Abstract
Ecologists urgently need a better ability to predict how environmental change affects biodiversity. We examine individual-based ecology (IBE), a research paradigm that promises better a predictive ability by using individual-based models (IBMs) to represent ecological dynamics as arising from how individuals interact with their environment and with each other. A key advantage of IBMs is that the basis for predictions—fitness maximization by individual organisms—is more general and reliable than the empirical relationships that other models depend on. Case studies illustrate the usefulness and predictive success of long-term IBE programs. The pioneering programs had three phases: conceptualization, implementation, and diversification. Continued validation of models runs throughout these phases. The breakthroughs that make IBE more productive include standards for describing and validating IBMs, improved and standardized theory for individual traits and behavior, software tools, and generalized instead of system-specific IBMs. We provide guidelines for pursuing IBE and a vision for future IBE research.
Keywords: ecology, fitness-maximization, individual-based, modeling, prediction
Ecological systems are under increasing pressure from environmental change, including climate change, habitat loss and fragmentation, and increasing human populations. To understand the consequences of environmental change, to minimize adverse impacts, and to prioritize actions, conservation managers and policymakers need to know how ecological systems will be affected (Evans 2012). Despite this need, predicting the consequences of environmental change for biodiversity has remained a challenge for ecologists. The reasons for this include the complexity, size, and slow dynamics of ecological systems, which usually prevent the use of controlled experiments (Grimm and Railsback 2012). Ecology therefore often relies on modeling, but most traditional models are too limited to predict the effect of future novel environmental change. They are usually focused on empirically determined demographic rates, such as birth and death rates—for example, using mark–recapture studies. A key limitation of this empirical approach is that the resulting demographic rates are valid only for the environmental conditions under which they were observed and may not hold for the new circumstances for which predictions are required (Evans 2012).
Individual-based ecology (IBE) provides a research paradigm for developing more-flexible and -predictive population models (Grimm and Railsback 2005). It views ecological populations as having properties (e.g., size, death rate, age distribution, space use) that arise from the behavioral traits and interactions (e.g., decision rules, behavior, physiology, genotype) of their constituent individuals, with the links among environment, individuals, and populations made through individual-based models (IBMs). IBMs explicitly represent discrete individuals within a population and their individual life cycles. The great potential of IBMs for understanding ecological systems has been known for a long time (e.g., Łomnicki 1978, DeAngelis et al. 1980), and they have been used widely in several fields. For example, IBMs have been used in forest research and management (Bugmann 2001), a suite of IBMs was developed to manage Everglades restoration (DeAngelis et al. 1998), and a long and diverse research program (for a summary, see Rose 2000) applied IBMs to many fisheries problems. Still, IBMs did not develop a record of successful predictions in their early decades (Grimm 1999). Rose (2000) concluded that the predictions would be more successful if IBMs were combined with life history theory and multidisciplinary research programs.
In IBE, IBMs are designed around two key criteria: emergence and fitness (Railsback 2001). Emergence means that the behavior underlying demographic rates results from the individuals’ behavioral decisions, which are based on fitness-related decision rules (Grimm and Railsback 2005). Consequently, the behavior of simulated individuals can resemble that of real individuals that are equipped with fitness-seeking traits (e.g., to minimize predation risk, to feed in locations in which their food consumption rate is maximized), because it is assumed that natural selection has produced behavior that maximizes individual fitness (Grimm and Railsback 2005). Model animals are expected to respond to environmental change as do real ones, because they use the same fitness-maximizing decision rules. The advantage over traditional methods is that IBMs require fewer historical data, and the basis of prediction—fitness maximization—is more likely to maintain its predictive power in new environments than are the empirical relationships of traditional methods. Adaptive behavior can also be represented implicitly, as behaviors or physiological processes that presumably evolved because they convey fitness (Sibly 2013). For example, in their IBM, Martin and colleagues (2013) used dynamic energy budget theory (Sousa et al. 2010) to represent evolved trade-offs in the allocation of food energy to maintenance, growth, and reproduction. Adaptive behavior can also be modeled by artificially evolving successful decision traits within a model (Huse et al. 1999, Giske et al. 2013).
