Abstract
The increased deposition of iron in gastric mucosa is known as gastric siderosis. It is believed that the only regulated step of the iron metabolism cycle occurs during absorption in the small intestine. Once this system becomes overwhelmed due to either local or widespread iron levels, then iron can be absorbed very quickly by a passive concentration-dependent mechanism. This excess iron is initially stored in the liver but later can be found in the pancreas, heart and joints. Excess iron is not expected to deposit in the gastric mucosa. This gastric deposition has been found in association with hemochromatosis, oral iron medications, alcohol abuse, blood transfusions, hepatic cirrhosis and spontaneous portacaval shunt with esophageal varices. The precise mechanism of this iron deposition in gastric epithelial and stromal cells is still not well understood; thus, identification of iron in gastric mucosa raises many questions. On histology, the pattern of deposition is variable, and recognition of the pattern is often useful to choose the appropriate workup for the patient and to diagnose and possibly treat the cause of iron overload. In this article, we have described a well-referenced review of this rare clinical entity with different histological patterns, diagnostic tests and the clinical significance of the different patterns of iron deposition.
Keywords: Gastric siderosis, iron metabolism, stomach, mucosal injury, hemochromatosis
Introduction and background
Iron is the most abundant trace mineral in the human body, and it is used mainly for the production of hemoglobin and myoglobin.1 The uptake of iron in the gastrointestinal (GI) tract is still not fully understood.2 It is believed that the only regulated step of the iron metabolism cycle occurs during absorption in the duodenum and jejunum.2,3 If the system becomes overwhelmed due to high iron levels (local or systemic), then iron can be absorbed very quickly in a passive concentration-dependent mechanism.4 Once there is an excess of iron in the body, it is initially stored as hemosiderin in the liver, and then later in the pancreas, heart and joints (Figure 1).2,5 Since ferrous and ferric ions are catalysts for the formation of reactive oxygen metabolites and highly reactive radicals, damage will occur to the various organs and joints where iron accumulates.2,4,6,7 When iron accumulates in the gastric mucosa, it is known as gastric siderosis (GS).2
Figure 1.
Pathophysiology of iron absorption, transport and storage.
Recently, in our Anatomic Pathology Department, two cases of GS were incidentally identified. Neither of these two patients were on oral iron therapy nor had a history of alcohol abuse, prior hospitalization or of blood transfusion. Their liver enzymes were within normal limits; and in both cases, endoscopy was performed for non-specific upper GI symptoms. Histopathological examination showed only mild reactive changes and was negative for Helicobacter pylori organisms. In both cases, on closer look, fine granular brown pigments were identified in fundic and antral glandular epithelium and later, these pigments were proven to be iron using Prussian blue stain. Since GS has been found in association with hemochromatosis, oral iron medications, alcohol abuse, blood transfusions, hepatic cirrhosis and spontaneous portacaval shunt with esophageal varices, it was interesting that in our cases none of these other factors were involved.2 Interestingly, the pattern of iron deposition of one of the patients did not meet the pattern previously described in the literature.2 We believe that identification of iron in gastric mucosa may have some clinical implications, and as such, recognition of this rare clinical entity will alert clinicians to investigate their patients further in order to determine the underlying causes.2,8
Materials and methods
To conduct an in-depth review of this rare entity, we performed an extensive literature search using PubMed, Google Scholar, Medline and Medscape to identify peer-reviewed original research, review articles and case reports using the phrases “gastric siderosis,” “hemosiderosis,” “iron deposition in gastric mucosa” and “hemochromatosis.” The search period included articles published up to March 2015. We have manually searched the references to identify additional relevant articles. We found 27 articles published in English literature, which are relevant to our index cases. We also extracted the information pertaining to the different histological patterns, diagnosis, clinical significance and management of this rare clinical entity.
