Abstract
An 11 year-old female developed heparin induced thrombocytopenia (HIT) with thrombosis during therapy for lower extremity deep vein thrombosis and pulmonary embolism. Transition from bivalirudin, a direct thrombin inhibitor (DTI), to warfarin resulted in extensive re-thrombosis, and fondaparinux therapy similarly failed. She was then treated with argatroban, and transitioned successfully to warfarin after nine weeks. The risk of re-thrombosis was ultimately reduced by allowing time for the thrombogenic potential to abate. The argatroban/warfarin transition was monitored with chromogenic factor X levels. This case highlights several difficult problems in pediatric thrombosis.
Keywords: Heparin induced thrombocytopenia, children, direct thrombin inhibitor, warfarin, fondaparinux
INTRODUCTION
Heparin induced thrombocytopenia (HIT) is a potentially life-threatening adverse effect of heparin therapy [1–3]. Marked by thrombocytopenia and associated with thrombosis in 20–50% of cases, HIT is rare in children (0.9–1.5% incidence) [4]. HIT is typically treated with a direct thrombin inhibitor (DTI) followed by conversion to oral warfarin upon platelet recovery [2–3]. We describe a child with HIT-associated thrombosis followed by multiple recurrent thrombotic events.
CASE REPORT
A previously healthy, obese 11 year-old female (weight 68 kg, >97th percentile) was treated for lobar pneumonia. One week later, still heparin-naive, she developed respiratory distress, fever, acute left leg occlusive deep vein thrombosis (DVT), and bilateral pulmonary emboli. Treatment with unfractionated heparin was initiated (70 unit/kg intravenous bolus, 18 units/kg/hr continuous infusion, goal activated partial thromboplastin time (PTT) 60–80 seconds) (Figure 1). The following day catheter-directed tissue plasminogen activator (tPA) was started at a total dose of 1 mg/hr (0.015 mg/kg/hr) via two Uni*Fuse™ catheters (AngioDynamics, Inc. Queensbury, NY) in the left lower extremity and superior vena cava (SVC). A retrievable inferior vena cava (IVC) filter was placed. The heparin infusion rate was lowered to prophylactic dosing of 10 units/kg/hr as generally accepted; however, later it was titrated to keep the PTT 1.5–2× normal. Heparin was changed to enoxaparin (target anti-Xa 0.5–1.0 U/mL) once off tPA. There were no bleeding complications. All thrombi resolved, but she remained on oxygen and had daily fevers of unclear etiology.
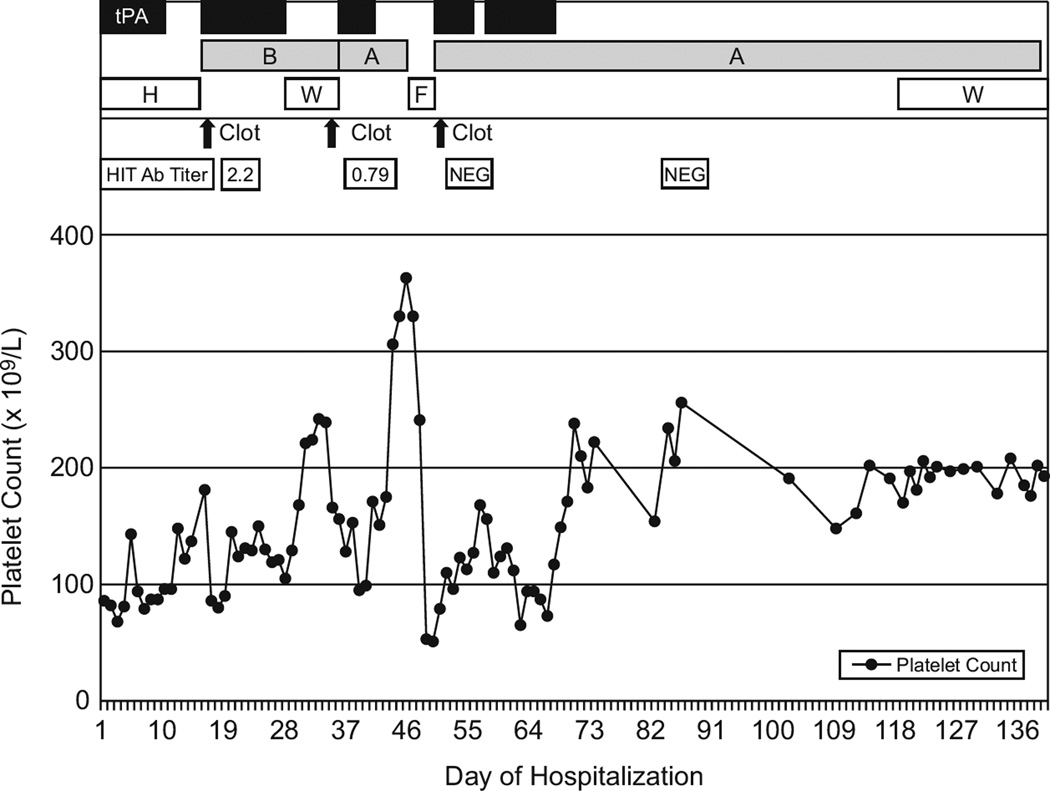
Figure 1.