In this article, we explain how IBE can be used to predict the effect of environmental change on animals and to provide evidence for conservation management and policy. We present two case studies of long-term research programs on IBE, of stream trout and coastal birds. Although we focus on animal populations, IBE applies to any organism, including plants (e.g., Berger and Hildenbrandt 2000) and microbes (e.g., Hellweger and Bucci 2009, Kreft et al. 2013). We identify three phases of IBE (figure 1): (1) Conceptualization: identifying the research questions and why IBE is an appropriate framework to answer them; (2) implementation: the development and validation of an IBM for the initial study system or systems; and (3) diversification: model simplification, generalization, and validation for a wider range of systems and questions. The case studies were independent and developed along different pathways, but there is considerable overlap and convergence of issues, approaches, and solutions, which makes it possible to extract general lessons. We provide general guidelines for pursuing a research program of IBE, highlight breakthroughs that have made IBE more productive, and present a future vision for IBE.
Figure 1.
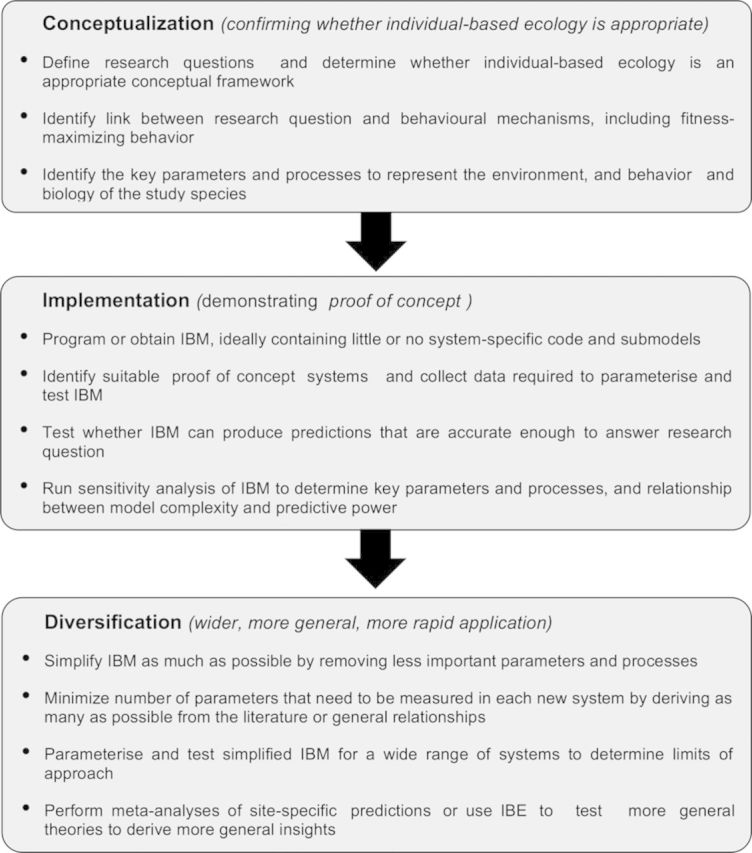
Three phases of individual-based ecology (IBE). Abbreviation: IBM, individual-based model.
Lessons from the individual-based ecology of stream trout
Our first case study of IBE is the development of inSTREAM, a model originally designed to improve predictions of how trout populations downstream of hydroelectric dams respond to alternative flow- and temperature-management policies (Railsback and Harvey 2002, Railsback et al. 2009). Such predictions are necessary at the many dams managed for both power production and conservation of downstream resources that include trout.
Conceptualization
Since the 1970s, the standard tool for assessing the effects of river flow on fish has been a habitat model (the physical habitat simulation of Bovee et al. 1998) that produces relations between flow and the areas of suitable habitat for separate species and life stages of fish. Suitable habitat has depths and velocities in ranges in which fish are often observed. This approach has well-known limitations (EPRI 2000), including that it does not produce testable predictions of population responses, only simulated changes in “suitable” habitat; it does not consider flow variation over time, which is a major emphasis of modern river management; and it does not link the different effects of flow on different life stages or multiple species into population- or community-level effects. In fact, whether animal populations are closely linked to the availability of suitable habitat has been strongly debated (see Railsback et al. 2003). Traditional methods of assessing the effects of water temperature are similarly limited, relying on a simple threshold approach. River managers often assume that temperatures are acceptable for trout as long as they are less than 20 degrees Celsius, a threshold for direct thermal stress. However, indirect and sublethal effects of temperature are important and well understood but are not incorporated in conventional assessments.
