Abstract
In peripheral tissues insulin activates signaling cascades to facilitate glucose uptake from the blood into tissues like liver, muscle and fat. While insulin appears to play a minor role in the regulation of glucose uptake in the central nervous system (CNS), insulin is known to play a major role in regulating synaptic plasticity in brain regions like the hippocampus. The concept that insulin regulates hippocampal neuroplasticity is further supported from animal models of type 2 diabetes (T2DM) and Alzheimer's disease (AD). The goal of this review is to provide an overview of these studies, as well as the studies that have examined whether deficits in hippocampal insulin signaling are amenable to intervention strategies.
Introduction
Insulin signaling in the CNS
Insulin is synthesized by pancreatic β cells and is released into circulation in response to increases in plasma glucose levels. Once released into circulation, insulin binds to and activates insulin receptors to promote glucose uptake and utilization in peripheral tissues such as liver, muscle and fat. While the central nervous system was once viewed as an insulin-insensitive organ, studies by Woods and coworkers demonstrated that intracerebroventricular administration of insulin suppressed food intake and body weight in a dose-dependent manner [1]. These seminal observations shed light on a role for insulin in the CNS and ultimately lead to a greater understanding of the role of hypothalamic peptide systems in the regulation of food intake, body weight and metabolism [2;3]. Beyond the hypothalamus, it is clear that the actions of insulin extend into other regions of the CNS including the hippocampus. Insulin receptors are expressed in the hippocampus [4;5] and activate similar signaling cascades as have been identified in peripheral tissues [6] In this regard, insulin crosses the blood-brain barrier (BBB) by a carrier facilitated process [7] and binds to insulin receptors expressed in the CNS, including the hippocampus. The insulin receptor is a heterotetrameric protein complex consisting on 2 α subunits that provide the insulin binding site and the membrane spanning β subunits that stimulate autophosphorylation of the β subunits. This initiates several signaling cascades, including the PI3-kinase/Akt pathway and MAPK/Erk signaling pathway (See Figure 1). It is important to note that these insulin-stimulated signaling cascades exhibit significant cross-talk and also that these signaling pathways are not the exclusive domain of insulin receptor activation. For example, leptin also facilitates hippocampal synaptic plasticity through activation of these pathways [8]. Conversely, hippocampal insulin resistance (i.e. ‘stop signs’ in insulin signaling; Figure 1) contributes to neuroplasticity deficits in T2DM, obesity and AD. The sections below will describe causes and consequences of this biphasic relationship between hippocampal insulin signaling and neuroplasticity, with a particular emphasis on how hippocampal insulin resistance contributes to the structural, functional and behavioral deficits observed in T2DM, obesity and AD.
Figure 1. Insulin receptor signaling in the hippocampus.
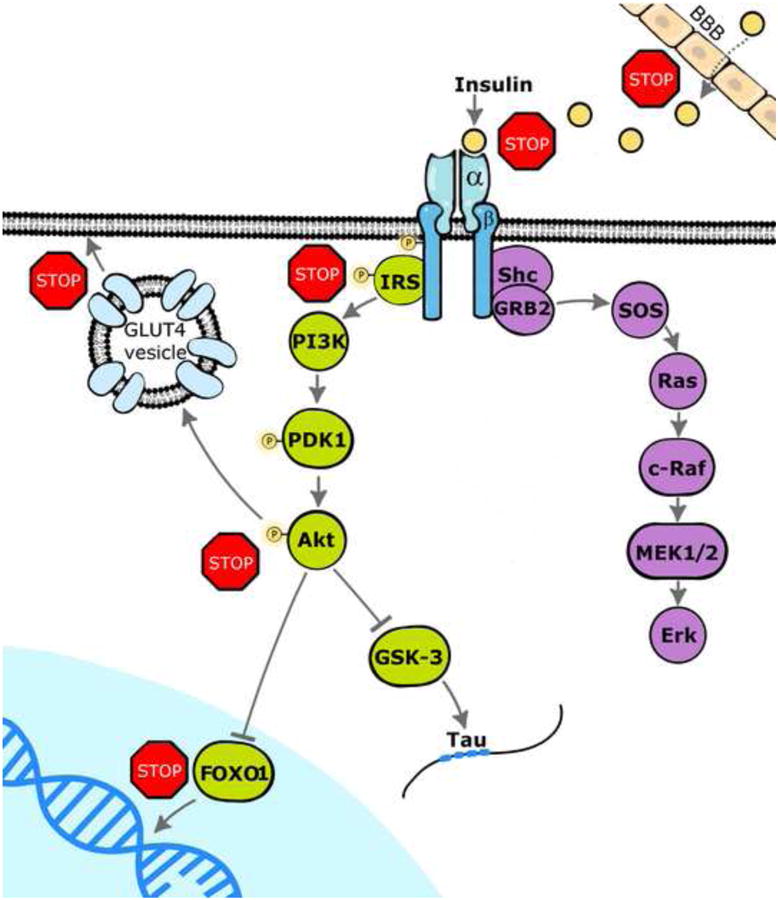
Insulin crosses the blood brain barrier (BBB) via a carrier facilitated process to activate insulin receptors in the CNS and stimulate similar signaling cascades as has been described in peripheral tissues such as skeletal muscle and adipose tissue. These insulin signaling cascades diverge following autophosphorylation of the β subunits of the insulin receptor and include the MEK/Erk pathway and the PI3/Akt pathway. Dysregulation of these pathways may occur at several sites (shown as Stop signs) and in doing so contribute to the development of hippocampal insulin resistance, including impaired BBB transport of insulin, decreased expression and/or activity of the insulin receptor, as well as modulation of the phosphorylation state of insulin receptor substrate (IRS) proteins. Decreased insulin-stimulated phosphorylation of Akt will also impact several downstream components of insulin signaling, including the trafficking of the insulin-sensitive glucose transporter GLUT4 and the activity of GSK-3β, which regulates the phosphorylation state of the microtubule associated protein Tau and the activity of FOX01 family of transcription factors. Figure adapted from [28].