Course of the patient’s platelet count, thrombotic events, anti-heparin:PF4 antibody titer (HIT Ab titer), and therapy.
tPA, tissue plasminogen activator; B, bivalirudin; A, argatroban; H, heparin/low molecular weight heparin; W, warfarin; F, fondaparinux; NEG, negative.
Risk factors for initial thrombosis were immobilization, obesity, and left-sided May-Thurner syndrome. A hypercoagulability work-up was normal for the following: protein C (PC), protein S, anti-thrombin, factor V Leiden, prothombin 20210, activated protein C resistance, homocysteine, factor VIII activity, lipoprotein (a), anti-phospholipid antibodies, and lupus anticoagulant.
On day 16 she developed recurrent DVT (in both legs), complete IVC occlusion, and new bilateral pulmonary emboli while retaining the IVC filter. Platelet counts dropped from 181 to 86×109/L. The pre-test probability for HIT (“four T” test [5]) was 7/8. Heparin-platelet antibodies by ELISA (Asserachrom, Diagnostica Stago, Parsippany, NJ) were positive at 2.2 (negative <0.46). Heparin was replaced with bivalirudin, 125 mcg/kg IV bolus followed by 125 mcg/kg/hr infusion monitored by PTT (goal 1.5–2× normal), for its potential ability to inhibit clot-bound thrombin [6]. Due to the extended thrombosis, site-directed tPA (to the left leg and IVC as described above) was given concurrently for 12 days until flow through bilateral leg veins and the IVC had improved markedly. The concomitant use of a DTI and tPA carries an increased risk of bleeding; however, it was judged clinically indicated.
On day 28, while continuing on bivalirudin, [platelet count 105×109/L, D-dimer >4618 ng/mL (normal <85), HIT-antibody titer 0.79, INR 2.76] oral warfarin was started at 5 mg every other day. It was held on day 34 due to an INR of 4.67. Co-administration of cefazolin (until day 31), ranitidine, and one dose of cholral hydrate, each known to increase the effect of warfain, complicated warfarin management. Without further warfarin, the INR increased to 7.3 on day 36, and despite having therapeutic PTTs on bivalirudin (240 mcg/kg/hr) and the elevated INR, she developed abdominal pain, hypertension, and increasing creatinine from 0.39 to 0.63 mg/dL. CT revealed complete re-thrombosis of the IVC, bilateral deep leg veins, and the right renal vein. Site-directed tPA (same method as above) was restarted, and, because of renal compromise, bivalirudin was replaced with argatroban at 2 mcg/kg/min (adjusted to 0.32–0.4 mcg/kg/min to maintain PTT 1.5–2× normal [7]). After six days of tPA and left external iliac vein angioplasty with stenting, she had patent lower extremity vessels and near total patency of the IVC. TPA was discontinued and argatroban continued at 0.4–7.9 mcg/kg/min.
To facilitate outpatient management, anti-coagulation was switched to subcutaneous fondaparinux, 7.5 mg daily, on day 47 [8]. Two days later, acute anxiety, respiratory distress, and decreasing platelets from 241 to 53×109/L heralded recurrent thrombotic IVC and bilateral leg vein occlusion and pulmonary emboli leading to respiratory failure. A fondaparinux-specific anti-Xa level was not available. HIT-antibodies were negative. Catheter-directed tPA (same method as above) and argatroban (goal PTT 1.5–2× normal, requiring increasing doses from 2 up to 17.5 mcg/kg/min) were restarted along with corticosteroids for an assumed, but never confirmed, immune mediated process. Additional complications included bilateral recurrent pneumothoraces, pulmonary hemorrhage (causing a 48 hour interruption in tPA therapy on day 54), Klebsiella pneumoniae sepsis, and severe pulmonary hypertension. At the end of tPA therapy on day 67, pulmonary perfusion had greatly improved, and the lower extremity veins and IVC were patent.
