Abstract
Calcium signaling in cells directs diverse physiological processes. The calcium waves triggered by fertilization is a highly conserved calcium signaling event essential for egg activation, and has been documented in every egg tested. This activity is one of the few highly conserved events of egg activation through the course of evolution. Echinoderm eggs, as well as many other cell types, have three main intracellular Ca2+ mobilizing messengers – IP3, cADPR and NAADP. Both cADPR and NAADP were identified as Ca2+ mobilizing messengers using the sea urchin egg homogenate, and this experimental system, along with the intact urchin and starfish oocyte/egg, continues to be a vital tool for investigating the mechanism of action of calcium signals. While many of the major regulatory steps of the IP3 pathway are well resolved, both cADPR and NAADP remain understudied in terms of our understanding of the fundamental process of egg activation at fertilization. Recently, NAADP has been shown to trigger Ca2+ release from acidic vesicles, separately from the ER, and a new class of calcium channels, the two-pore channels (TPCs), was identified as the likely targets for this messenger. Moreover, it was found that both cADPR and NAADP can be synthesized by the same family of enzymes, the ADP-rybosyl cyclases (ARCs). In this context of increasing amount of information, the potential coupling and functional roles of different messengers, intracellular stores and channels in the formation of the fertilization calcium wave in echinoderms will be critically evaluated.
Introduction
Fertilization is the “simple” process of sperm and egg fusion used in all sexually reproducing animals and plants. Yet, the molecular mechanisms of sperm-egg interaction, the morphology of each species’ sperm and eggs, and the process of developing into an egg are wildly diverse. Formally, an egg is a female gamete that is in a state capable of fertilization – it is a functional, not a morphological, distinction from its earlier stages of being an oocyte in development (e.g. Chiba, 2011). As in all organisms, oocytes and sperm undergo a meiotic reduction division to yield a single, haploid content of chromosomes. When the oocyte completes meiosis relative to being fertilized, though, is different for all species – some eggs are fertilized having finished meiosis (as in sea urchins and Cnidarians), while others have yet to begin meiosis (as in dogs and fox) or they may complete this process anywhere in between (as in humans, usually having finished one round of meiosis) (summarized in Austin, 1965, Gilbert, 2010). Often fertilization re-initiates the meiotic progression and sometimes even multiple sperm enter the egg for activation, all but one eventually dying (Carré and Sardet, 1984).
Oocyte development is equally variable depending on the species. Oogenesis, by definition, is the complete process of differentiation of the female germ line stem cell into a mature oocyte, the egg, competent to be fertilized (Grudzinskas and Yovich, 1995). Oocyte maturation refers to the transition from an oocyte to an egg. In echinoderms, meiosis starts when one germ cell enters meiotic division I and then shortly arrests at prophase I. During this arrest period, the primary oocyte grows by massive accumulation of macromolecules (mainly yolk) generating a typically complex cytoplasm contained in a large cell (100-300 μm). Primary oocytes complete meiotic division I to form a small polar body and a large secondary oocyte, which proceeds to metaphase of meiotic division II (Grudzinskas and Yovich, 1995). There, were it a sea urchin, the oocyte would complete meiosis forming a haploid nucleus called the pronucleus, and would then be capable of activation by sperm. Were the species instead a sea star, also an echinoderm, the early events of oogenesis are similar, but the egg is fertilized during meiosis, any time after beginning the meiotic reduction divisions, seen first by breakdown of the specialized oocyte nucleus, the germinal vesicle (Kishimoto, 2011; Chiba, 2011; see also Silvestre et al., 2011, for summary of regulation in mammalian oocyte-cumulus complex). The egg activation pathway is referred to as the collection of intracellular signals that will activate the egg, whether it is triggering of meiosis resumption in some animals, to starting the early developmental program of the embryo. Even with all the tremendous variation in reproductive strategies used to finally get to an embryo, some of the pathways used to activate the egg are highly conserved. The main early event of the egg activation cascade is the generation of a global Ca2+ oscillation in the cytoplasm, well known as the fertilization calcium wave (e.g. Gilkey et al., 1978; Striker, 1999). Later events in the activation cascade include the conclusion of meiosis, changes in cytoplasmic pH, pro-nuclei formation and DNA synthesis for the first cleavages (Whitaker, 2006). It is remarkable that in the midst of all the variation used by animals and plants to get to the point of initiating embryonic development, that the calcium activation pathway is used universally.
The ion Ca2+ is a most versatile intracellular messenger found in eukaryotic cells. Its concentration within the cytoplasm is spatially and temporally controlled by ion channels, exchangers, and pumps, which in total are able to elevate calcium levels over 1000 fold in less than a second, and then promptly restore their resting levels. The Ca2+ transporting proteins are located in the plasma membrane and in the membranes of organelles such as the endoplasmic reticulum, the mitochondria and the lysosomes, which play specific roles in the cellular homeostasis of Ca2+. In general, the transduction of the Ca2+ signals is mediated by reversible binding to specific classes of proteins that act as Ca2+ sensors. The decoding operation is based on conformational changes in the sensor proteins regulating the interaction with specific targets (Berridge et al., 2000; Carafoli et al., 2001). Ca2+ is the most ubiquitous of the intracellular messengers, and most cell types contain very similar machinery of calcium signaling elements, being present in somatic cells and also in the germ line: in both sperm and eggs. This review will focus on the calcium signaling events involved at echinoderm fertilization.
Echinoderms and the fertilization calcium waves
Echinoderms are marine animals that represent the ancient - at least 450 million years old - phylum echinodermata with thousands of known living species present throughout the world's oceans (Ettensohn et al., 2004). Due to rather useful conveniences, sea urchin gametes have been massively used for the past 150 years as a valuable experimental model for studies on fertilization, Ca2+ signaling and development (Vacquier, 2011). Some of the many advantages of working with echinoderm eggs include the separated sexes of the animals, facility to obtain large amounts of eggs and sperm, both of which have completed meiosis, external fertilization – therefore, easy to manipulate, synchronous development and the fact that animals are easy to keep in the lab at relatively low costs.
Historically, muscle contraction was the first physiological event to be recognized as a calcium modulated process (Ringer, 1882; see Szent-Györgyi, 1975), and thereafter, fertilization and nerve functions emerged as examples of the widespread roles of calcium. Thus, many of the fundamental calcium signaling machinery were first described and/or heavily studied using sea urchin eggs as a model. The first fertilization calcium wave to be discovered was visualized by the luminescent calcium-stimulated photoprotein aequorin, microinjected into eggs of the fish Oryzias latipes (Gilkey et al., 1978). Ten years later, in the mid- 80's, aequorin was used to detect calcium waves at fertilization in sea urchin (Eisen et al., 1984, Eisen and Reynolds, 1985) and starfish (Eisen and Reynolds, 1984), the main representatives of echinoderms used for calcium signaling investigations. Later, the development of confocal fluorescence imaging and the increasing availability of fluorescent calcium probes defined the characteristics of the echinoderm fertilization calcium wave as we know them today: they originate at the point of sperm entry and cross the egg with a spherical wave front. Differently from ascidians and mammalian eggs (that produce repetitive calcium transients), echinoderm eggs produce one single wave after fertilization, which takes about 20 s to cross the egg (Hafner et al., 1988; Jaffe, 1995) with a constant velocity of 5-50 μm/s (Jaffe and Creton, 1998).
The fertilization calcium wave can be formed by eggs or oocytes independently from the sperm. They can be triggered in vitro by microinjection of calcium messengers, ionophores or even by a needle prick (Jaffe, 1995; Whitaker et al., 2006). However, it is accepted that the sperm-egg interaction has a role on the triggering of a signal transduction pathway that initiates the calcium transient in vivo. Although most of the molecular detail of this pathway is still uncertain, activation of PLC-γ of starfish oocytes and sea urchin eggs occurs during fertilization and stimulates the production of IP3 (Swann and Whitaker, 1986; Carroll et al., 1997; Carroll et al., 1999; Shearer et al., 1999). In general, the consensus holds that PLC activation is central to the initiation of the fertilization calcium transient. In echinoderms, the PLC is likely to be PLC-γ, activated by a src-like kinase (Runft et al., 2002; McGinnis et al., 2011) whereas in mammals, the key PLC (PLC-ζ) is an isoform unique to, and contributed by the sperm (Saunders et al., 2002; Ito et al., 2011).