Insulin, cognition and behavior
Clinical and preclinical studies support the hypothesis that insulin is a critical regulator of structural and functional plasticity in the hippocampus, including cognitive function. In this regard, studies by Alkon and co-workers demonstrated that spatial learning increases insulin receptor expression and signaling in the rat hippocampus [9]. In addition, a number of studies have reported that increasing hippocampal insulin levels increases behavioral performance in control rats. For example, intracerebroventicular insulin administration improves behavioral performance in the passive avoidance task [10], while intrahippocampal administration of insulin improves spatial learning and memory in control rodents [11-13]. Intranasal insulin administration also increases both short and long term memory retrieval in mice [14]. Interestingly, these studies also demonstrated that activation of hippocampal insulin signaling participates in the memory-enhancing properties of insulin [11] and that increasing brain insulin levels does not impact peripheral glucose metabolism [12;14]. These rodent studies correlate nicely with clinical studies demonstrating that the cognitive-enhancing effects of intranasal insulin are not associated with changes in peripheral glucose homeostasis in humans [15].
Stop signs in CNS insulin signaling
The accumulated data from both clinical and preclinical studies support the hypothesis that hippocampal insulin resistance contributes to neuroplasticity deficits in T2DM, obesity and AD. For example, deficits in hippocampal insulin receptor signaling (i.e. hippocampal insulin resistance) have been identified at each step in this cascade, including impairments in BBB transport of insulin [16-18], reduced insulin-stimulated phosphorylation events [19-22] and reduced trafficking of insulin sensitive glucose transporters [20;23;24]. In addition, increased serine phosphorylation of insulin receptor substrate 1 (IRS-1), which is a hallmark feature of peripheral insulin resistance, is also observed in the hippocampus of mice fed a high fat diet [22]. Collectively, these studies demonstrate that deficits in insulin receptor signaling can occur at all steps in the cascade, from the transport of insulin into the CNS to the level of transcription. More importantly, these results support the hypothesis that hippocampal insulin resistance is a keystone mechanistic mediator of neuroplasticity deficits observed in experimental models of T2DM and obesity.
Cellular and Molecular underpinnings of hippocampal insulin resistance
Diabetes and obesity are characterized by a wide variety of metabolic and endocrine abnormalities, many of which may elicit hippocampal insulin resistance (Figure 2). This includes dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis that is characterized by elevated levels of glucocorticoids [23;25]. Our previous studies have demonstrated that exposure to glucocorticoids elicits peripheral insulin resistance, as well as hippocampal insulin resistance [20]. Additional support is provided by Stranahan and coworkers, who showed that deficits in hippocampal synaptic plasticity observed in db/db mice could be reversed by adrenalectomy and replacement with physiological levels of glucocorticoids [26]. In addition to HPA axis dysregulation, impairment in mitochondrial function leading to increased production of reactive oxygen species (ROS) and lipid peroxidation products is also suggested to contribute to the neurological complications associated with metabolic disorders [27], including the development of hippocampal insulin resistance [28]. Pro-inflammatory cytokines may also elicit hippocampal insulin resistance; indeed, chronic mild inflammation is a characteristic feature of metabolic disorders [29]. In metabolic disorders associated with increases in adiposity, it is proposed that macrophages infiltrate adipocytes thereby leading to increases in the production and secretion of pro-inflammatory cytokines. Increases in pro-inflammatory cytokines are proposed to transduce their signal across the BBB and induce neuroinflammation. In support of this hypothesis, immunohistochemical indices of increased microglial activation and increases in pro-inflammatory cytokines are observed in the hippocampus of experimental models of T2DM and obesity [30;31]. More recent studies have identified a critical role for IL-1 signaling in the behavioral deficits associated with obesity and diabetes phenotypes [32•]. Interestingly, neuroinflammation-induced insulin resistance has also been proposed as a potential mechanism that links metabolic disorders with AD [33]. Amyloid β oligomers (AβO) may similarly link insulin resistance in T2DM with AD. In this regard, intrahippocampal AβO administration decreases spatial memory performance [34•] and mechanistically these impairments in spatial learning are proposed to result from AβO-mediated decreases in hippocampal insulin receptor expression and signaling [34•,35••,36]. While it is likely that these factors also act more directly to impair hippocampal synaptic plasticity, these studies identify HPA axis dysregulation, mitochondrial dysfunction, neuroinflammation and AβO as factors that elicit hippocampal insulin resistance.
Figure 2. Hippocampal neuroplasticity deficits in experimental models of IR: causes and consequences.
