Summary
Evidence from the RV144 HIV‐1 vaccine trial implicates anti‐HIV‐1 antibody‐dependent cellular cytotoxicity (ADCC) in vaccine‐conferred protection from infection. Among effector cells that mediate ADCC are natural killer (NK) cells. The ability of NK cells to be activated in an antibody‐dependent manner is reliant upon several factors. In general, NK cell‐mediated antibody‐dependent activation is most robust in terminally differentiated CD57+ NK cells, as well as NK cells educated through ontological interactions between inhibitory killer immunoglobulin‐like receptors (KIR) and their major histocompatibility complex class I [MHC‐I or human leucocyte antigen (HLA‐I)] ligands. With regard to anti‐HIV‐1 antibody‐dependent NK cell activation, previous research has demonstrated that the epidemiologically relevant KIR3DL1/HLA‐Bw4 receptor/ligand combination confers enhanced activation potential. In the present study we assessed the ability of the KIR2DL1/HLA–C2 receptor/ligand combination to confer enhanced activation upon direct stimulation with HLA‐I‐devoid target cells or antibody‐dependent stimulation with HIV‐1 gp140‐pulsed CEM.NKr‐CCR5 target cells in the presence of an anti‐HIV‐1 antibody source. Among donors carrying the HLA‐C2 ligand for KIR2DL1, higher interferon (IFN)‐γ production was observed within KIR2DL1+ NK cells than in KIR2DL1– NK cells upon both direct and antibody‐dependent stimulation. No differences in KIR2DL1+ and KIR2DL1– NK cell activation were observed in HLA‐C1 homozygous donors. Additionally, higher activation in KIR2DL1+ than KIR2DL1– NK cells from HLA–C2 carrying donors was observed within less differentiated CD57– NK cells, demonstrating that the observed differences were due to education and not an overabundance of KIR2DL1+ NK cells within differentiated CD57+ NK cells. These observations are relevant for understanding the regulation of anti‐HIV‐1 antibody‐dependent NK cell responses.
Keywords: AIDS, killer immunoglobulin‐like receptors, natural killer cells
Introduction
A prophylactic vaccine is desired to reduce the number of new HIV‐1 infections. Anti‐HIV‐1 antibodies capable of triggering antibody‐dependent cellular cytotoxicity (ADCC) might be important to elicit through vaccination. Following binding of viral epitopes on the surface of HIV‐1‐infected cells, the constant regions of ADCC antibodies engage the CD16 constant region receptor on innate immune cells, such as natural killer (NK) cells and monocytes. Engagement of CD16 can result in the lysis of the HIV‐1‐infected target cell 1, 2, 3. Additionally, NK cells activated upon stimulation through CD16 release chemokines and cytokines 4, 5. Chemokines produced by activated NK cells can directly inhibit HIV‐1 replication 6. In the context of vaccination, ADCC would allow for the elimination of autologous cells that become infected upon HIV‐1 exposure, as well as HIV‐1‐infected allogeneic cells delivered within infected bodily fluids. The modestly successful RV144 vaccine trial has indicated a role for ADCC‐competent antibodies in vaccine‐conferred protection against HIV‐1 infection 7, 8, 9. Indeed, ADCC antibodies were associated with a lower likelihood of infection in vaccinees who carried low levels of anti‐envelope immunoglobulin (Ig)A that could compete with IgG for antigen binding and block anti‐HIV‐1 ADCC 7, 9. This observation highlights the need for further research into the factors regulating the ability of innate immune effector cells to mediate antibody‐dependent functions, facilitating utilization of the full potential of ADCC antibodies in HIV‐1 vaccine development.
The ability of NK cells to mediate anti‐HIV‐1 antibody‐dependent functions is dependent upon several NK cell factors, including the education status and state of differentiation of the cell 10, 11. The education status of an NK cell is determined through interactions of activating and inhibitory NK cell receptors with self‐major histocompatibility complex class I [MHC‐I or human leucocyte antigen (HLA)‐I] ligands, which tunes the functional potential of the NK cell 12, 13, 14. In general, NK cells that express inhibitory receptors capable of binding self‐HLA‐I are tuned for higher functional potential, whereas NK cells lacking inhibitory receptors or expressing inhibitory receptors that do not recognize self HLA‐I are tuned for reduced functional potential 12, 14. Furthermore, NK cells expressing activating receptors capable of binding self HLA‐I are tuned for lower functional potential 13. The education status of NK cells is linked to their ability to become activated by antibody‐dependent and independent stimuli 12. With regard to anti‐HIV‐1 antibody‐dependent NK cell activation, our group has demonstrated previously that educated NK cells expressing the inhibitory killer immunoglobulin‐like receptor 3DL1 (KIR3DL1), derived from donors carrying the HLA‐Bw4 ligand, exhibit a functional advantage over autologous KIR3DL1– NK cells and allogeneic KIR3DL1+ NK cells, derived from donors lacking the HLA‐Bw4 ligand 11. In addition to education, NK cells undergo a differentiation process independently whereby they progress phenotypically from CD56brightCD16–CD57– to CD56dimCD16+CD57–, and finally develop into CD56dimCD16+CD57+NK cells 15. Along with changes in phenotype, the functional profile of NK cells is also altered by differentiation. Indeed, NK cells expressing the CD57 differentiation marker exhibit more robust activation upon stimulation through CD16 10, 16. Differentiated CD57+ NK cells are also more likely to express inhibitory KIRs 16. Although differentiated NK cells expressing inhibitory KIR for self‐HLA‐I would be educated for higher functional potential, the contributions of NK cell education and differentiation to NK cell antibody‐dependent functional potential appear to be at least partially distinct 10.