How the eggs form a calcium wave
It is known that the calcium wave can propagate even when the egg or oocyte is immersed in calcium free media, indicating that the calcium is released from internal stores (Crossley et al., 1988, Schmidt et al., 1982). In echinoderms eggs as in many other cell types, , the ER is the main organelle involved in calcium storage and calcium release events. Calcium accumulation in the ER is driven by a SERCA pump (Smooth endoplasmic reticulum calcium ATPase pump) and elementary release events from the ER either by IP3 or RyR receptors – transmembrane calcium channels that open upon binding their specific ligand enabling calcium to diffuse out along a concentration gradient (Carafoli et al, 2001). The architecture of the ER can have profound effects on the spatiotemporal characteristics of calcium signals. In sea urchins, the cortical ER morphology is transiently altered during the peak of the calcium oscillation at fertilization, with the formation of transient vesicles (as opposed to its original lamellar-reticulate morphology) that may be important for shaping calcium signals and the formation of the wave itself. Most of that work was accomplished by visualizing the ER at fertilization using lipophilic fluorescent dyes (Terasaki et al., 1984; Terasaki and Jaffe, 1991) and GFP probes (Terasaki et al., 1996), directly showed that the ER is a key element in shaping calcium signals that rely on release of calcium from internal stores at fertilization. It is possible; however, that a Ca2+ influx from the external media may play a role in the first steps of the signaling cascade that eventually leads to the global calcium release from the ER and possibly other internal stores. In echinoderms, this is still a matter of debate (Runft et al., 2002). Ca2+ influx was demonstrated during the period between fertilization and the initiation of the Ca2+ wave in sea urchin (Creton and Jaffe, 1995). However, this evidence has been underestimated by several authors, since the eggs can be activated in free-Ca2+ media (Runft et al., 2002). Nevertheless, in starfish, prophase I-arrested oocytes Ca2+ influx may participate in oocyte activation (Santella et al., 2004), and this process may involve coupling of different calcium messengers and stores, as will be later discussed. Moreover, what does happen and what can happen may be significantly different, especially when invoking feedback steps within an overlapping pathway.
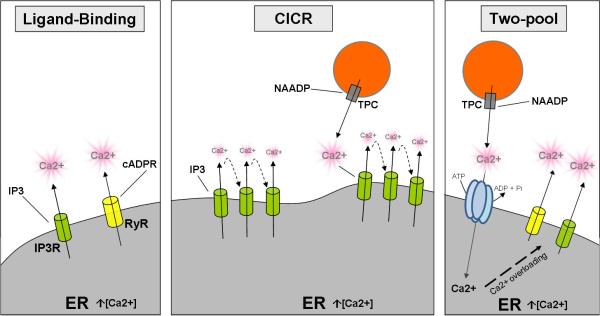
Several models have been proposed to explain the formation of global spatially-regulated Ca2+ oscillations. These can be classified by the number of Ca2+ stores required (one or two) and control by positive and/or negative feedback of Ca2+ on Ca2+ induced calcium release channels (CICR) (Berridge and Galione, 1988; Carafoli et al., 2001). The CICR model is based on the fact that IP3 and RyR channels are known to be biphasically regulated by Ca2+ concentrations i.e.: low calcium concentrations activate the channels, triggering calcium flow into the cytoplasm, whereas higher concentrations inhibit the channels and stop calcium movement. Thus, calcium released from one receptor can diffuse to neighboring receptors, triggering further calcium release (Galione et al., 1991, Patel and Brailoiu, 2012) forming a trail along a cluster of channels. Note that a regulated variation on IP3 concentrations may be added to this scenario, further regulating the signal in a localization-specific manner. Moreover, in the so called two pool mechanism, calcium released from one compartment (pool 1) may be taken up by a second compartment (pool 2), which would overload and spontaneously release Ca2+. This is a mechanism used, for example, by the RyR receptors (Fill and Copello, 2002). Evidence for “all of the above” mechanisms exist in the fertilization calcium wave in echinoderms. The fundamental evidence for this is: 1) microinjection of calcium or calcium ionophores in sea urchin eggs can trigger a propagating wave (Hamaguchi and Hiramoto, 1981, Mohri and Hamaguchi, 1991) and; 2) Long-term Ca2+ oscillations were reported by releasing Ca2+ from a ER-separated store, resulting in cycles of Ca2+ overloading, release and reuptake in sea urchin egg homogenates (Churchill and Galione, 2001) (Figure 1).
Figure 1. Calcium-release mechanisms in the endoplasmic reticulum (ER).
Ligand-binding: Specific ligands, IP3 and cADPR, bind to their channels (IP3 and RyR receptors, respectively), triggering Ca2+-release. Calcium-induced calcium release (CICR): IP3 and RyR receptors in the ER are biphasically regulated by Ca2+ concentrations - low calcium concentrations activate the channels, whereas higher concentrations inhibit the channels. Thus, calcium released from one receptor can diffuse to neighboring receptors, triggering further calcium release, forming a trail along a cluster of channels, and amplifying the original Ca2+ signal. Two-pool mechanism: Ca2+ released from one compartment may be taken up by a second compartment, which would overload and spontaneously release Ca2+.
In many species the fertilization calcium oscillation(s) is mainly carried by the IP3 receptor (Stricker, 1999). In echinoderm eggs, however, it appears to be a substantial coupling between IP3 and RyR receptors in order to shape the calcium wave, since antagonists of the RyR receptors considerably reduce its traveling velocity and structure (Galione et al., 1993a, 1993b; Whitakeer, 2006). RyR receptors are gated by cADPR, a metabolite nucleotide from NAD+. Indeed, the calcium mobilizing activity of cADPR was first demonstrated in sea urchin eggs and shown to be independent of the IP3 mechanism, acting via CICR (Galione et al., 1991; Lee, 1993, Lee, 2012). cADPR is enzymatically synthesized by enzymes belonging to the ADP-rybosil cyclase family, which were also first detected in sea urchin egg homogenates and subsequently found to be widespread in animal and plant tissues.
cADPR and the ADP-rybosil cyclase family in echinoderms
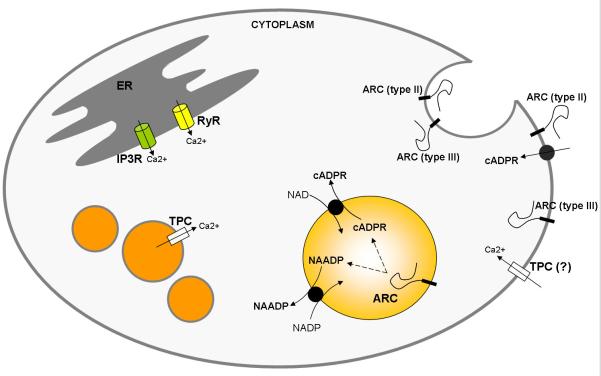
ARC enzymes are quite remarkable in several features, which may potentially influence the regulation and shape of Ca2+ signals during fertilization, oocyte maturation, and early embryo development. One of the interesting aspects of ARCs is their so called localization paradox (De Flora et al., 2004) (Figure 2). cADPR is known and well studied as a cytosolic messenger, and ARCs, in general, have a “targeted for secretion” signal peptide, being found in the plasma membrane or in the lumen of secretory vesicles (close to the plasma membrane). The question of how an ectocellular (or luminal, shielded from the cytoplasm) enzyme works on the production of a cytoplasmic messenger is still to be elucidated. In sea urchin (S. purpuratus) eggs, three isoforms of ARCs have initially been described (Davis et al., 2008). Their subcellular localization has been found to differ from the mammalian homologs CD38 and CD157, which are strict extracellular enzymes (Liu et al., 2005; Lee, 2012). Although the three SpARCs are membrane bound (either by a GPI anchor or a TM domain), ARCs beta and gamma appear to be within the lumen of acidic organelles present in the cortex of the egg. This orientation makes them contiguous with the extracellular space – at least in as much as to get into this lumen they needed to be synthesized and processed through the secretory pathway. Interestingly, ARC-beta (or ARC-2) is recovered in fractions of cortical granules and co-localized with hyalin, a protein of the cortical granules. Their activity (in vitro, measured via production of radioactive cADPR) is indeed better at acidic pH – consistent with an acidic vesicle location (Davis et al., 2008).