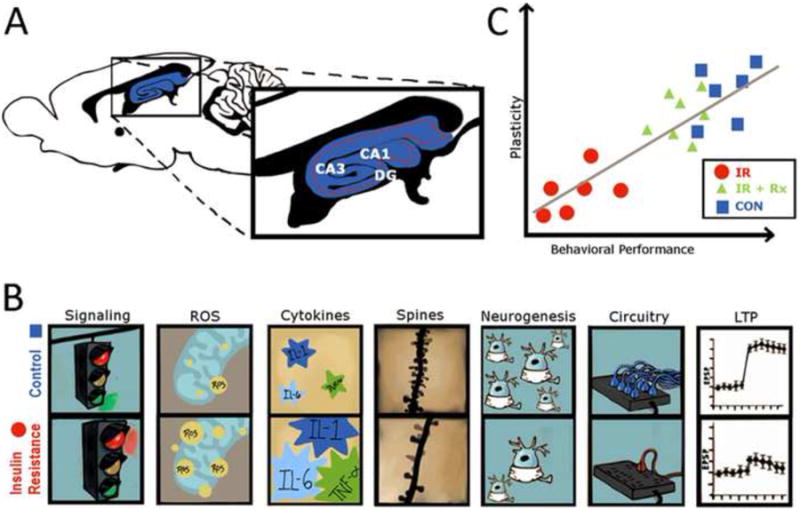
In experimental models, the complications of peripheral insulin resistance extend to the central nervous system and elicit neuroplasticity deficits in the hippocampus, including impairments in the dentate gyrus (DG) and the CA1 and CA3 regions of Ammon's Horn (Panel A). An important mechanism in hippocampal neuroplasticity deficits is the development of hippocampal IR, which results from a combination of decreases in BBB transport of insulin and insulin receptor signaling (Panel B). The cellular underpinnings of hippocampal IR are also likely to include mitochondrial dysfunction leading to increased production of reactive oxygen species (ROS) and neuroinflammation, including increased levels of pro-inflammatory cytokines. The consequences of hippocampal IR include morphological changes (decreases in spine density and neurogenesis and disruption of neuronal circuitry), as well as deficits in synaptic transmission (LTP). While these IR-induced hippocampal neuroplasticity deficits are associated with impairments in the performance of hippocampal-dependent behaviors (Panel C), a variety of intervention strategies are known to effectively restore neuroplasticity in models of hippocampal IR. See text for details.
Behavioral consequences of hippocampal insulin resistance
The concept that hippocampal insulin resistance contributes to neurobehavioral deficits is supported by rodent models of insulin resistance. This includes studies in rodents fed a high fat diet (HFD), experimental models of T2DM/obesity (such as Zucker rats, db/db and ob/ob mice) and molecular approaches to reduce brain insulin receptor expression. Behavioral deficits in hippocampal-dependent learning are consistently observed in these experimental models of T2DM and obesity [28], including deficits in spatial learning in the water maze [37-40], object recognition testing [26;41], discrimination testing [42;43], contextual fear conditioning [44] and the variable interval delayed alternation task [23;45-47]. In addition to deficits in learning and memory tasks, increases in depressive-like behaviors and anxiety-like behaviors are observed in animal models of T2DM and obesity [48-51]. These findings are consistent with clinical observations that patients with metabolic disorders exhibit cognitive deficits [52] and also have increased risk of developing neuropsychiatric disorders like depressive illness [53;54]. Importantly, these studies support the concept that hippocampal insulin resistance contributes to deficits in hippocampal-dependent function.
Structural and functional consequences of hippocampal insulin resistance
Additional studies have begun to identify the structural and functional neuroplasticity deficits that may contribute to the behavioral deficits observed in experimental models of insulin resistance, including decreases in neurogenesis in the dentate gyrus [55;56], decreases in spine density [57], alterations in synapse formation [44] and decreased BBB integrity [58]. Importantly, these structural changes are associated with behavioral deficits in diabetes/obesity phenotypes [38;39;43;44]. Similarly, functional deficits in hippocampal synaptic transmission, specifically impairments in stimulus-evoked long term potentiation (LTP), are associated with spatial memory deficits in experimental models of insulin resistance, including Zucker rats [37;40], db/db mice [26;40] and rodents fed a high fat diet [38]. Insulin receptor haploinsufficient (i.e. IR+/-) mice also exhibit deficits in LTP that are accompanied by spatial learning deficits [41]. These electrophysiological deficits are likely driven in part by alterations in glutamate receptor expression and subunit composition (For reviews see [59;60]). Histochemical features normally associated with AD-like pathology, such as hyperphosphorylated tau protein, are also observed in the hippocampus of rodents with insulin resistance [61-64], which provides another example of where the causes and consequences of insulin resistance may overlap in AD and T2DM.
Causal relationship for hippocampal insulin resistance and neuroplasticity deficits
While these studies involving experimental models illustrate that hippocampal insulin resistance is correlated with structural, functional and behavioral deficits, they cannot demonstrate a causal link between hippocampal insulin resistance and these neuroplasticity deficits. As noted above, experimental models of T2DM and obesity exhibit a wide array of metabolic and endocrine changes in addition to insulin resistance, including leptin resistance, glucose intolerance, HPA axis dysregulation and increases in pro-inflammatory cytokines. Moreover, the key loci of insulin resistance that mediate hippocampal neuroplasticity deficits in diabetes/obesity phenotypes remain unclear. More simply, does peripheral insulin resistance, CNS insulin resistance or a combination of peripheral and central insulin resistance contribute to hippocampal neuroplasticity deficits in metabolic disorders like T2DM? To address this issue, we recently developed an animal model of hippocampal-specific insulin resistance. Using virus-mediated gene transfer, we downregulated insulin receptor expression in the hippocampus without affecting hypothalamic insulin receptor expression. In addition, rats with hippocampal-specific insulin resistance did not exhibit changes in body weight, body adiposity or peripheral insulin sensitivity. However, rats with hippocampal-specific insulin resistance exhibited changes in glutamate receptor subunit expression, decreases in stimulus-evoked LTP and impairments in spatial learning [65••]. These results demonstrate that hippocampal insulin resistance may occur independent of peripheral insulin resistance and glucose dysregulation and supports the concept that hippocampal insulin resistance contributes to the development of cognitive deficits observed in patients with metabolic disorders like T2DM and obesity.
