Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
Key Points
Unlicensed NK cells release GM-CSF upon allogeneic MHCI recognition, which promotes donor allogeneic BMC engraftment.
Abstract
Natural killer (NK) cells exist as subsets based on expression of inhibitory receptors that recognize major histocompatibility complex I (MHCI) molecules. NK cell subsets bearing MHCI binding receptors for self-MHCI have been termed as “licensed” and exhibit a higher ability to respond to stimuli. In the context of bone marrow transplantation (BMT), host licensed-NK (L-NK) cells have also been demonstrated to be responsible for the acute rejection of allogeneic and MHCI-deficient BM cells (BMCs) in mice after lethal irradiation. However, the role of recipient unlicensed-NK (U-NK) cells has not been well established with regard to allogeneic BMC resistance. After NK cell stimulation, the prior depletion of host L-NK cells resulted in a marked increase of donor engraftment compared with the untreated group. Surprisingly, this increased donor engraftment was reduced after total host NK cell depletion, indicating that U-NK cells can actually promote donor allogeneic BMC engraftment. Furthermore, direct coculture of U-NK cells with allogeneic but not syngeneic BMCs resulted in increased colony-forming unit cell growth in vitro, which was at least partially mediated by granulocyte macrophage colony-stimulating factor (GM-CSF) production. These data demonstrate that host NK cell subsets exert markedly different roles in allogeneic BMC engraftment where host L- and U-NK cells reject or promote donor allogeneic BMC engraftment, respectively.
Introduction
The biology of natural killer (NK) cells has become more complex after the description of “licensing,” which allowed the functional differentiation of subsets based on the expression of inhibitory receptors (IRs).1-3 Licensed-NKs (L-NK) were reported to be more rapidly functional than unlicensed-NKs (U-NK).1 In agreement with these studies, we demonstrated that host L-NKs were responsible for the preferential elimination of both allogeneic4-7 and MHCI-deficient bone marrow cells (BMCs)8 during BM transplantation (BMT). Similarly, L-NKs primarily eliminated murine cytomegalovirus–infected cells after both allogeneic- (alloBMT)9 and syngeneic-BMT (synBMT).10 However, the role of host U-NKs in alloBMT has been unclear. Because of the importance of NKs in the protection against virus pathology and the regulation of T-cell responses,11 we were interested in analyzing the impact of the host-NK subsets on the engraftment of alloBMCs and whether differential functions were observed. The unique cytokine profiles observed after infection allows us to hypothesize that U-NKs can have a beneficial effect on donor reconstitution after alloBMCs and can possibly be useful in the application of NK-based therapy to improve outcomes in clinical BMT.
Methods
BMT studies
The work presented in this manuscript was performed under protocols approved by the University of California, Davis Institutional Animal Care and Use Committee. Eight- to 10-week-old female B6, B10.D2, BALB/c, NSG, or MHCI-deficient (β2m−/− B6) mice were purchased from The Jackson Laboratory and maintained in AAALAC-approved specific pathogen-free facilities. BMTs were done as described.8 For adoptive transfer studies, 3 × 106 donor B6 or BALB/c BMCs plus 1 × 106 of B6 IL2-activated sorted L- or U-NKs12,13 were injected IV. into lethally irradiated (240cGy) NSG mice. Donor cell reconstitution was analyzed by splenic colony-forming unit (CFU-c) assay and/or flow cytometry at day 7 post-BMT.8,14
In vitro assays
Activated sorted L- or U-NK subsets were cocultured with allo- or synBMCs at 1:1 ratio for 24 hours. Treatment with 50 μg/mL anti–granulocyte macrophage colony-stimulating factor (GM-CSF) (Peprotech), 10 μg/mL sodium stibogluconate (SSG) (Sigma-Aldrich, St. Louis, MO), or vehicle control was done when indicated. BMCs or adherent NKs were collected and CFU-c assay8 or reverse transcription polymerase chain reaction (RT-PCR)15 was performed, respectively. Recombinant-murine GM-CSF was not used for CFU-c assay except otherwise indicated. For RT-PCR, BMCs were irradiated (3 Gy) before coculture.