Figure 2. ARC topological paradox.
NAADP and cADPR are cytosolic messengers targeting channels in the ER (RyR receptors) or in acidic vesicles (TPCs). Paradoxically, ARCs in general, have a “targeted for secretion” signal peptide and are found in the plasma membrane or in the lumen of secretory vesicles (Type II transmembrane protein orientation). Putative nucleoside transporters would be responsible for transporting the messengers to the cytoplasm. In human lymphocytes, ARCs were also found in an alternative orientation (Type III), with the C-terminal facing the cytoplasm.
In mammalian cells, nucleoside transporters that would transport newly synthesized cADPR to the cytoplasm after its extracellular production were described (Guida et al., 2002). Remarkably, Zhao et al., (2012) reported that the human CD38 can also be found in an alternative orientation in lymphocytes - with the catalytic C-terminal facing the cytoplasm - thus suggesting that the flipping of the catalytic domain may be a mechanism for regulating the production of the cytoplasmic messengers. Although the mechanism is yet unknown, the alternative orientation was more active at producing cADPR, and the changed orientation could be artificially conferred with a single amino acid change. This finding may reflect a broader functionality for flippases or similar functioning membrane proteins.
In sea urchin eggs, the topological paradox was addressed by using specific nucleoside transporters inhibitors, and evidence surfaced that substrate and product are indeed transported into and out of the vesicles that enclose ARC-beta (Davis et al., 2010), although the physiological role or relevance of this arrangement is still uncertain. Another intriguing feature of ARCs is that individual ARCs show remarkable catalytic differences in the face of marked structural similarity. For example, using NAD as substrate, the sea slug Aplysia ARC (the first ARC to be described and structurally resolved) produces essentially cADPR as a product, while CD38 (Human homolog for ARC) produces mainly ADP ribose and only a small amount of cADPR (2-3% of the reaction products) (Howard et al., 1993; Takasawa et al., 1993; Kim et al., 1993). The molecular determinants of that change have to do with the affinity of the docking site to the newly synthesized cADPR. In normal conditions, cADPR has a finite possibility of reentering the active site, resulting in its hydrolysis to ADP ribose. It is thought that protein factors or membrane lipids interacting with amino acids exposed to the surface of the enzyme (e.g. Asp147, in the catalytic dipeptide Glu146Asp147) can induce conformational changes and thus alter the catalysis. It is important to note that ADP-ribose is an activator of the TRPM2 (transient receptor potential channel, subfamily M, member 2) channels, being therefore a Ca2+ signaling messenger itself (Kolisek et al., 2005; Lange et al., 2008). Moreover, the multi-functionality of ARCs goes beyond cyclization to form cADPR and hydrolysis of cADPR to form ADP ribose. Using NADP as substrate in the presence of nicotinic acid (NA), ARCs are also able to switch to another catalysis mode, the base-exchange reaction, to produce a third messenger for calcium signaling, nicotinic acid adenine dinucleotide phosphate (NAADP). The nicotinamide group of NADP (formed by a NAD kinase) is exchanged for a nicotinic acid group (NA), requiring a half maximum concentration of NA of 5 mM and an acidic pH (at neutral pH it converts NADP to another metabolite - cADPR 2 phosphate) (Lee, 2012).
Interestingly, a fourth ARC was described in sea urchin eggs, further expanding the ADP-ribosyl cyclase family in echinoderms. Although its subcellular localization in the eggs was not addressed, exogenous expression of that enzyme in mammalian cell lines and Xenopus oocytes showed that one single residue exchange (Tyr149) is enough to alter the preference for product formation from cADPR to NAADP (Ramakrishnan et al., 2010). Although not much is know about the mechanisms of ARC catalysis in echinoderms, given their different localizations (peripheral secretory vesicles versus plasma membrane), indications are that ARCs are key players in the triggering and regulation of calcium signals, since they can produce three (cADPR, ADP-ribose and NAADP) calcium messengers, depending on conditions like substrate availability, pH, and potential interactions with other factors in the membrane. How the messenger products are regulated in vivo is still not clear, but it is certainly an essential topic to be resolved by further investigation.
NAADP – multiple calcium messengers in the eggs
The most recently discovered Ca2+-mobilizing messenger is NAADP (Lee and Aarhus, 1994; Galione, 2011, Hooper and Patel, 2012). The Ca2+-mobilizing properties of NAADP were first recognized by Lee and colleagues using sea urchins while they were investigating the effects of various pyridine nucleotides on calcium release from egg homogenates. Most of the subsequent work in the early days of NAADP study was accomplished with the use of the sea urchin egg homogenates. This approach is advantageous since the lysate can be prepared simply from eggs, are remarkably stable and able to release/uptake calcium when challenged with different messengers and inhibitors (Morgan and Galione 2008). However, much less is known about this pathway compared to IP3 and cADPR pathways.
The channels/receptors activated by NAADP are intriguing, including their characteristic lack of positive feedback by Ca2+ (CICR) and the self-inactivation of calcium release response when NAADP is added at concentrations less than those required to activate detectable Ca2+ release (Aarhus et al., 1996; Genazzani et al., 1996; Lee et al., 1997). The later is thought to be related with the fact that once bound to its receptor radiolabelled NAADP cannot be displaced by non-labeled NAADP (Aarhus et al., 1996; Billington and Genazzani, 2000; Patel et al., 2000). As stated before, synthesis of NAADP in vivo is thought to be accomplished by ARCs, and its turnover (to NAAD) can also be carried out by a cytoplasmic alkaline phosphatase (Schimid et al., 2012). Thus, a balance of NAADP calcium-potentiating ligand may be in constant flux, of synthesis and degradation (recycled to an inactive form) depending on the physiology and signaling inputs on the cell.
Multiple messengers, new calcium stores
Accumulating evidence suggests that the primary Ca2+ stores targeted by NAADP are distinct from the endoplasmic reticulum. The initial reports of Ca2+ release evoked by metabolites of pyridine nucleotides already showed that the reactive subcellular fractions in egg homogenates were largely separate from the microsomal/ER fraction sensitive to IP3 and cADPR (Clapper et al, 1987), and that blocking of Ca2+ storage by the ER only partially reduced Ca2+ release evoked by NAADP in both sea urchin egg homogenates (Genazzani and Galione, 1996) and intact eggs (Churchill and Galione, 2001). Later, subcellular fractionation experiments in sea urchin eggs recovered a “reserve granule” fraction presenting NAADP but not IP3/cADPR-evoked Ca2+ release. This fraction was enriched in lysosomal markers and presented ATP-dependent Ca2+ sequestration - sensitive to preincubation with bafilomycin and the ionophore nigericin. Furthermore, in intact sea urchin eggs, treatment with glycyl-phenylalanine 2-naphthylamide (GPN, a lysosomotropic agent), caused the lysis of acidic vesicles, resulting in release of calcium in the cytoplasm, consistent with Ca2+ storage (Churchill and Galione, 2002). From these data, it was proposed that in sea urchins the primary targets of NAADP are the acidic stores, rather than the ER.
