Summary
Both dengue NS1 antigen and serum interleukin (IL)‐10 levels have been shown to associate with severe clinical disease in acute dengue infection, and IL‐10 has also been shown to suppress dengue‐specific T cell responses. Therefore, we proceeded to investigate the mechanisms by which dengue NS1 contributes to disease pathogenesis and if it is associated with altered IL‐10 production. Serum IL‐10 and dengue NS1 antigen levels were assessed serially in 36 adult Sri Lankan individuals with acute dengue infection. We found that the serum IL‐10 levels correlated positively with dengue NS1 antigen levels (Spearman's r = 0·47, P < 0·0001), and NS1 also correlated with annexin V expression by T cells in acute dengue (Spearman's r = 0·63, P = 0·001). However, NS1 levels did not associate with the functionality of T cell responses or with expression of co‐stimulatory molecules. Therefore, we further assessed the effect of dengue NS1 on monocytes and T cells by co‐culturing primary monocytes and peripheral blood mononuclear cells (PBMC), with varying concentrations of NS1 for up to 96 h. Monocytes co‐cultured with NS1 produced high levels of IL‐10, with the highest levels seen at 24 h, and then declined gradually. Therefore, our data show that dengue NS1 appears to contribute to pathogenesis of dengue infection by inducing IL‐10 production by monocytes.
Keywords: dengue infection, IL‐10, monocytes, NS1 antigen, T cell function
Introduction
Dengue viral infections are one of the most rapidly emerging mosquito‐borne viral infections in the world, resulting in a huge economic burden in affected countries 1. It is estimated that 390 million individuals are infected by the virus each year, resulting in 96 million apparent infections 2. Approximately 70% of these infections occur in Asian countries, which have inadequate resources to handle such vast patient numbers 2, 3. Currently there is no licensed vaccine to prevent dengue, nor an effective specific drug for its treatment.
In the majority of infected individuals, infection with the dengue virus (DENV) results in asymptomatic infection or causes an undifferentiated febrile illness. However, some individuals develop dengue fever or severe clinical disease, which manifests as dengue haemorrhagic fever (DHF) or dengue shock syndrome (DSS) 4. The hallmark of severe clinical disease is vascular leak, which results in reduced blood pressure, pleural effusions, ascites and, if severe, impaired organ perfusion and shock 5. Although the exact mechanisms that lead to severe clinical disease are not clear, it appears to occur due to the complex interaction between the DENV and the host immune response 5, 6. Our previous studies showed that serum IL‐10 levels were elevated significantly in patients with severe clinical disease and interleukin (IL)‐10 suppressed DENV‐specific T cell responses, possibly contributing to disease pathogenesis 7, 8. It has also been shown that dengue NS1 protein was associated with severe clinical disease and that a level of > 600 ng/ml in the first 72 h of illness was associated with the development of DHF 9. We also found that dengue NS1 antigen persisted in patients with DHF and that it could be possibly used as a marker of severe dengue 10.
Dengue NS1 is a 50 kDa non‐structural protein, which is a lipoprotein that also exists in the secreted form 11, 12. Because NS1 carries lipids in its secretory form it is thought to have many implications in disease pathogenesis, as lipoproteins are important in coagulation pathways and are associated with vascular inflammation 11. Indeed, it was shown recently that NS1 of all four DENV serotypes induced vascular leak in dengue mouse models by inducing endothelial barrier dysfunction 13. It was also shown that dengue NS1 stimulated cytokine production from innate immune cells by acting through Toll‐like receptor (TLR)‐4 14. The immune complexes formed by secretory NS1 have been shown to activate complement, which is also thought to contribute to disease pathogenesis 15. Therefore, although the main effects of NS1 in the pathogenesis of dengue infections are believed to occur due to immune complexes and anti‐NS1 antibodies 16, 17, 18, 19, more recent studies have shown that NS1 is pathogenic by itself 13, 14. However, a recent study of 13 autopsy cases showed that neither dengue NS1 nor dengue NS1 antibodies were detected in the endothelium of patients who died due to dengue infection, questioning the hypothesis of the role of NS1 antibodies in the pathogenesis of acute dengue 20.
As dengue NS1 circulates in patients with acute dengue in the secretory form and binds to both infected and non‐infected cells, and also from the data originating from the dengue mice models 13, it is likely that NS1 could have direct effects on the immune system apart from binding to anti‐NS1 antibodies. As studies have shown that higher NS1 levels are found in patients with more severe forms of clinical disease 9, in this study we proceeded to investigate the potential effects of NS1 on monocytes and also on the functionality of the T cell responses and T cell apoptosis. We found that in acute infection, NS1 levels correlated with serum IL‐10 levels and that dengue NS1 induces production of IL‐10 from primary monocytes in a dose‐dependent manner. In addition, we found that dengue NS1 also correlated with annexin V expression of T cells in acute dengue infection and that expression of annexin V was increased in T cells of some individuals, when their peripheral blood mononuclear cells (PBMCs) were co‐cultured with NS1. Therefore, dengue NS1 appears to contribute to the pathogenesis of dengue infection by inducing production of immunosuppressive cytokines.
Materials and methods
Patients
As our initial aim was to determine if dengue NS1 was associated with severe clinical disease, we first determined the kinetics of dengue NS1 levels and serum IL‐10 levels in 36 adult individuals with acute dengue infection from the Colombo South Teaching Hospital, Sri Lanka following informed written consent.
In order to determine changes in the kinetics of dengue NS1 antigen levels and serum IL‐10 levels, serial blood samples were taken in the morning (6 a.m.) and again at 1.00 p.m., from the time of admission to the time of discharge from hospital. The duration of illness at the time of admission was based on the number of days the patient was febrile before admission to hospital. For instance, if the patient had fever for 4 days prior to admission, the patient's duration of illness was considered to be 96 h. All clinical features, such as presence of fever, abdominal pain, vomiting, bleeding manifestations, hepatomegaly, blood pressure, pulse pressure and evidence of fluid leakage, were recorded several times each day. The clinical disease severity was classified according to the 2011 World Health Organization (WHO) dengue diagnostic criteria 4. Accordingly, in patients with a rise in haematocrit greater than ≥20% of the baseline haematocrit or clinical or ultrasound scan evidence of plasma leakage were classified as having DHF 4. Shock was defined as having cold clammy skin, along with a narrowing of pulse pressure of ≤20 mmHg. According to the WHO 2011 disease classification, 25 patients were classified as having DHF, as they had clinical or laboratory evidence of fluid leakage, and 11 patients were classified as DF.