Statistical analysis
Each experiment was repeated at least 2 times. Statistical significance was determined as previously described.14
Results and discussion
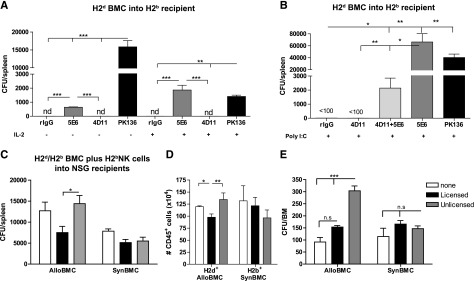
Numerous studies have demonstrated the predominant role of L-NKs in mediating alloBMCs rejection in mice.6,8,9 Prior NK activation was also demonstrated to augment rejection overall, and L- and U-NKs were equally responsive and capable of rejecting MHCI-deficient BMCs.8 Here, we analyzed the impact of activated host-NK subsets in the engraftment of MHCI+ BMCs. We observed that in vivo administration of IL2 or polyI:C in B6 (H2b) mice before alloBMT to augment host-NK activity led to markedly differential outcomes on alloBMC engraftment depending on the host NK subset depleted. As previously reported, removal of all host-NK cells (via anti-NK1.1) resulted in improved alloBMC engraftment, measured by CFU-c, indicating the net effect of host-NKs is to reject alloBMCs. In addition, the prior depletion of host Ly49C/I+ L-NKs (binds H2b and therefore licensed) by anti-Ly49C/I (5E6) resulted in a significant increase of donor (H2d) alloBMC engraftment as determined by CFU-c (Figure 1A-B). Interestingly, prior treatment with IL2 or polyI:C significantly also markedly augmented the growth-promoting effect of the host L-NK subset depletion, being comparable with and even superior to total NK depletion (Figure 1A-B). These data indicate that the lack of allogeneic BMC rejection by host L-NKs as a result of prior depletion of this subset is surprisingly also accompanied by a promotion of alloBMC engraftment by the remaining NKs subsets. Despite this, approximately 30% of NKs are unlicensed-Ly49G2+ (binds H2d) in B6 mice, and no effect on allogeneic donor engraftment was observed after depletion with anti-Ly49G2 (4D11) at any given condition. However, the concurrent depletion of L- and U-NKs reduced the proportion of alloBMC engraftment compared with depletion of L-NKs alone (Figure 1B), suggesting an antagonistic function between the subsets where the U-NK are growth-promoting rather than inhibiting for the donor alloBMCs. Importantly, it does reveal an unknown stimulatory function of U-NKs in the promotion of alloBMC engraftment. This effect was not strain-dependent because the same pattern was observed when B10 (H2b) or B10.D2 (H2d) mice were used for alloBMT (supplemental Figure 1A-B, available on the Blood Web site), whereas no differences were found after synBMT (supplemental Figure 1C).
Figure 1.
Activated unlicensed U-NK cells promote donor BMC engraftment after allogeneic BMT. B6 mice were treated with mAb against licensed L-Ly49C/I+ NK cells (5E6) and/or unlicensed U-Ly49G2+ NK cells (4D11) with or without NK cell stimulation (IL2 or Poly I:C) 2 days before allogeneic BMT of 3 × 106 B10.D2 donor BMCs. Anti-NK1.1 (PK136) was used as positive control of total engraftment. (A-B) The hematopoietic progenitor content of spleens (Total CFU-c/spleen) of IL2- (A) or Poly I:C-treated (B) host NK cells was assessed 7 days post-allogeneic BMT. Lethally irradiated NSG mice were IV injected with 3 × 106 BALB/c (allogeneic: alloBMCs) or B6 (syngeneic: synBMCs) BMCs plus 1 × 106 of in vitro activated licensed (CD45+CD3–CD122+Ly49G2–C/I+) or unlicensed (CD45+CD3–CD122+Ly49G2+Ly49C/I–) sorted NK cells. (C) CFU-c/spleen is shown 7 days post-BMT of NSG mice. (D) Total number of engrafted donor BMCs (H2d+ alloBMCs or H2b+ synBMCs) in NSG mice measured by flow cytometry is shown for the spleen. Activated sorted licensed or unlicensed B6 NK cells were also cultured in vitro with allogeneic (B10.D2) or syngeneic (B6) BMCs at a 1:1 ratio for 24 hours and then CFU-c was assessed in the absence of rGM-CSF. (E) CFU-c/BM for in vitro assay is shown. Data are representative of at least 2 experiments with 3 mice per group (A-D) or by triplicate (E) (mean ± SEM). One-way analysis of variance was used to assess significance (*P < .05, **P < .01, ***P < .001; n.d., not detected, n.s., not significant).