Acidic stores, such as lysosomes, have been shown to sequester Ca2+ by mechanisms dependent on their low luminal pH (Patel and Docampo, 2010), and have been increasingly implicated in elementary calcium signals in several models (Patel and Mualem, 2011), including sea urchin eggs (Morgan, 2011). In egg homogenates, as in other cell types, the rationale for calcium storage/release into/out of acidic vesicles has to do with the recurrent observations that inhibition of the vacuolar H+-ATPase decreases proton uptake, and, if their membrane is sufficiently leaky (sometimes a low concentration of valinomycin – a potassium ionophore - have to be added to assure a counter ion transport), that alkalinization results in Ca2+ release (Morgan and Galione, 2007a; 2007b; Ramos et al., 2010). Thus, calcium uptake is thought to be driven by the proton gradient, probably coupled to a Ca2+/H+ exchanger. Although the detailed mechanisms are not well understood, it has been extensively shown that different acidic vesicles are able to store/ release calcium in sea urchin eggs (Morgan, 2011). In fact, experiments in sea urchin egg homogenates employing luminal pH indicators such as acridine orange or lysosensor also have shown that NAADP uniquely among Ca2+ mobilizing messengers also causes the alkalinization of acidic stores, representing another possible signaling mechanism for this molecule (Morgan and Galione, 2007).
The two pore channels – candidate NAADP targeted channels
Following the accumulating amount of biochemical/physiological data regarding NAADP, a new family of calcium channels has emerged as the likely targets for this new messenger response, the two-pore channels (TPCs) – members of the superfamily of the voltage gated channels. The hypothesis of TPCs as the NAADP receptors and/or responders has emerged due to complementary genetic based results from several different laboratories, as well as their localization with a lysosomal marker, LAMP1, when heterologously expressed in HEK293 cells, an increased response to NAADP when overexpressed, and the evidence that a TPC in A. thaliana (AtTPC1) localized to plant vacuoles, the major acidic organelle in plants (Guse, 2012; Lee, 2012; Galione, 2011; Patel and Muallem, 2011; Peiter et al. 2005). TPC topologies are predicted as two domains, each containing six transmembrane regions (TMs), connected by a cytosolic loop. The N-terminus and C-terminus are predicted to be cytosolic whereas the short loops between TMs 1-2, 3-4 and the putative reentrant loop between TMs 5-6 (for each domain) are predicted to be luminal. Sea urchin eggs express three isoforms of TPCs (1, 2 and 3) (Ruas et al., 2010; Hooper et al., 2012), and over expression of those channels were shown to be related with effects on endolysosomal structures and dynamics, implicating a role for NAADP in the regulation of vesicular trafficking in sea urchin eggs (Ruas et al., 2010). Most of the evidence directing TPCs as the NAADP receptors is related to experiments where TPC-overexpressing cells lineages co-precipitate more radiolabelled NAADP than in wild type cells (Calcraft et al., 2010, Brauiloiu et al., 2010). Recently, however, contradicting findings that human TPCs are preferably sodium-selective channels activated by the phosphoinositide PI(3,5,)P2 (elegantly accomplished by patch-clamping intact enlarged vacuolin-treated lysosomes) (Wang et al., 2012) have increased the debate regarding the potential in vivo functional roles of those channels in different animals and cell lines. Additionally, photoaffinity labeling of NAADP binding proteins in sea urchin and mammalian cells showed that NAADP does not bind directly to TPCs in sea urchin (Walseth et al., 2011) and mammalian cells (Lin-Moshier et al., 2011). Instead, NAADP binds to another set of unidentified proteins (with 30, 40 and 45 kDa) that co-precipitate with SpTPC1 and SpTPC3 (Walseth et al., 2012). Thus, the identification of the TPC-interacting NAADP binding proteins (or NAADP receptor(s)) has emerged as a crucial aspect to understand the function of this second messenger. Nevertheless, evidence for NAADP targeting RyR and transient receptor potential channel, subtype mucolipin 1 (TRP-ML1) was obtained in mammalian cells (Gerasimenko et al., 2003; Dammermann and Guse, 2005; Dammermann et al., 2009; Zhang et al., 2011) raising the suggestion that NAADP may be targeting multiple channels in different cell types (Guse, 2012).
ARCs/ NAADP/ TPCs at echinoderm fertilization
Echinoderm eggs depolarize at fertilization (Steinhardt et al., 1971). The depolarization is a physiological response to the first interacting sperm that reduces the probability of interaction with additional sperm, the so-called fast block to polyspermy (Jaffe, 1976; Whitaker, 2006). It is fast, compared with the other time scales at fertilization - within 20 ms and is due to activation of voltage-gated channels in the plasma membrane (Chambers and de Armendi, 1979). The absolute block to polyspermy is provided a few seconds later by the calcium-dependent exocytosis of cortical granules that cause elevation of the fertilization envelope, which functions as a mechanical barrier to polyspermy (Whitaker, 2006; Wessel and Wong, 2009; Vacquier, 2011).
Scarce information is available on the trigger of the sperm evoked initial depolarization, which in turn will activate the voltage gated calcium channels to allow calcium influx. In echinoderm eggs, many calcium indicator dyes detect a calcium influx in the cell cortex that occurs when the egg depolarizes (McDougall et al, 1993; Shen and Buck, 1993), referred to as the cortical flash. In starfish oocytes the cortical flash and wave initiation occur within a few seconds of one another (Moccia et al., 2004), whereas in sea urchin eggs, a remarkably long time elapses between the cortical flash and the initiation of the fertilization calcium wave (10-15s, depending on the species) (Shen and Buck, 1993). Interestingly, in starfish, electrophysiological studies have demonstrated that NAADP participates in the response to sperm by triggering a fertilization potential which depolarizes the membrane to the threshold of activation of the voltage gated-Ca2+ channels, allowing the Ca2+ influx for the cortical flash. The injection of NAADP elicits a cortical Ca2+ flash which is not affected by the down-regulation of IP3 receptors (Santella et al., 2000; Lim et al., 2001) and, even more interestingly, a link between NAADP-dependent fertilization membrane potential and the onset of the Ca2+ wave was suggested. Evidence has accumulated that desensitization of NAADP receptors either prevents Ca2+ release or impairs the pattern of starfish oocyte activation. Thus, Ca2+ influx during the early phase of the NAADP-induced fertilization potential may interact with the initiation of the Ca2+ wave at fertilization (Moccia et al., 2006; Moccia et al., 2006). According to those findings, NAADP may be in fact the messenger that generates the first local calcium signal at fertilization in echinoderms, triggering the cascade that will eventually lead to the formation of the global calcium wave.
NAADP in the sperm
The molecular link between sperm interaction and the calcium wave is still mostly nebulous and whether or not the sperm activates a signal transduction receptor (much as a hormone) or delivers an “activating messenger” to the egg - or both - is still a matter of debate in most egg activation studies. In mammals, substantial evidence now exists that an isoform of PLC (PLC-ζ) is necessary and sufficient to activate the egg, and delivery of this enzyme by the sperm presents strong arguments of it being the sperm factor for that group of animals. PLC-ζ however only appears to be present in most vertebrates (Saunders et al., 2002; Ito et al., 2011). In keeping with the remarkable theme of eggs, sperm, fertilization, and evolutionary diversity, most animals then must use a different method to initiate the calcium flux.
In echinoderms, the idea that the sperm is the vehicle that transmits an activating messenger to the egg once sperm egg fusion occurs has been a recurrent theme in the field (Galione et al., 1997; Wilding and Dale, 1997; Runft et al., 2002, Santella et al., 2004), mostly based on the observations that extracts of sperm cytoplasm will induce calcium transients when microinjected into eggs. Suggestively, NAADP is present in sea urchin sperm (Billington et al., 2002, Vasudevan et al., 2008) and is rapidly synthesized upon exposure to the egg jelly (Churchill et al., 2003). Initially, it was suggested that NAADP is produced in the sperm entirely for transference into the sea urchin egg to activate it (Churchill et al., 2003). Such a messenger role for NAADP is supported by the data on fertilization in starfish (Moccia et al., 2004; Moccia et al., 2006; Moccia et al., 2006b). Later, a direct role for NAADP was also investigated in the sperm per se. NAADP produced after contact with the egg jelly would act on its receptor/ channel on the acrosome to release Ca2+ to signal to vesicle fusion and exocytosis (Vasudevan, 2010). Because the acrosome is a lysosome-related organelle (Dell’ Angelica et al., 2000), and sperm lack an endoplasmic reticulum, it is possible that NAADP is the messenger responsible for calcium signals related to acrosomal exocytosis, rather than IP3 or cADPR.