After determining the kinetics of dengue NS1 antigen levels and serum IL‐10 levels, in order to investigate the functionality of T cell responses we recruited an additional 24 adult individuals with acute dengue infection. The first blood samples were collected during days 3–5 of illness (day 1 was considered as the first day of fever) in all patients and the second samples were collected 2 days later. In order to determine the severity of dengue infection, serial recordings of clinical features and laboratory investigations (platelet counts, haematocrits, white cell counts) were also made in this cohort. Clinical disease severity was classified according to the WHO 2011 dengue guidelines, as in the earlier cohort of 36 patients from whom we collected serial blood samples 4. Of these 24 patients who were recruited for T cell studies, 18 patients were classified as having DHF based on the 2011 WHO criteria, as they had clinical or laboratory evidence of fluid leakage, and six patients were classified as having DF. Blood was also collected from 13 healthy dengue‐seropositive volunteers.
Ethics statement
Ethics approval was obtained by the Ethics Review Committee of the Faculty of Medical Sciences, University of Sri Jayawardenapura. All patients were recruited following informed written consent.
Confirmation of dengue infection and determining dengue NS1 antigen levels
Acute dengue infection was confirmed in the serum samples using the NS1 early dengue enzyme‐linked immunosorbent assay (ELISA) (PanBio, Brisbane, QLD, Australia) or with the commercial capture‐immunoglobulin (Ig)M and IgG ELISA (PanBio). The ELISA was performed and the results were interpreted according to the manufacturer's instructions. This ELISA assay has been validated as both sensitive and specific for primary and secondary dengue virus infections 21, 22. In order to determine the kinetics of dengue NS1 antigen levels, the NS1 antigen levels were determined in serial blood samples and the levels expressed as PanBio units. Those with clinical features of a suggestive dengue infection who were either positive for dengue NS1 or were positive for dengue IgM antibody were considered to be having an acute dengue infection.
Quantification of cytokines
IL‐10 quantitative cytokine assays (Mabtech, Nacka Strand, Sweden) were performed in duplicate on serum samples of patients with acute infection, healthy volunteers and also in serum samples obtained during the convalescent phase, according to the manufacturer's instructions. It was also performed in cultured monocyte supernatants stimulated with different concentrations of DENV1‐NS1 recombinant protein expressed in both Escherichia coli and mammalian cells. All reactions were carried out in duplicate.
Determining the effect of dengue NS1 antigen on monocytes
Monocytes were isolated from fresh PBMCs in four dengue‐seronegative donors using magnetic‐activated cell sorting (MACS) columns with anti‐human CD14 microbeads (Miltenyi Biotec, Auburn, CA, USA), followed by magnetic separation. The purity of positively selected monocytes was again assessed by flow cytometry using CD14 fluorescein isothiocyanate (FITC) (Biolegend, San Diego, CA, USA) and CD3 allophycocyanin (APC) (Biolegend). The purity was found to be >98%. Monocytes, 2 × 106/ml, were co‐cultured with varying concentrations of E. coli‐derived dengue NS1 recombinant protein, which was certified as free from lipopolysaccharide (LPS) (Abcam, Cambridge, UK) for up to 96 h. The monocytes were cultured in RPMI‐1640 with 5% autologous serum and then dispensed in a volume of 200 μl per well into individual wells of 96‐well round‐bottomed plates (Corning, Acton, MA, USA). E. coli‐derived recombinant DENV1–NS1 protein (Abcam) was used at concentrations of 250, 500 and 1000 ng/ml. A well containing only media was added as the control for each time‐point. Cells were centrifuged and the supernatants harvested at 24, 48, 72 and 96 h to determine IL‐10 levels.
Although we used an E. coli‐derived NS1 of recombinant protein which was certified as free from LPS from DENV1, we repeated the above experiments using a NS1 recombinant protein from DENV3 expressed from a mammalian cell line (Abcam) in order to compare the results obtained with DENV1‐NS1 protein on monocytes. As possible bacterial contaminants in NS1 recombinant protein derived from E. coli could stimulate IL‐10 production from monocytes, we used a mock protein (PRF full‐length protein) generated by the same method, which is of equal molecular weight as the E. coli and mammalian‐derived dengue NS1 protein from the same manufacturer (Abcam).
Determining the effects of dengue immune sera on NS1 antigen
Monocytes were isolated from fresh PBMCs in four dengue seronegative donors using MACS columns with anti‐human CD14 microbeads (Miltenyi Biotec) followed by magnetic separation. As both E. coli‐derived NS1 protein from DENV1 and mammalian‐derived NS1 from DEN3 showed comparable results, E. coli‐derived DENV1‐NS1 was used in these experiments. E. coli‐derived DENV1‐NS1 (Abcam) was used at a final concentration of 250 ng/ml per well.
The DENV immune serum pool for all four DENV serotypes was prepared for experiments for determining whether or not antibodies cause enhancement (antibody‐dependent enhancement, ADE) of the effects of NS1 on monocytes. The ADE experiments were performed as described previously 23. In order to determine the effects of DENV‐specific antibodies, sera from healthy volunteers with known past infecting serotypes were collected and pooled together 23, 24, 25. Pooled sera from these healthy individuals were heat‐inactivated before being used in ADE experiments. The sera were incubated in dilutions of 1 : 5, 1 : 10 and 1 : 100 in RPMI media (Gibco, Life Technologies, Grand Island, NY, USA) containing DENV1‐NS1 at 37°C for 1 h prior to incubation with the purified monocytes. A well containing only media, only serum and only NS1 at the same concentration (250 ng/ml) was used as the controls for each time‐point. Cells were centrifuged and the supernatants harvested at 24, 48, 72 and 96 h to determine levels of IL‐10 in the supernatant.
Determining annexin V expression levels in T cells in acute dengue infection
In order to investigate if annexin V expression in T cells in patients with acute dengue was associated with dengue NS1 antigen, expression of annexin V was determined in 22 adult patients with confirmed acute dengue infection. PBMCs were stained with annexin V FITC, IL‐10R phycoerythrin (PE), 7‐aminoactinomycin D (7‐AAD) and CD3 APC and acquired on a Partec Cyflow Cube 6 and analysed with De Novo FCS Express software version 4.