To delineate that this promoting effect on allogeneic engraftment was solely caused by U-NKs, lethally irradiated, immunodeficient NSG mice received BALB/c (H2d) BMCs (alloBMT) or B6 BMCs (synBMT) with activated B6 sorted Ly49CI+ L- or Ly49G2+ U-NK cells (supplemental Figure 2). The adoptive transfer of U-NKs significantly increased alloBMC engraftment compared with L-NK infusion and, although not significant, a trend toward increased alloBMC engraftment was also observed when compared with mice that did not receive NK infusion (Figure 1C). As expected, no differences were observed in the engraftment capabilities of synBMCs. These results on donor engraftment were also confirmed by analysis of H2d+ cell engraftment in the spleen (Figure 1D). This, combined with previous data demonstrating rejection of MHCI-negative BMCs by U-NKs,8 indicates that triggering of the U-NK subset with the appropriate MHCI (H2d for Ly49G2) was required for the hematopoietic growth-promoting effects. In addition, the prior coculture of alloBMCs with activated U-NKs improved in vitro BMC growth, indicating a direct role in promoting engraftment (Figure 1E). When the supernatant of these cocultures was used in the CFU-c assays to assess effects on the growth of synBMCs, an increase of CFU-c was observed if supernatants came from the U-NK:alloBMC cocultures (supplemental Figure 3). These data suggest that the interaction between the inhibitory receptor (IR) of U-NKs with the MHCI of alloBMC leads to the production of cytokines that favor alloBMC engraftment and proliferation.
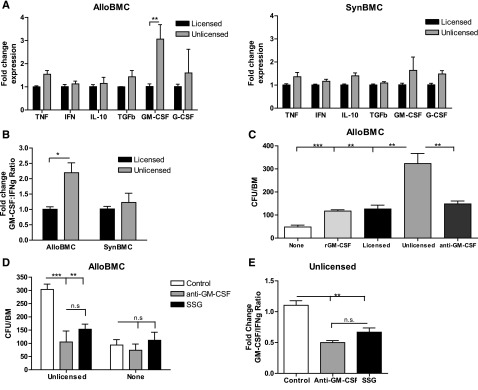
GM-CSF and granulocyte (G)-CSF are secreted by multiple immune cells including NKs and are involved in hematopoiesis.16 We then analyzed the cytokine profile of L- or U-NKs from H2b or H2d mice after coculture with alloBMCs or synBMCs. Compared with L-NK, U-NK had a significant increase of GM-CSF mRNA expression, whereas there were no differences in IFNg or tumor necrosis factor mRNA expression (Figure 2A-B and supplemental Figure 4). Interestingly, human NK cells produce GM-CSF after IL2 treatment and TLR9 signaling or Candida albicans infection.17-19 However, high levels of interferon and tumor necrosis factor were also detected under these stimulatory conditions, leading to an activating phenotype that does not coincide with the phenotype displayed by U-NKs during alloBMT settings. To further demonstrate the GM-CSF–dependent hematopoietic role of U-NKs, neutralizing antibodies to GM-CSF were used during cocultures and indeed prevented alloBMC growth (Figure 2C). It is likely that GM-CSF production occurs after allogeneic MHCI interaction with the IRs of U-NKs because of activation of Scr homology protein tyrosine phosphatase-I (SHP-1). We therefore blocked phosphorylation of SHP-1 using SSG, a protein tyrosine phosphatase inhibitor,20 to prevent the signaling cascade mediated by IRs-MHCI interactions. Blocking GM-CSF and SHP-1 reduced alloBMC growth to a similar extent (Figure 2D). SSG caused a reduction of GM-CSF on U-NKs exemplified by reduction of the fold change ratio between GM-CSF and IFNg mRNA compared with vehicle control after U-NK:alloBMC cocultures (Figure 2E). These data demonstrate that activated U-NKs produce higher amounts of GM-CSF upon alloBMC:IR interactions, which correlates with augmented engraftment and implies a clinical application of host-derived U-NK cells in allogeneic-BMT settings. Yu et al demonstrated that human U-NKs (with killer immunoglobulin-like receptors for non-self-HLA) were functional immediately after BMT, possibly because of the conditioning regimen, and had potent antitumor responses on target cells that did not express ligands for those killer immunoglobulin-like receptors.21,22 We are thus describing a previously unreported role of host U-NK cells in the promotion of donor allogeneic BMC engraftment. We are also proposing a dual role of SHP-1, because its phosphorylation after MHCI-IR encounter can lead to NK suppression in resting conditions or GM-CSF release during stimulatory conditions. A different SHP1 function upon activation was also described for NK and mast cells.23,24 These results indicate profound functional differences in the ability of host-NK subsets to affect donor alloBMC engraftment based on licensing, suggesting their potential use in clinical BMT.