Conclusions
Cells have developed various ways to control free cytosolic Ca2+ concentrations using highly regulated spatial-temporal protein regulators. Through the course of evolution, signaling at fertilization has been shown to be exceptionally conserved, where calcium is the main intracellular signal molecule that wakes the egg from its dormancy state after the sperm-egg interaction. The molecular pathway of that signal transduction is mostly unknown, but recent evidence points to rather complex arrangements including the coupling functions of multiple calcium stores and messengers (Berridge, 2000; Carafoli, 2001; Whitaker, 2006; Galione, 2011; Patel, 2011).
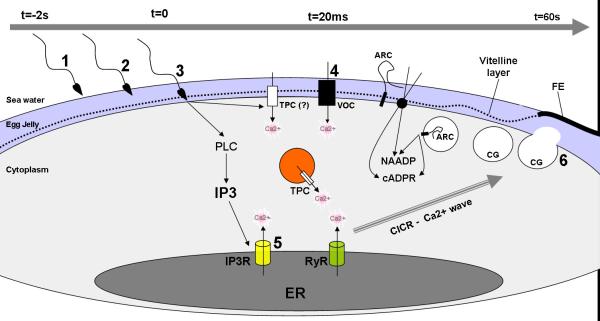
In echinoderms, our understanding regarding calcium at fertilization is essentially depicted as two separated - but possibly interacting – calcium events: the cortical flash and the calcium wave, and given the latest findings it is seems reasonable to assume that most (if not all) of the calcium stores and messengers known so far play a coordinated role in the formation and shaping of those signals. The current conviction is that NAADP is the messenger that acts “upstream” of IP3 and RyR receptors, triggering local calcium signals that can be amplified by ER channels either by CICR or overloading two-pool mechanisms (Figure 3). Furthermore, one may add to the considerations the potential functional role of the sperm in producing NAADP for the acrosomal reaction and/or for delivering it to activate the egg at fertilization (Vasudevan, 2010); the findings that modulation of ARCs and its topology (type II × type III) may modulate the production either cADPr, NAADP or even a third calcium messenger ADP ribose (Lee, 2011; Zhao et al., 2012); and the assumption that Ca2+ storage and uptake into the acidic vesicles may also be a reflection of their pH (H+ pumping activity) (Patel and Docampo, 2010, Morgan, 2011). By any means, however complex that process may be, further studies addressing the coupling actions of NAADP/TPCs and the ER channels are likely to be very helpful in our understanding of calcium signals at fertilization and in general cellular functionality.
Figure 3. Calcium signaling events at fertilization in echinoderms - coupling of activity of multiple Ca2+ stores, channels and messengers.
Sperm contact with the egg jelly triggers production of NAADP (1), which has been implicated in vesicle membrane fusion for the acrosomal reaction (2) and/or suggested to be delivered as the activating molecule to the eggs. Sperm-egg contact (3) has been shown to trigger a NAADP-dependent Ca2+ current that activate voltage-operated Ca2+ channels (VOC) in the membrane allowing Ca2+ influx for the cortical Ca2+ flash and the membrane depolarization responsible for the fast block to polyspermy (4). Coordinately, sperm egg interaction also triggers PLC activation and IP3 production, leading to IP3 receptors activation and amplification of the Ca2+ signal to form the wave by coupling with RyR receptors via CICR (5). The Ca2+ wave triggers the exocytosis of the cortical granules leading to the elevation of the fertilization envelope (FE) (6), which works as a shield for additional sperm, the slow block to polyspermy mechanism. Additionally, TPCs in acidic vesicles are found in the egg periphery and may play a role in local Ca2+ release events triggered by NAADP. ARCs are located in secretory vesicles, cortical granules and/or the plasma membrane, and may be responsible for differential production of either cADPR or NAADP.
Abbreviations
- SERCA
Sarco/endoplasmic reticulum calcium-ATPase
- IP3
Inositol-3-phoshate
- cADPR
Cyclic ADP ribose
- NAADP
Nicotinic acid adenine dinucleotide-phosphate
- TPC
Two-pore channel
- ARC
ADP-ribosyl cyclase
- SpARCs
S. purpuratus ARC
- PLC
Phospholipase
- ER
Endoplasmic reticulum
- CICR
Calcium-induced calcium release
- RyR
Ryanodine receptor
- VOC
Voltage operated calcium channels
- TRP
Transient receptor potential ion channels
References
- Aarhus R, Dickey DM, Graeff RM, Gee KR, Walseth TF, Lee HC. Activation and inactivation of Ca2+ release by NAADP+. J Biol Chem. 1996;271:8513–8516. doi: 10.1074/jbc.271.15.8513. [DOI] [PubMed] [Google Scholar]
- Austin CR. Fertilization. Prentice-Hall; Englewood Cliffs, NJ.: 1965. [Google Scholar]
- Berridge MJ, Galione A. Cytosolic calcium oscillators. FASEB J. 1988;2:3074–3082. doi: 10.1096/fasebj.2.15.2847949. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Billington RA, Genazzani AA. Characterization of NAADP(+) binding in sea urchin eggs. Biochem Biophys Res Commun. 2000;276:112–116. doi: 10.1006/bbrc.2000.3444. [DOI] [PubMed] [Google Scholar]
- Billington RA, Ho A, Genazzani AA. Nicotinic acid adenine dinucleotide phosphate (NAADP) is present at micromolar concentrations in sea urchin spermatozoa. The Journal of Physiology. 2002;544:107–112. doi: 10.1113/jphysiol.2002.030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu E, Rahman T, Churamani D, Prole DL, Brailoiu GC, Hooper R, Taylor CW, Patel S. An NAADP-gated two-pore channel targeted to the plasma membrane uncouples triggering from amplifying Ca2+ signals. J Biol Chem. 2010;285:38511–38516. doi: 10.1074/jbc.M110.162073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang KT, et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E, Santella L, Branca D, Brini M. Generation, control, and processing of cellular calcium signals. Crit Rev Biochem Mol Biol. 2001;36:107–260. doi: 10.1080/20014091074183. [DOI] [PubMed] [Google Scholar]
- Carré D, Sardet C. Fertilization and early development in Beroe ovata. Dev Biol. 1984;105(1):188–95. doi: 10.1016/0012-1606(84)90274-4. [DOI] [PubMed] [Google Scholar]
- Carroll DJ, Albay DT, Terasaki M, Jaffe LA, Foltz KR. Identification of PLCgamma-dependent and -independent events during fertilization of sea urchin eggs. Dev Biol. 1999;206:232–247. doi: 10.1006/dbio.1998.9145. [DOI] [PubMed] [Google Scholar]
- Chambers EL, de Armendi J. Membrane potential, action potential and activation potential of eggs of the sea urchin, Lytechinus variegatus. Experimental cell research. 1979;122:203–218. doi: 10.1016/0014-4827(79)90575-5. [DOI] [PubMed] [Google Scholar]
- Chebotareva T, Taylor J, Mullins JJ, Wilmut I. Rat eggs cannot wait: Spontaneous exit from meiotic metaphase-II arrest. Mol.Reprod.Dev. 2011;78:795–807. doi: 10.1002/mrd.21385. [DOI] [PubMed] [Google Scholar]
- Chiba K. Evolution of the acquisition of fertilization competence and polyspermy blocks during meiotic maturation. 2011;78:808–813. doi: 10.1002/mrd.21378. [DOI] [PubMed] [Google Scholar]
- Churchill GC, Galione A. NAADP induces Ca2+ oscillations via a two-pool mechanism by priming IP3- and cADPR-sensitive Ca2+ stores. Embo J. 2001a;20:2666–2671. doi: 10.1093/emboj/20.11.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill GC, Galione A. Prolonged inactivation of nicotinic acid adenine dinucleotide phosphate-induced Ca2+ release mediates a spatiotemporal Ca2+ memory. J Biol Chem. 2001b;276:11223–11225. doi: 10.1074/jbc.M009335200. [DOI] [PubMed] [Google Scholar]