IBE was appealing for this flow and temperature assessment problem for several reasons that were already well understood at the time at which inSTREAM's precursor (Van Winkle et al. 1998) was conceptualized. Foremost was that the key effects of flow and temperature on individual trout were already well understood. Drift-feeding (sit-and-wait predation, the foraging mode typically used by trout) models had already been shown to predict how water velocity and depth affect fish food intake and swimming speed (e.g., Hughes 1992). Bioenergetics models then link food intake and temperature to growth. Second, an IBM could combine the multiple effects of flow and temperature on individual growth and survival and combine them into testable predictions of population and community responses. Third, an IBM operating at a daily time step could predict the effects of naturally variable flow and temperature regimes. Finally, trout are known to use at least one key behavior, habitat selection, to adapt to changes in flow and temperature; representing behavior and its effects are exactly the realm of IBE.
Implementation
The first IBM for this problem was published by Van Winkle and colleagues (1998). inSTREAM retains some basic structure of the first IBM but was completely redesigned. inSTREAM was also implemented in new software, especially to provide the graphical displays that are essential for testing and understanding complex spatial models (figure 2a, table 1).
Figure 2.
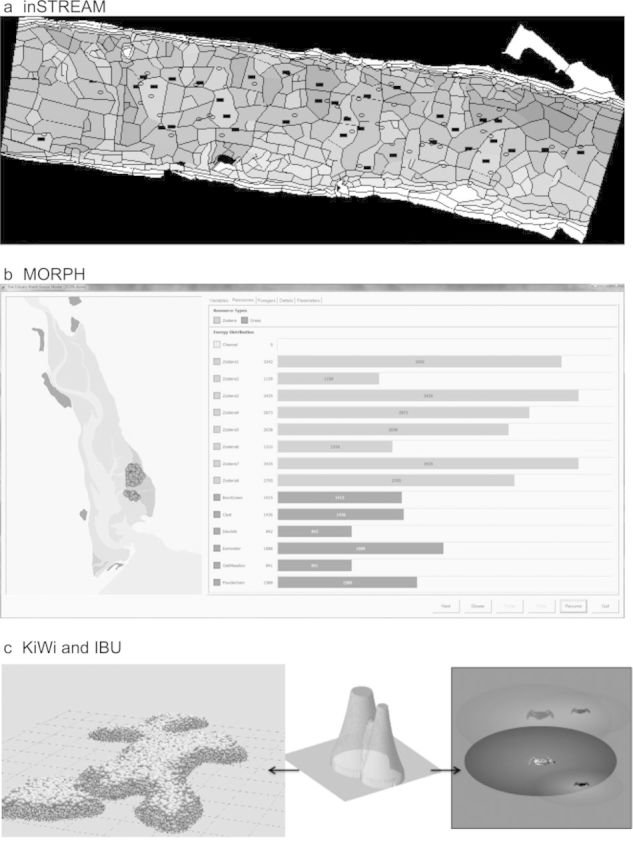
Graphical output of individual-based models. (a) inSTREAM. Habitat cells are shaded by depth. Adult trout are depicted as black rectangles in the cells in which they feed; redds (egg nests) appear as ovals. Users can click on the display to view and even change state variables of individual cells, fish, and redds. (b) MORPH (here, simulating Brent geese [Branta bernicla L.] in the Exe Estuary, United Kingdom). The distribution of patches and foragers (circles) are displayed to the left (different types of forager can be represented in different colors). The tabs to the right display the values of state variables (here, food resources) graphically. The “Details” tab shows the numerical value of each global, patch, and forager state variable during each time step. Individual foragers can be selected by double clicking either in the display or on the “Details” tab; the forager can then be followed through the simulation. The buttons at the bottom right allow the simulation to be paused, slowed down, speeded up, or progressed one time step at a time. (c) KiWi (left; Piou et al. 2008) simulates mangrove forests, and IBU (right; Piou et al. 2007) simulates competition between crabs (Ucides cordatus). KiWi and IBU use the field-of-neighborhood concept as a standard way to model neighborhood interactions among sessile and nonsessile organisms (center; the height of the zone represents the strength of interaction).
Table 1.
Overview of the inSTREAM and MORPH models.