Restoration of hippocampal insulin signaling
Restoration of insulin signaling has shown promise in reversing hippocampal neuroplasticity deficits in rodents with insulin resistance. For example, while insulin sensitivity was reduced in diet-induced obese rats provided a high fat diet, higher doses of intrahippocampal insulin effectively reversed spatial learning in this model of insulin resistance [11]. Several studies have also examined the ability of anti-diabetic drugs to reverse hippocampal synaptic plasticity deficits, with mixed results. Specifically, the PPAR-γ ligand rosiglitazone reversed HFD-induced deficits in spatial memory [66] while metformin administration did not rescue operant learning behavioral deficits in HFD rats [67]. Studies aimed at increasing CNS activity of the incretins (i.e. glucagon-like peptide and glucose dependent insulinotropic polypeptide) have yielded more consistent findings. In this regard, incretin analogs restore brain insulin activity and behavioral performance in experimental models of insulin resistance [68-70]. Similarly, inhibition of dipeptidyl peptidase-4 activity, which is responsible for incretin degradation, reversed HFD-induced decreases in hippocampal insulin signaling and impairments in spatial learning [71]. Collectively, these pharmacological approaches support the concept that restoration of behavioral performance in experimental models of insulin resistance involves enhancement of hippocampal insulin activity.
Conclusions and translational perspectives
While this review has focused on experimental models of diabetes and obesity, these findings provide insight into the observations in patients with metabolic disorders. Indeed, structural and functional deficits are observed in the hippocampus of patients with insulin resistance, including cognitive dysfunction [28]. These rodent studies also support the concept that activation of hippocampal insulin signaling contributes to the pro-cognitive effects of intranasal insulin administration in normal healthy adults [72;73] in T2DM patients [74;75••] and in AD patients [76;77]. Similarly, the pro-cognitive effects of the incretin analogs may result at least in part from the ability of these compounds to cross the BBB and activate hippocampal incretin signaling to promote cognition in patients with metabolic disorders [78;79]. Increased physical activity [57;80;81] and weight loss approaches [82-84] also restore behavioral performance in rodent models of T2DM and obesity, thereby supporting data indicating that lifestyle interventions enhance cognitive performance in patients with metabolic disorders. This includes aerobic exercise [85;86] and weight loss achieved through bariatric surgical procedures [87•]. While these lifestyle approaches improved peripheral insulin sensitivity in these patient populations, it remains to be determined whether the pro-cognitive effects of these interventions involves enhancement of hippocampal insulin signaling. However, if the preclinical studies are truly ‘translatable’, the rodent studies support the hypothesis that restoration of hippocampal insulin signaling is responsible for improved cognitive performance in these clinical settings. More importantly, these studies illustrate that pharmacological and lifestyle approaches represent promising treatment strategies that have the potential to attenuate or reverse cognitive deficits in T2DM, obesity and AD.
Highlights.
Insulin signaling in the central nervous system (CNS) facilitates cognition
CNS insulin resistance (IR) reduces cognition and neuroplasticity
Hippocampal IR impairs cognition and neuroplasticity independent of peripheral IR
Restoration of hippocampal insulin activity improves cognition and neuroplasticity
Acknowledgments
The authors would like to thank Victoria Macht for design and preparation of figures. The work of JRF is supported by the National Institutes of Health (R01AG030646 and R01AG050518) and the University of South Carolina Research Foundation. The work of LPR is supported by the Department of Veterans Affairs [I21 BX002085 and IO1 BX001804] and the University of South Carolina Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Woods SC, Lotter EC, McKay LD, Porte D., Jr Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282:503–505. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 3.Levin BE, Routh VH. Role of the brain in energy balance and obesity. Am J Physiol. 1996;271:R491–R500. doi: 10.1152/ajpregu.1996.271.3.R491. [DOI] [PubMed] [Google Scholar]
- 4.Marks JL, Porte D, Jr, Stahl WL, Baskin DG. Localization of insulin receptor mRNA in rat brain by in situ hybridization. Endocrinology. 1991;127:3234–3236. doi: 10.1210/endo-127-6-3234. [DOI] [PubMed] [Google Scholar]
- 5.Dore S, Kar S, Rowe W, Quirion R. Distribution and levels of [125I]IGF-I, [125I]IGF-II and [125I]insulin receptor binding sites in the hippocampus of aged memory-unimpaired and -impaired rats. Neuroscience. 1997;80:1033–1040. doi: 10.1016/s0306-4522(97)00154-1. [DOI] [PubMed] [Google Scholar]
- 6.Leto D, Saltiel AR. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat Rev Mol Cell Biol. 2012;13:383–396. doi: 10.1038/nrm3351. [DOI] [PubMed] [Google Scholar]