Results and discussion
GS has been previously described in patients with hemochromatosis, alcoholic cirrhosis, esophageal varices, history of multiple blood transfusions and those taking excessive therapeutic oral iron formulations.2 However, the clinical significance of these findings and the precise mechanism of this iron deposition in gastric epithelial and stromal cells are still not well understood.2 Iron is stored intracellularly as a storage protein either in the form of ferritin or hemosiderin. It is initially deposited as hemosiderin in the liver and when this storage exceeds the capacity, it is also deposited in other sites, such as the heart, large joints and the pancreas leading to cell damage and organ dysfunction.2 The stomach has no known contribution in iron metabolism, including absorption and storage. Hence, identification of hemosiderin deposition in gastric mucosa is certainly interesting and raises many questions.2
Iron metabolism: from uptake to storage
Dietary iron (1–2 mg daily) is mainly absorbed through the jejunal and duodenal mucosa.2,4,9 It is believed to be the only regulated step of iron metabolism in the body.2 The dietary oxidized iron (Fe3+) must be enzymatically changed to the reduced form (Fe2+) by ferric reductases.7,9 This reduced iron is chelated and it can then bind to the divalent metal transporter 1 (DMT1) and translocate, using an energy-dependent, carrier-mediated system, across the apical surface of the mucosal cells of the micro-villi in the jejunum and duodenum.2,4,9,10 It then travels through the cell and exits from the basolateral surface through the iron exporter ferroportin 1 (Fnp1) to enter circulation.2,9 Some paracellular movement through tight junctions between cells also occurs to move iron into circulation.4 Once it is in the blood, it is re-oxidized to Fe3+ via a membrane-bound ferroxidase called hephaestin.7 The oxidized iron then joins the labile pool and can travel unbound or bound to transferrin2 to various sites of the body for storage, including red blood cells, macrophages, muscle cells and liver cells.9 The transferrin binds to transferrin receptor 1 (trf1) or transferrin receptor 2 (trf2) allowing the iron to enter the cell via endocytosis; trf2 is present only in liver cells, duodenal crypt cells and erythroid cells.2,7,9 Once iron is inside a cell, it can be stored in one of two ways: as ferritin or as hemosiderin (Figure 1).2,3 This absorption of iron into the cell is regulated by the balance between intracellular ferritin and transferrin.11 In times of high iron intake or under inflammatory conditions, a molecule known as hepcidin is produced which acts to decrease dietary absorption of iron and retain iron inside macrophages.7 The loss of iron from the body occurs through the sloughing of mucosal and skin cells, during menstruation, or from other losses of blood.7,9 Thus, regulating the absorption of dietary iron in the small intestine is critical to maintaining the appropriate iron balance and for preventing iron overload.
Diagnosis and evaluation
Clinical history
GS has been found in association with multiple clinical conditions. It is of prime importance to obtain all relevant clinical information from the patient.2 Pertinent areas to explore include family history and past medical history, in particular about hemochromatosis, gastritis, conditions that required multiple blood transfusions as a part of treatment or any other significant liver disease. It is also important to ask the patient about alcohol abuse and to get a detailed medication history including iron supplements, non-steroidal anti-inflammatory drugs (NSAIDs) or proton pump inhibitors (PPIs).2 NSAIDs and oral iron supplements have been found to be associated with GS. However, this observation has not been validated in a statistically significant number of cases. Serum iron studies or liver function tests are usually within normal limits unless the patient has hemochromatosis or another systemic iron overload syndrome. It is interesting to note that both of our cases were clinically asymptomatic with normal serum iron studies and liver function tests.
Endoscopic findings
The endoscopic appearance of a patient with GS is highly variable and is usually described as yellow-brown discoloration of the mucosa that is often associated with shallow to frank ulcerations or regenerative polyps.12,13 This yellow-brown discoloration of the mucosa resembles the cutaneous iron deposition that causes skin hyperpigmentation or “bronzing” in hemochromatosis.13
Histopathological evaluation
The tissue sample obtained during endoscopy is usually stained with routine hematoxylin and eosin (H&E) to evaluate the tissue for inflammation, ulceration and H. pylori infection.1,2 Observation of pigmented materials is a key finding for GS in the routine histological evaluation of all gastric biopsies. Once any pigmented material is identified, regardless of its location, further evaluation should be prompted. However, it is important to classify the location of deposition as intra- or extracellular and to check other tissue components, such as glandular structures, macrophages and even stromal cells.
