | D | 2.06e–05 |
aNumbers beginning with ‘ko’ indicate a functional pathway; those beginning with an ‘M’ indicate a functional module.
bSee Methods.
cEnriched (E) or depleted (D) in CF.
dWilcoxon rank-sum test for CF vs. non-CF.
Because the CF fecal dysbiosis we observed previously3 was characterized by a marked relative enrichment for the Proteobacterium E. coli, particularly among the samples taken at earlier ages, we additionally set out to examine the contribution of E. coli to the functional differences in fatty acid metabolism reported above. To this end, we considered only the 63 samples that had a relative abundance of E. coli of <5% (since a substantial number of healthy samples, 7 out of 52, had at least 5% E. coli) and again used comparative analysis to identify differentially abundant pathways. We found that, despite the decreased sample size of this analysis, both the enrichment for genes encoding fatty acid degradation and the depletion of those encoding fatty acid biosynthesis in CF samples remained significant (p < 0.02 and p < 0.002, respectively, FDR <5%), indicating that the marked shifts in the abundance of fatty acid degradation and biosynthetic genes in the CF metagenomes could not be attributed solely to the differential abundance of E. coli, but rather involved the wider microbiota. In addition, we confirmed that the depletion of the fatty acid biosynthesis pathway in CF samples is not solely driven by E. coli by using an alternative sequence-based alignment analysis and removing short reads originating from E. coli genomes (see Methods). As for our taxonomic analyses described above, we further confirmed that our findings are not affected by excluding samples collected after antibiotic treatment or during breastfeeding (Supplementary Table S10).
Pediatric CF fecal microbiomes have increased capacities for breakdown of the anti-inflammatory small-chain fatty acids butyrate and propionate, which correlate with fecal measures of inflammation.
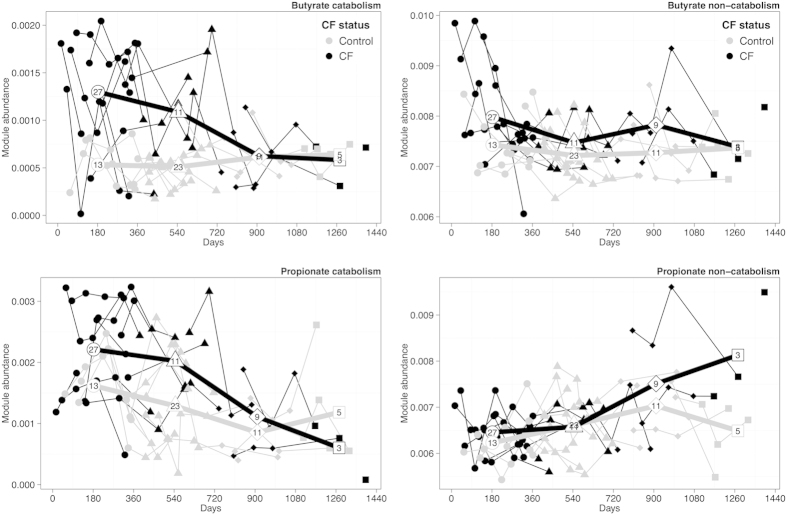
In addition to the above general shifts in fatty acid metabolism, we identified CF-associated enrichment specifically in the metabolism of butyrate and propionate – two short-chain fatty acids (SCFAs) produced and metabolized by GI microbiota and important for intestinal health26. In defining these pathways, however, KEGG does not distinguish between synthetic and degrading (or catabolic) processes. Considering the difference observed above between fatty acid biosynthesis and catabolism in relation to CF, we conducted a literature survey to manually partition the genes in each of these two SCFA pathways into two modules, one representing genes known to be associated with catabolism and the other representing genes encoding other (i.e., non-catabolic) enzymatic functions (Methods; see Supplementary Tables S11–S12 for full lists). In contrast to the trend observed above for fatty-acid metabolism, both the butyrate catabolism module and the butyrate non-catabolism module were enriched in CF samples; however, the CF enrichment level of the catabolism module was markedly more pronounced (p < 10−6 vs. p < 10−3 for catabolism and non-catabolism, respectively; Supplementary Table S13). Moreover, comparing the ratio between the average abundance of each of these modules in CF vs. non-CF samples, we detected a markedly more pronounced increase in the abundance of the catabolic module in CF vs. that of the non-catabolic module (1.95-fold vs. 1.07-fold for catabolism and non-catabolism, respectively). For propionate, only the catabolism module was enriched in CF, and again its relative abundance in CF vs. non-CF samples was much higher than that of the non-catabolic module (p < 10−3, 1.47-fold, vs. p = 0.56, 1.02-fold, for catabolism and non-catabolism, respectively; Supplementary Table S13). Plotting the relative abundance of these SCFA modules in CF and non-CF samples over time highlighted the more pronounced CF-associated enrichment of the catabolic modules (compared to the non-catabolic modules), again suggesting that these differences in SCFA metabolism diminish with age (Fig. 3).