- 7.Banks WA, Owen JB, Erickson MA. Insulin in the brain: there and back again. Pharmacol Ther. 2012;136:82–93. doi: 10.1016/j.pharmthera.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harvey J. Leptin regulation of neuronal excitability and cognitive function. Curr Opin Pharmacol. 2007;7:643–647. doi: 10.1016/j.coph.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao W, Chen H, Xu H, Moore E, Meiri N, Quon MJ, Alkon DL. Brain insulin receptors and spatial memory. Correlated changes in gene expression, tyrosine phosphorylation, and signaling molecules in the hippocampus of water maze trained rats. J Biol Chem. 1999;274:34893–34902. doi: 10.1074/jbc.274.49.34893. [DOI] [PubMed] [Google Scholar]
- 10.Park CR, Seely RJ, Craft S, Woods SC. Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiol Behav. 2000;68:509–514. doi: 10.1016/s0031-9384(99)00220-6. [DOI] [PubMed] [Google Scholar]
- 11.McNay EC, Ong CT, McCrimmon RJ, Cresswell J, Bogan JS, Sherwin RS. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol Learn Mem. 2010;93:546–553. doi: 10.1016/j.nlm.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moosavi M, Naghdi N, Maghsoudi N, Zahedi AS. The effect of intrahippocampal insulin microinjection on spatial learning and memory. Horm Behav. 2006;50:748–752. doi: 10.1016/j.yhbeh.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 13.Moosavi M, Naghdi N, Choopani S. Intra CA1 insulin microinjection improves memory consolidation and retrieval. Peptides. 2007;28:1029–1034. doi: 10.1016/j.peptides.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Marks DR, Tucker K, Cavallin MA, Mast TG, Fadool DA. Awake intranasal insulin delivery modifies protein complexes and alters memory, anxiety, and olfactory behaviors. J Neurosci. 2009;29:6734–6751. doi: 10.1523/JNEUROSCI.1350-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benedict C, Hallschmid M, Schmitz K, Schultes B, Ratter F, Fehm HL, Born J, Kern W. Intranasal insulin improves memory in humans: superiority of insulin aspart. Neuropsychopharmacology. 2007;32:239–243. doi: 10.1038/sj.npp.1301193. [DOI] [PubMed] [Google Scholar]
- 16.Urayama A, Banks WA. Starvation and triglycerides reverse the obesity-induced impairment of insulin transport at the blood-brain barrier. Endocrinology. 2008;149:3592–3597. doi: 10.1210/en.2008-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baskin DG, Stein LJ, Ikeda H, Woods SC, Figlewicz DP, Porte D, Jr, Greenwood MR, Dorsa DM. Genetically obese Zucker rats have abnormally low brain insulin content. Life Sci. 1985;36:627–633. doi: 10.1016/0024-3205(85)90166-3. [DOI] [PubMed] [Google Scholar]
- 18.Stein LJ, Dorsa DM, Baskin DG, Figlewicz DP, Porte D, Jr, Woods SC. Reduced effect of experimental peripheral hyperinsulinemia to elevate cerebrospinal fluid insulin concentrations of obese Zucker rats. Endocrinology. 1987;121:1611–1615. doi: 10.1210/endo-121-5-1611. [DOI] [PubMed] [Google Scholar]
- 19.Mielke JG, Taghibiglou C, Liu L, Zhang Y, Jia Z, Adeli K, Wang YT. A biochemical and functional characterization of diet-induced brain insulin resistance. J Neurochem. 2005;93:1568–1578. doi: 10.1111/j.1471-4159.2005.03155.x. [DOI] [PubMed] [Google Scholar]
- 20.Piroli GG, Grillo CA, Reznikov LR, Adams S, McEwen BS, Charron MJ, Reagan LP. Corticosterone impairs insulin-stimulated translocation of GLUT4 in the rat hippocampus. Neuroendocrinology. 2007;85:71–80. doi: 10.1159/000101694. [DOI] [PubMed] [Google Scholar]
- 21.Pratchayasakul W, Kerdphoo S, Petsophonsakul P, Pongchaidecha A, Chattipakorn N, Chattipakorn SC. Effects of high-fat diet on insulin receptor function in rat hippocampus and the level of neuronal corticosterone. Life Sci. 2011;88:619–627. doi: 10.1016/j.lfs.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 22•.Arnold SE, Lucki I, Brookshire BR, Carlson GC, Browne CA, Kazi H, Bang S, Choi BR, Chen Y, McMullen MF, Kim SF. High fat diet produces brain insulin resistance, synaptodendritic abnormalities and altered behavior in mice. Neurobiol Dis. 2014;67:79–87. doi: 10.1016/j.nbd.2014.03.011. Study illustrates that access to a high fat diet elicits hippocampal insulin resistance that is associated with structural and functional deficits in hippocampal neuroplasticity measures in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winocur G, Greenwood CE, Piroli GG, Grillo CA, Reznikov LR, Reagan LP, McEwen BS. Memory Impairment in Obese Zucker Rats: An Investigation of Cognitive Function in an Animal Model of Insulin Resistance and Obesity. Behav Neurosci. 2005;119:1389–1395. doi: 10.1037/0735-7044.119.5.1389. [DOI] [PubMed] [Google Scholar]
- 24.Piroli GG, Grillo CA, Hoskin EK, Znamensky V, Katz EB, Milner TA, McEwen BS, Charron MJ, Reagan LP. Peripheral glucose administration stimulates the translocation of GLUT8 glucose transporter to the endoplasmic reticulum in the rat hippocampus. J Comp Neurol. 2002;452:103–114. doi: 10.1002/cne.10368. [DOI] [PubMed] [Google Scholar]
- 25.Plotsky PM, Thrivikraman KV, Watts AG, Hauger RL. Hypothalamic-pituitary-adrenal axis function in the Zucker obese rat. Endocrinology. 1992;130:1931–1941. doi: 10.1210/endo.130.4.1312431. [DOI] [PubMed] [Google Scholar]
- 26.Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci. 2008;11:309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattson MP. Roles of the lipid peroxidation product 4-hydroxynonenal in obesity, the metabolic syndrome, and associated vascular and neurodegenerative disorders. Exp Gerontol. 2009;44:625–633. doi: 10.1016/j.exger.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biessels GJ, Reagan LP. Hippocampal insulin resistance and cognitive dysfunction. Nat Rev Neurosci. 2015;16:660–671. doi: 10.1038/nrn4019. [DOI] [PubMed] [Google Scholar]