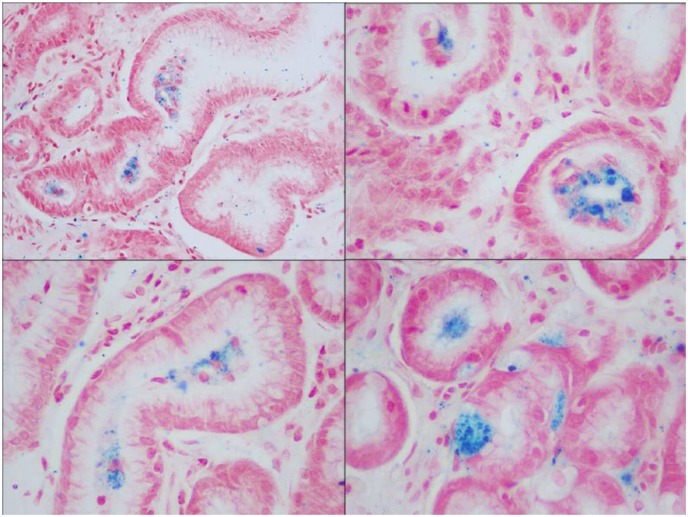
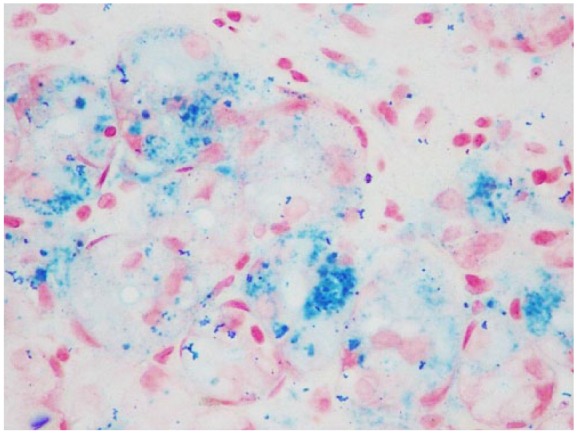
In both of our patients, the GS was found in the antrum of the stomach which is concordant with the histological findings of other published articles that showed the antrum more frequently involved than the body or fundus. Histological findings in one of our index case are concordant with a pattern described by Marginean et al., as pattern C (Figure 2). Iron tends to have an intracellular location and show a fine, granular consistency.2,4 Oxidized ferrous sulfate has a different appearance from hemosiderin. It tends to be in the form of a globule with a dark core with a surrounding lighter ring that represents the portion of ferrous sulfate that was oxidized.2 Iron from ferrous sulfate tablets has a characteristic dark brown, clumpy fibrillary and crystalline appearance.4,6,14 Whether granular or fibrillary, both forms of iron deposits are refractile and non-polarizable.4,6,14 When stained with Perls’ iron (Prussian blue) stain, hemosiderin deposits appear as fine or clumped deposits, ranging from patchy to diffuse in distribution, and are located in the cytoplasm of stromal and glandular cells with varying intensity (Figure 3).1,2,14 Histological findings in our second index case appear to be compatible with the pattern described as types A and B, where the deposition is predominantly extracellular, mostly in macrophages and stromal cells, and glandular cells as well (Figure 4). Mild to moderate non-specific gastric inflammation and mild reactive/regenerative gastropathy may also be found in association; however, H. pylori infection is not usually associated with GS. Neither of our index cases had any evidence of inflammation or H. pylori infection. In fact, in one of our index cases, the deposition of iron pigments was predominantly in the luminal aspect without any associated gastropathy or H. pylori infection (Figure 5). Although, this observation is somewhat interesting considering the previously described patterns of hemosiderin deposition that has not been addressed yet in any published literature. We believe it is an unusual variation that is beyond any explanation by our current knowledge and perhaps, clinically insignificant.
Figure 2.