Figure 3. Temporal patterns in the abundance of butyrate and propionate modules in fecal samples from children with (black) vs. without (gray) CF.
The abundance of each module is defined as the sum of the relative abundances of all the KOs associated with that module and is plotted by age of source subject. Lines connect samples from the same subject. The bold lines illustrate the average abundance of the module for all CF vs. all non-CF samples within a year of age (i.e., 0 to 365 days, 366 to 730 days, etc.), with each average plotted at the midpoint for each year. The number of samples available to calculate each average is shown inside the marker.
The above results indicate that pediatric CF fecal microbiota have altered capacities for metabolism of fatty acids in general, and SCFAs in particular. Importantly, both butyrate and propionate are produced by the GI microbiota during fermentation of non-digestible starches and other carbohydrates26. In turn, both SCFAs (particularly butyrate) play important roles in GI epithelial health, including enterocyte nourishment and development, as well as ameliorating intestinal inflammation, reinforcing the epithelial defense barrier, and regulating intestinal motility, all of which are dysfunctional in humans and/or animals with CF mutations2. The observed enrichment of genes involved in catabolism of butyrate and propionate in the pediatric CF microbiota, which is likely to result in increased breakdown of these SCFAs, would be predicted to increase GI inflammation. In support of this prediction, we showed previously that measures of both fecal fat and inflammation in CF were highly correlated with the magnitude of CF-associated E. coli dysbiosis3. To directly explore the link between SCFA metabolism, fat content, and inflammation, we calculated the correlation between the overall abundance of genes for metabolism of butyrate and propionate, fecal fat content, and fecal calprotectin. We found a significant positive correlation between the fecal abundance of both the butyrate and propionate catabolism modules and fecal fat content (r = 0.61, p < 10−4 and r = 0.47, p < 10−4, respectively; Supplementary Table S14). We similarly found a significant positive correlation between the fecal abundance of these two modules and calprotectin (r = 0.5, p < 10−4 and r = 0.45, p < 10−4, respectively). Notably, the two corresponding non-catabolism modules were not significantly correlated with calprotectin (Supplementary Table S14). Combined, these findings could indicate that GI luminal fat selects for microbiota that, in turn, are pro-inflammatory, as schematized in the model in Supplementary Fig. S5.
Discussion
Nutrient malabsorption, intestinal dysfunction, and malnutrition are among the most important and troubling early manifestations of CF. The malabsorption of fats in CF is largely due to inadequate secretion of the enzyme pancreatic lipase into the intestinal lumen, with contributions from other mechanisms27, resulting not only in fatty stools, but also to loss of nutritionally important dietary fat and fat-soluble vitamins. Our results suggest a model wherein excess dietary fat within the CF GI lumen also plays an indirect role in the intestinal inflammation that characterizes childhood CF GI disease by selecting for microbiota that preferentially degrade the SCFAs butyrate and propionate, important molecules for enteric health (Supplementary Fig. S5).