Our previous research regarding the contribution of NK cell education to the antibody‐dependent functional potential of NK cells focused upon the KIR3DL1/HLA‐Bw4 receptor/ligand combination 10, 11, 17. The results of those KIR3DL1/HLA‐Bw4 studies corroborate epidemiological studies that have linked allelic combinations of this receptor/ligand pair to protection from HIV‐1 infection and progression to AIDS 18, 19. Several recent studies have now suggested that the inhibitory KIR2DL1 receptor might also be important for understanding susceptibility to HIV‐1 infection and HIV‐1 pathogenesis. The KIR2DL1 receptor binds a subset of HLA‐C alleles, termed HLA‐C2, which are characterized by the presence of lysine at amino acid position 80 20. Non‐HLA‐C2 alleles are termed HLA‐C1 and are characterized by the presence of asparagine at amino acid position 80. An association between the KIR2DL1/HLA‐C2 receptor/ligand combination and protection from HIV‐1 infection has been provided by the observation that exposed but uninfected Senegalese carry the education‐competent KIR2DL1/HLA‐C2 combination, while their infected partners lack HLA‐C2 ligands that could inhibit the recognition and cytolysis of HIV‐1‐infected allogeneic leukocytes 21. A potential link has also been noted between KIR2DL1 and HIV‐1 disease progression through the demonstration by Korner et al. that KIR2DL1+ NK cells are expanded in primary HIV‐1 infection in individuals carrying the HLA‐C2 ligand 22. These expanded KIR2DL1+ NK cells also exhibited a functional advantage over KIR2DL1– NK cells upon stimulation with HLA‐I‐devoid target cells. Interestingly, the frequency of KIR2DL1+ NK cells appears to wane during chronic HIV‐1 infection, further indicating a role in HIV‐1 pathogenesis. Indeed, this phenomenon has been demonstrated in HIV‐1 clade C‐infected South Africans and clades A‐ and D‐infected Ugandans 23, 24. Additional research has demonstrated that while NK cells expressing the HLA‐C binding KIR2DL1/2/3 receptors contribute to activation upon stimulation with HLA‐I‐devoid target cells in HIV‐1‐uninfected donors, this contribution is lower in chronically HIV‐1‐infected donors 25.
Given that recent evidence has implied that educated KIR2DL1+ NK cells might play a role in protection from HIV‐1 infection and suppressing viral replication during primary HIV‐1 infection, we sought to determine if education of NK cells through KIR2DL1/HLA‐C2 interactions enhanced the ability of NK cells to become activated in an anti‐HIV‐1 antibody‐dependent manner. We now present data demonstrating that education of NK cells through KIR2DL1/HLA‐C2 interactions enhances NK cell activation upon exposure to antibody‐dependent and antibody‐independent stimuli. Furthermore, we demonstrate, through assessing CD57– NK cells, that the functional advantage of educated KIR2DL1+ NK cells upon antibody‐dependent stimulation is not a bystander effect of NK cell differentiation. These results enhance our understanding of the regulation of anti‐HIV‐1 antibody‐dependent NK cell responses.
Materials and methods
Participants
Blood was collected from 13 HIV‐1‐uninfected donors by forearm venepuncture into vacuettes containing sodium heparin anti‐coagulant. Ficoll Paque PLUS (GE Healthcare Life Sciences, Parramatta, NSW, Australia) density gradient centrifugation was employed to obtain peripheral blood mononuclear cells (PBMCs) from whole blood. These PBMCs were utilized as effector cells in NK cell activation assays. As a source of anti‐HIV‐1 antibodies, plasma was obtained from an HIV‐1‐infected client of the Melbourne Sexual Health Centre. This HIV‐1‐infected donor's plasma has been shown previously to carry antibodies capable of activating NK cells in an anti‐HIV‐1‐dependent manner 26. All donors provided informed consent prior to collection of biological samples and the ethics committees of the participating institutions approved all performed experiments.
Cell lines
The CD4+ CEM.NKr‐CCR5 T cell line was obtained from the NIH AIDS Reagent Program, Division of AIDS, NIAID (Germantown, MD, USA). The HLA‐I‐devoid 721.221 cell line was kindly provided by Dr Andrew Brooks (Department of Microbiology and Immunology, University of Melbourne).
HLA‐C typing and KIR2DL1 expression
The Victorian Transplantation and Immunogenetics Service at The Australian Red Cross Blood Service performed sequence‐based typing of HLA‐C alleles to four‐digit resolution for all 13 donors. Expression of KIR2DL1 by NK cells from all 13 donors was demonstrated by staining with fluorescein isothiocyanate (FITC)‐conjugated anti‐KIR2DL1 antibody (clone: 143221; R&D Systems, Minneapolis, MN, USA) and detection by flow cytometry using a BD LSR Fortessa (BD Biosciences, San Jose, CA, USA). This antibody clone detects KIR2DL1 and exhibits no cross‐reactivity with KIR2DL2/3 or KIR3DL1 gene products. FlowJo version 9·2 (Tree Star, Ashland, OR, USA) was utilized for analysis of flow cytometry data.
NK cell activation assays
To study anti‐HIV‐1 antibody‐specific NK cell activation, we used a previously described flow cytometric assay to detect NK cell interferon (IFN)‐γ expression 17. Briefly, CEM.NKr‐CCR5 target cells were prepared by coating with HIV‐1 gp140AD8 (3 µg/1·0 × 106 cells in 1 ml of solution) for 90 min at 4°C. The HIV‐1 gp140AD8 was prepared as described previously 27. Next, PBMC effector cells were combined with gp140‐pulsed CEM.NKr‐CCR5 target cells at a 10 : 1 effector to target ratio in the presence or absence of a 1 : 2000 final dilution of plasma from an HIV‐1‐infected donor, brefeldin A (5 µg/ml) (Sigma‐Aldrich, Castle Hill, NSW, Australia) and monensin (6 µg/ml) (BD Biosciences). Co‐cultures were incubated for 5 h at 37°C. To demonstrate that the assay detects specifically anti‐HIV‐1 antibody‐dependent responses, three donors were assessed for NK cell activation in the presence of 1 : 2000 dilutions of both HIV‐1‐uninfected and HIV‐1‐infected plasma. The utilized plasma dilution was implemented due to a previously reported prozone effect in assays measuring anti‐HIV‐1 ADCC 1. Following incubation, cells were surface‐stained with peridinin chlorophyll (PerCP)‐conjugated anti‐CD3 (clone: SK7; BD Biosciences), phycoerythrin‐cyanin 7 (PE‐Cy7)‐conjugated anti‐CD56 (clone: NCAM16.2; BD Biosciences), FITC‐conjugated anti‐KIR2DL1 (clone: 143221; R&D Systems) and Pacific Blue‐conjugated anti‐CD57 (clone: HCD57; Biolegend/Australian Biosearch, Karrinyup, WA, Australia) antibodies. Next, cells were fixed in formaldehyde, permeabilized with 1× Perm solution (BD Biosciences) and stained with Alexa Fluor 700‐conjugated anti‐IFN‐γ antibody (clone: b27; BD Biosciences). Lastly, cells were fixed in formaldehyde and acquired using a BD LSR Fortessa (BD Biosciences). Data were analysed with FlowJo version 9·2. Antibody‐independent NK cell activation was also assessed in an identical manner as anti‐HIV‐1 antibody‐dependent NK cell activation as described above, except that PBMCs were cultured with the HLA‐I‐devoid 721.221 cell line in the absence of any antibody sources.