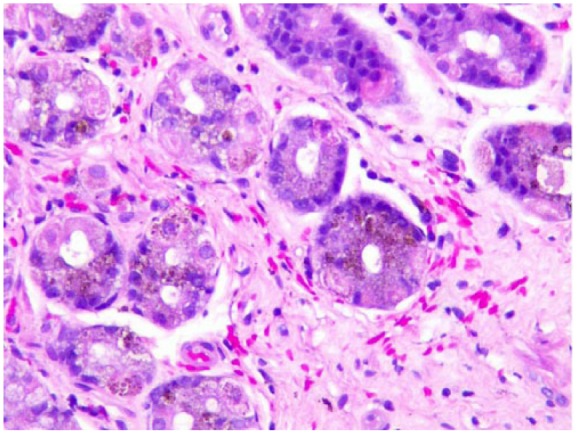
Index case 1: Patchy hemosiderin deposition in gastric glandular cells that appear as dark brown pigments in the absence of gastritis (Hematoxylin and eosin stain, 200× magnification).
Figure 3.

Fine granular and clumped hemosiderin deposition highlighted by Prussian blue stain (400× magnification).
Figure 4.
Prussian blue stain showing hemosiderin deposition in glandular, as well as, stromal cells (400× magnification).
Figure 5.
Index case 2: Hemosiderin deposition predominantly in lumen of gastric glands (Prussian blue stain, 100× magnification).
Patterns of iron deposition in GS and their clinical significance
Iron deposition in duodenal and jejunal mucosa in acquired and genetic hemochromatosis patients was initially studied by Astaldi et al.15 in 1968. Duodenal siderosis and GS were also reported later, mostly in alcoholic and hemochromatosis patients, by Conte et al.16 in 1987. Nonetheless, iron deposition was not observed in otherwise healthy individuals. In 2004, Hattori et al. observed that 25% of their patients (n = 723) with cirrhosis had GS, although any correlation of this hepatic cirrhosis and GS was not mentioned in their study. Recently, Marginean et al.2 described three main patterns of iron deposition which include (A) iron deposition in macrophages, stroma and in epithelium; (B) mostly extracellular deposition with some focal deposition in blood vessels, macrophages and epithelium; and finally, (C) mostly in glandular epithelium of antral and fundic cells.14 In the first two patterns mentioned, the iron was mainly deposited in the surface epithelium, in a focal or patchy distribution. The third pattern showed diffuse, deeply stained iron deposition in the deep gastric glands (foveolar, antral, chief or parietal).2,14
In patterns A and C, hemosiderin deposits were visualized in the cytoplasm as free-floating entities and in the form of siderosomes.2 In pattern B, the iron deposits consist of dense, refractile, non-polarizable, extracellular material that stained purple/brown and appears as large clumps of coarse, somewhat fibrillar material. It was found that in pattern A, 9% of patients took oral ferrous sulfate tablets; in pattern B, all patients took oral ferrous sulfate tablets, and in pattern C, no one took oral ferrous sulfate tablets.2 The authors described pattern A as “non-specific GS” and found that it was the most common and the most likely to be associated with gastric inflammation, ulceration or possibly prior hemorrhages.2 We believe, the most likely explanation in identifying iron pigments deposited in gastric mucosa is prior mucosal hemorrhage of any etiology. Following hemorrhage, iron as a hemoglobin degradation product is picked up by reticuloendothelial cells. This may explain the presence of iron in stromal cells, mostly in macrophages. Pattern B is designated as “iron pill gastritis” because it was associated with consumption of oral iron supplements. These two aforementioned (types A and B) patterns, although Marginean et al.,2 described as two different patterns; however, they are etiologically somewhat similar considering the underlying mechanisms. The histopathological features are also overlapping when there is only epithelial/glandular involvement. In the absence of a history of oral iron pill ingestion, and identification of brown crystalline materials on the mucosal surface, those two patterns share many histomorphologic features. We believe that these patterns of GS are mostly due to local effects following mucosal micro-hemorrhage or direct corrosive effects of iron. It is interesting to note that oral pill gastritis/gastropathy is not usually observed in liquid iron preparations. Finally, pattern C was named as “gastric glandular siderosis” and it appears to have a frequent association with systemic iron overload from hereditary hemochromatosis, multiple blood transfusions or possibly portal hypertension and cirrhosis.2,14
What makes our index cases more interesting is that neither of our patients had any recent history of upper GI symptoms, ingestion of oral iron or any evidence of abnormal liver function tests. Considering the possible etiologic factors of GS, our two cases most likely fit with an “idiopathic” variant of GS which was never proposed in the literature; however, morphologically they share some features of the “non-specific GS,” a variant or pattern proposed by Marginean et al.2 as type A.