SCFAs are known to have multiple positive effects in the GI tract. For example, butyrate has both growth-promoting and anti-inflammatory effects on enteric epithelia26,28, and it was shown to ameliorate intestinal inflammation in animal models of colitis by promoting the differentiation of homeostatic regulatory T cells20. Many of its effects in the GI tract apparently are conveyed by inhibiting the activation of both NF-κB signaling and histone deacetylation29. Propionate, by contrast, is used as a substrate for gluconeogenesis and regulates cholesterol synthesis in the liver, potentially impacting nutritional status, but with less of a defined effect on inflammation30,31. SCFAs are produced by carbohydrate fermentation in the large intestine, largely by Firmicute bacteria of the order Clostridiales, including those in the genera Eubacterium, Faecalibacterium, Ruminococcus, and Roseburia31,32, many of which are depleted in human inflammatory bowel diseases33, and all of which were less abundant in the current study among CF microbiota (Supplementary Table S2). Moreover, SCFAs are important sources of energy salvage in people with malabsorption due to pancreatic insufficiency, a key manifestation of CF GI dysfunction34. While the enrichment for genes involved in butyrate and propionate catabolism relative to biosynthesis among CF metagenomes likely reflects an altered ratio of SCFA-producing versus SCFA-consuming taxa, it is challenging to determine exactly which species are responsible for the relative enrichment of catabolic genes in CF. Butyrate degradation, for example, is known to be associated with methanogenic archaea in the human colon35. An altered abundance of such archaea would have been reflected in our metagenomic (and thus gene content), but not taxonomic, analyses, as the taxonomic reference database had relatively little representation of archaea. Sulfate-reducing bacteria, including species of the genus Desulfovibrio, can also oxidize butyrate, and Desulfovibrio species are present in human feces36. Sulfate-reducing bacteria in the feces of diverse human populations have been shown to be capable of fermenting butyrate and propionate ex vivo37. Many of these sulfate-reducing bacteria have been shown to oxidize butyrate and other SCFAs using sulfate or nitrate as electron acceptors12,38,39; both sulfate and nitrate are present in the human GI tract38,40,41,42. The GI tracts of children with CF also contain elevated levels of the potential electron acceptor nitric oxide43. Therefore, the CF GI luminal environment would be predicted to be favorable for microbial butyrate catabolism, lending further support for our model. Furthermore, a previous metaproteomic study of CF fecal samples found evidence for a relative depletion of butyrate-producing bacteria, in support of our findings, but the species responsible were not identified44. The laboratory isolation and/or cultivation of most of these species is technically very challenging, and for some impossible, rendering further study of these concepts difficult.
Similarly, because butyrate and propionate are volatile acids, measurement of their abundances must be performed either on freshly collected fecal samples or on those that have been appropriately processed and stored in airtight containers45, neither of which was the case for our samples. In addition, 98% of SCFAs in the colon are absorbed rather than excreted34, and inflammatory and malabsorptive GI conditions are often associated with decreased colonic butyrate uptake and utilization46. Accordingly, a study comparing GI luminal SCFA measurements in children with vs. without CF would be required to test our model. Nevertheless, there is strong supportive evidence from animal models. For example, mice fed a high-fat diet had lower colonic abundances of butyrate-producing microbes, including Roseburia, and higher abundances of Escherichia and Desulfovibrio, than did mice on a normal diet, resulting in significant reductions in fecal butyrate and compromised GI host defenses that normalized with oral butyrate administration47.
While this study focused on bacteria, the GI tract microbiota clearly also includes both viruses and fungi, each of which could conceivably contribute to GI microbial community metabolism48,49. For example, the fungus Aspergillus nidulans has been shown to express a transporter for SCFAs (albeit with low affinity for either butyrate or propionate50). Therefore, future work will be required to define the contribution of non-bacterial taxa on community metabolism.
While people with CF frequently receive antibiotics to treat their respiratory disease51, and antibiotic treatment can at least transiently deplete butyrate-producing microbiota in the GI tract52, we showed previously that pediatric CF fecal dysbiosis was independent of recent antibiotic exposure within the prior 30 days3. In this study, we again confirmed that our findings were not likely impacted by antibiotic exposure by excluding all samples that were collected within 30 days of antibiotic treatment (15 samples; Supplementary Table S1), or even those collected within 60 days (20 samples), and repeating our analysis (see Supplementary Table S10). Nevertheless, antibiotics would be likely to contribute to functional depletion of butyrate production capacity by the microbiome. We also confirmed that restricting our analysis to a single sample from each individual did not markedly impact our findings (Supplementary Table S10). Finally, since breastfeeding is known to impact the composition of the GI microbiota24, we additionally confirmed that restricting our analysis to samples from non-breastfed infants did not significantly affected our results (Supplementary Table S10).