Determining effect of dengue NS1 antigen on annexin V expression on T cells
These experiments were carried out in 10 healthy dengue‐seropositive individuals. PBMCs were incubated for 72 h with E. coli‐derived DENV1‐NS1 glycoprotein protein (Abcam) at 37°C in 5% CO2. Dengue NS1 antigen was used at concentrations ranging from 250 to 500 ng/ml. Annexin V expression was determined at 24, 48 and 72 h. Every 24 h cells were washed and stained for surface staining using annexin V FITC (Biolegend), CD8 PE (Biolegend), CD4 peridinin chlorophyll (PerCP) (Biolegend) and CD3 APC (Biolegend). Cells were acquired on a Partec Cyflow Cube 6 and analysed with De Novo FCS Express software version 4.
Peptides
The DENV‐NS3 peptides were 20 mer peptides overlapping by 10 amino acids, which spanned the whole length of the DENV3‐NS3 protein. These were 20 mer peptides which were synthesized in‐house in an automated synthesizer using F‐MOC chemistry. The purity of the peptides was determined to be greater than 90% by high‐pressure liquid chromatography analysis and mass spectrometry. The synthetic NS3 20 mer peptides were pooled together to represent the whole NS3 protein. The FEC peptides (synthesized in‐house) that were used contained a panel of 23, 8–11 amino acid CD8+ T cell epitopes of Epstein–Barr virus (EBV), influenza and cytomegalovirus (CMV) viruses and have been used as quality control in enzyme‐linked immunospot (ELISPOT) assays.
Surface staining and intracellular cytokine assays
In order to determine interferon (IFN)‐γ production by T cells, PBMCs were isolated from whole blood and these ex‐vivo PBMCs were stimulated at 1 × 106 to 2 × 106/ml in RPMI‐1640 plus 10% fetal calf serum (FCS) with DENV‐NS3 overlapping peptides and phorbol myristate acetate (PMA) and ionomycin for 16 h, according to the manufacturer's instructions in the presence of brefeldin A (Biolegend). To determine CD107a expression, staining for CD107a was carried out prior to adding peptides. Prior to permeabilization of cells, a surface stain was carried out to determine the expression of cytotoxic T lymphocyte antigen‐4 (CTLA‐4) APC (Biolegend), T cell immunoglobulin and mucin domain containing protein‐3 (TIM‐3) APC (Biolegend), programmed death (PD)‐1 FITC (Biolegend) and CD28 (FITC). For intracellular staining, cells were permeabilized and fixed with Cytofix/Cytoperm (Biolegend) and then stained with IFN‐γ APC. Propidium iodide (PI) was used in the CD107a detection assays to gate out dead cells. Isotype‐matched controls were included in each experiment. Cells were acquired on a Partec Cyflow Cube 6 and analysed with De Novo FCS Express software version 4.
Statistical analysis
Statistical analysis was performed using GraphpPad Prism version 6. As the data were not distributed normally, differences in means were compared using the Mann–Whitney U‐test (two‐tailed). The degree of association between serum NS1 antigen levels and serum IL‐10 levels was analysed using Spearman's correlation. Power calculations to determine if NS1 antigen levels were higher in patients with DHF when compared to DF were calculated by the mean and standard deviation (s.d.) of the population being assessed. The means and s.d. of NS1 antigen levels (PanBio units) for this study were derived from the values obtained from a previous large study of 186 patients 10.
Results
Dengue NS1 antigen has been shown previously to associate with DHF, and indeed we found that it could also be considered as a marker of severe clinical disease 10. In addition, our previous studies showed that serum IL‐10 levels were also associated with severe dengue 7, 8. Therefore, we proceeded to investigate the kinetics of both dengue NS1 antigen and serum IL‐10 levels in 36 patients with acute dengue infection throughout the course of their illness. We found that although dengue NS1 antigen levels were not different between those with DHF (n = 25) and those with DF (n = 11) during early infection, dengue NS1 levels remained high in those with DHF (Fig. 1). By 144 h of illness, 11 of 25 (44%) of the patients with DHF and three of 11 (27·2%) patients with DF were positive for dengue NS1. Of these 36 patients, six of 25 patients with DHF had a primary dengue infection and three of 11 patients with DF had a primary dengue infection. All three patients with DF who were still positive for dengue NS1 at 144 h had a primary dengue infection, while six of 11 of the patients with DHF who were positive for dengue NS1 at 144 h had a primary dengue infection. Although the NS1 antigen levels were very much lower in patients with DF at 168 h, as the majority of patients with DF were discharged from hospital due to the milder nature of their illness at this time‐point, we did not have sufficient numbers to calculate if the differences between NS1 antigen levels in DHF and DF were significant.
Figure 1.
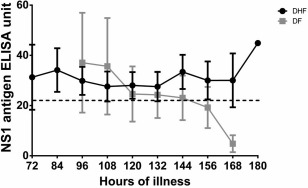
Kinetics of dengue NS1 levels in acute dengue: levels of serum dengue NS1 antigen were measured twice a day by enzyme‐linked immunosorbent assay (ELISA) in 25 patients with dengue haemorrhagic fever (DHF) and 11 patients with dengue fever (DF) from the time of admission until discharge. The means are shown along with error bars indicate the standard error of the mean; (–) indicates the titre above in which the NS1 ELISA gives a positive result.
Relationship between dengue NS1 antigen and serum IL‐10 levels
We then proceeded to determine the relationship between dengue NS1 antigen levels and serum IL‐10 levels in this cohort of patients throughout the course of the illness. In measuring dengue NS1 antigen levels, we used the NS1 early dengue ELISA (PanBio) and the NS1 antigen levels were expressed as PanBio units. Serum IL‐10 levels correlated significantly (P < 0·0001) with dengue NS1 antigen levels (Spearman's r = 0.47) (Fig. 2a). Furthermore, the patterns of variation in serum IL‐10 levels and dengue NS1 antigen levels were very similar in patients with DHF and DF. For instance, the IL‐10 levels decreased at 132–144 h, closely following the patterns of dengue NS1 in patients with DF (Fig. 2b). However, in patients with DHF, similar to the observations with dengue NS1, serum IL‐10 levels persisted for longer periods (Fig. 2c).