| inSTREAM | MORPH | |
|---|---|---|
| Purpose | To predict effects of changes in flow, temperature, turbidity and channel characteristics on river trout communities (e.g., abundance and relative abundance) | To predict how environmental change (e.g., habitat loss, disturbance) affects population processes (e.g., mortality rate, emigration) within foraging animal populations |
| Time scales | One-day time steps | Fixed time steps (e.g., hours) |
| Simulation durations of weeks to decades | Simulation durations of months for coastal birds | |
| Spatial scales | One or more reaches, representative pieces of stream typically hundreds to thousands of meters long; | Uniform patches of fixed location and area |
| reaches typically contain hundreds to thousands of polygonal cells up to tens of square meters in area | ||
| Decisionmaking | Trout select a cell and food resource to maximize expected fitness over a future time window | Foragers select a patch and food resource to maximize perceived fitness |
| Entities and state variables | Reaches: Daily values of flow, temperature, and turbidity | Global environment: Variables that apply across the modeled system (e.g., time of day) |
| Cells: Depth and velocity that depend on flow; static variables for availability of hiding and feeding cover and spawning gravel | Patches: Local state variables (e.g., availability of prey) | |
| Trout: Species, sex, age, length, and weight | Resources (types of food consumed by foragers, e.g., prey species and size classes): One or more components | |
| Redds (nests of trout eggs): The number of eggs, and how developed the eggs are | Components (elements of resources that are assimilated by foragers, e.g., energy): User-defined variables | |
| Foragers (animals of one or more species/types): User-defined variables such as size, energy stores | ||
| Processes (in the order executed each time step) | Habitat update: Temperature, turbidity, and cell depths and velocities are updated | Resource update: Changes in the density of patch resources caused by consumption by the foragers or other factors; changes in resource component density |
| Spawning: Any female trout ready to spawn creates a redd | Forager immigration into the system | |
| Habitat selection: Trout (from largest to smallest) select a cell and deplete its food and cover | Forager movement among patches | |
| Growth: Trout gain or lose weight and length, depending on food intake and metabolic costs | Forager consumption: Transfer of components into foragers when resources are consumed | |
| Survival: Trout may die from risks (e.g., predation, starvation) that depend on trout and habitat states | Forager physiology: Change forager component reserves due to consumption metabolic costs | |
| Redd mortality: Eggs may die due to extreme temperature and other risks | Forager emigration from the system | |
| Birth: When fully developed, eggs become new juvenile trout | Forager mortality | |
| Validation (predictions that have been compared to observed patterns) | Changes in trout distribution in response to changes in flow | Changes in biomass of prey species due to consumption by birds |
| Changes in trout distribution in response to presence of larger competitors | Range of prey species and size of prey included in bird diets | |
| Changes in distribution due to presence of piscivorous fish | Rate at which birds consume prey species from different habitats | |
| Seasonal changes in selected water velocities | Distribution of birds among intertidal habitat patches | |
| Changes in habitat selection in response to reduced food availability | Proportion of birds using terrestrial habitats to supplement food from intertidal habitats | |
| A critical period of high mortality among newly hatched juveniles | Proportion of time spent feeding by birds | |
| Fewer large trout in the absence of pools | Body mass and rate of mass gain of birds | |
| Differences in individual growth between reduced-flow and control habitat units | Mortality rate of birds during non-breeding season | |
| Population biomass above and below a flow diversion |
For inSTREAM, validation—the process of showing that a model is useful for its intended purpose—started with testing its main adaptive behavior. Modeling the habitat selection behavior of trout was a significant challenge, because conventional foraging theory was not directly applicable to this IBM (Railsback and Harvey 2013). Trout clearly adapt to changes in flow and temperature by selecting different feeding sites. However, their decisions depend on predation risk as well as energy intake, both of which depend on habitat conditions that vary unpredictably day to day, and on the numbers of other fish. Many previous IBMs included adaptive foraging behavior but assumed that foraging was driven only by growth or by risk; the few models in which both were considered achieved this via assumptions too simple for the trout model. In behavioral ecology, the theory for risk–growth trade-offs was well established but only for situations in which the future is known and unaffected by the individuals (e.g., Mangel and Clark 1986). However, in the trout IBM's population context, individuals compete for limited resources, so current decisions depend on future conditions that depend on the behavior of other individuals, as well as on unknown future flows and temperatures. The solution was to assume that model trout use the simple prediction that current conditions will persist over a future time horizon and then to select the habitat that provides the highest expected survival rates against both starvation (a function of energy intake and current weight) and predation over the time horizon (Railsback et al. 1999, Railsback and Harvey 2013). Updating the prediction and decision daily lets individuals continually adapt to changing situations.
The validation of inSTREAM's predictions was initially focused on showing that its habitat selection behavior was adequate. The model was shown to reproduce a variety of observed patterns in how trout change habitat selection in response to factors such as competition and predation, flow and temperature, and food availability (Railsback and Harvey 2002). A separate set of simulation experiments (Railsback et al. 2002) showed inSTREAM to reproduce patterns often observed at the population level, such as high rates of density-dependent mortality in early juveniles, density-dependent growth, and fewer old fish when pool habitat is rare. Parameter sensitivity and uncertainty analysis identified the parameters to which the results are sensitive but also showed that the model's relative ranking of flow management alternatives can be quite robust to parameter uncertainty (Railsback et al. 2009).