CF is caused by dysfunction of the epithelial transmembrane ion channel, the CF transmembrane regulator (CFTR); interestingly, butyrate has been shown to increase the expression of CFTR on the epithelial apical surface53,54. Therefore, should GI luminal butyrate abundance be decreased in children with CF, treatments that address this imbalance (such as therapies that modify the GI microbiota or replete luminal butyrate concentrations) could improve CF GI function and nutritional outcomes, and subsequent long-term health, through multiple mechanisms.
In conclusion, we found that the fecal microbiomes from children with CF exhibited taxonomic and functional differences from those of children without CF. Computational analysis of these microbiomes indicates that the pediatric CF GI microbiota are selected, at least in part, by the high abundance of unabsorbed luminal fatty acids, and that these CF microbiota are predicted to yield lower amounts of health-promoting SCFAs in the GI lumen (Supplementary Fig. S5). Future research will be required to verify the predicted depletion of butyrate and propionate in the GI tracts of children with CF, and to determine whether treatments to manipulate their microbiota lead to improved outcomes.
Additional Information
How to cite this article: Manor, O. et al. Metagenomic evidence for taxonomic dysbiosis and functional imbalance in the gastrointestinal tracts of children with cystic fibrosis. Sci. Rep. 6, 22493; doi: 10.1038/srep22493 (2016).
Supplementary Material
Footnotes
Author Contributions L.R.H., E.B., O.M., R.L., B.W.R. and S.I.M. conceived and planned the project. C.E.P., H.S.H. and K.R.H. performed all laboratory-based analyses. R.C., M.C.R and M.J.B. processed and annotated the sequence data. O.M. and R.L. analyzed the data and generated all the figures and tables. S.L.H. performed statistical analysis. O.M., L.R.H. and E.B. wrote the manuscript. All authors reviewed the manuscript and provided comments.
References
- Davis P. B. Cystic fibrosis since 1938. Am. J. Respir. Crit. Care Med. 173, 475–482 (2006). [DOI] [PubMed] [Google Scholar]
- De Lisle R. C. & Borowitz D. The Cystic Fibrosis Intestine. Cold Spring Harb. Perspect. Med. (2013). doi: 10.1101/cshperspect.a009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman L. R. et al. Escherichia coli Dysbiosis Correlates With Gastrointestinal Dysfunction in Children With Cystic Fibrosis. Clin. Infect. Dis. 58, 396–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenhower C. et al. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abubucker S. et al. Metabolic reconstruction for metagenomic data and its application to the human microbiome. Plos Comput. Biol. 8, e1002358 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata N. et al. Metagenomic microbial community profiling using unique clade-specific marker genes. Nat. Methods 9, 811–814 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M., Goto S., Sato Y., Furumichi M. & Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 40, D109–14 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr R. & Borenstein E. Comparative analysis of functional metagenomic annotation and the mappability of short reads. Plos One 9, e105776 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor O. & Borenstein E. MUSiCC: a marker genes based framework for metagenomic normalization and accurate profiling of gene abundances in the microbiome. Genome Biol. 16, 53 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. & Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblum S., Carr R. & Borenstein E. Extensive Strain-Level Copy-Number Variation across Human Gut Microbiome Species. Cell 160, 583–94 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen P. H. & Schink B. Pathway of butyrate catabolism by Desulfobacterium cetonicum. J. Bacteriol. 177, 3870–3872 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y., Okamura-Ikeda K. & Tanaka K. Purification and characterization of short-chain, medium-chain, and long-chain acyl-CoA dehydrogenases from rat liver mitochondria. Isolation of the holo- and apoenzymes and conversion of the apoenzyme to the holoenzyme. J. Biol. Chem. 260, 1311–1325 (1985). [PubMed] [Google Scholar]