Conditions associated with iron deposition
Acute overdose of iron
An acute overdose of iron is rare and tends to be limited to the pediatric population.1,14,17 Doses of 3–10 g were reported as being lethal.17 When it occurs, it causes mucosal injury in the upper GI tract due to its corrosive nature and can cause distal stenosis of the stomach.4,6,8,14 The corrosive nature of iron can lead to hemorrhagic necrosis, ulceration and even perforation resulting in shock from hemorrhage and fluid loss.4,6,8,17 In addition, iron can cause mucosal injury in the form of epithelial distortion and ischemia.6 The ischemia has been found to occur due to submucosal venous thrombosis.4,8 The iron, in these cases, deposits in necrotic membranes, blood vessels and within thrombi.6,8 Reissman et al.17 found that in their study of iron toxicity in a dog model, there was less necrosis found in the intestinal membranes as compared to prior studies of children who ingested toxic doses. This finding may be attributed to the enteric-coated iron tablets that the children ingested, as opposed to the aqueous solution given to the dogs.17 It is also of note that iron can be fatal even without any noticeable necrosis of the intestinal membrane.17
Therapeutic doses of iron
Therapeutic doses of oral iron are commonly employed for the treatment of iron deficiency anemia but can also cause local effects, primarily due to prolonged impaction of tablets in patients with underlying infectious, mechanical, toxic or systemic medical conditions.1,4,6,14,18 Abraham et al.4 reported that the prevalence of therapeutic oral iron-associated erosive mucosal injury is 0.7% of all GI mucosal biopsies. It may occur within a few hours to days after iron tablet ingestion. In a study by Kaye et al.,12 it was shown that in patients taking therapeutic doses of iron, 16.1% had detectable levels of iron found in the tissue biopsy.
Inorganic iron forms a brown-black crystalline coating on the epithelial surface that can cause gastric mucosal injury.19 The exact pathogenesis of this mucosal injury and the reason why inorganic iron bypasses the normal iron absorption pathway and depositions in this fashion is not clear yet.6,19 Also, it is unknown whether the iron deposition in gastric mucosa is a primary cause or secondary to its corrosive nature.6 It has been postulated that disruption in normal energy-dependent absorption by inorganic ferric iron leads to rapid passive uptake of iron in a concentration-dependent manner. These ferrous and ferric ions then lead to the formation of reactive oxygen metabolites causing mucosal injury.20 This inorganic iron also interferes with ulcer healing, increase inflammation, promotes thrombosis and leads to progression of strictures.4,19,21 In addition, malabsorption and deposition of inorganic iron may be caused by prior or concurrent gastric mucosal injury, delayed gastric emptying, achlorhydria and reduced gastric mucin secretion.6,8
There have been two types of iron-induced lesions described in the literature.8 The first involves a chemical burn, usually in the esophagus or stomach, composed of brown-black crystalline material and a layer of the sloughed epithelium overlying the region.6,8,14,22 In this injured mucosa, there is encrusted iron material, distortion of the epithelium, mucosal erosion, as well as some focal inflammatory exudate may be seen.6 In histological tissue sections, hemosiderin pigment may be visualized in the deep fundic glands of the stomach.6 This tends to occur in an older patient who has multiple medications, decreased saliva production or spends the majority of time in a horizontal position.8 In some of these patients, they may even have preceding ulcerations that are simply aggravated further by the ingestion of iron.6 The second pattern of injury includes patients with oral iron intake or blood transfusion-related iron overload.8 Typical endoscopic findings of iron pill-associated gastric mucosal damage include erosions, erythema and yellowish-brown discoloration of the mucosa.14 The histological findings in these patients show iron deposited in the epithelium, lamina propria, in glandular lumens and even in micro-thrombi.8,14 It may also be associated with a giant cell reaction.14