Diversification
inSTREAM has been in continual use and development since 1999 and has evolved to increase its applicability to a variety of sites and questions (table 2). It has been applied to over 20 sites, ranging from very small creeks to major rivers. Its software has been improved to automate or ease common tasks, such as setting up and running simulation experiments, and to keep up with improvements in the hydraulic models used to generate habitat input.
Table 2.
Example issues to which inSTREAM and MORPH have been applied.
| Theoretical questions | Management predictions | ||
|---|---|---|---|
| inSTREAM | MORPH | inSTREAM | MORPH |
| Adaptive trade-offs: How can we model decisions (e.g., habitat and foraging effort selection) that trade off growth and risk, when future growth and risk is unknown and subject to feedbacks of this behavior? | Decision rules: How do alternative forager decision rules (e.g., rate maximization or risk minimizing) influence their distribution and survival? | Stream flow assessment: Effects of alternative policies for flow releases from dams. | Shellfishing: Shellfishing quotas that account for biomass required by shorebirds. |
| Habitat selection modeling: How useful is habitat selection modeling for predicting population response to habitat alteration? | Competition and individual variation: How do individual variation, depletion and interference competition affect survival and distribution? | Stream temperature assessment: Effects of changes in water temperature regimes. | Disturbance from humans: Impacts of increased disturbance due to housing near the coast. |
| Food limitation: How useful is the traditional concept that food “limits” populations only when relatively scarce? | Spatial scale: When does spatial variation in food abundance and availability need to be incorporated into models? | Turbidity assessment: Effects of turbidity regimes, e.g., from alternative forest harvest management policies. | Sea level rise: Effects of future sea level rise on shorebirds via reduced habitat area. |
| Habitat restoration project design and assessment: Benefits of restoration actions such as re-shaping channels and adding spawning gravel or hiding cover. | Port development: Impacts of habitat loss caused by port development. | ||
| Flow fluctuation assessment: Effects of hydropower “load following” that causes flow to change multiple times per day. | Tidal barrages: Impacts of changes in habitat quality and tidal exposure due to tidal power barrages. | ||
| Barrier assessment: Effects of barriers that prevent trout movement up- or downstream. | Wind farms: Effects of wind farms on diving sea ducks. | ||
| Facultative anadromy: Effects of river management on production of anadromous individuals in species with individuals that decide adaptively whether to migrate to the ocean. | Bridges: Effects of bridge-construction disturbance on sea ducks. | ||
| Nuclear power stations: Effects of warm-water outflows on shorebirds via changes in prey species in intertidal habitats. | |||
| Mitigation for developments: Benefits of habitat creation to offset habitat loss or disturbance through development. | |||
Two applications required modifications that substantially diversified inSTREAM's usefulness. One new application was the prediction of trout population responses to subdaily flow releases: hourly changes in flow that allow hydropower projects to match short-term electricity demand. Modeling trout at an hourly time step required adding another adaptive behavior: how they shift between feeding and hiding behaviors between day and night. The modified model reproduced many observed patterns in such daily activity and habitat shifts (Railsback et al. 2005). The second new application turned inSTREAM into a model of the freshwater life stages of salmon, by adding the arrival at spawning sites of adults migrating from the ocean and the decision by juveniles of when to migrate out of their natal stream toward the ocean (Railsback et al. 2013).
Although inSTREAM was not originally designed as a virtual ecosystem for exploring and testing general hypotheses and assumptions, it contains enough natural complexity to be useful for this additional purpose. Perhaps the most important general contribution from inSTREAM is that the habitat selection method developed for it became an important illustration of the theory for how individuals make adaptive trade-off decisions when future conditions are uncertain and subject to feedbacks from behavior. Such a theory is crucial for modern ecological concepts, such as trait-mediated trophic interactions, but has been elusive (Railsback and Harvey 2013). inSTREAM was also used to explore how well traditional habitat selection models can predict a population's response even under ideal conditions. Habitat selection models were developed from observations of virtual trout within inSTREAM (so that the data used to model the habitat selection were 100% complete and error free) and were tested in subsequent simulation experiments in which habitat availability was manipulated. The experiment identified eight reasons for which the habitat selection models were unreliable (Railsback et al. 2003).