- Suvorova I. A., Ravcheev D. A. & Gelfand M. S. Regulation and evolution of malonate and propionate catabolism in proteobacteria. J. Bacteriol. 194, 3234–3240 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka T. et al. Reconstruction and regulation of the central catabolic pathway in the thermophilic propionate-oxidizing syntroph Pelotomaculum thermopropionicum. J. Bacteriol. 188, 202–210 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin L. G., Tucci V., Cercenado E., Eliopoulos G. & Isenberg H. D. Vancomycin-resistant Enterococcus faecium in hospitalized children. Infect. Control Hosp. Epidemiol. 13, 700–705 (1992). [DOI] [PubMed] [Google Scholar]
- Paulsen I. T. et al. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science 299, 2071–2074 (2003). [DOI] [PubMed] [Google Scholar]
- Brook I. & Fink R. Transtracheal aspiration in pulmonary infection in children with cystic fibrosis. Eur. J. Respir. Dis. 64, 51–57 (1983). [PubMed] [Google Scholar]
- Atarashi K. et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa Y. et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450 (2013). [DOI] [PubMed] [Google Scholar]
- Stanley J. D., Bartlett J. G., Dart B. W. & Ashcraft J. H. Clostridium difficile infection. Curr. Probl. Surg. 50, 302–337 (2013). [DOI] [PubMed] [Google Scholar]
- Koenig J. E. et al. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. USA 108 Suppl, 4578–4585 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallani M. et al. Determinants of the human infant intestinal microbiota after the introduction of first complementary foods in infant samples from five European centres. Microbiology 157, 1385–92 (2011). [DOI] [PubMed] [Google Scholar]
- Fallani M. et al. Intestinal microbiota of 6-week-old infants across Europe: geographic influence beyond delivery mode, breast-feeding, and antibiotics. J. Pediatr. Gastroenterol. Nutr. 51, 77–84 (2010). [DOI] [PubMed] [Google Scholar]
- Fouhy F. et al. High-throughput sequencing reveals the incomplete, short-term recovery of infant gut microbiota following parenteral antibiotic treatment with ampicillin and gentamicin. Antimicrob. Agents Chemother. 56, 5811–5820 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havenaar R. Intestinal health functions of colonic microbial metabolites: a review. Benef. Microbes 2, 103–114 (2011). [DOI] [PubMed] [Google Scholar]
- Peretti N., Marcil V., Drouin E. & Levy E. Mechanisms of lipid malabsorption in Cystic Fibrosis: the impact of essential fatty acids deficiency. Nutr. Metab. (Lond). 2, 11 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canani R. B. et al. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. WJG 17, 1519–1528 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer H. M. et al. Review article: the role of butyrate on colonic function. Aliment. Pharmacol. Ther. 27, 104–119 (2008). [DOI] [PubMed] [Google Scholar]
- Wong J. M. W., de Souza R., Kendall C. W. C., Emam A. & Jenkins D. J. A. Colonic health: fermentation and short chain fatty acids. J. Clin. Gastroenterol. 40, 235–243 (2006). [DOI] [PubMed] [Google Scholar]
- Puertollano E., Kolida S. & Yaqoob P. Biological significance of short-chain fatty acid metabolism by the intestinal microbiome. Curr. Opin. Clin. Nutr. Metab. Care 17, 139–144 (2014). [DOI] [PubMed] [Google Scholar]
- Louis P., Young P., Holtrop G. & Flint H. J. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ. Microbiol. 12, 304–314 (2010). [DOI] [PubMed] [Google Scholar]
- Sokol H., Lepage P., Seksik P., Doré J. & Marteau P. Molecular comparison of dominant microbiota associated with injured versus healthy mucosa in ulcerative colitis. Gut 56, 152–154 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owira P. M. O. & Winter T. A. Colonic energy salvage in chronic pancreatic exocrine insufficiency. JPEN. J. Parenter. Enteral Nutr. 32, 63–71 (2008). [DOI] [PubMed] [Google Scholar]
- Abell G. C. J., Conlon M. A. & McOrist A. L. Methanogenic archaea in adult human faecal samples are inversely related to butyrate concentration. Microb. Ecol. Health Dis. 18, 154–160 (2006). [Google Scholar]