Autologous whole blood antibody‐dependent NK cell activation assay
To assess anti‐HIV‐1 antibody‐dependent NK cell activation in an autologous setting, we utilized a previously described assay measuring antibody‐dependent NK cell activation by autologous CD4+ cells that have bound HIV‐1 envelope and antibodies derived from HIV‐1‐infected plasma 26. Briefly, 150 µl of HIV‐1‐uninfected whole blood was combined with 50 µl of HIV‐1‐infected plasma, 1 µg/ml HIV‐1 gp140AD8, brefeldin A (5 µg/ml) and monensin (6 µg/ml). Control conditions containing whole blood alone or whole blood plus HIV‐1‐infected plasma were conducted simultaneously. All conditions were incubated for 5 h at 37°C. Following incubation, blood was incubated with PerCP‐conjugated anti‐CD3 (clone: SK7; BD Biosciences), PE‐Cy7‐conjugated anti‐CD56 (clone: NCAM16.2; BD Biosciences) and FITC‐conjugated anti‐KIR2DL1 (clone: 143221; R&D Systems) antibodies. Next, red blood cells were lysed with 1× lysis buffer (BD Biosciences), permeabilized with 1× Perm solution (BD Biosciences) and stained with Alexa Fluor 700‐conjugated anti‐IFN‐γ antibody (clone: b27; BD Biosciences). Samples were fixed in formaldehyde and acquired using a BD LSR Fortesa (BD Biosciences). Data analysis was performed with FlowJo version 9·2.
Assessment of CD16 expression on KIR2DL1+ and KIR2DL1– NK cells
Whole blood from nine donors was incubated with PerCP‐conjugated CD3 (clone: SK7; BD Biosciences), PE‐Cy7‐conjugated CD56 (clone: NCAM16.2; BD Biosciences), allophycocyanin (APC)‐conjugated anti‐KIR2DL1 (clone: 143221; R&D Systems) and FITC‐conjugated anti‐CD16 (clone: 3G8; BD Biosciences). Next, red blood cells were lysed with 1× lysis buffer, washed and fixed in formaldehyde. Samples were acquired using a BD LSR Fortessa (BD). Data were analysed with FlowJo version 9·2.
Statistics
GraphPad Prism version 4·0 was used for statistical analyses. Within‐group differences were compared using the Wilcoxon matched‐pairs test. Data throughout the paper are presented in the [median (range) versus median (range)] format.
Results
Direct and anti‐HIV‐1 antibody‐dependent activation of NK cells educated through KIR2DL1
The functional advantage of educated KIR2DL1+ NK cells over the KIR2DL1– population, which contains both uneducated NK cells and cells educated through other HLA/KIR combinations, has been observed upon direct stimulation for both HIV‐1‐infected and uninfected donors, and non‐HIV‐1 antibody‐dependent stimulation for HIV‐1‐uninfected donors 12, 22, 28. The role of education through KIR2DL1 on anti‐HIV‐1 antibody‐dependent activation potential, however, has not yet been investigated. To address this issue we stimulated NK cell effectors within PBMCs, obtained from eight HLA‐C2‐carrying donors and five donors homozygous for HLA‐C1 alleles (Table 1), with HIV‐1AD8 gp140‐coated CEM.NKr‐CCR5 T cells in the presence of plasma from an HIV‐1‐infected donor. This assay specifically detects anti‐HIV‐1 antibody‐dependent NK cell activation, as activation is observed in the presence of HIV‐1‐infected plasma but not in the presence of HIV‐1‐uninfected plasma (Supporting information, Fig. S1) Simultaneously, in order to demonstrate that the utilized HLA‐C2‐carrying donors, but not the HLA‐C1 homozygous donors, exhibit the previously reported functional advantage within the educated KIR2DL1+ population upon direct stimulation, we stimulated NK cells within PBMC with the HLA‐I‐devoid 721.221 cell line. Following stimulation, samples were assessed by flow cytometry. The gating procedure used to identify KIR2DL1+ and KIR2DL1– NK cells, as well as the percentage of NK cells within each population that became activated to produce IFN‐γ, is depicted in Fig. 1a. As expected, upon stimulation with 721.221 targets the percentage of NK cells activated to produce IFN‐γ was higher in the KIR2DL1+ population than in the KIR2DL1– population for HLA‐C2 carrying donors [16·2% (3·6–28·9%) versus 10·4% (3·4–12·9%), P = 0·0078] (Fig. 1b). No differences in IFN‐γ production was observed between these NK cell populations in donors homozygous for HLA‐C1 alleles [10·7% (5·2–16·9%) versus 8·5% (7·3–14%), P = 1·00] (Fig. 1b). Similarly, when NK cells were stimulated in an anti‐HIV‐1 antibody‐dependent manner, HLA‐C2‐carrying donors exhibited higher percentages of IFN‐γ‐producing NK cells in the KIR2DL1+ population than the KIR2DL1– population [6·6% (1·9–16·2%) versus 3·5% (0·9–5·7%), P = 0·0078] (Fig. 1c). No differences were observed between these NK cell populations upon anti‐HIV‐1 antibody‐dependent stimulation in donors homozygous for HLA‐C1 alleles [6·2% (3·0–9·0%) versus 6·2% (2·8–6·9%), P = 0·6250] (Fig. 1c). As antibody‐dependent NK cell activation is triggered through CD16, we next questioned if these differences in antibody‐dependent NK cell activation could be attributed simply to preferentially higher expression levels of CD16 on KIR2DL1+ NK cells in HLA‐C2‐carrying donors. Although we noted higher CD16 median fluorescence intensity (MFI) on KIR2DL1+ than KIR2DL1– NK cells, this expression pattern was seen in both HLA‐C2 carriers [3227 (1118–5549) versus 2814 (877–4405); n = 5] and HLA‐C1 homozygotes [2994 (1134–8652) versus 2534 (993–7355); n = 4]. As such, CD16 expression levels do not account for the preferential activation of KIR2DL1+ NK cells in HLA‐C2‐carrying donors upon antibody‐dependent stimulation. These data reaffirm a role for NK cell education through KIR2DL1 in determining the ability of NK cells to exhibit activation upon direct stimulation. Further, the data suggest that the role of education in determining NK cell functional potential extends to the ability of NK cells to exhibit anti‐HIV‐1 antibody‐dependent NK cell activation.