- 29.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boitard C, Cavaroc A, Sauvant J, Aubert A, Castanon N, Laye S, Ferreira G. Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats. Brain Behav Immun. 2014 doi: 10.1016/j.bbi.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Dinel AL, Andre C, Aubert A, Ferreira G, Laye S, Castanon N. Cognitive and emotional alterations are related to hippocampal inflammation in a mouse model of metabolic syndrome. PLoS One. 2011;6:e24325. doi: 10.1371/journal.pone.0024325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32•.Erion JR, Wosiski-Kuhn M, Dey A, Hao S, Davis CL, Pollock NK, Stranahan AM. Obesity elicits interleukin 1-mediated deficits in hippocampal synaptic plasticity. J Neurosci. 2014;34:2618–2631. doi: 10.1523/JNEUROSCI.4200-13.2014. The study reported that surgical removal of fat pads restored behavioral performance, stimulus-evoked LTP and dendritic spine density in db/db mice, while fat transplantation to wild type mice induced hippocampal neuroplasticity deficits. This study also more selectively identified IL-1β as an essential proinflammatory cytokine in neuroplasticity deficits in db/db mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Felice FG, Ferreira ST. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes. 2014;63:2262–2272. doi: 10.2337/db13-1954. [DOI] [PubMed] [Google Scholar]
- 34••.Pearson-Leary J, McNay EC. Intrahippocampal administration of amyloid-beta(1-42) oligomers acutely impairs spatial working memory, insulin signaling, and hippocampal metabolism. J Alzheimers Dis. 2012;30:413–422. doi: 10.3233/JAD-2012-112192. This study demonstrated that acute administration of β-amyloid oligomers elicited deficits in hippocampal-dependent behaviors that were associated with decreases in hippocampal insulin receptor signaling, thereby supporting the concept that insulin resistance is a common pathological mechanisms in the etiology of metabolic disorders and Alzheimer's disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35••.Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel JC, Decker H, Silverman MA, Kazi H, Melo HM, McClean PL, Holscher C, Arnold SE, Talbot K, Klein WL, Munoz DP, Ferreira ST, De Felice FG. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer's disease- associated Abeta oligomers. J Clin Invest. 2012;122:1339–1353. doi: 10.1172/JCI57256. This study demonstrated that insulin resistance induced by exposure to amyloid β oligomers is associated with deficits in hippocampal neuroplasticity, including deficits in spatial learning. Additionally, this study reported that the GLP analog exendin-4 restored hippocampal insulin signaling and reversed the amyloid β oligomers-induced neuroplasticity deficits. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao WQ, De Felice FG, Fernandez S, Chen H, Lambert MP, Quon MJ, Krafft GA, Klein WL. Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J. 2008;22:246–260. doi: 10.1096/fj.06-7703com. [DOI] [PubMed] [Google Scholar]
- 37.Kamal A, Ramakers GM, Gispen WH, Biessels GJ. Hyperinsulinemia in rats causes impairment of spatial memory and learning with defects in hippocampal synaptic plasticity by involvement of postsynaptic mechanisms. Exp Brain Res. 2013;226:45–51. doi: 10.1007/s00221-013-3409-4. [DOI] [PubMed] [Google Scholar]
- 38.Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18:1085–1088. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boitard C, Etchamendy N, Sauvant J, Aubert A, Tronel S, Marighetto A, Laye S, Ferreira G. Juvenile, but not adult exposure to high-fat diet impairs relational memory and hippocampal neurogenesis in mice. Hippocampus. 2012;22:2095–2100. doi: 10.1002/hipo.22032. [DOI] [PubMed] [Google Scholar]
- 40.Li XL, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience. 2002;113:607–615. doi: 10.1016/s0306-4522(02)00162-8. [DOI] [PubMed] [Google Scholar]
- 41.Nistico R, Cavallucci V, Piccinin S, Macri S, Pignatelli M, Mehdawy B, Blandini F, Laviola G, Lauro D, Mercuri NB, D'Amelio M. Insulin Receptor beta-Subunit Haploinsufficiency Impairs Hippocampal Late-Phase LTP and Recognition Memory. Neuromolecular Med. 2012 doi: 10.1007/s12017-012-8184-z. [DOI] [PubMed] [Google Scholar]
- 42.Kanoski SE, Meisel RL, Mullins AJ, Davidson TL. The effects of energy-rich diets on discrimination reversal learning and on BDNF in the hippocampus and prefrontal cortex of the rat. Behav Brain Res. 2007;182:57–66. doi: 10.1016/j.bbr.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanoski SE, Zhang Y, Zheng W, Davidson TL. The effects of a high-energy diet on hippocampal function and blood-brain barrier integrity in the rat. J Alzheimers Dis. 2010;21:207–219. doi: 10.3233/JAD-2010-091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grillo CA, Piroli GG, Junor L, Wilson SP, Mott DD, Wilson MA, Reagan LP. Obesity/hyperleptinemic phenotype impairs structural and functional plasticity in the rat hippocampus. Physiol Behav. 2011;105:138–144. doi: 10.1016/j.physbeh.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greenwood CE, Winocur G. Cognitive impairment in rats fed high-fat diets: a specific effect of saturated fatty-acid intake. Behav Neurosci. 1996;110:451–459. doi: 10.1037//0735-7044.110.3.451. [DOI] [PubMed] [Google Scholar]
- 46.Winocur G, Greenwood CE. The effects of high fat diets and environmental influences on cognitive performance in rats. Behav Brain Res. 1999;101:153–161. doi: 10.1016/s0166-4328(98)00147-8. [DOI] [PubMed] [Google Scholar]