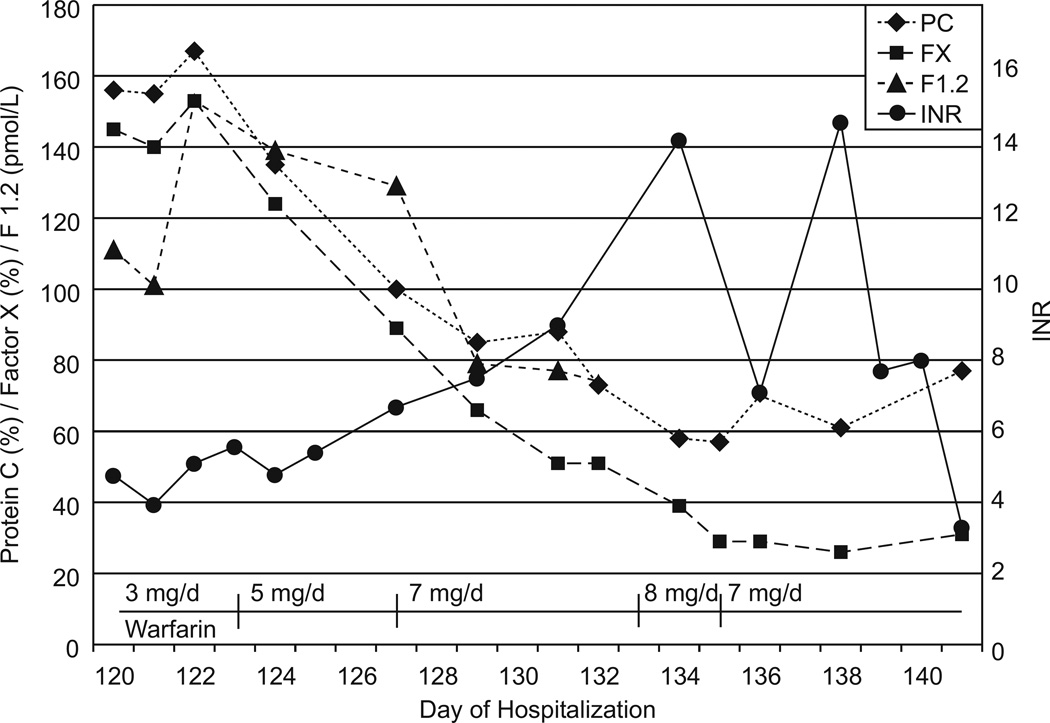
The patient remained on argatroban and steroids until day 120. After near-normalization of D-dimers at 0.81 mcg FEU/mL (normal <0.36), transition from argatroban to warfarin was started at a low dose of 0.05 mg/kg/day. Chromogenic factor X, PC, D-dimer, and prothrombin fragment 1.2 (F1.2) were followed to monitor the effect of warfarin (FX goal 20–40%) and estimate thrombogenicity (Figure 2). Thrombogenicity was estimated using levels of PC, D-dimer, and F1.2 compared to normal and their rate of change with relation to their normal half-life. Rapid decrease of PC, increase of F1.2 and/or D-dimer triggered holding or decreasing warfarin; no decrease in PC while decreasing or near-normal F1.2 and/or D-dimer triggered increasing warfarin by 1 mg/day every 3–5 days. At the desired FX level of 20–40%, argatroban was weaned over 7 days without complications. Steroids were discontinued, and the patient was discharged on day 141. Currently, she is on warfarin with normal pulmonary function, no evidence of pulmonary hypertension, normal creatinine, and mild post-thrombotic syndrome (leg edema, increased lower extremity vascularity without functional limitations). The IVC filter remains in place, due to parental preference, with planned removal 12 months after discharge.
Figure 2.
Changes in protein C activity (PC), chromogenic factor X level (FX), prothrombin fragment 1.2 (F1.2), INR, and warfarin dose changes during the transition from argatroban to warfarin. D-dimer is not shown but started at 0.81 FEU/mL (normal <0.36) and consistently decreased to 0.28 FEU/mL (normal <0.45) over this time period.
DISCUSSION
Although HIT is rare in children [4], this patient had compatible clinical findings and a positive ELISA for HIT antibodies. No confirmatory serotonin release assay was available, but given the high pre-test probability and second positive ELISA result, HIT was the most probable diagnosis [5]. Unexpectedly, re-thrombosis occurred when converting from bivalirudin to warfarin and from argatroban to fondaparinux. Conversion from argatroban to warfarin was achieved during a lowered thrombogenic state and by monitoring chromogenic FX levels.