Table 1.
Human leucocyte antigen (HLA)‐C typing of killer immunoglobulin‐like receptor (KIR)2DL1 carrying effector cell donors.
| Donor ID | HLA‐C alleles | HLA‐C1/C2 typing |
|---|---|---|
| 1 | 04:01, 14:02 | C1/C2 |
| 2 | 07:02, 17:01 | C1/C2 |
| 3 | 02:02, 03:04 | C1/C2 |
| 4 | 07:02, 07:02 | C1/C1 |
| 5 | 05:01, 12:03 | C1/C2 |
| 6 | 07:04, 12:03 | C1/C1 |
| 7 | 04:03, 07:02 | C1/C2 |
| 8 | 04:01, 07:01 | C1/C2 |
| 9 | 03:03, 07:02 | C1/C1 |
| 10 | 03:04, 07:01 | C1/C1 |
| 11 | 07:01, 16:02 | C1/C2 |
| 12 | 01:02, 01:02 | C1/C1 |
| 13 | 04:01, 12:02 | C1/C2 |
Figure 1.
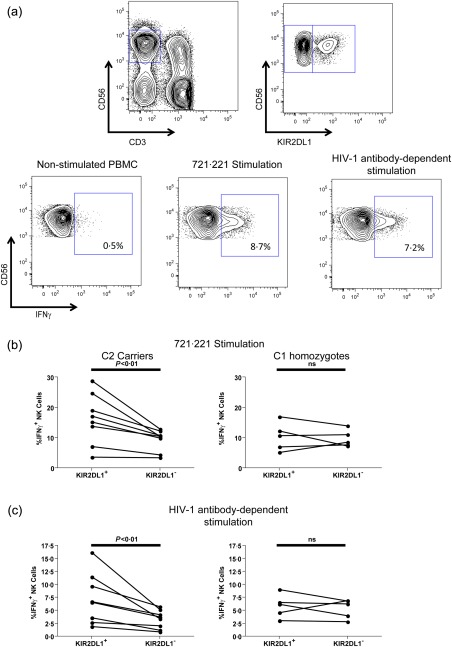
Direct and anti‐HIV‐1 antibody‐dependent activation of killer immunoglobulin‐like receptor (KIR)2DL1+ and KIR2DL1– natural killer (NK) cells from human leucocyte antigen (HLA)‐C2+ and HLA‐C1 homozygous donors. (a) Direct and anti‐HIV‐1 antibody‐dependent activation of NK cell effectors within peripheral blood mononuclear cells (PBMC) was accomplished by stimulation with the HLA‐I‐devoid 721.221 cell line or HIV‐1AD8 gp140‐pulsed CEM.NKr‐CCR5 in the presence of anti‐HIV‐1 antibodies, respectively. Following stimulation PBMCs were stained with fluorochrome‐conjugated antibodies and assessed by flow cytometry. The fluorescence activated cell sorter (FACS) plots depict progressive gating upon CD3–CD56+ NK cells (top left), KIR2DL1+ and KIR2DL1– NK cell subsets (top right) and the assessment of gated cells for interferon (IFN)‐γ production in the non‐stimulated (bottom left), 721.221‐stimulated (bottom middle) and anti‐HIV‐1 antibody‐dependent stimulated conditions (bottom right). (b) Graphs depict the relative activation of the KIR2DL1+ and KIR2DL1– NK cell subsets upon activation by the 721.221 cell line in eight donors carrying HLA‐C2 alleles (left) and five HLA‐C1 homozygotes (right). (c) Graphs depict the relative activation of the KIR2DL1+ and KIR2DL1– NK cell subsets upon anti‐HIV‐1 antibody‐dependent activation in eight donors carrying HLA‐C2 alleles (left) and five HLA‐C1 homozygotes (right).