Figure 2.
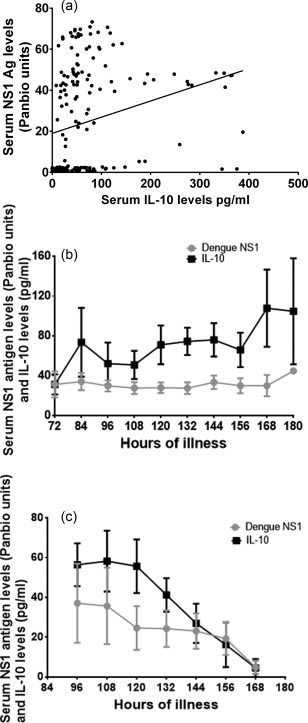
Relationship between dengue NS1 and serum interleukin (IL)‐10 levels. (a) Dengue NS1 antigen levels (PanBio units) and serum IL‐10 levels were measured in serial blood samples in 36 patients with acute dengue infection. (b) Dengue NS1 antigen levels and serum IL‐10 levels were measured by enzyme‐linked immunosorbent assay (ELISA) in 25 patients with dengue haemorrhagic fever (DHF) from the time of admission to discharge. The means are shown along with error bars indicating the standard error of the mean. The dengue NS1 antigens levels are expressed in PanBio ELISA units and IL‐10 levels are expressed as pg/ml. (c) Dengue NS1 antigen levels and serum IL‐10 levels were measured by ELISA in 11 patients with dengue fever (DF) from the time of admission to discharge. The means are shown along with error bars indicating the standard error of the mean. The dengue NS1 antigen levels are expressed in PanBio ELISA units and IL‐10 levels are expressed as pg/ml.
Dengue NS1 antigen stimulated IL‐10 production by monocytes
Our previous studies showed that monocytes were an important source of IL‐10 in acute dengue infection 8. As we found that serum IL‐10 concentrations correlated well with dengue NS1 levels, we investigated if NS1 stimulated IL‐10 production by monocytes. Freshly isolated monocytes from four dengue seronegative individuals were co‐cultured with varying concentrations of dengue NS1, which were comparable to concentrations reported in vivo in patients in previous studies 9. We found that monocytes co‐cultured with dengue NS1 antigen (E. coli‐derived, certified as LPS free) produced high levels of IL‐10 at 24 h, which decreased gradually by 96 h (Fig. 3a). No IL‐10 production was seen in monocytes cultured in media alone. Although production of IL‐10 was higher when monocytes were cultured with 1000 ng/ml of dengue NS1 when compared to lower concentrations, this was not significant (Fig. 3a). However, although the E. coli‐derived NS1 recombinant protein was certified as LPS free, as other bacterial contaminants could induce IL‐10 production from monocytes, we used a mock protein (PRF full‐length protein), which was purchased from the same manufacturer, and was produced in the same manner and was of similar molecular weight. We found that primary monocytes of four individuals did not produce any IL‐10 in the presence of PRF 250 ng/ml, while monocytes that we co‐cultured with dengue NS1 at a similar concentration (250 ng/ml) produced IL‐10 (data not shown).
Figure 3.
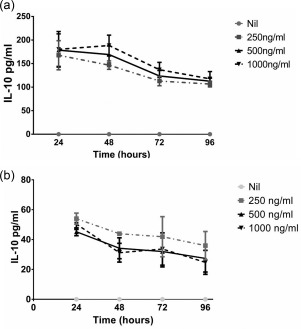
Production of interleukin (IL)‐10 by monocytes co‐cultured with varying concentrations of dengue NS1. (a) Monocytes isolated from four dengue seronegative individuals were incubated with media or Escherichia coli expressed [certified as lipopolysaccharide (LPS)‐free] dengue NS1 protein of dengue virus (DENV) serotype 1 at concentrations of 250 ng/ml, 500 ng/ml or 1000 ng/ml for 96 h. All experiments were performed in duplicate. The IL‐10 levels in the supernatants were measured every 24 h by enzyme‐linked immunosorbent assay (ELISA). (b) Monocytes isolated from four dengue seronegative individuals were incubated with media or mammalian expressed dengue NS1 protein of DENV serotype 3 at concentrations of 250 ng/ml, 500 ng/ml or 1000 ng/ml for 96 h. All experiments were performed in duplicate. The IL‐10 levels in the supernatants were measured every 24 h by ELISA.
Although the E. coli‐expressed recombinant NS1 protein from DENV1 was certified as LPS free, in order to further exclude possible effects of E. coli contamination we repeated these experiments in four individuals using NS1 recombinant protein from DENV3, which was expressed in a mammalian cell line. We found that mammalian expressed DENV3 NS1 protein also induced IL‐10 from monocytes (Fig. 3b).
DENV‐specific antibodies and their effect on NS1 on monocytes
DENV‐specific antibodies have been shown to enhance disease severity which is thought to be mediated by ADE 23, 26, 27. NS1‐specific antibodies are believed to contribute to disease pathogenesis by activating complement and also leading to endothelial dysfunction 15, 17. Therefore, in order to investigate if DENV‐NS1‐specific antibodies could potentiate the effects of NS1, we used DENV immune sera in ADE experiments as described previously 23. We found that there was a trend towards DENV immune sera potentiating the effects of NS1 on monocytes and the production of IL‐10 was higher, especially at 48 h (Fig. 4). Primary monocytes isolated from four healthy individuals incubated with DENV seronegative serum alone did not produce any IL‐10 up to 96 h (data not shown).
Figure 4.
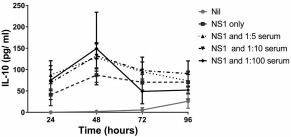
Effect of dengue immune sera on effects of NS1 on monocytes. Monocytes isolated from five individuals were incubated with media or Escherichia coli expressed [certified as lipopolysaccharide (LPS)‐free] dengue NS1 protein of dengue virus (DENV) serotype 1 at concentrations of 250 ng/ml and dengue immune sera of 1 : 5, 1 : 10 and 1 : 100 dilutions for 96 h. All experiments were performed in duplicate. The interleukin (IL)‐10 levels in the supernatants were measured every 24 h by enzyme‐linked immunosorbent assay (ELISA).