Figure 2.
Unlicensed U-NK cells promote allogeneic BMC engraftment through increased GM-CSF production that requires MHCI interaction and consequent SHP-1 phosphorylation. Twenty-four hours after coculture of irradiated allo- or synBMC cells with activated sorted B6 licensed and unlicensed NK cells, adherent NK cells were collected and RT-PCR–assessed. (A) Fold change of cytokine expression is shown for licensed and unlicensed NK cells that were exposure to irradiated allo- or synBMCs. Data are represented as fold change mRNA expression of L-NK data. (B) Fold change ratio between GM-CSF and IFNg is shown. To study engraftment, BMCs were collected after 24 hours of culture with NK cells, and CFU-c per BM was determined. (C) Blockade of GM-CSF with anti–GM-CSF prevents the unlicensed NK cell–dependent engraftment. (D) Inhibition of SHP-1 with SSG prevents alloBMC engraftment Ly49G2+ NK cell–dependent to a similar extent than GM-CSF blockade measured by CFU-c/BM. (E) Fold change of GM-CSF:IFNg ratio is shown for unlicensed NK cells exposed to allogeneic BMCs after GM-CSF blockade and SHP-1 inhibition. Data are representative of at least 2 experiments with n = 3 (mean ± SEM). One-way analysis of variance was used to assess significance (*P < .05, **P < .01, ***P < .001; n.d., not detected, n.s., not significant).
Acknowledgments
The authors thank Weihong Ma and Monja Metcalf for their technical assistance, and Gail Sckisel and Antonio Pierini for critical revision of the manuscript.
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grant R01-HL089905 and National Science Foundation of China grant No. 81273259 (K.S.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.A. and W.J.M. had experimental oversight, analyzed data, and wrote the manuscript; and K.S. conducted initial BMT studies.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: William J. Murphy, Departments of Dermatology and Internal Medicine, University of California, Davis School of Medicine, TSC-IRC Suite 1630, 2921 Stockton Blvd, Sacramento, CA 95817; e-mail: wmjmurphy@ucdavis.edu.
References
- 1.Kim S, Poursine-Laurent J, Truscott SM, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436(7051):709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105(11):4416–4423. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anfossi N, André P, Guia S, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25(2):331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Barao I, Hanash AM, Hallett W, et al. Suppression of natural killer cell-mediated bone marrow cell rejection by CD4+CD25+ regulatory T cells. Proc Natl Acad Sci USA. 2006;103(14):5460–5465. doi: 10.1073/pnas.0509249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raziuddin A, Bennett M, Winkler-Pickett R, Ortaldo JR, Longo DL, Murphy WJ. Synergistic effects of in vivo depletion of Ly-49A and Ly-49G2 natural killer cell subsets in the rejection of H2(b) bone marrow cell allografts. Blood. 2000;95(12):3840–3844. [PubMed] [Google Scholar]
- 6.Raziuddin A, Longo DL, Bennett M, Winkler-Pickett R, Ortaldo JR, Murphy WJ. Increased bone marrow allograft rejection by depletion of NK cells expressing inhibitory Ly49 NK receptors for donor class I antigens. Blood. 2002;100(8):3026–3033. doi: 10.1182/blood.V100.8.3026. [DOI] [PubMed] [Google Scholar]
- 7.Koh CY, Welniak LA, Murphy WJ. Lack of correlation between an assay used to determine early marrow allograft rejection and long-term chimerism after murine allogeneic bone marrow transplantation: effects of marrow dose. Biol Blood Marrow Transplant. 2005;11(4):252–259. doi: 10.1016/j.bbmt.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Sun K, Alvarez M, Ames E, et al. Mouse NK cell-mediated rejection of bone marrow allografts exhibits patterns consistent with Ly49 subset licensing. Blood. 2012;119(6):1590–1598. doi: 10.1182/blood-2011-08-374314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sungur CM, Tang-Feldman YJ, Zamora AE, Alvarez M, Pomeroy C, Murphy WJ. Murine NK-cell licensing is reflective of donor MHC-I following allogeneic hematopoietic stem cell transplantation in murine cytomegalovirus responses. Blood. 