Impact of NK cell differentiation on differences in activation between KIR2DL1+ and KIR2DL1– NK cells
The ability of NK cells to exhibit activation upon stimulation through CD16 is higher in NK cells expressing the CD57 differentiation marker 10, 16. As higher percentages of CD57+ NK cells express KIRs, the relative contributions of education and differentiation to antibody‐dependent NK cell activation can become unclear upon assessments of the total NK cell population 10, 16. We therefore assessed the degree to which NK cell differentiation influences the apparent antibody‐dependent activation advantage of educated KIR2DL1+ NK cells. Coinciding with previously published data, we observed higher anti‐HIV‐1 antibody‐dependent NK cell activation in the CD57+ NK cells than the CD57– NK cells in all 13 donors [6·5% (1·4–13%) versus 2·4% (0·6–4·4%), P = 0·0002] (Fig. 2a) 10. Additional assessments of CD57+ and CD57– NK cells revealed a higher frequency of KIR2DL1 expression in the CD57+ population across all 13 donors [25·3% (8·4–50·4%] versus 12·7% (3·5–32·1%), P = 0·0002] (Fig. 2b). Lastly, we assessed if the differences in anti‐HIV‐1 antibody‐dependent activation observed between KIR2DL1+ and KIR2DL1– NK cells in HLA‐C2 carrying donors were due to education, and not a bystander effect of NK cell differentiation. We compared the anti‐HIV‐1 antibody‐dependent activation of CD57–KIR2DL1+ and CD57–KIR2DL1– NK cells within HLA‐C2‐carrying donors and HLA‐C1 homozygous donors. As depicted in Fig. 2c, within HLA‐C2‐carrying donors KIR2DL1+ NK cells within the CD57– population exhibited higher levels of IFN‐γ production upon anti‐HIV‐1 antibody‐dependent stimulation than the KIR2DL1– population [4·6% (0·8–9·4%) versus 2·2% (0·4–3·0%), P = 0·0156]. No differences were observed between the CD57–KIR2DL1+ and CD57–KIR2DL1– populations in donors homozygous for HLA‐C1 [3·1% (0·1–3·5%) versus 2·4% (1·3–4·5%), P = 0·3125] (Fig. 2c). These data further confirm a role for NK cell education through KIR2DL1 in determining the ability of NK cells to exhibit anti‐HIV‐1 antibody‐dependent activation.
Figure 2.
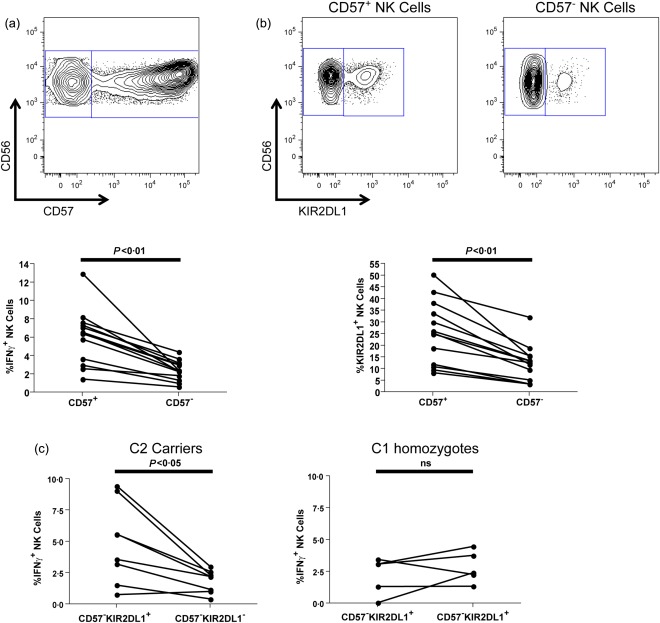
The anti‐HIV‐1 antibody‐dependent activation advantage of differentiated natural killer (NK) cells and the role of differentiation in the activation advantage of educated killer immunoglobulin‐like receptor (KIR)2DL1+ NK cells. (a) The fluorescence activated cell sorter (FACS) plot depicts the gating utilized to identify the differentiated CD57+ and less differentiated CD57– CD56dim NK cell populations. The graph highlights the relative ability of CD57+CD56dim and CD57–CD56dim NK cells from all 13 donors to exhibit anti‐HIV‐1 antibody‐dependent activation. (b) FACS plots depict the gating implemented to identify KIR2DL1+ and KIR2DL1– NK cells within the CD57+ (left) and CD57– (right) NK cell subsets. The graph depicts the relative percentages of CD57+CD56dim and CD57–CD56dim NK cells expressing the KIR2DL1 receptor in all 13 donors. (c) The graphs depict the relative anti‐HIV‐1 antibody‐dependent activation of KIR2DL1+ and KIR2DL1– NK cells within the CD57–CD56dim NK cell population in eight HLA‐C2+ donors (left) and five HLA‐C1 homozygotes (right).
Anti‐HIV‐1 antibody‐dependent activation of educated KIR2DL1+ NK cells against autologous targets
Although the preferential activation of educated KIR2DL1+ NK cells against gp140‐coated CEM.NKr‐CCR5 target cells demonstrates a role for NK cell education in determining NK cell activation potential, this experimental system does not determine if target cells expressing the HLA‐C2 ligand can activate KIR2DL1+ NK cells. Indeed, CEM.NKr‐CCR5 target cells are HLA‐C1 homozygous (Dr Nicole F Bernard, personal communication). To address this issue we performed an autologous whole blood anti‐HIV‐1 antibody‐dependent NK cell activation assay. This assay combines HIV‐1‐uninfected whole blood with HIV‐1 gp140AD8 and HIV‐1‐infected plasma. This allows CD4+ cells to bind to the viral envelope, which binds anti‐HIV‐1 antibodies and activates NK cells. Stimulation of NK cells within whole blood from five HLA‐C2 carriers resulted in activation of both the KIR2DL1+ and KIR2DL1– NK cell subsets (Fig. 3). Intriguingly, four of the five donors screened exhibited higher activation in the KIR2DL1+ [2·1% (1·8–18%) versus 1·8% (1·3–10·7%)] than the KIR2DL1– NK cell subset, while the fifth donor exhibited equal activation in both NK cell subsets. These data, demonstrating that educated KIR2DL1+ NK cells can become activated in an anti‐HIV‐1 antibody‐dependent manner against target cells expressing the HLA‐C2 ligand, even maintaining a functional advantage over KIR2DL1– NK cells in a majority of donors, highlight that anti‐HIV‐1 antibody‐dependent stimulation at least partially overcomes inhibitory signals through KIR2DL1/HLA‐C2 receptor ligand combinations.
Figure 3.