Railsback and Harvey (2011) tested the concept of food limitation often used by fisheries and wildlife managers: that if food is sufficiently available, it no longer limits population size. Within inSTREAM, adaptive behavior lets trout convert food availability into predation avoidance: When more food was available, the fish fed less often and in safer places and, therefore, survived longer, so the population size continued to increase. Instead of food availability reaching a level at which it no longer limited population growth, the effect of food on population size continued to increase as food availability increased.
Lessons from the individual-based ecology of coastal birds
Our second case study concerns the shorebirds and wildfowl that occur in vast numbers in coastal habitats and that have international protection. To advise on issues concerning their conservation, ecologists need to predict how changes to the environment will affect either population size or the demographic processes, such as survival rate, that determine population size.
Conceptualization
Despite the need, it has proven difficult to use traditional techniques (e.g., population models or habitat selection models) to accurately predict how changes to the environment influence either the population size or the survival rate of coastal birds (Stillman and Goss-Custard 2010). The difficulties include the fact that environmental changes to sites are often novel, and so there are rarely historical data to predict how the population size within a site will be influenced by these changes, and the fact that measuring survival in these species is complex and time consuming, which means that survival rates have been measured at relatively few sites.
IBE has proven to be an appropriate solution because population-level processes in coastal birds can be understood as arising from individual behavioral mechanisms that can be accurately measured or predicted. Furthermore, there is a good understanding of the fitness-related factors on which these species can base their decisions (e.g., starvation risk can be reduced by maximizing the rate of consuming prey). Starvation and body condition depend on the adaptive behavior of individuals (e.g., their choice of diet and feeding location); the number of birds present within a site; variations in foraging efficiency and the dominance of individuals; local competitive interactions among individuals; the area, quality, and spatial arrangement of feeding habitat; the time for which the feeding habitat is exposed by the tide; and the effects of food and competitor density on the rate at which birds consume food. IBE has been successfully applied to these species because it has been possible to accurately measure or predict these processes and to integrate them within IBMs to predict population-level responses. The important advantages of these IBMs over alternative models are that their predictions are derived from fitness-based decisionmaking, which is more likely to persist when the birds encounter novel environments than are the empirical relationships within habitat association models, and that IBMs directly predict survival and body condition, which are closely linked to factors determining population size.
Implementation
The first coastal bird IBM was developed for the Eurasian oystercatcher (Haematopus ostralegus) feeding on mussels (Mytilus edulis) during the nonbreeding season on the Exe Estuary in England (Goss-Custard et al. 1995, Stillman et al. 2000a, 2001). The Exe Estuary was chosen as the study site because of its relatively small size, accessibility, and relative isolation from neighboring estuaries. The oystercatcher was an ideal study species, because its large size and large prey make its competitive processes easy to observe. Important steps toward the development and validation of an IBM were the quantification of the food supply and its population dynamics, the measurement of individual variation in foraging efficiency and dominance and how these factors determine individual feeding rates, the measurement of seasonal changes in the spatial distribution of birds and the time at which birds move to more marginal habitats, the measurement of the nonbreeding survival of the birds (Durell et al. 2001), and the programming of the IBM.
A build-up approach was adopted for the development of the IBM. Processes and parameters needed to make the model reproduce the mortality rates observed in the wild over five calibration winters (1976–1977 through 1980–1981) were included, step-by-step, testing the accuracy of the model as each new parameter or process was included. Once the model was able to predict the observed mean mortality rate recorded over the 5 calibration years and the underlying behavior from which mortality was predicted, it was used to generate a density-dependent mortality function (Stillman et al. 2000a). The model predicted that mortality would be density dependent, and this prediction was supported when the winter mortality rate was measured in 6 subsequent years (Durell et al. 2001). This quantitative agreement between prediction and observation, in combination with the fact that density dependence was predicted by the model before its presence in nature had been demonstrated, provided strong validation of the approach.
Diversification
To allow coastal bird IBMs to be applied more rapidly to a wider range of systems, three major breakthroughs were required to cope with the following problems: model complexity, software designs that were too specific, and requirements for prohibitively large data sets.
The original IBM represented the foraging behavior of the birds in great detail. A sensitivity analysis of the model showed that many of the foraging parameters had less influence on predictions than did broader parameters representing factors such as the average rate of consuming food or overall energy demands. Furthermore, accurate values of such detailed parameters were unlikely to be available for systems less intensively studied than the Exe Estuary. Therefore, one of the main ways in which the IBM was simplified was representing foraging behavior more simply than in the original model, so fewer parameters need to be estimated for each new study system.