2013;122(8):1518–1521. doi: 10.1182/blood-2013-02-483503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sungur CM, Tang-Feldman YJ, Ames E, et al. Murine natural killer cell licensing and regulation by T regulatory cells in viral responses. Proc Natl Acad Sci USA. 2013;110(18):7401–7406. doi: 10.1073/pnas.1218767110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alvarez M, Bouchlaka MN, Sckisel GD, Sungur CM, Chen M, Murphy WJ. Increased antitumor effects using IL-2 with anti-TGF-β reveals competition between mouse NK and CD8 T cells. J Immunol. 2014;193(4):1709–1716. doi: 10.4049/jimmunol.1400034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu B, He Y, Wu Y, et al. Activated allogeneic NK cells as suppressors of alloreactive responses. Biol Blood Marrow Transplant. 2010;16(6):772–781. doi: 10.1016/j.bbmt.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 13.Barao I, Alvarez M, Ames E, et al. Mouse Ly49G2+ NK cells dominate early responses during both immune reconstitution and activation independently of MHC. Blood. 2011;117(26):7032–7041. doi: 10.1182/blood-2010-11-316653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarez M, Sungur CM, Ames E, Anderson SK, Pomeroy C, Murphy WJ. Contrasting effects of anti-Ly49A due to MHC class I cis binding on NK cell-mediated allogeneic bone marrow cell resistance. J Immunol. 2013;191(2):688–698. doi: 10.4049/jimmunol.1300202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouchlaka MN, Sckisel GD, Chen M, et al. Aging predisposes to acute inflammatory induced pathology after tumor immunotherapy. J Exp Med. 2013;210(11):2223–2237. doi: 10.1084/jem.20131219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Y, Liu CH, Roberts AI, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don’t know. Cell Res. 2006;16(2):126–133. doi: 10.1038/sj.cr.7310017. [DOI] [PubMed] [Google Scholar]
- 17.Levitt LJ, Nagler A, Lee F, Abrams J, Shatsky M, Thompson D. Production of granulocyte/macrophage-colony-stimulating factor by human natural killer cells. Modulation by the p75 subunit of the interleukin 2 receptor and by the CD2 receptor. J Clin Invest. 1991;88(1):67–75. doi: 10.1172/JCI115306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez JM, Marchicio J, López M, et al. PyNTTTTGT and CpG immunostimulatory oligonucleotides: effect on granulocyte/monocyte colony-stimulating factor (GM-CSF) secretion by human CD56+ (NK and NKT) cells. PLoS One. 2015;10(2):e0117484. doi: 10.1371/journal.pone.0117484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voigt J, Hünniger K, Bouzani M, et al. Human natural killer cells acting as phagocytes against Candida albicans and mounting an inflammatory response that modulates neutrophil antifungal activity. J Infect Dis. 2014;209(4):616–626. doi: 10.1093/infdis/jit574. [DOI] [PubMed] [Google Scholar]
- 20.Pathak MK, Yi T. Sodium stibogluconate is a potent inhibitor of protein tyrosine phosphatases and augments cytokine responses in hemopoietic cell lines. J Immunol. 2001;167(6):3391–3397. doi: 10.4049/jimmunol.167.6.3391. [DOI] [PubMed] [Google Scholar]
- 21.Yu J, Venstrom JM, Liu XR, et al. Breaking tolerance to self, circulating natural killer cells expressing inhibitory KIR for non-self HLA exhibit effector function after T cell-depleted allogeneic hematopoietic cell transplantation. Blood. 2009;113(16):3875–3884. doi: 10.1182/blood-2008-09-177055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarek N, Le Luduec JB, Gallagher MM, et al. Unlicensed NK cells target neuroblastoma following anti-GD2 antibody treatment. J Clin Invest. 2012;122(9):3260–3270. doi: 10.1172/JCI62749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viant C, Fenis A, Chicanne G, Payrastre B, Ugolini S, Vivier E. SHP-1-mediated inhibitory signals promote responsiveness and anti-tumour functions of natural killer cells. Nat Commun. 2014;5:5108. doi: 10.1038/ncomms6108. [DOI] [PubMed] [Google Scholar]
- 24.Nakata K, Yoshimaru T, Suzuki Y, et al. Positive and negative regulation of high affinity IgE receptor signaling by Src homology region 2 domain-containing phosphatase 1. J Immunol. 2008;181(8):5414–5424. doi: 10.4049/jimmunol.181.8.5414. [DOI] [PubMed] [Google Scholar]