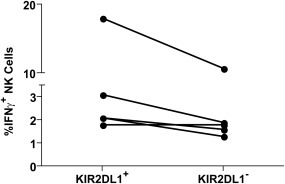
Anti‐HIV‐1 antibody‐dependent activation of killer immunoglobulin‐like receptor (KIR)2DL1+ and KIR2DL1− natural killer (NK) cells from human leucocyte antigen (HLA)‐C2 carrying donors by autologous target cells. NK cells within whole blood from five HLA‐C2 carrying donors were stimulated using the autologous whole blood anti‐HIV‐1 antibody‐dependent activation assay. Data were collected by flow cytometry and CD3−CD56+KIR2DL1+ and CD3−CD56+KIR2DL1– NK cells were assessed for interferon (IFN)‐γ production. The graph depicts the relative production of IFN‐γ by KIR2DL1+ and KIR2DL1− NK cells from each donor.
Discussion
The data presented in this paper provide the first demonstration that education of NK cells through KIR2DL1/HLA‐C2 combinations enhances the ability of NK cells to respond upon anti‐HIV‐1 antibody‐dependent activation. Additionally, and in concordance with previous studies, we provide data demonstrating an activation advantage of the KIR2DL1+ NK cell population upon antibody‐independent stimulation with HLA‐I‐devoid target cells 12, 22. These observations are interesting in the context of recent studies implicating KIR2DL1/HLA‐C2 combinations in providing protection from HIV‐1 infection, or contributing to inhibiting viral replication during primary HIV‐1 infection 21, 22. Indeed, the data presented in the current paper could be of importance for understanding mechanisms contributing to protective outcomes upon HIV‐1 exposure or infection.
Jennes et al. suggested recently that KIR2DL1/HLA‐C2 combinations could contribute to protection from HIV‐1 infection 21. In a cohort of Senegalese couples concordant and discordant for HIV‐1 infection the authors observed cognate ligand matches between inhibitory KIR in HIV‐1 recipients of concordant couples and HLA‐I in the HIV‐1 donors. In the discordant couples, cognate ligand mismatches were observed between the inhibitory KIR of the uninfected partner and the HLA‐I of the infected partner. The HIV‐1 uninfected partners were observed to carry the education‐competent KIR2DL1/HLA‐C2 combination, while their HIV‐1 infected partners tended to be HLA‐C1 homozygous. The authors demonstrated further that NK cells carrying KIR mismatched to HLA‐I expressed on CD4+ T‐cells were capable of killing allogeneic CD4+ T cells, suggesting that lack of ligands for inhibitory KIRs can result in enhanced direct recognition of allogeneic target cells and potentially offer protection from HIV‐1 acquisition. The data presented in the current paper, showing that educated KIR2DL1+ NK cells have an activation advantage for anti‐HIV‐1 antibody‐dependent activation, might explain further the protection observed in Senegalese serodiscordant couples. Although HIV‐1 exposed uninfected individuals do not carry anti‐HIV‐1 IgG within their sera, it has been shown recently that antibodies provided passively by HIV‐1‐infected mothers to their children via breast milk can protect against virus transmission 29. We and others have observed recently anti‐HIV‐1 antibodies capable of activating NK cells and/or triggering ADCC in seminal plasma and vaginal fluids 30, 31. As these antibodies are exchanged between HIV‐1‐infected donors and their uninfected partners upon exposure to HIV‐1, it is possible that the NK cells within the exposed individual could utilize these antibodies to eliminate infected allogeneic lymphocytes or autologous lymphocytes infected early after exposure.
Although the prospect of eliminating HIV‐1‐infected allogeneic lymphocytes via ADCC is supported by the observation that KIR/HLA‐I mismatched NK cells can directly kill allogeneic CD4+ T cells, the notion that autologous infected CD4+ T cells can be targeted is complicated by the presence of cognate HLA‐I ligands on CD4+ T cells for the inhibitory KIRs expressed by educated NK cells. Indeed, Ward et al. demonstrated that the presence of HLA‐C and HLA‐E on HIV‐1‐infected CD4+ T cells inhibits NK cell‐mediated ADCC via triggering inhibitory signals through KIR2DL1/2/3 and NKG2A 32. Despite this observation, several additional studies assessing ADCC or antibody‐dependent NK cell activation, triggered by polyclonal anti‐HIV‐1 antibodies or therapeutic anti‐tumour monoclonal antibodies, have demonstrated that antibody‐dependent NK cell stimulation can at least partially overcome inhibitory signals through HLA‐I/KIR combinations 11, 17, 33, 34. Indeed, we have demonstrated recently that educated KIR3DL1+ NK cells from HLA‐Bw4‐carrying donors exhibit higher anti‐HIV‐1 antibody‐dependent activation than KIR3DL1– NK cells upon stimulation with allogeneic CD4+ T cells expressing HLA‐Bw4 17. Additionally, we now show that KIR2DL1+ NK cells can at least partially overcome inhibitory signals conferred through KIR2DL1/HLA‐C2 combinations to become activated in an anti‐HIV‐1 antibody‐dependent manner against autologous targets (Fig. 3). Further research is required to resolve why some previous research has indicated robust inhibition of anti‐HIV‐1 antibody‐dependent NK cell functions occurs through HLA‐C and HLA‐E interactions with KIR2DL1/2/3 and NKG2A, while we have reported anti‐HIV‐1 antibody‐dependent stimulation at least partially overcomes inhibition through HLA‐Bw4/KIR3DL1 and HLA‐C2/KIR2DL1 combinations. It is perhaps most likely that methodological differences account for the discrepancy. To assess anti‐HIV‐1 antibody‐dependent responses, Ward et al. utilized a pool of four monoclonal antibodies, while our studies implemented polyclonal anti‐HIV‐1 serum 11, 17, 32. Smalls‐Mantey et al. demonstrated that pooled monoclonal anti‐HIV‐1 antibodies mediate poor anti‐HIV‐1 ADCC compared to the polyclonal mixtures found in sera 35. We hypothesize that the strength of the signal through CD16 determines the susceptibility of antibody‐dependent NK cell responses to inhibition via inhibitory NK cell receptors. The differential ability of monoclonal and polyclonal antibodies to activate NK cells in an antibody‐dependent manner is likely to determine if the preferential activation of educated NK cells reported in the current paper extends to antibody‐dependent responses triggered against different antigenic targets. Future studies should assess the relative activation of educated and uneducated NK cells upon antibody‐dependent stimulation against additional infectious disease targets, such as influenza, or by monoclonal antibodies utilized for therapy of malignancies.