It is important to note that these iron-induced erosive injuries to the gastric mucosa are usually secondary to oral solid iron tablets and do not pertain to the liquid formulations.8 The liquid iron does not have the same side effect as the solid iron of causing mucosal injury because it lacks the concentration effect necessary to cause damage.18 Of the solid oral formulations, ferrous sulfate was the most damaging to the upper GI tract followed by ferrous gluconate, ferrous succinate, and finally, ferrous carbonate tablets.4,6 There have been some cases described in which the iron is seen deposited without any visible erosions or ulcerations on the mucosa.4,17 This has been further demonstrated in studies on rabbits and dogs, after ingestion of liquid iron formulations, these animals were found to have lethal levels of iron in their serum without any evidence of erosion of the GI tract.1,4,17 This shows that the mucosal damage secondary to iron pills/medications is potentially reversible.4 This was further confirmed by Benoni et al.23 in which greater mucosal injury was shown in rats after 3 h of ingestion of a large dose of iron versus in those that ingested iron 24 h prior.4
Over the last 15 years, there were four published series that have shown an association between therapeutic doses of iron and mucosal damage.14 Particularly since oral iron tablets can cause side effects such as nausea or dyspepsia, it is beneficial to consider using formulations that are different from the standard oral ferrous sulfate tablets.14 Kaye et al.12 also found an association between PPI use and iron deposition. It is unclear whether this finding is due to an increase in PPI use for the symptoms caused by iron-induced erosion, or whether the alkaline environment caused by the PPI may aid in iron deposition in the gastric mucosa.12
History of multiple blood transfusions
Iron deposition in the gastric mucosa is also observed in patients with a history of multiple blood transfusions. This transfusion-related iron deposition first occurs in the reticuloendothelial system, and the parenchymal organs are affected only if the transfusion is chronic and multiple in number.24 Wang and McDermott25 described gastric epithelial hemosiderin deposition in patients undergoing treatment for acute lymphoblastic leukemia (ALL) with a history of multiple blood transfusions (average transfusion of 16.75 units). The authors believed that finding iron deposition in gastric mucosa in this group was most likely secondary to transfusion-related systemic iron overload.
Hereditary hemochromatosis
Hereditary hemochromatosis is an autosomal recessive disorder associated with common mutations of the HFE gene: C282Y and H63D.2,3 These mutations are present in 90%–95% of North European population with hemochromatosis, about 80% of Southern European populations and are absent in most Asian and African populations.2,3 Hereditary hemochromatosis is more common in males than in females due to a greater loss of iron during menstruation.3 In hemochromatosis, patients develop a toxic accumulation of iron in their system due to the loss of homeostatic mechanisms of iron absorption.3 Even when in excess, iron continues to be absorbed from the GI tract due to the lack of gastroferrin, an iron binding substance in gastric juice. Gastroferrin is negligible or absent in hemochromatosis patients.3,26 Accumulation of excess iron begins in the liver, but as the hepatic storage protein becomes saturated, iron begins to deposit in the pancreas, joints, skin, heart and the gonadotrophin-secreting cells of the anterior pituitary. Cases of GS have been described in patients with hemochromatosis, but it is important to note that the patients with known hemochromatosis do not always develop GS.2 It has been postulated that the presence of GS represents an early manifestation of non-HFE mutation iron overload syndromes.2
Hepatic cirrhosis
Hattori24 found hemosiderin deposition in 26% of patients with cirrhosis as compared to 4% of patients without cirrhosis, suggesting an association between cirrhosis and GS. In addition, gender had no significant effect on gastric iron deposition among patients with and without cirrhosis. The authors also reported that patients who progressed to hepatocellular carcinoma (HCC) from cirrhosis were not found to have any increase in the gastric iron deposition. Thus, the association with cirrhosis and GS is not influenced by gender or progression to HCC.24 The authors described that the hemosiderin deposition in such patients occurred secondarily to spontaneous portacaval shunt caused by hepatic cirrhosis, micro-hemorrhage in the gastric mucosa, with a long turnover time of gastric glands.24