Dengue NS1 and annexin V expression by T cells
Massive apoptosis of T cells is known to occur in acute dengue infection 28, 29, 30. Our previous studies have shown that serum IL‐10 levels were associated with annexin V expression on T cells 29. However, subsequent studies showed that IL‐10 did not cause apoptosis of T cells in vitro 8. Because, in this study, we found that serum IL‐10 correlates with dengue NS1, we investigated if dengue NS1 caused apoptosis of T cells. Initially, we determined annexin V expression and dengue NS1 levels in 22 patients with acute dengue infection. We found that dengue NS1 antigen levels (PanBio units) correlated positively with annexin V expression on T cells (Spearman's r = 0·63, P = 0·001) (Fig. 5a). As annexin V expression on T cells in patients with acute dengue correlated with dengue NS1 antigen levels, we then investigated if NS1 caused apoptosis of T cells. PBMCs of 10 healthy individuals were co‐cultured with varying concentrations of dengue NS1 for 24–72 h and annexin V expression was assessed. Although not significant, a trend towards an increase in annexin V expression at higher doses of NS1 was seen in both CD4+ and CD8+ T cells (Fig. 5b). For instance, although not significant, the mean annexin V expression in CD8+ T cells at NS1 antigen concentrations of 250 ng/ml (mean 18·09, s.d. ± 8.6% expression) was higher than CD8+ T cells cultured with media alone (mean 13·6, s.d. ± 3.8% expression) (Fig. 5b). Annexin V expression was even higher in CD8+ T cells at NS1 antigen at 500 ng/ml concentrations (mean 22·9, s.d. ± 13·1% expression) (Fig. 5b). However, when co‐cultured with NS1 antigen, the increase in the expression of annexin V in CD8+ and CD4+ T cells varied widely between individuals.
Figure 5.
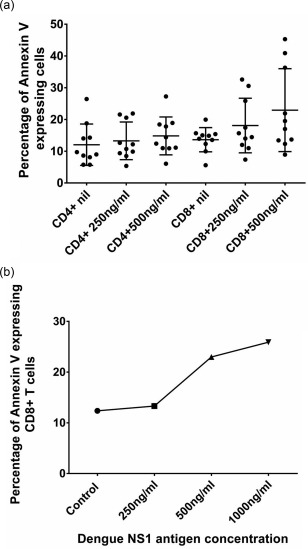
Effect of dengue NS1 on annexin V expression by T cells. (a) Dengue NS1 antigen levels (PanBio units) and annexin V expression was assessed in 22 patients with acute dengue. NS1 antigen levels (PanBio units) correlated positively with the proportion of annexin V‐expressing T cells (Spearman's r = 0·63, P = 0·001). (b) Annexin V expression by CD4+ and CD8+ T cells following co‐culturing of peripheral blood mononuclear cells (PBMCs) with dengue NS1. Annexin V expression on CD4+ and CD8+ T cells of 10 healthy individuals was determined by flow cytometry following co‐culture with media alone or dengue NS1 concentrations of 250 ng/ml and 500 ng/ml for 24 h.
Dengue NS1 and functionality of the T cell responses in acute dengue
Because dengue NS1 appears to associate with apoptosis of T cells, we went on to determine if dengue NS1 had any other effects on the functionality of T cells. In addition, as the functionality of T cell responses and the expression of co‐stimulatory markers have not been studied in detail previously, we also investigated the expression of PD‐1, CTLA‐4, CD28 and TIM‐3 in 24 patients (the second cohort of patients) with acute dengue and 13 healthy individuals. In order to assess the functionality of T cell responses, intracellular cytokine assays were performed for IFN‐γ and CD107a expression following stimulation by dengue NS3 overlapping peptides. We found that dengue NS1 antigen levels (PanBio units) had no correlation with the frequency of NS3‐specific IFN‐γ‐producing T cells or NS3‐specific CD107a‐expressing T cells. Dengue NS1 antigen levels also did not show any correlation with expression of PD‐1, CTLA‐4, TIM‐3 or CD28 levels (data not shown).
Although PD‐1 is known to be associated with T cell dysfunction and is thought to be a negative regulator of T cell function, its expression has been shown to be up‐regulated in acute viral infections, driven by responses to viral antigens 31, 32, 33. We found that, although not significant (P = 0·12), patients with acute dengue had a higher percentage of PD‐1‐expressing T cells [mean 65·2, s.d. ± 17·6 median fluorescence intensity (MFI)] when compared to healthy individuals (mean 57·6, s.d. ± 10·2 MFI) (Fig. 6a). There was no difference in expression of PD‐1 in the general T cell population (mean 65·2, s.d. ± 17·6 median fluorescence intensity) when compared to DENV‐NS3‐specific T cells (mean 65.3, s.d. ± 17.3 MFI). There was no difference in the expression of PD‐1 in the first sample taken at days 3–5 of illness and the second sample taken at days 6–8 of illness (data not shown). Again, there was no difference in the expression of CTLA‐4 or TIM‐3 in patients with acute dengue infection and healthy individuals. The expression levels were not different between the first and second blood samples (data not shown).
Figure 6.
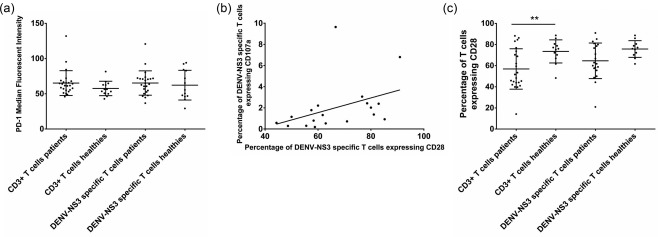
Expression of co‐stimulatory molecules in T cells in patients with acute dengue. (a) Expression of programmed death (PD)−1 in the second cohort of patients with acute dengue (n = 24) and healthy individuals (n = 12). PD‐1 expression in dengue virus (DENV)‐NS3‐specific T cells was determined by flow cytometric‐guided gating on interferon (IFN)‐γ‐producing T cells following stimulation with DENV‐NS3 overlapping peptides. (b) Correlation of DENV‐NS3‐specific CD28 expressing T cells with DENV‐NS3‐specific CD107a expressing T cells with acute dengue infection (n = 20). DENV‐NS3‐specific T cells were determined by flow cytometric‐guided gating on CD107a‐producing T cells following stimulation with DENV‐NS3 overlapping peptides. Spearman's r = 0·59, P = 0·007. (c) Expression of CD28 by DENV‐NS3‐specific T and total T cells was determined by flow cytometry in patients with acute dengue (n = 20) and healthy individuals (n = 12).