- Churchill GC, O'Neill JS, Masgrau R, Patel S, Thomas JM, Genazzani AA, Galione A. Sperm deliver a new second messenger: NAADP. Curr Biol. 2003;13:125–128. doi: 10.1016/s0960-9822(03)00002-2. [DOI] [PubMed] [Google Scholar]
- Churchill GC, Okada Y, Thomas JM, Genazzani AA, Patel S, Galione A. NAADP mobilizes Ca(2+) from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell. 2002;111:703–708. doi: 10.1016/s0092-8674(02)01082-6. [DOI] [PubMed] [Google Scholar]
- Crossley I, Swann K, Chambers E, Whitaker M. Activation of sea urchin eggs by inositol phosphates is independent of external calcium. Biochem J. 1988;252:257–262. doi: 10.1042/bj2520257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammermann W, Guse AH. Functional ryanodine receptor expression is required for NAADP-mediated local Ca2+ signaling in T-lymphocytes. J Biol Chem. 2005;280:21394–21399. doi: 10.1074/jbc.M413085200. [DOI] [PubMed] [Google Scholar]
- Dammermann W, Zhang B, Nebel M, Cordiglieri C, Odoardi F, Kirchberger T, Kawakami N, Dowden J, Schmid F, Dornmair K, et al. NAADP-mediated Ca2+ signaling via type 1 ryanodine receptor in T cells revealed by a synthetic NAADP antagonist. Proc Natl Acad Sci U S A. 2009;106:10678–10683. doi: 10.1073/pnas.0809997106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LC, Morgan AJ, Ruas M, Wong JL, Graeff RM, Poustka AJ, Lee HC, Wessel GM, Parrington J, Galione A. Ca(2+) signaling occurs via second messenger release from intraorganelle synthesis sites. Curr Biol. 2008;18:1612–1618. doi: 10.1016/j.cub.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Flora A, Zocchi E, Guida L, Franco L, Bruzzone S. Autocrine and paracrine calcium signaling by the CD38/NAD+/cyclic ADP-ribose system. Ann N Y Acad Sci. 2004;1028:176–191. doi: 10.1196/annals.1322.021. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica EC, Mullins C, Caplan S, Bonifacino JS. Lysosome-related organelles. FASEB J. 2000;14:1265–1278. doi: 10.1096/fj.14.10.1265. [DOI] [PubMed] [Google Scholar]
- Eisen A, Kiehart DP, Wieland SJ, Reynolds GT. Temporal sequence and spatial distribution of early events of fertilization in single sea urchin eggs. J Cell Biol. 1984;99:1647–1654. doi: 10.1083/jcb.99.5.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen A, Reynolds GT. Calcium transients during early development in single starfish (Asterias forbesi) oocytes. J Cell Biol. 1984;99:1878–1882. doi: 10.1083/jcb.99.5.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen A, Reynolds GT. Source and sinks for the calcium released during fertilization of single sea urchin eggs. J Cell Biol. 1985;100:1522–1527. doi: 10.1083/jcb.100.5.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettensohn CA, Wessel GM, Wray G. Development of Sea Urchins, Ascidians and Other Non-Vertebrate Deuterostomes: An Experimental Analysis. Academic Press; 2004. The invertebrate deuterostomes: An introduction to their phylogeny, reproduction, development, and genomics. pp. 1–13. [DOI] [PubMed] [Google Scholar]
- Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- Galione A. Cyclic ADP-ribose: a new way to control calcium. Science. 1993;259:325–326. doi: 10.1126/science.8380506. [DOI] [PubMed] [Google Scholar]
- Galione A. NAADP receptors. Cold Spring Harbor perspectives in biology. 2011;3:a004036. doi: 10.1101/cshperspect.a004036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galione A, Jones KT, Lai FA, Swann K. A cytosolic sperm protein factor mobilizes Ca2+ from intracellular stores by activating multiple Ca2+ release mechanisms independently of low molecular weight messengers. J Biol Chem. 1997;272:28901–28905. doi: 10.1074/jbc.272.46.28901. [DOI] [PubMed] [Google Scholar]
- Galione A, Lee HC, Busa WB. Ca(2+)-induced Ca2+ release in sea urchin egg homogenates: modulation by cyclic ADP-ribose. Science. 1991;253:1143–1146. doi: 10.1126/science.1909457. [DOI] [PubMed] [Google Scholar]
- Galione A, McDougall A, Busa WB, Willmott N, Gillot I, Whitaker M. Redundant mechanisms of calcium-induced calcium release underlying calcium waves during fertilization of sea urchin eggs. Science. 1993;261:348–352. doi: 10.1126/science.8392748. [DOI] [PubMed] [Google Scholar]
- Genazzani AA, Empson RM, Galione A. Unique inactivation properties of NAADP-sensitive Ca2+ release. J Biol Chem. 1996;271:11599–11602. doi: 10.1074/jbc.271.20.11599. [DOI] [PubMed] [Google Scholar]
- Genazzani AA, Galione A. Nicotinic acid-adenine dinucleotide phosphate mobilizes Ca2+ from a thapsigargin-insensitive pool. Biochem J. 1996;315(Pt 3):721–725. doi: 10.1042/bj3150721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko JV, Maruyama Y, Yano K, Dolman NJ, Tepikin AV, Petersen OH, Gerasimenko OV. NAADP mobilizes Ca2+ from a thapsigargin-sensitive store in the nuclear envelope by activating ryanodine receptors. J Cell Biol. 2003;163:271–282. doi: 10.1083/jcb.200306134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert S. Developmental Biology. Sinauer Associates; Sunderland MA.: 2010. [Google Scholar]
- Gilkey JC, Jaffe LF, Ridgway EB, Reynolds GT. A free calcium wave traverses the activating egg of the medaka, Oryzias latipes. J Cell Biol. 1978;76:448–466. doi: 10.1083/jcb.76.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grudzinskas JG, Yovich JL, editors. Gametes - The Oocyte. Cambridge University Press; Cambridge England: 1995. [Google Scholar]
- Guida L, Bruzzone S, Sturla L, Franco L, Zocchi E, De Flora A. Equilibrative and concentrative nucleoside transporters mediate influx of extracellular cyclic ADP-ribose into 3T3 murine fibroblasts. J Biol Chem. 2002;277:47097–47105. doi: 10.1074/jbc.M207793200. [DOI] [PubMed] [Google Scholar]
- Hafner M, Petzelt C, Nobiling R, Pawley JB, Kramp D, Schatten G. Wave of free calcium at fertilization in the sea urchin egg visualized with fura-2. Cell motility and the cytoskeleton. 1988;9:271–277. doi: 10.1002/cm.970090309. [DOI] [PubMed] [Google Scholar]
- Hamaguchi Y, Hiramoto Y. Activation of sea urchin eggs by microinjection of calcium buffers. Experimental cell research. 1981;134:171–179. doi: 10.1016/0014-4827(81)90474-2. [DOI] [PubMed] [Google Scholar]
- Hooper R, Churamani D, Brailoiu E, Taylor CW, Patel S. Membrane topology of NAADP-sensitive two-pore channels and their regulation by N-linked glycosylation. J Biol Chem. 2011;286:9141–9149. doi: 10.1074/jbc.M110.189985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper R, Patel S. NAADP on target. Advances in experimental medicine and biology. 2012;740:325–347. doi: 10.1007/978-94-007-2888-2_14. [DOI] [PubMed] [Google Scholar]
- Howard M, Grimaldi JC, Bazan JF, Lund FE, Santos-Argumedo L, Parkhouse RM, Walseth TF, Lee HC. Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science. 1993;262:1056–1059. doi: 10.1126/science.8235624. [DOI] [PubMed] [Google Scholar]
- Ito J, Parrington J, Fissore RA. PLCζ and its role as a trigger of development in vertebrates. Mol.Reprod.Dev. 2011;78:846–853. doi: 10.1002/mrd.21359. [DOI] [PubMed] [Google Scholar]
- Jaffe LA. Fast block to polyspermy in sea urchin eggs is electrically mediated. Nature. 1976;261:68–71. doi: 10.1038/261068a0. [DOI] [PubMed] [Google Scholar]