- 47.Greenwood CE, Winocur G. Learning and memory impairment in rats fed a high saturated fat diet. Behav Neural Biol. 1990;53:74–87. doi: 10.1016/0163-1047(90)90831-p. [DOI] [PubMed] [Google Scholar]
- 48.Collin M, Hakansson-Ovesjo ML, Misane I, Ogren SO, Meister B. Decreased 5-HT transporter mRNA in neurons of the dorsal raphe nucleus and behavioral depression in the obese leptin-deficient ob/ob mouse. Brain Res Mol Brain Res. 2000;81:51–61. doi: 10.1016/s0169-328x(00)00167-4. [DOI] [PubMed] [Google Scholar]
- 49.Grillo CA, Piroli GG, Kaigler KF, Wilson SP, Wilson MA, Reagan LP. Downregulation of hypothalamic insulin receptor expression elicits depressive-like behaviors in rats. Behav Brain Res. 2011;222:230–235. doi: 10.1016/j.bbr.2011.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharma AN, Elased KM, Garrett TL, Lucot JB. Neurobehavioral deficits in db/db diabetic mice. Physiol Behav. 2010;101:381–388. doi: 10.1016/j.physbeh.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharma S, Fulton S. Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry. Int J Obes (Lond) 2013;37:382–389. doi: 10.1038/ijo.2012.48. [DOI] [PubMed] [Google Scholar]
- 52.Koekkoek PS, Kappelle LJ, van den BE, Rutten GE, Biessels GJ. Cognitive function in patients with diabetes mellitus: guidance for daily care. Lancet Neurol. 2015;14:329–340. doi: 10.1016/S1474-4422(14)70249-2. [DOI] [PubMed] [Google Scholar]
- 53.Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2006;23:1165–1173. doi: 10.1111/j.1464-5491.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- 54.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 55.Hwang IK, Kim IY, Kim DW, Yoo KY, Kim YN, Yi SS, Won MH, Lee IS, Yoon YS, Seong JK. Strain-specific differences in cell proliferation and differentiation in the dentate gyrus of C57BL/6N and C3H/HeN mice fed a high fat diet. Brain Res. 2008;1241:1–6. doi: 10.1016/j.brainres.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 56.Lindqvist A, Mohapel P, Bouter B, Frielingsdorf H, Pizzo D, Brundin P, Erlanson-Albertsson C. High-fat diet impairs hippocampal neurogenesis in male rats. Eur J Neurol. 2006;13:1385–1388. doi: 10.1111/j.1468-1331.2006.01500.x. [DOI] [PubMed] [Google Scholar]
- 57.Stranahan AM, Lee K, Martin B, Maudsley S, Golden E, Cutler RG, Mattson MP. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. 2009;19:951–961. doi: 10.1002/hipo.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Freeman LR, Granholm AC. Vascular changes in rat hippocampus following a high saturated fat and cholesterol diet. J Cereb Blood Flow Metab. 2012;32:643–653. doi: 10.1038/jcbfm.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nelson TJ, Sun MK, Hongpaisan J, Alkon DL. Insulin, PKC signaling pathways and synaptic remodeling during memory storage and neuronal repair. Eur J Pharmacol. 2008;585:76–87. doi: 10.1016/j.ejphar.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 60.Huang CC, Lee CC, Hsu KS. The role of insulin receptor signaling in synaptic plasticity and cognitive function. Chang Gung Med J. 2010;33:115–125. [PubMed] [Google Scholar]
- 61.Bhat NR, Thirumangalakudi L. Increased tau phosphorylation and impaired brain insulin/IGF signaling in mice fed a high fat/high cholesterol diet. J Alzheimers Dis. 2013;36:781–789. doi: 10.3233/JAD-2012-121030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim B, Backus C, Oh S, Hayes JM, Feldman EL. Increased tau phosphorylation and cleavage in mouse models of type 1 and type 2 diabetes. Endocrinology. 2009;150:5294–5301. doi: 10.1210/en.2009-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schubert M, Gautam D, Surjo D, Ueki K, Baudler S, Schubert D, Kondo T, Alber J, Galldiks N, Kustermann E, Arndt S, Jacobs AH, Krone W, Kahn CR, Bruning JC. Role for neuronal insulin resistance in neurodegenerative diseases. Proc Natl Acad Sci U S A. 2004;101:3100–3105. doi: 10.1073/pnas.0308724101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wrighten SA, Piroli GG, Grillo CA, Reagan LP. A look inside the diabetic brain: Contributors to diabetes-induced brain aging. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbadis.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65••.Grillo CA, Kelly SJ, Lawrence RC, Wrighten SA, Green AJ, Wilson SP, Sakai RR, Wilson MA, Mott DD, Reagan LP. Hippocampal insulin resistance impairs spatial learning and synaptic plasticity. Diabetes. 2015;64:3927–3936. doi: 10.2337/db15-0596. This study demonstrated that hippocampal insulin resistance, in the absence of changes in peripheral insulin sensitivity or glucose homeostasis, can induce neuroplasticity deficits in the hippocampus, including impairments in spatial learning and memory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pathan AR, Gaikwad AB, Viswanad B, Ramarao P. Rosiglitazone attenuates the cognitive deficits induced by high fat diet feeding in rats. Eur J Pharmacol. 2008;589:176–179. doi: 10.1016/j.ejphar.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 67.McNeilly AD, Williamson R, Balfour DJ, Stewart CA, Sutherland C. A high-fat-diet-induced cognitive deficit in rats that is not prevented by improving insulin sensitivity with metformin. Diabetologia. 2012;55:3061–3070. doi: 10.1007/s00125-012-2686-y. [DOI] [PubMed] [Google Scholar]
- 68.Porter DW, Kerr BD, Flatt PR, Holscher C, Gault VA. Four weeks administration of Liraglutide improves memory and learning as well as glycaemic control in mice with high fat dietary-induced obesity and insulin resistance. Diabetes Obes Metab. 2010;12:891–899. doi: 10.1111/j.1463-1326.2010.01259.x. [DOI] [PubMed] [Google Scholar]