CD28 is essential for anti‐viral responses 34. We found that in patients with acute dengue, CD28 expression by DENV‐NS3‐specific T cells correlated positively with CD107a expressing DENV‐NS3‐specific T cells (Spearman's r = 0·59, P = 0·007) (Fig. 6b). However, no such correlation was observed between CD28 expression by DENV‐NS3‐specific T cells and IFN‐γ production (data not shown). Although, CD28 has been shown to be required for the development of early anti‐viral responses, we found that CD28 expression was significantly lower (P = 0·01) in T cells of patients with acute dengue (mean 56·8, s.d. ± 19·04% of CD3+ T cells) when compared to healthy individuals (mean 73·48, s.d. ± 10·94% of CD3+ T cells) (Fig. 6c).
Discussion
In this study, our initial observation, that serum IL‐10 levels correlated with dengue NS1 antigen levels, led to the experiments that showed that dengue NS1 induced IL‐10 production by monocytes and was associated with apoptosis of T cells. It has been shown previously that a dengue NS1 antigen level of more than 600 ng/ml in the first 72 h was associated with the development of DHF 9. In addition, patients with DHF have been shown to have higher viral loads and a delay in clearance of the virus 35, 36. Although secretory NS1 circulates independently of the virus, it has been shown that dengue NS1 antigen levels correlate with viral loads in acute infection 37. Therefore, persistent NS1 antigenaemia could be a reflection of delayed clearance of the virus. We also found that dengue NS1 antigen positivity at days 5–6 of the illness was associated with the development of DHF and shock 10. However, as NS1 circulates independently of the virus, and has shown to be pathogenic by itself 13, we proceeded to investigate the changes in NS1 levels and clinical disease severity. In this study, as NS1 antigen levels were measured serially in patients with acute dengue infection, we found that by 144 h (day 6 of illness) 11 of 25 patients with DHF and three of 11 patients were positive. However, all three patients with DF and six of 11 patients with DHF who were positive had a primary dengue infection. This is consistent with the finding that dengue NS1 persists for a longer time in primary dengue infection 38.
Our previous studies and studies conducted by others have shown that serum IL‐10 was associated with severe disease 7, 39, 40. In addition, we reported previously that IL‐10 inhibits DENV‐specific T cell responses 8. As dengue NS1 was also shown to associate with severity, we determined the association of serum IL‐10 and dengue NS1 antigen levels (PanBio units) in serial blood samples collected throughout the course of the illness. We found that serum IL‐10 levels correlated significantly with dengue NS1 antigen levels. In our subsequent experiments we also found that dengue NS1 stimulates production of IL‐10. The highest levels of IL‐10 were observed when monocytes were co‐cultured with NS1 of 1000 ng/ml. Therefore, these experiments show that dengue NS1 may contribute to disease pathogenesis by stimulating IL‐10 production by monocytes. Although we carried out our experiments using both recombinant dengue NS1 derived from E. coli and mammalian‐expressed protein, because other bacterial contaminants may stimulate IL‐10 production we also used a mock protein generated by E. coli which was of similar molecular weight. No IL‐10 was detected in monocytes co‐cultured with this mock protein. It was shown recently that NS1 is recognized via TLR‐4 14. Although this group did not assess IL‐10 production, they have shown that dengue NS1 acts through TLR‐4 and induced transcription of many proinflammatory cytokine genes 14. It has been shown that LPS induces production of IL‐10 from macrophages through TLR‐4 activation 41. As NS1 was shown to have LPS‐like activity and act through TLR‐4, it is likely that production of IL‐10 by monocytes could also occur due to similar mechanisms. Therefore, it would be crucial to determine if the virulence of the DENVs are also dependent upon the LPS‐like activity of NS1.
Although dengue NS1 was thought previously to contribute to disease pathogenesis by forming immune complexes with anti‐NS1 antibody, thus leading to complement activation 15, 18, other effects of NS1 such as stimulation of immunosuppressive cytokine production have not been reported until recently. However, apart from anti‐NS1 antibody immune complexes resulting in complement activation, there is a possibility of NS1‐specific antibodies contributing to disease pathogenesis by increased IL‐10 production, as suggested in our experiments. However, although we observed higher levels of IL‐10 production when NS1 was incubated with varying concentrations of DENV‐specific antibodies, it is possible that other components in human serum apart from DENV antibodies could potentiate the effects of NS1. However, we did not observe any IL‐10 production by monocytes co‐cultured with non‐dengue immune serum alone, suggesting that serum alone did not induce IL‐10 production.
Due to the association of dengue NS1 levels with serum IL‐10 levels, we carried out further experiments to determine possible other roles of NS1 in acute dengue. Massive apoptosis of T cells is known to occur in acute dengue infection and many genes associated with apoptosis are up‐regulated 28, 29, 42. Although serum IL‐10 was associated with apoptosis of T cells in acute illness, in‐vitro experiments showed that IL‐10 did not induce apoptosis in T cells in healthy individuals or in patients 29. In this study we found that dengue NS1 also correlated with annexin V expression by T cells and our in‐vitro studies on PBMCs of healthy individuals showed that, although not significant, dengue NS1 resulted in increased expression of annexin V in both CD4+ and CD8+ T cells. As, these experiments suggest that NS1 could be contributing to disease pathogenesis by causing apoptosis of T cells, whether NS1 is indeed a cause of T cell apoptosis should be investigated further. However, the increase in annexin V expression in CD4+ and CD8+ T cells in PBMCs co‐cultured with dengue NS1 varied markedly between individuals. Therefore, it is now important to investigate further if NS1 causes T cell apoptosis in only some individuals and, if so, the mechanisms by which this occurs. As the majority of dengue infections result in asymptomatic infection, it would be interesting to investigate whether the susceptibility of T cells to apoptosis by dengue NS1 was associated with clinical disease severity and higher viraemia.