Several elements of the Exe Estuary and shorebirds had been hard coded into the original IBM and its software implementation; it contained shorebird-specific assumptions and assumptions that were only applicable to certain coastal sites. Therefore, new software, MORPH (figure 2b, table 1), was developed to address these limitations (Stillman 2008). MORPH contains a basic framework to describe animal physiology and foraging behavior and the distribution and abundance of resources. Its key assumption is that individuals behave to maximize a specific fitness-related factor—for example, the rate of consuming food. Allowing the user to specify this factor is a more general way of modeling decisionmaking than was used in the original model. MORPH achieves its flexibility by using equations specified by the user rather than having hard-coded equations. MORPH therefore learns about a system from the information provided to it. This adaptive design of MORPH means that new systems can be modeled without new software.
Much of the time-consuming research underlying the original IBM measured the relationship between the feeding rate of birds and the density of food and competitors; in ecology, this is called the functional response. The functional response is important because it determines how starvation and body condition are influenced by the amount of food and the population size of birds. Finding new ways to quickly parameterize the functional response allowed models to be more rapidly applied to new species and systems. The influence of competitor density on the feeding rates in different shorebird–prey systems was determined using a combination of field observations and small-scale IBMs (Stillman et al. 2000b, 2002). This combination allowed the functional response to be predicted in three ways: (1) from previous empirical studies of a range of species, (2) from a separate IBM of interference, and (3) from the foraging behavior of the bird species and the mobility and antipredator escape responses of the prey. The empirical and modeling developments allowed the functional response to be predicted for different bird species, prey, and sites without the need for many years of time-consuming fieldwork.
These breakthroughs accelerated the rate at which IBMs can be parameterized and validated for coastal and wetland bird systems. Since 2000, these IBMs have been applied to over 35 systems and have been used to advise conservationists on the potential impact of environmental changes caused by sea level rise, habitat loss, shellfishing, disturbance from humans, tidal barrages, wind farms, nuclear power stations, and changes in agriculture and hunting (table 2; Stillman et al. 2003, Stillman and Goss-Custard 2010). Meta-analyses of model predictions have been used to show the threshold amounts of shellfish food required to support oystercatchers throughout the nonbreeding system in different sites (Goss-Custard et al. 2004). Although MORPH has been mainly applied to shorebirds, it has also been applied to grazing wildfowl (Duriez et al. 2009), diving ducks, and wetland birds (Stillman and Goss-Custard 2010). Learning from previous experience has been a key factor in increasing the speed with which new models can be developed. The increased speed of model development has not come at the cost of reduced predictive ability; the models have typically been able to predict the real systems with sufficient accuracy to usefully advise conservation (Stillman and Goss-Custard 2010).
Learning from the lessons: Guidelines for individual-based ecology
We use the lessons learned from the trout and bird studies and from other applications of IBE to provide guidelines for undertaking a general research program of IBE (figure 1).
Conceptualization
IBE is an appropriate conceptual framework when variation among individuals, local interactions among individuals, adaptive behavior, or the presence of dynamic or spatially heterogeneous resources are important in determining population processes. Alternative approaches are likely to be more simplistic (e.g., population models or habitat selection models) and less dependent on mechanistic understanding. For both river trout and coastal birds, the alternative approaches essentially related the abundance (or density) of the animals to habitat features, using either empirical relationships or simple assumptions about the influence of habitat on distribution. The main advantage of IBE was that the basis of its predictions—representing individual behavior as the consequence of fitness-seeking decisions—was more likely to hold for new environmental conditions than were the empirical relationships within the statistical models. IBE is a useful conceptual framework if the processes that drive the population, such as survival and reproduction, can be understood in terms of behavioral mechanisms, which, themselves, can be measured or modeled with sufficient precision to make meaningful predictions. It is also important that the link between behavior and fitness be understood sufficiently well to incorporate appropriate decisions within the models. IBE's intended basis for predictive models are first principles, in the sense that all population and community-level structures and dynamics emerge from what individuals decide to do, which, in turn, is based on evolutionary, physiological, and physicochemical principles.
IBE is a useful conceptual framework for understanding the ecology of any fitness-seeking organism. For example, it has proven valuable for understanding the importance of individual behavior on the dynamics of plant populations and communities. Lin and colleagues (2013) used an IBM to demonstrate that changes in the population-level self-thinning trajectory are dominated by internal physiological mechanisms at the level of organisms, but only if the competition among neighboring plants is asymmetric rather than symmetric. Piou and colleagues (2008) used the KiWi IBM of Berger and Hildenbrandt (2000) to identify how mangrove forest succession patterns are determined by underlying mechanisms, including the competing theories of the tidal sorting of seeds and species physiological adaptations to nutrient availability and salinity.