Esophageal varices
Hemosiderin deposition in the gastric mucosa is also described in patients with a history of esophageal varices. Hattori et al. showed that the amount of hemosiderin deposition to be 19% in patients with cirrhosis alone, as compared to 40% in those with cirrhosis and esophageal varices. There was not much difference in the amount of deposition when ruptured varices were compared to intact varices.24 Possibly, the GS in patients with esophageal varices occurs because gastric cells are exposed to higher iron concentrations from the portacaval shunting.14 In some cases, finding of hemosiderin accumulation in gastric glands can be a sign of chronically increased portal flow in the gastroenteric micro-vasculature; early findings before varices have developed. Further studies needed to determine whether the finding of GS of cirrhotic patients can be used to predict the future development of varices.24
Conclusion
GS is a rare and under-recognized clinical entity. The pathophysiology and clinical implications of GS have not been studied well due to the paucity of case series.2,24 It is usually identified in patients with hemochromatosis, iron pill medication, multiple transfusions, hepatic cirrhosis, spontaneous portacaval shunting with esophageal varices, and also, in alcoholics.2,24,27 Due to lack of clinical correlation of this entity, it is still unclear whether GS is an unexplained local morphologic change or a rare, unusual sign of systemic iron overload. Three types of GS were recently proposed based on patterns of iron deposition in a very few published articles although a precise correlation between the pattern of deposition and clinical correlation is still lacking and therefore, not established yet. Many clinicians believe that GS may not represent a clinical disease by itself, rather an unexplained association with several clinical entities, hence do not offer any specific diagnostic tests or treatment to these patients with GS. Although, some gastroenterologists believe that patients with this rare finding may need further workup to exclude any abnormalities in the body’s iron metabolism. We believe GS is an interesting but unusual morphologic change seen in glandular and stromal cells, most likely secondary to local mucosal injury or hemorrhage.
Acknowledgments
J.P.K. contributed to the concept and design, acquisition of available literature, and drafting of the review article; R.A. contributed to the acquisition of reference articles and preparing the histological pictures; M.K. contributed to the preparation of the illustrative image of iron transport, drafting of the article, and manuscript proofreading; A.M. contributed to the literature search and provided technical support in preparation of images; J.C. contributed to the manuscript proofreading and critical review; and S.G. contributed to the concept and design, drafting and critical revision of the manuscript for important intellectual content, and preparing legends for the histological pictures.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
References
- 1. Mahjoub F, Saffar H, Najafi M, et al. Iron deposition in duodenal mucosa: a review and report of three cases in pediatric age group. Iran J Pediatr 2011; 21: 235–238. [PMC free article] [PubMed] [Google Scholar]
- 2. Marginean EC, Bennick M, Cyczk J, et al. Gastric siderosis: patterns and significance. Am J Surg Pathol 2006; 30: 514–520. [DOI] [PubMed] [Google Scholar]
- 3. Griffiths WJH. Haemochromatosis. Medicine 2011; 39: 597–601. [Google Scholar]
- 4. Abraham SC, Yardley JH, Wu TT. Erosive injury to the upper gastrointestinal tract in patients receiving iron medication: an underrecognized entity. Am J Surg Pathol 1999; 23: 1241–1247. [DOI] [PubMed] [Google Scholar]