Because NS1 appeared to associate with T cell apoptosis in at least some individuals, we further proceeded to investigate if it had any effects on the function of DENV‐specific T cells or on expression of co‐stimulatory molecules. We found that dengue NS1 was not associated with the functionality of the DENV‐specific T cell response and did not associate with expression of co‐stimulatory molecules such as CTLA‐4, CD28 or PD‐1. However, although not significant, an increased expression of PD‐1 was observed in patients with acute dengue when compared to healthy individuals. Although PD‐1 is a negative regulator of T cell responses and is associated with T cell dysfunction, it is up‐regulated in activated T cells 33, 43, 44. Therefore, increased expression of PD‐1 in patients with acute dengue is most likely to be due to viral antigen‐induced activation.
CD28 is a potent inducer of anti‐viral T cell responses 34, and we also found that CD28 expression by DENV‐NS3‐specific T cells correlated significantly with T cell degranulation. However, we also found that CD28 expression was significantly less in T cells in patients with acute dengue infection when compared to healthy individuals. It has been shown that, during T cell differentiation and proliferation, T cells reduce expression of CD28 and CD27 in viral infections 45. In influenza A virus infection (H1N1 infection) it was also shown that CD8+ T cells expressing CD28 were low during acute illness 46, 47. Therefore, it would be important to determine if lower expression of CD28 in T cells in patients with acute dengue infection was due to proliferation and differentiation of DENV‐specific T cells in acute dengue.
In summary, we found that dengue NS1 antigen levels correlated with serum IL‐10 levels and were higher in patients with DHF. Dengue NS1 appears to contribute to disease pathogenesis by inducing IL‐10 production by monocytes. Therefore, it would be crucial to investigate further the role of NS1 in the pathogenesis of dengue by determining the mechanisms by which NS1 induces IL‐10 production by monocytes and also the mechanisms by which it possibly induces apoptosis of T cells. As persistence of dengue NS1 was associated with severe clinical disease and also with primary dengue infection, NS1‐targeted treatments could be useful in reducing disease pathogenesis.
Acknowledgements
Funding was provided by the Centre for Dengue Research, University of Sri Jayawardenapura, Sri Lanka and the Medical Research Council, UK and NIHR Biomedical Research Centre Programme.
Disclosure
The authors have no disclosures to declare.
Author contributions
T. N. A., L. G., A. K., M. S. and N. W. were involved in carrying out experiments. T. N. A. was involved in writing the manuscript and analysing the data. N. L. A. S. and N. W. were involved in patient recruitment and obtaining data from patients. G. S. O. and G. N. M. were involved in the conceptual design, writing the manuscript, analysing the data and funding.
References
- 1. Shepard DS, Undurraga EA, Betancourt‐Cravioto M et al Approaches to refining estimates of global burden and economics of dengue. PLOS Negl Trop Dis 2014; 8:e3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bhatt S, Gething PW, Brady OJ et al The global distribution and burden of dengue. Nature 2013; 496:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shepard DS, Undurraga EA, Halasa YA. Economic and disease burden of dengue in Southeast Asia. PLOS Negl Trop Dis 2013; 7:e2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization (WHO) , editor. Comprehensive Guidelines for Prevention and Control of Dengue Fever and Dengue Haemorrhagic Fever. SEARO, New Delhi, India: WHO, 2011.
- 5. Martina BE, Koraka P, Osterhaus AD. Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev 2009; 22:564–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Malavige GN, Ogg GS. T cell responses in dengue viral infections. J Clin Virol 2013; 58:605–11. [DOI] [PubMed] [Google Scholar]
- 7. Malavige GN, Gomes L, Alles L et al Serum IL‐10 as a marker of severe dengue infection. BMC Infect Dis 2013; 13:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Malavige GN, Jeewandara C, Alles KM et al Suppression of virus specific immune responses by IL‐10 in acute dengue infection. PLOS Negl Trop Dis 2013; 7:e2409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Libraty DH, Young PR, Pickering D et al High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J Infect Dis 2002; 186:1165–8. [DOI] [PubMed] [Google Scholar]
- 10. Paranavitane SA, Gomes L, Kamaladasa A et al Dengue NS1 antigen as a marker of severe clinical disease. BMC Infect Dis 2014; 14:570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gutsche I, Coulibaly F, Voss JE et al Secreted dengue virus nonstructural protein NS1 is an atypical barrel‐shaped high‐density lipoprotein. Proc Natl Acad Sci USA 2011; 108:8003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Avirutnan P, Zhang L, Punyadee N et al Secreted NS1 of dengue virus attaches to the surface of cells via interactions with heparan sulfate and chondroitin sulfate E. PLOS Pathog 2007; 3:e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beatty PR, Puerta‐Guardo H, Killingbeck SS, Glasner DR, Hopkins K, Harris E. Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci Transl Med 2015; 7:304ra141. [DOI] [PubMed] [Google Scholar]
- 14. Modhiran N, Watterson D, Muller DA et al Dengue virus NS1 protein activates cells via Toll‐like receptor 4 and disrupts endothelial cell monolayer integrity. Sci Transl Med 2015; 7:304ra142. [DOI] [PubMed] [Google Scholar]
- 15. Avirutnan P, Punyadee N, Noisakran S et al Vascular leakage in severe dengue virus infections: a potential role for the nonstructural viral protein NS1 and complement. J Infect Dis 2006; 193:1078–88. [DOI] [PubMed] [Google Scholar]
- 16. Cheng HJ, Lei HY, Lin CF et al Anti‐dengue virus nonstructural protein 1 antibodies recognize protein disulfide isomerase on platelets and inhibit platelet aggregation. Mol Immunol 2009; 47:398–406. [DOI] [PubMed] [Google Scholar]
- 17. Falconar AK. The dengue virus nonstructural‐1 protein (NS1) generates antibodies to common epitopes on human blood clotting, integrin/adhesin proteins and binds to human endothelial cells: potential implications in haemorrhagic fever pathogenesis. Arch Virol 1997; 142:897–916. [DOI] [PubMed] [Google Scholar]