In addition to potentially modulating susceptibility to HIV‐1 acquisition, the enhanced function of educated KIR2DL1+ NK cells has been suggested to contribute to control of HIV‐1 replication during primary HIV‐1 infection 22. Indeed, educated KIR2DL1+ NK cells are expanded during primary HIV‐1 infection, and these cells exhibit potent activation, compared to KIR2DL1– NK cells, upon direct stimulation with HLA‐I‐devoid 721.221 target cells. Future research should assess the ability of educated KIR2DL1+ NK cells expanded during primary HIV‐1 infection to mediate anti‐HIV‐1 antibody‐dependent activation. As anti‐HIV‐1 antibodies capable of activating NK cells are detected during primary HIV‐1 infection, the anti‐HIV‐1 antibody‐dependent activation potential of educated KIR2DL1+ NK cells might play a role in controlling early viral replication and establishing a lower viral set point 36.
The data presented in this paper add to a growing body of literature on the importance of NK cell education and the interplay of activating and inhibitory receptors in determining the potential of NK cells to mediate anti‐viral functions. Although highly important for assisting with understanding the regulation of NK cell activation potential, the data presented in the current paper might also be useful in designing future research to enhance our understanding of HIV‐1 susceptibility and pathogenesis.
Disclosure
The authors declare no disclosures.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Anti‐HIV‐1‐specific antibody‐dependent activation of natural killer (NK) cells against gp140‐coated CEM.NKr‐CCR5 target cells. NK cell effectors within peripheral blood mononuclear cells (PBMC) were stimulated with gp140‐coated CEM.NKr‐CCR5 target cells in the presence 1 : 2000 dilutions of HIV‐1+ or HIV‐1– plasma. Antibody‐dependent NK cell activation was assessed by flow cytometry. The fluorescence activated cell sorter (FACS) plot at the top depicts gating to identify CD3–CD56+ NK cells. The FACS plots on the bottom depict the relative amounts of NK cell activation, measured as interferon (IFN)‐γ production, observed when NK cells and gp140‐coated CEM.NKr‐CCR5 cells were co‐cultured in the presence of HIV‐1– (left plot) or HIV‐1+ (right plot) plasma. The data shown from this single PBMC donor is representative of three independent PBMC donors tested.
References
- 1. Pollara J, Hart L, Brewer F et al High‐throughput quantitative analysis of HIV‐1 and SIV‐specific ADCC‐mediating antibody responses. Cytometry A 2011; 79:603–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smalls‐Mantey A, Connors M, Sattentau QJ. Comparative efficiency of HIV‐1‐infected T cell killing by NK cells, monocytes and neutrophils. PLOS ONE 2013; 8:e74858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Veillette M, Desormeaux A, Medjahed H et al Interaction with cellular CD4 exposes HIV‐1 envelope epitopes targeted by antibody‐dependent cell‐mediated cytotoxicity. J Virol 2014; 88:2633–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chung AW, Rollman E, Center RJ, Kent SJ, Stratov I. Rapid degranulation of NK cells following activation by HIV‐specific antibodies. J Immunol 2009; 182:1202–10. [DOI] [PubMed] [Google Scholar]
- 5. Fauriat C, Long EO, Ljunggren HG, Bryceson YT. Regulation of human NK‐cell cytokine and chemokine production by target cell recognition. Blood 2010; 115:2167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Song R, Lisovsky I, Lebouche B, Routy JP, Bruneau J, Bernard NF. HIV protective KIR3DL1/S1‐HLA‐B genotypes influence NK cell‐mediated inhibition of HIV replication in autologous CD4 targets. PLOS Pathog 2014; 10:e1003867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haynes BF, Gilbert PB, McElrath MJ et al Immune‐correlates analysis of an HIV‐1 vaccine efficacy trial. N Engl J Med 2012; 366:1275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rerks‐Ngarm S, Pitisuttithum P, Nitayaphan S et al Vaccination with ALVAC and AIDSVAX to prevent HIV‐1 infection in Thailand. N Engl J Med 2009; 361:2209–20. [DOI] [PubMed] [Google Scholar]
- 9. Tomaras GD, Ferrari G, Shen X et al Vaccine‐induced plasma IgA specific for the C1 region of the HIV‐1 envelope blocks binding and effector function of IgG. Proc Natl Acad Sci USA 2013; 110:9019–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parsons MS, Loh L, Gooneratne S, Center RJ, Kent SJ. Role of education and differentiation in determining the potential of natural killer cells to respond to antibody‐dependent stimulation. AIDS 2014; 28:2781–6. [DOI] [PubMed] [Google Scholar]
- 11. Parsons MS, Wren L, Isitman G et al. HIV infection abrogates the functional advantage of natural killer cells educated through KIR3DL1/HLA‐Bw4 interactions to mediate anti‐HIV antibody‐dependent cellular cytotoxicity. J Virol 2012; 86:4488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anfossi N, Andre P, Guia S et al Human NK cell education by inhibitory receptors for MHC class I. Immunity 2006; 25:331–42. [DOI] [PubMed] [Google Scholar]
- 13. Fauriat C, Ivarsson MA, Ljunggren HG, Malmberg KJ, Michaelsson J. Education of human natural killer cells by activating killer cell immunoglobulin‐like receptors. Blood 2010; 115:1166–74. [DOI] [PubMed] [Google Scholar]
- 14. Kim S, Sunwoo JB, Yang L et al HLA alleles determine differences in human natural killer cell responsiveness and potency. Proc Natl Acad Sci USA 2008; 105:3053–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Solana R, Tarazona R, Gayoso I, Lesur O, Dupuis G, Fulop T. Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans. Semin Immunol 2012; 24:331–41. [DOI] [PubMed] [Google Scholar]