- 5. Walker R, Dymock I, Ansell I, et al. Synovial biopsy in haemochromatosis arthropathy: histological findings and iron deposition in relation to total body iron overload. Ann Rheum Dis 1972; 31: 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ji H, Yardley JH. Iron medication-associated gastric mucosal injury. Arch Pathol Lab Med 2004; 128: 821–822. [DOI] [PubMed] [Google Scholar]
- 7. Wang J, Pantopoulos K. Regulation of cellular iron metabolism. Biochem J 2011; 434: 365–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang X, Ouyang J, Wieczorek R, et al. Iron medication-induced gastric mucosal injury. Pathol Res Pract 2009; 205: 579–581. [DOI] [PubMed] [Google Scholar]
- 9. Siah CW, Ombiga J, Adams LA, et al. Normal iron metabolism and the pathophysiology of iron overload disorders. Clin Biochem Rev 2006; 27: 5–16. [PMC free article] [PubMed] [Google Scholar]
- 10. Gastearena MI, Gil A, Azqueta A, et al. A comparative study on the gastroduodenal tolerance of different antianaemic preparations. Hum Exp Toxicol 2003; 22: 137–141. [DOI] [PubMed] [Google Scholar]
- 11. Francanzani A, Fargion S, Romano R, et al. Immunohistochemical evidence for a lack of ferritin in duodenal absorptive epithelial cells in idiopathic hemochromatosis. Gastroenterology 1989; 96: 1071–1078. [DOI] [PubMed] [Google Scholar]
- 12. Kaye P, Abdulla K, Wood J, et al. Iron-induced mucosal pathology of the upper gastrointestinal tract: a common finding in patients on oral iron therapy. Histopathology 2008; 53: 311–317. [DOI] [PubMed] [Google Scholar]
- 13. Levy A, Bongiorno C, Myint MA, et al. The endoscopic appearance of focal gastric hemosiderosis as a brown macular patch resembling that of cutaneous iron deposition in hemochromatosis. Am J Gastroenterol 2008; 103: 246–248. [DOI] [PubMed] [Google Scholar]
- 14. De Petris G, Gatius Caldero S, Chen L, et al. Histopathological changes in the gastrointestinal tract due to drugs: an update for the surgical pathologist (part I of II). Int J Surg Pathol 2014; 22: 120–128. [DOI] [PubMed] [Google Scholar]
- 15. Astaldi G, Meardi G, Lisino T. The iron content of jejunal mucosa obtained by Crosby’s biopsy in hemochromatosis and hemosiderosis. Blood 1966; 28: 70–82. [PubMed] [Google Scholar]
- 16. Conte D, Velio P, Brunelli L, et al. Stainable iron in gastric and duodenal mucosa of primary hemochromatosis patients and alcoholics. Am J Gastroenterol 1987; 82: 237–240. [PubMed] [Google Scholar]
- 17. Reissman KR, Coleman TJ, Budai BS, et al. Acute intestinal iron intoxication, I: iron absorption, serum iron and autopsy findings. Blood 1955; 10: 35–45. [PubMed] [Google Scholar]
- 18. Hashash JG, Proksell S, Kuan SF, et al. Iron pill-induced gastritis. ACG Case Rep J 2013; 1: 13–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haig A, Driman DK. Iron-induced mucosal injury to the upper gastrointestinal tract. Histopathology 2006; 48: 808–812. [DOI] [PubMed] [Google Scholar]
- 20. Mladěnka P, Šimůnek T, Hübl M, et al. The role of reactive oxygen and nitrogen species in cellular iron metabolism. Free Radic Res 2006; 40: 263–272. [DOI] [PubMed] [Google Scholar]
- 21. Eckstein RP, Symons P. Iron tablets cause histopathologically distinctive lesions in mucosal biopsies of the stomach and esophagus. Pathology 1996; 28: 142–145. [DOI] [PubMed] [Google Scholar]
- 22. Parfitt JR, Driman DK. Pathological effects of drugs on the gastrointestinal tract: a review. Hum Pathol 2007; 38: 527–536. [DOI] [PubMed] [Google Scholar]
- 23. Benoni G, Cuzzolin L, Zambreri D, et al. Gastrointestinal effects of single and repeated doses of ferrous sulphate in rats. Pharmacol Res 1993; 27: 73–80. [DOI] [PubMed] [Google Scholar]
- 24. Hattori H. High prevalence of haemosiderin accumulation in the cytoplasm of gastric glands in patients with liver cirrhosis. J Clin Pathol 2004; 57: 621–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang LM, McDermott M. Gastric epithelial siderosis in acute lymphoblastic leukemia. Am J Surg Pathol 2007; 31: 646–647. [DOI] [PubMed] [Google Scholar]
- 26. Luke CG, Davis PS, Deller DJ. Gastric iron binding in haemochromatosis, secondary iron overload, cirrhosis, and diabetes. Lancet 1968; 2: 844–846. [DOI] [PubMed] [Google Scholar]
- 27. Morton D, Wimsatt WA. Distribution of iron in the gastrointestinal tract of the common vampire bat: evidence for macrophage-linked iron clearance. Anat Rec 1980; 198: 183–192. [DOI] [PubMed] [Google Scholar]