- 18. Lin CF, Wan SW, Chen MC et al Liver injury caused by antibodies against dengue virus nonstructural protein 1 in a murine model. Lab Invest 2008; 88:1079–89. [DOI] [PubMed] [Google Scholar]
- 19. Lin CF, Lei HY, Shiau AL et al Antibodies from dengue patient sera cross‐react with endothelial cells and induce damage. J Med Virol 2003; 69:82–90. [DOI] [PubMed] [Google Scholar]
- 20. Aye KS, Charngkaew K, Win N et al Pathologic highlights of dengue hemorrhagic fever in 13 autopsy cases from Myanmar. Hum Pathol 2014; 45:1221–33. [DOI] [PubMed] [Google Scholar]
- 21. Vaughn DW, Nisalak A, Solomon T et al Rapid serologic diagnosis of dengue virus infection using a commercial capture ELISA that distinguishes primary and secondary infections. Am J Trop Med Hyg 1999; 60:693–8. [DOI] [PubMed] [Google Scholar]
- 22. Sang CT, Cuzzubbo AJ, Devine PL. Evaluation of a commercial capture enzyme‐linked immunosorbent assay for detection of immunoglobulin M and G antibodies produced during dengue infection. Clin Diagn Lab Immunol 1998; 5:7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chaichana P, Okabayashi T, Puiprom O et al Low levels of antibody‐dependent enhancement in vitro using viruses and plasma from dengue patients. PLOS ONE 2014; 9:e92173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boonnak K, Slike BM, Donofrio GC, Marovich MA. Human FcgammaRII cytoplasmic domains differentially influence antibody‐mediated dengue virus infection. J Immunol 2013; 190:5659–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luo YY, Feng JJ, Zhou JM et al Identification of a novel infection‐enhancing epitope on dengue prM using a dengue cross‐reacting monoclonal antibody. BMC Microbiol 2013; 13:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chau TN, Hieu NT, Anders KL et al Dengue virus infections and maternal antibody decay in a prospective birth cohort study of Vietnamese infants. J Infect Dis 2009; 200:1893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Halstead SB, Porterfield JS, O'Rourke EJ. Enhancement of dengue virus infection in monocytes by flavivirus antisera. Am J Trop Med Hyg 1980; 29:638–42. [DOI] [PubMed] [Google Scholar]
- 28. Mongkolsapaya J, Dejnirattisai W, Xu XN et al Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med 2003; 9:921–7. [DOI] [PubMed] [Google Scholar]
- 29. Malavige GN, Huang LC, Salimi M, Gomes L, Jayaratne SD, Ogg GS. Cellular and cytokine correlates of severe dengue infection. PLOS ONE 2012; 7:e50387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Myint KS, Endy TP, Mongkolsirichaikul D et al Cellular immune activation in children with acute dengue virus infections is modulated by apoptosis. J Infect Dis 2006; 194:600–7. [DOI] [PubMed] [Google Scholar]
- 31. Barber DL, Wherry EJ, Masopust D et al Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006; 439:682–7. [DOI] [PubMed] [Google Scholar]
- 32. Kasprowicz V, Schulze Zur Wiesch J, Kuntzen T et al High level of PD‐1 expression on hepatitis C virus (HCV)‐specific CD8+ and CD4+ T cells during acute HCV infection, irrespective of clinical outcome. J Virol 2008; 82:3154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Greenough TC, Campellone SC, Brody R et al Programmed Death‐1 expression on Epstein Barr virus specific CD8+ T cells varies by stage of infection, epitope specificity, and T‐cell receptor usage. PLOS ONE 2010; 5:e12926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fang M, Sigal LJ. Direct CD28 costimulation is required for CD8+ T cell‐mediated resistance to an acute viral disease in a natural host. J Immunol 2006; 177:8027–36. [DOI] [PubMed] [Google Scholar]
- 35. Guilarde AO, Turchi MD, Siqueira JB Jr et al Dengue and dengue hemorrhagic fever among adults: clinical outcomes related to viremia, serotypes, and antibody response. J Infect Dis 2008; 197:817–24. [DOI] [PubMed] [Google Scholar]
- 36. Wang WK, Chen HL, Yang CF et al Slower rates of clearance of viral load and virus‐containing immune complexes in patients with dengue hemorrhagic fever. Clin Infect Dis 2006; 43:1023–30. [DOI] [PubMed] [Google Scholar]
- 37. Thomas L, Najioullah F, Verlaeten O et al Relationship between nonstructural protein 1 detection and plasma virus load in Dengue patients. Am J Trop Med Hyg 2010; 83:696–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Duyen HT, Ngoc TV, Ha do T et al Kinetics of plasma viremia and soluble nonstructural protein 1 concentrations in dengue: differential effects according to serotype and immune status. J Infect Dis 2011; 203:1292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brasier AR, Ju H, Garcia J et al A three‐component biomarker panel for prediction of dengue hemorrhagic fever. Am J Trop Med Hyg 2012; 86:341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ju H, Brasier AR. Variable selection methods for developing a biomarker panel for prediction of dengue hemorrhagic fever. BMC Res Notes 2013; 6:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Iyer SS, Ghaffari AA, Cheng G. Lipopolysaccharide‐mediated IL‐10 transcriptional regulation requires sequential induction of type I IFNs and IL‐27 in macrophages. J Immunol 2010; 185:6599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sun P, Garcia J, Comach G et al Sequential waves of gene expression in patients with clinically defined dengue illnesses reveal subtle disease phases and predict disease severity. PLOS Negl Trop Dis 2013; 7:e2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Duraiswamy J, Ibegbu CC, Masopust D et al Phenotype, function, and gene expression profiles of programmed death‐1(hi) CD8 T cells in healthy human adults. J Immunol 2011; 186:4200–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Parry RV, Chemnitz JM, Frauwirth KA et al CTLA‐4 and PD‐1 receptors inhibit T‐cell activation by distinct mechanisms. Mol Cell Biol 2005; 25:9543–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Borthwick NJ, Lowdell M, Salmon M, Akbar AN. Loss of CD28 expression on CD8(+) T cells is induced by IL‐2 receptor gamma chain signalling cytokines and type I IFN, and increases susceptibility to activation‐induced apoptosis. Int Immunol 2000; 12:1005–13. [DOI] [PubMed] [Google Scholar]
- 46. Fox A, Le NM, Horby P et al Severe pandemic H1N1 2009 infection is associated with transient NK and T deficiency and aberrant CD8 responses. PLOS ONE 2012; 7:e31535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Appay V, Dunbar PR, Callan M et al Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med 2002; 8:379–85. [DOI] [PubMed] [Google Scholar]