Bivalirudin, used by itself at reported dosing, successfully protected this patient against re-thrombosis [6,9]. Conversion from bivalirudin to warfarin was hampered by probable concomitant drug/warfarin interferences and alteration of warfarin metabolism during severe illness, resulting in early elevated INRs. Bivalirudin did not protect against re-thrombosis after three doses of warfarin were administered. At that time, the hypercoagulable effect of warfarin likely was high with insufficient anticoagulant effect. The patient’s thrombogenic state (elevated D-dimers and only partially recovered platelet count) may have potentiated the hypercoagulable effect of warfarin (e.g. via low PC). The 2008 ACCP guidelines now recommend warfarin transition at >150 ×109/L platelets [10].
The combination of argatroban and warfarin causes supratherapeutic INRs not correlating with the anticoagulant effect [11][12] nor the risk of bleeding [13]. The use of chromogenic FX assays instead of INRs has been suggested because warfarin anticoagulates chiefly by inhibiting the synthesis of factors II and X, and a clot-based FX assay is inaccurate in the presence of argatroban [14–15]. On warfarin monotherapy FX levels of 20–40% correlate with therapeutic INRs [16]. Chromogenic FX levels of <45% in adults on argatroban and warfarin were predictive of a therapeutic INR once off argatroban [14]. We successfully used FX monitoring during the argatroban/warfarin conversion while also following PC, F1.2, and D-dimer to estimate thrombogenicity. A low initial dose of warfarin was intended to slow the decrease of PC.
The reason for the failure of fondaparinux remains unclear. Data on its use in children are scarce, but it remains an option in children unable to take warfarin. Fondaparinux-associated HIT was described in adults [17–18] although, in vitro, fondaparinux does not cause platelet activation in the presence of HIT-antibodies [1,5]. In this patient, a concurrent HIT-antibody titer was negative.
In addition, this case adds to the controversy of using an IVC filter with pulmonary embolism because efficiency is unclear and complications, such as thrombosis, may be serious [19]. The placement of a temporary IVC filter is currently only recommended for lower extremity DVT when anticoagulation is contraindicated, and it should be removed when anticoagulation becomes possible [20]. Also, the concurrent use of DTIs or therapeutic heparin and tPA carries an increased risk of bleeding, and is not standard.
This case combined several difficult and, in children, scarcely studied problems: the rare event of HIT, the limited data on the use of DTIs or fondaparinux, the limited data on the efficiency of IVC filters, and the complexity of warfarin management in a sick child on multiple medications and in conjunction with an alternative anticoagulant. Apart from warranting further study of HIT in children, this case indicates that awaiting a lowered thrombogenic potential prior to transitioning to warfarin can be beneficial. The use of chromogenic FX levels together with parameters measuring thrombogenicity should be considered for guiding an argatroban/warfarin transition.
Acknowledgments
Russell Ware for critical review of the manuscript; Eddie Hankins for reviewing warfarin drug interactions.
Contract grant sponsor: Supported in part by The American Lebanese Syrian Associated Charities (ALSAC)
REFERENCES
- 1.Dager WE, Dougherty JA, Nguyen PH, et al. Heparin-Induced Thrombocytopenia: Treatment Options and Special Considerations. Pharmacotherapy. 2007;27:564–587. doi: 10.1592/phco.27.4.564. [DOI] [PubMed] [Google Scholar]
- 2.Prechel M, Walenga JM. The Laboratory Diagnosis and Clinical Management of Patients with Heparin-Induced Thrombocytopenia: An Update. Semin Thromb Haemost. 2008;34:86–96. doi: 10.1055/s-2008-1066027. [DOI] [PubMed] [Google Scholar]