- Balamurugan R., Rajendiran E., George S., Samuel G. V. & Ramakrishna B. S. Real-time polymerase chain reaction quantification of specific butyrate-producing bacteria, Desulfovibrio and Enterococcus faecalis in the feces of patients with colorectal cancer. J. Gastroenterol. Hepatol. 23, 1298–1303 (2008). [DOI] [PubMed] [Google Scholar]
- Gibson G. R., Macfarlane G. T. & Cummings J. H. Occurrence of sulphate-reducing bacteria in human faeces and the relationship of dissimilatory sulphate reduction to methanogenesis in the large gut. J. Appl. Bacteriol. 65, 103–111 (1988). [DOI] [PubMed] [Google Scholar]
- Willis C. L., Cummings J. H., Neale G. & Gibson G. R. Nutritional aspects of dissimilatory sulfate reduction in the human large intestine. Curr. Microbiol. 35, 294–298 (1997). [DOI] [PubMed] [Google Scholar]
- Elefsiniotis P., Wareham D. G. & Smith M. O. Use of volatile fatty acids from an acid-phase digester for denitrification. J. Biotechnol. 114, 289–297 (2004). [DOI] [PubMed] [Google Scholar]
- Parham N. J. & Gibson G. R. Microbes involved in dissimilatory nitrate reduction in the human large intestine. FEMS Microbiol. Ecol. 31, 21–28 (2000). [DOI] [PubMed] [Google Scholar]
- Saul R. L., Kabir S. H., Cohen Z., Bruce W. R. & Archer M. C. Reevaluation of nitrate and nitrite levels in the human intestine. Cancer Res. 41, 2280–2283 (1981). [PubMed] [Google Scholar]
- Florin T., Neale G., Gibson G. R., Christl S. U. & Cummings J. H. Metabolism of dietary sulphate: absorption and excretion in humans. Gut 32, 766–773 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzese E. et al. Disrupted intestinal microbiota and intestinal inflammation in children with cystic fibrosis and its restoration with Lactobacillus GG: a randomised clinical trial. Plos One 9, e87796 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debyser G. et al. 123 A shotgun metaproteomics approach to study the faecal microbiome of patients with cystic fibrosis reveals a reduction of butyrate-producing bacteria. J. Cyst. Fibros. 12, S80 (2013). [Google Scholar]
- Treem W. R., Ahsan N., Shoup M. & Hyams J. S. Fecal short-chain fatty acids in children with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 18, 159–164 (1994). [DOI] [PubMed] [Google Scholar]
- Thibault R. et al. Butyrate utilization by the colonic mucosa in inflammatory bowel diseases: A transport deficiency. Inflamm. Bowel Dis. 16, 684–695 (2010). [DOI] [PubMed] [Google Scholar]
- Schulz M. D. et al. High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature 514, 508–512 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim E. S. et al. Early life dynamics of the human gut virome and bacterial microbiome in infants. Nat. Med. 21, 1228–1234 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister E. B., Gao C. & Versalovic J. Compositional and functional features of the gastrointestinal microbiome and their effects on human health. Gastroenterology 146, 1449–1458 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sá-Pessoa J., Amillis S., Casal M. & Diallinas G. Expression and specificity profile of the major acetate transporter AcpA in Aspergillus nidulans. Fungal Genet. Biol. Fg B 76, 93–103 (2015). [DOI] [PubMed] [Google Scholar]
- Gibson R. L., Burns J. L. & Ramsey B. W. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 168, 918–951 (2003). [DOI] [PubMed] [Google Scholar]
- Bartosch S., Fite A., Macfarlane G. T. & McMurdo M. E. T. Characterization of Bacterial Communities in Feces from Healthy Elderly Volunteers and Hospitalized Elderly Patients by Using Real-Time PCR and Effects of Antibiotic Treatment on the Fecal Microbiota. Appl. Environ. Microbiol. 70, 3575–3581 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita M., Kongo H. & Shiba Y. Molecular dissection of the butyrate action revealed the involvement of mitogen-activated protein kinase in cystic fibrosis transmembrane conductance regulator biogenesis. Mol. Pharmacol. 66, 1248–1259 (2004). [DOI] [PubMed] [Google Scholar]
- Cheng S. H. et al. Functional activation of the cystic fibrosis trafficking mutant delta F508-CFTR by overexpression. Am. J. Physiol. 268, L615–624 (1995). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.