- Jaffe LF. Calcium waves and development. Ciba Foundation symposium. 1995;188:4–12. doi: 10.1002/9780470514696.ch2. discussion 12-17. [DOI] [PubMed] [Google Scholar]
- Jaffe LF, Creton R. On the conservation of calcium wave speeds. Cell calcium. 1998;24:1–8. doi: 10.1016/s0143-4160(98)90083-5. [DOI] [PubMed] [Google Scholar]
- Kim H, Jacobson EL, Jacobson MK. Synthesis and degradation of cyclic ADP-ribose by NAD glycohydrolases. Science. 1993;261:1330–1333. doi: 10.1126/science.8395705. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. A primer on meiotic resumption in starfish oocytes: The proposed signaling pathway triggered by maturation-inducing hormone. Mol.Reprod.Dev. 2011;78:704–707. doi: 10.1002/mrd.21343. [DOI] [PubMed] [Google Scholar]
- Kolisek M, Beck A, Fleig A, Penner R. Cyclic ADP-ribose and hydrogen peroxide synergize with ADP-ribose in the activation of TRPM2 channels. Molecular cell. 2005;18:61–69. doi: 10.1016/j.molcel.2005.02.033. [DOI] [PubMed] [Google Scholar]
- Lange I, Penner R, Fleig A, Beck A. Synergistic regulation of endogenous TRPM2 channels by adenine dinucleotides in primary human neutrophils. Cell calcium. 2008;44:604–615. doi: 10.1016/j.ceca.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC. Potentiation of calcium- and caffeine-induced calcium release by cyclic ADP-ribose. J Biol Chem. 1993;268:293–299. [PubMed] [Google Scholar]
- Lee HC. Mechanisms of calcium signaling by cyclic ADP-ribose and NAADP. Physiol Rev. 1997;77:1133–1164. doi: 10.1152/physrev.1997.77.4.1133. [DOI] [PubMed] [Google Scholar]
- Lee HC. Cyclic ADP-ribose and Nicotinic Acid Adenine Dinucleotide Phosphate (NAADP) as Messengers for Calcium Mobilization. J Biol Chem. 2012;287:31633–31640. doi: 10.1074/jbc.R112.349464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC, Aarhus R. A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose. J Biol Chem. 1995;270:2152–2157. doi: 10.1074/jbc.270.5.2152. [DOI] [PubMed] [Google Scholar]
- Lim D, Kyozuka K, Gragnaniello G, Carafoli E, Santella L. NAADP+ initiates the Ca2+ response during fertilization of starfish oocytes. FASEB J. 2001;15:2257–2267. doi: 10.1096/fj.01-0157com. [DOI] [PubMed] [Google Scholar]
- Lin-Moshier Y, Walseth TF, Churamani D, Davidson SM, Slama JT, Hooper R, Brailoiu E, Patel S, Marchant JS. Photoaffinity labeling of nicotinic acid adenine dinucleotide phosphate (NAADP) targets in mammalian cells. J Biol Chem. 2012;287:2296–2307. doi: 10.1074/jbc.M111.305813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Kriksunov IA, Graeff R, Munshi C, Lee HC, Hao Q. Crystal structure of human CD38 extracellular domain. Structure. 2005;13:1331–1339. doi: 10.1016/j.str.2005.05.012. [DOI] [PubMed] [Google Scholar]
- McDougall A, Gillot I, Whitaker M. Thimerosal reveals calcium-induced calcium release in unfertilised sea urchin eggs. Zygote. 1993;1:35–42. doi: 10.1017/s0967199400001271. [DOI] [PubMed] [Google Scholar]
- McGinnis LK, Carroll DJ, Kinsey WH. Protein tyrosine kinase signaling during oocyte maturation and fertilization. Mol.Reprod.Dev. 2011;78:831–845. doi: 10.1002/mrd.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moccia F, Billington RA, Santella L. Pharmacological characterization of NAADP-induced Ca2+ signals in starfish oocytes. Biochem Biophys Res Commun. 2006a;348:329–336. doi: 10.1016/j.bbrc.2006.05.157. [DOI] [PubMed] [Google Scholar]
- Moccia F, Lim D, Kyozuka K, Santella L. NAADP triggers the fertilization potential in starfish oocytes. Cell calcium. 2004;36:515–524. doi: 10.1016/j.ceca.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Moccia F, Nusco GA, Lim D, Ercolano E, Gragnaniello G, Brown ER, Santella L. Ca2+ signalling and membrane current activated by cADPr in starfish oocytes. Pflugers Arch. 2003;446:541–552. doi: 10.1007/s00424-003-1076-1. [DOI] [PubMed] [Google Scholar]
- Moccia F, Nusco GA, Lim D, Kyozuka K, Santella L. NAADP and InsP3 play distinct roles at fertilization in starfish oocytes. Dev Biol. 2006b;294:24–38. doi: 10.1016/j.ydbio.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Mohri T, Hamaguchi Y. Propagation of transient Ca2+ increase in sea urchin eggs upon fertilization and its regulation by microinjecting EGTA solution. Cell structure and function. 1991;16:157–165. doi: 10.1247/csf.16.157. [DOI] [PubMed] [Google Scholar]
- Morgan AJ. Sea urchin eggs in the acid reign. Cell calcium. 2011;50:147–156. doi: 10.1016/j.ceca.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Morgan AJ, Galione A. Fertilization and nicotinic acid adenine dinucleotide phosphate induce pH changes in acidic Ca(2+) stores in sea urchin eggs. J Biol Chem. 2007a;282:37730–37737. doi: 10.1074/jbc.M704630200. [DOI] [PubMed] [Google Scholar]
- Morgan AJ, Galione A. NAADP induces pH changes in the lumen of acidic Ca2+ stores. Biochem J. 2007b;402:301–310. doi: 10.1042/BJ20060759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AJ, Galione A. Investigating cADPR and NAADP in intact and broken cell preparations. Methods. 2008;46:194–203. doi: 10.1016/j.ymeth.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Patel S, Brailoiu E. Triggering of Ca2+ signals by NAADP-gated two-pore channels: a role for membrane contact sites? Biochem Soc Trans. 2012;40:153–157. doi: 10.1042/BST20110693. [DOI] [PubMed] [Google Scholar]
- Patel S, Churchill GC, Galione A. Unique kinetics of nicotinic acid-adenine dinucleotide phosphate (NAADP) binding enhance the sensitivity of NAADP receptors for their ligand. Biochem J 352 Pt. 2000;3:725–729. [PMC free article] [PubMed] [Google Scholar]
- Patel S, Docampo R. Acidic calcium stores open for business: expanding the potential for intracellular Ca2+ signaling. Trends Cell Biol. 2010;20:277–286. doi: 10.1016/j.tcb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Muallem S. Acidic Ca(2+) stores come to the fore. Cell calcium. 2011;50:109–112. doi: 10.1016/j.ceca.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Peiter E, Maathuis FJ, Mills LN, Knight H, Pelloux J, Hetherington AM, Sanders D. The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature. 2005;434:404–408. doi: 10.1038/nature03381. [DOI] [PubMed] [Google Scholar]