- 16. Lopez‐Verges S, Milush JM, Pandey S et al CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK‐cell subset. Blood 2010; 116:3865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gooneratne SL, Richard J, Lee WS, Finzi A, Kent SJ, Parsons MS. Slaying the Trojan horse: natural killer cells exhibit robust anti‐HIV‐1 antibody‐dependent activation and cytolysis against allogeneic T cells. J Virol 2015; 89:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boulet S, Kleyman M, Kim JY et al A combined genotype of KIR3DL1 high expressing alleles and HLA‐B*57 is associated with a reduced risk of HIV infection. AIDS 2008; 22:1487–91. [DOI] [PubMed] [Google Scholar]
- 19. Martin MP, Qi Y, Gao X et al Innate partnership of HLA‐B and KIR3DL1 subtypes against HIV‐1. Nat Genet 2007; 39:733–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Williams AP, Bateman AR, Khakoo SI. Hanging in the balance. KIR and their role in disease. Mol Interv 2005; 5:226–40. [DOI] [PubMed] [Google Scholar]
- 21. Jennes W, Verheyden S, Mertens JW et al Inhibitory KIR/HLA incompatibility between sexual partners confers protection against HIV‐1 transmission. Blood 2013; 121:1157–64. [DOI] [PubMed] [Google Scholar]
- 22. Korner C, Granoff ME, Amero MA et al Increased frequency and function of KIR2DL1‐3(+) NK cells in primary HIV‐1 infection are determined by HLA‐C group haplotypes. Eur J Immunol 2014; 44:2938–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eller MA, Eller LA, Ouma BJ et al Elevated natural killer cell activity despite altered functional and phenotypic profile in Ugandans with HIV‐1 clade A or clade D infection. J Acquir Immune Defic Syndr 2009; 51:380–9. [DOI] [PubMed] [Google Scholar]
- 24. Wong AH, Williams K, Reddy S et al Alterations in natural killer cell receptor profiles during HIV type 1 disease progression among chronically infected South African adults. AIDS Res Hum Retroviruses 2010; 26:459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kamya P, Tallon B, Melendez‐Pena C et al Inhibitory killer immunoglobulin‐like receptors to self HLA‐B and HLA‐C ligands contribute differentially to natural killer cell functional potential in HIV infected slow progressors. Clin Immunol 2012; 143:246–55. [DOI] [PubMed] [Google Scholar]
- 26. Parsons MS, Tang CC, Jegaskanda S et al Anti‐HIV antibody‐dependent activation of NK cells impairs NKp46 expression. J Immunol 2014; 192:308–15. [DOI] [PubMed] [Google Scholar]
- 27. Center RJ, Wheatley AK, Campbell SM et al Induction of HIV‐1 subtype B and AE‐specific neutralizing antibodies in mice and macaques with DNA prime and recombinant gp140 protein boost regimens. Vaccine 2009; 27:6605–12. [DOI] [PubMed] [Google Scholar]
- 28. Charoudeh HN, Schmied L, Gonzalez A et al Quantity of HLA‐C surface expression and licensing of KIR2DL+ natural killer cells. Immunogenetics 2012; 64:739–45. [DOI] [PubMed] [Google Scholar]
- 29. Mabuka J, Nduati R, Odem‐Davis K, Peterson D, Overbaugh J. HIV‐specific antibodies capable of ADCC are common in breastmilk and are associated with reduced risk of transmission in women with high viral loads. PLOS Pathog 2012; 8:e1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Batraville LA, Richard J, Veillette M et al Short communication: anti‐HIV‐1 envelope immunoglobulin Gs in blood and cervicovaginal samples of Beninese commercial sex workers. AIDS Res Hum Retroviruses 2014; 30:1145–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parsons MS, Madhavi V, Ana‐Sosa‐Batiz F et al Seminal plasma anti‐HIV antibodies trigger antibody‐dependent cellular cytotoxicity: implications for HIV transmission. JAIDS 2016; 71:17–23. [DOI] [PubMed] [Google Scholar]
- 32. Ward JP, Bonaparte MI, Barker E. HLA‐C and HLA‐E reduce antibody‐dependent natural killer cell‐mediated cytotoxicity of HIV‐infected primary T cell blasts. AIDS 2004; 18:1769–79. [DOI] [PubMed] [Google Scholar]
- 33. Lang P, Pfeiffer M, Handgretinger R et al Clinical scale isolation of T cell‐depleted CD56+ donor lymphocytes in children. Bone Marrow Transplant 2002; 29:497–502. [DOI] [PubMed] [Google Scholar]
- 34. Terszowski G, Klein C, Stern M. KIR/HLA interactions negatively affect rituximab‐ but not GA101 (obinutuzumab)‐induced antibody‐dependent cellular cytotoxicity. J Immunol 2014; 192:5618–24. [DOI] [PubMed] [Google Scholar]
- 35. Smalls‐Mantey A, Doria‐Rose N, Klein R et al Antibody‐dependent cellular cytotoxicity against primary HIV‐infected CD4+ T cells is directly associated with the magnitude of surface IgG binding. J Virol 2012; 86:8672–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Forthal DN, Landucci G, Daar ES. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural‐killer effector cells. J Virol 2001; 75:6953–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Anti‐HIV‐1‐specific antibody‐dependent activation of natural killer (NK) cells against gp140‐coated CEM.NKr‐CCR5 target cells. NK cell effectors within peripheral blood mononuclear cells (PBMC) were stimulated with gp140‐coated CEM.NKr‐CCR5 target cells in the presence 1 : 2000 dilutions of HIV‐1+ or HIV‐1– plasma. Antibody‐dependent NK cell activation was assessed by flow cytometry. The fluorescence activated cell sorter (FACS) plot at the top depicts gating to identify CD3–CD56+ NK cells. The FACS plots on the bottom depict the relative amounts of NK cell activation, measured as interferon (IFN)‐γ production, observed when NK cells and gp140‐coated CEM.NKr‐CCR5 cells were co‐cultured in the presence of HIV‐1– (left plot) or HIV‐1+ (right plot) plasma. The data shown from this single PBMC donor is representative of three independent PBMC donors tested.