- 3.Greinacher A, Warkentin TE. Recognition, Treatment, and Prevention of Heparin-Induced Thrombocytopenia: Review and Update. Thromb Res. 2006;118:165–176. doi: 10.1016/j.thromres.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Klenner AF, Lubenow N, Raschke R, Greinacher A. Heparin-Induced Thrombocytopenia in Children: 12 New Cases and Review of the Literature. Thromb Haemost. 2004;91:719–724. doi: 10.1160/TH03-09-0571. [DOI] [PubMed] [Google Scholar]
- 5.Warkentin TE. Heparin-Induced Thrombocytopenia: Pathogenesis and Management. Br J Haematol. 2003;121:535–555. doi: 10.1046/j.1365-2141.2003.04334.x. [DOI] [PubMed] [Google Scholar]
- 6.Young G, Tarantino MD, Wohrley J, et al. Pilot Dose-Finding and Safety Study of Bivalirudin in Infants <6 Months of Age with Thrombosis. J Thromb Haemost. 2007;5:1654–1659. doi: 10.1111/j.1538-7836.2007.02623.x. [DOI] [PubMed] [Google Scholar]
- 7.Hursting M, Dubb J. Argatroban Anticoagulation in Pediatric Patients: a literature analysis. J Pediatr Hematol Oncol. 2006;28:4–10. doi: 10.1097/01.mph.0000195296.48319.38. [DOI] [PubMed] [Google Scholar]
- 8.Papadopoulos S, Flynn J, Lewis D. Fondaparinux as a Treatment Option in Heparin-Induced Thrombocytopenia. Pharmacotherapy. 2008;27:921–926. doi: 10.1592/phco.27.6.921. [DOI] [PubMed] [Google Scholar]
- 9.Rayapudi S, Torres A, Jr, Deshpande G, et al. Bivalirudin for Anticoagulation in Children. Pediatr Blood Cancer. 2008;51:798–801. doi: 10.1002/pbc.21731. [DOI] [PubMed] [Google Scholar]
- 10.Warkentin TE, Greinacher A, Koster A, et al. Treatment and Prevention of Heparin-Induced Thrombocytopenia: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:340–380. doi: 10.1378/chest.08-0677. [DOI] [PubMed] [Google Scholar]
- 11.Hursting M, Zehnder J, Becker J, et al. The International Normalized Ratio During Concurrent Warfarin and Argatroban Anticoagulation: Differential Contributions of Each Agent and Effects of the Choice on Thromboplastin Used. Clin Chem. 1999;45:409–411. [PubMed] [Google Scholar]
- 12.Sheth S, DiCicco M, Hursting M, et al. Interpreting the International Normalized Ratio (INT) in Individuals Receiving Argatroban and Warfarin. Thromb Haemost. 2001;85:435–440. [PubMed] [Google Scholar]
- 13.Hursting MJ, Lewis BE, Macfarlane DE. Transitioning from Argatroban to Warfarin Therapy in Patients with Heparin-Induced Thrombocytopenia. Clin Appl Thrombosis/Hemostasis. 2005;11:279–287. doi: 10.1177/107602960501100306. [DOI] [PubMed] [Google Scholar]
- 14.Arpino PA, Demirjian Z, Van Cott EM. Use of the Chromogenic Factor X Assay to Predict the International Normalized Ratio in Patients Transitioning from Argatroban to Warfarin. Pharmacotherapy. 2005;25:157–164. doi: 10.1592/phco.25.2.157.56950. [DOI] [PubMed] [Google Scholar]
- 15.Walenga JM, Fasanella A, Iqbal O, et al. Coagulation Laboratory Testing in Patients Treated with Argatroban. Semin Thromb Haemost. 1999;25(Suppl 1):61–66. [PubMed] [Google Scholar]
- 16.Ciavarella N, Coccheri S, Gensini G, et al. Multicenter Evaluation of a New Chromogenic Factor X Assay in Plasma of Patients on Oral Anticoagulants. Thromb Res. 1980;19:493–502. doi: 10.1016/0049-3848(80)90022-5. [DOI] [PubMed] [Google Scholar]
- 17.Warkentin TE, Maurer BT, Aster RH. Heparin-Induced Thrombocytopenia Associated with Fondaparinux. N Engl J Med. 2007;356:2653–2654. doi: 10.1056/NEJMc070346. [DOI] [PubMed] [Google Scholar]
- 18.Rota E, Bazzan M, Fantino G. Fondaparinux-Related Thrombocytopenia in a Previous Low-Molecular-Weight Heparin (LMWH)-Induced Heparin-Induced Thrombocytopenia (HIT) Thromb Haemost. 2008;99:779–781. doi: 10.1160/TH07-09-0573. [DOI] [PubMed] [Google Scholar]
- 19.Raffini L, Cahill AM, Hellinger J, et al. A Prospective Observational Study of IVC Filters in Pediatric Patients. Pediatr Blood Cancer. 2008;51:517–520. doi: 10.1002/pbc.21622. [DOI] [PubMed] [Google Scholar]
- 20.Monagle P, Chalmers E, Chan A, et al. Antithrombotic Therapy in Neonates and Children: American College of Chest Physicians Evidence-Based Clinical Guidelines (8th Edition) Chest. 2008;133:887s–968s. doi: 10.1378/chest.08-0762. [DOI] [PubMed] [Google Scholar]