- Prasad GS, McRee DE, Stura EA, Levitt DG, Lee HC, Stout CD. Crystal structure of Aplysia ADP ribosyl cyclase, a homologue of the bifunctional ectozyme CD38. Nature structural biology. 1996;3:957–964. doi: 10.1038/nsb1196-957. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan L, Muller-Steffner H, Bosc C, Vacquier VD, Schuber F, Moutin MJ, Dale L, Patel S. A single residue in a novel ADP-ribosyl cyclase controls production of the calcium-mobilizing messengers cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate. J Biol Chem. 2010;285:19900–19909. doi: 10.1074/jbc.M110.105312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos IB, Miranda K, Pace DA, Verbist KC, Lin FY, Zhang Y, Oldfield E, Machado EA, de Souza W, Docampo R. Calcium and polyphosphate-containing acidic granules of sea urchin eggs are similar to acidocalcisomes but are not the targets for NAADP. Biochem J. 2010;429(3):485–95. doi: 10.1042/BJ20091956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringer S. Concerning the influence exerted by each of the constituents of the blood on the contraction of the ventricle. J Physiol (Lond) 1882;3:380–383. doi: 10.1113/jphysiol.1882.sp000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruas M, Rietdorf K, Arredouani A, Davis LC, Lloyd-Evans E, Koegel H, Funnell TM, Morgan AJ, Ward JA, Watanabe K, et al. Purified TPC isoforms form NAADP receptors with distinct roles for Ca(2+) signaling and endolysosomal trafficking. Curr Biol. 2010;20:703–709. doi: 10.1016/j.cub.2010.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runft LL, Jaffe LA, Mehlmann LM. Egg activation at fertilization: where it all begins. Dev Biol. 2002;245:237–254. doi: 10.1006/dbio.2002.0600. [DOI] [PubMed] [Google Scholar]
- Santella L, Kyozuka K, Genazzani AA, De Riso L, Carafoli E. Nicotinic acid adenine dinucleotide phosphate-induced Ca(2+) release. Interactions among distinct Ca(2+) mobilizing mechanisms in starfish oocytes. J Biol Chem. 2000;275:8301–8306. doi: 10.1074/jbc.275.12.8301. [DOI] [PubMed] [Google Scholar]
- Santella L, Lim D, Moccia F. Calcium and fertilization: the beginning of life. Trends Biochem Sci. 2004;29:400–408. doi: 10.1016/j.tibs.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development. 2002;129:3533–3544. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- Schmid F, Fliegert R, Westphal T, Bauche A, Guse AH. Nicotinic Acid Adenine Dinucleotide Phosphate (NAADP) Degradation by Alkaline Phosphatase. J Biol Chem. 2012;287:32525–32534. doi: 10.1074/jbc.M112.362715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt T, Patton C, Epel D. Is there a role for the Ca2+ influx during fertilization of the sea urchin egg? Dev Biol. 1982;90:284–290. doi: 10.1016/0012-1606(82)90377-3. [DOI] [PubMed] [Google Scholar]
- Shearer J, De Nadai C, Emily-Fenouil F, Gache C, Whitaker M, Ciapa B. Role of phospholipase Cgamma at fertilization and during mitosis in sea urchin eggs and embryos. Development. 1999;126:2273–2284. doi: 10.1242/dev.126.10.2273. [DOI] [PubMed] [Google Scholar]
- Silvestre F, Boni R, Fissore RA, Tosti E. Ca2+ signaling during maturation of cumulus–oocyte complex in mammals. Mol.Reprod.Dev. 2011;78:744–756. doi: 10.1002/mrd.21332. [DOI] [PubMed] [Google Scholar]
- Shen SS, Buck WR. Sources of calcium in sea urchin eggs during the fertilization response. Dev Biol. 1993;157:157–169. doi: 10.1006/dbio.1993.1120. [DOI] [PubMed] [Google Scholar]
- Steen M, Kirchberger T, Guse AH. NAADP mobilizes calcium from the endoplasmic reticular Ca(2+) store in T-lymphocytes. J Biol Chem. 2007;282:18864–18871. doi: 10.1074/jbc.M610925200. [DOI] [PubMed] [Google Scholar]
- Steinhardt RA, Lundin L, Mazia D. Bioelectric responses of the echinoderm egg to fertilization. Proc Natl Acad Sci U S A. 1971;68:2426–2430. doi: 10.1073/pnas.68.10.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker SA. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev Biol. 1999;211:157–176. doi: 10.1006/dbio.1999.9340. [DOI] [PubMed] [Google Scholar]
- Swann K, Whitaker M. The part played by inositol trisphosphate and calcium in the propagation of the fertilization wave in sea urchin eggs. J Cell Biol. 1986;103:2333–2342. doi: 10.1083/jcb.103.6.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szent-Györgyi AG. Calcium regulation of muscle contraction. Biophys. J. 1975;15:707–723. doi: 10.1016/S0006-3495(75)85849-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasawa S, Tohgo A, Noguchi N, Koguma T, Nata K, Sugimoto T, Yonekura H, Okamoto H. Synthesis and hydrolysis of cyclic ADP-ribose by human leukocyte antigen CD38 and inhibition of the hydrolysis by ATP. J Biol Chem. 1993;268:26052–26054. [PubMed] [Google Scholar]
- Terasaki M, Jaffe LA. Organization of the sea urchin egg endoplasmic reticulum and its reorganization at fertilization. J Cell Biol. 1991;114:929–940. doi: 10.1083/jcb.114.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki M, Jaffe LA, Hunnicutt GR, Hammer JA., 3rd Structural change of the endoplasmic reticulum during fertilization: evidence for loss of membrane continuity using the green fluorescent protein. Dev Biol. 1996;179:320–328. doi: 10.1006/dbio.1996.0263. [DOI] [PubMed] [Google Scholar]
- Terasaki M, Song J, Wong JR, Weiss MJ, Chen LB. Localization of endoplasmic reticulum in living and glutaraldehyde-fixed cells with fluorescent dyes. Cell. 1984;38:101–108. doi: 10.1016/0092-8674(84)90530-0. [DOI] [PubMed] [Google Scholar]
- Vacquier VD. Laboratory on sea urchin fertilization. Mol Reprod Dev. 2011;78:553–564. doi: 10.1002/mrd.21360. [DOI] [PubMed] [Google Scholar]
- Vasudevan SR, Galione A, Churchill GC. Sperm express a Ca2+-regulated NAADP synthase. Biochem J. 2008;411:63–70. doi: 10.1042/BJ20071616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan SR, Lewis AM, Chan JW, Machin CL, Sinha D, Galione A, Churchill GC. The calcium-mobilizing messenger nicotinic acid adenine dinucleotide phosphate participates in sperm activation by mediating the acrosome reaction. J Biol Chem. 2010;285:18262–18269. doi: 10.1074/jbc.M109.087858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walseth TF, Lin-Moshier Y, Jain P, Ruas M, Parrington J, Galione A, Marchant JS, Slama JT. Photoaffinity labeling of high affinity nicotinic acid adenine dinucleotide phosphate (NAADP)-binding proteins in sea urchin egg. J Biol Chem. 2012;287:2308–2315. doi: 10.1074/jbc.M111.306563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang X, Dong XP, Samie M, Li X, Cheng X, Goschka A, Shen D, Zhou Y, Harlow J, et al. TPC Proteins Are Phosphoinositide- Activated Sodium-Selective Ion Channels in Endosomes and Lysosomes. Cell. 2012;151:372–383. doi: 10.1016/j.cell.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel GM, Wong J. Cell surface changes in the egg at fertilization. Mol. Reprod. Devel. 2009;76:942–953. doi: 10.1002/mrd.21090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker M. Calcium at fertilization and in early development. Physiol Rev. 2006;86:25–88. doi: 10.1152/physrev.00023.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker MJ, Steinhardt RA. Evidence in support of the hypothesis of an electrically mediated fast block to polyspermy in sea urchin eggs. Dev Biol. 1983;95:244–248. doi: 10.1016/0012-1606(83)90024-6. [DOI] [PubMed] [Google Scholar]
- Wilding M, Dale B. Sperm factor: what is it and what does it do? Molecular human reproduction. 1997;3:269–273. doi: 10.1093/molehr/3.3.269. [DOI] [PubMed] [Google Scholar]
- Zhang F, Xu M, Han WQ, Li PL. Reconstitution of lysosomal NAADPTRP-ML1 signaling pathway and its function in TRP-ML1(−/−) cells. American journal of physiology. Cell physiology. 2011;301:C421–430. doi: 10.1152/ajpcell.00393.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YJ, Lam CM, Lee HC. The membrane-bound enzyme CD38 exists in two opposing orientations. Science signaling. 2012;5:ra67. doi: 10.1126/scisignal.2002700. [DOI] [PubMed] [Google Scholar]