- 69.Porter DW, Irwin N, Flatt PR, Holscher C, Gault VA. Prolonged GIP receptor activation improves cognitive function, hippocampal synaptic plasticity and glucose homeostasis in high-fat fed mice. Eur J Pharmacol. 2011;650:688–693. doi: 10.1016/j.ejphar.2010.10.059. [DOI] [PubMed] [Google Scholar]
- 70.Agrawal R, Zhuang Y, Cummings BP, Stanhope KL, Graham JL, Havel PJ, Gomez-Pinilla F. Deterioration of plasticity and metabolic homeostasis in the brain of the UCD-T2DM rat model of naturally occurring type-2 diabetes. Biochim Biophys Acta. 2014;1842:1313–1323. doi: 10.1016/j.bbadis.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pipatpiboon N, Pintana H, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. DPP4-inhibitor improves neuronal insulin receptor function, brain mitochondrial function and cognitive function in rats with insulin resistance induced by high-fat diet consumption. Eur J Neurosci. 2013;37:839–849. doi: 10.1111/ejn.12088. [DOI] [PubMed] [Google Scholar]
- 72.Kern W, Born J, Schreiber H, Fehm HL. Central nervous system effects of intranasally administered insulin during euglycemia in men. Diabetes. 1999;48:557–563. doi: 10.2337/diabetes.48.3.557. [DOI] [PubMed] [Google Scholar]
- 73.Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 2004;29:1326–1334. doi: 10.1016/j.psyneuen.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 74.Novak V, Milberg W, Hao Y, Munshi M, Novak P, Galica A, Manor B, Roberson P, Craft S, Abduljalil A. Enhancement of vasoreactivity and cognition by intranasal insulin in type 2 diabetes. Diabetes Care. 2014;37:751–759. doi: 10.2337/dc13-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75••.Zhang H, Hao Y, Manor B, Novak P, Milberg W, Zhang J, Fang J, Novak V. Intranasal insulin enhanced resting-state functional connectivity of hippocampal regions in type 2 diabetes. Diabetes. 2015;64:1025–1034. doi: 10.2337/db14-1000. Study demonstrated that a single acute administration of intranasal insulin increased functional connections of the hippocampus with the medial frontal cortex in type 2 diabetes patients and that these enhancements were correlated with cognitive performance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cholerton B, Baker LD, Craft S. Insulin, cognition, and dementia. Eur J Pharmacol. 2013;719:170–179. doi: 10.1016/j.ejphar.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D, Gerton B. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69:29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Felice FG. Alzheimer's disease and insulin resistance: translating basic science into clinical applications. J Clin Invest. 2013;123:531–539. doi: 10.1172/JCI64595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Irwin N, Gault V, Flatt PR. Therapeutic potential of the original incretin hormone glucose-dependent insulinotropic polypeptide: diabetes, obesity, osteoporosis and Alzheimer's disease? Expert Opin Investig Drugs. 2010;19:1039–1048. doi: 10.1517/13543784.2010.513381. [DOI] [PubMed] [Google Scholar]
- 80.Molteni R, Wu A, Vaynman S, Ying Z, Barnard RJ, Gomez-Pinilla F. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience. 2004;123:429–440. doi: 10.1016/j.neuroscience.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 81.Noble EE, Mavanji V, Little MR, Billington CJ, Kotz CM, Wang C. Exercise reduces diet-induced cognitive decline and increases hippocampal brain-derived neurotrophic factor in CA3 neurons. Neurobiol Learn Mem. 2014;114C:40–50. doi: 10.1016/j.nlm.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grillo CA, Piroli GG, Evans AN, Macht VA, Wilson SP, Scott KA, Sakai RR, Mott DD, Reagan LP. Obesity/hyperleptinemic phenotype adversely affects hippocampal plasticity: Effects of dietary restriction. Physiol Behav. 2011;104:235–241. doi: 10.1016/j.physbeh.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grillo CA, Mulder P, Macht VA, Kaigler KF, Wilson SP, Wilson MA, Reagan LP. Dietary restriction reverses obesity-induced anhedonia. Physiol Behav. 2014;128:126–132. doi: 10.1016/j.physbeh.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grayson BE, Fitzgerald MF, Hakala-Finch AP, Ferris VM, Begg DP, Tong J, Woods SC, Seeley RJ, Davidson TL, Benoit SC. Improvements in hippocampal-dependent memory and microglial infiltration with calorie restriction and gastric bypass surgery, but not with vertical sleeve gastrectomy. Int J Obes (Lond) 2014;38:349–356. doi: 10.1038/ijo.2013.100. [DOI] [PubMed] [Google Scholar]
- 85.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 86.Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Cholerton BA, Plymate SR, Fishel MA, Watson GS, Duncan GE, Mehta PD, Craft S. Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer's disease. J Alzheimers Dis. 2010;22:569–579. doi: 10.3233/JAD-2010-100768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87•.Alosco ML, Spitznagel MB, Strain G, Devlin M, Cohen R, Paul R, Crosby RD, Mitchell JE, Gunstad J. Improved memory function two years after bariatric surgery. Obesity (Silver Spring) 2014;22:32–38. doi: 10.1002/oby.20494. This study reported that a number of cognitive measures were improved as quickly as 12 weeks following bariatric surgery and that these cognitive improvements were maintained at a 24 month follow up assessment. [DOI] [PMC free article] [PubMed] [Google Scholar]