Implementation
This phase puts the conceptual ideas of IBE into practice by developing an IBM and testing it to determine whether key processes are modeled well enough to make predictions useful for research questions or conservation problems. Typically, the first IBM will be developed for a relatively simple or well-studied system, for which general functional relationships are established in the literature or can be observed in a relatively straightforward manner. The programming and adaptation of the initial IBM will most likely be undertaken in parallel with data collection (which can include lab experiments on individuals, as well as field observations), incorporating new understanding of the system as it emerges. The interaction between data collection and model development is a key element of IBE: Model development is guided by empirical knowledge of the system, whereas knowledge gaps and model predictions direct data collection.
The comparison of model results to data is just as important in IBE as it is elsewhere, but IBMs also need to be validated by showing that their individual-level processes are sufficiently realistic. Validation is central to both the implementation and the diversification phases. Pattern-oriented modeling, illustrated by the validation of individual behavior and population dynamics of inSTREAM and MORPH (table 1), is a strategy for designing and testing models of complex systems by comparing the observations and predictions of multiple processes, at multiple levels, from the individual to the population and community (Grimm and Railsback 2012). IBMs should be designed to reproduce not just one but multiple patterns observed in the real system at different scales and levels of organization. Doing so reduces the risk that a model reproduces the right pattern for the wrong reason, because, in real systems, different patterns are linked to each other in ways that reflect the systems’ internal organization. Each pattern serves as a filter for falsifying unsuitable versions of submodels and unsuitable parameter combinations. In contrast, traditional population models that lack internal mechanisms are often focused only on single patterns, such as population growth rates or cycles.
Gaining a thorough understanding of behavior—and, in particular, adaptive decisionmaking—is a key step in the implementation of IBE. The trout and coastal bird IBMs were based on existing knowledge of the behavior of these well-studied species but still required new theories of decisionmaking and new data. The early phases of IBE will be more productive if they are based on species or sites for which behavioral parameters are already available, can be collected, or can be predicted from existing theory (e.g., optimal foraging behavior, state-dependent trade-offs). However, an alternative is to evolve behavioral rules in the computer, assuming that rules conveying fitness to simulated individuals adequately represent the naturally evolved behaviors of real organisms (e.g., Giske et al. 1998, Huse and Giske 1998, Bauer and Klaassen 2013).
Diversification
Near the end of the implementation phase, a parameterized and validated IBM will exist for one or more systems. Two possible issues exist, however. The IBM may be relatively complicated and overfitted to the test system, and it may contain parameters or processes that cannot be measured in other systems with reasonable effort. In contrast, early IBMs can also lack processes that are important in other systems to which they could otherwise be applied. Sensitivity and robustness analyses are therefore required to determine the extent to which the IBM can be simplified without adversely affecting its predictive power or how it must include more detail to be more general (Evans et al. 2013). Sensitivity analyses are used to examine the sensitivity of model results to uncertainties in model parameters. Robustness analysis varies and simplifies model structure and processes to identify essential and inessential elements of the model. The need for robustness analysis has long been known (Grimm 1999, Grimm and Railsback 2005) but is yet to be fully acknowledged. It certainly requires resources but pays off in making models more generally applicable. Continued validation throughout the diversification phase is key and must not only compare the final results to data but also show that individual-level mechanisms are realistic enough to be useful.
The need for an eventual payoff is important, because it increases the productivity of IBE by increasing the speed and efficiency with which IBMs can be applied to an increasing number of systems and problems. In fact, the practice of IBE has already produced protocols, tools, and knowledge that have become widely used and accepted as making research and applications much more efficient and effective (table 3). Software tools for IBE have improved rapidly. Projects such as the ones that we profiled here have produced software, such as MORPH, that are applicable to wide classes of systems. At the same time, general IBM platforms, such as NetLogo (http://ccl.northwestern.edu/netlogo) have evolved with the practice of IBE to allow even inexperienced programmers to develop powerful software for IBMs rapidly (Railsback and Grimm 2012).
Table 3.
Major developments that have made individual-based modeling (IBM) and ecology (IBE) more productive.
| Development | Benefits |
|---|---|
| ODD protocol for describing IBMs (Grimm et al. 2010); TRACE (Grimm et al. 2014) protocol for documenting model development, analysis, and application | Standardized and thorough methods for describing IBMs and the modeling process make models and their results easier to understand and replicate; protocols also improve model design by providing a comprehensive list of concepts that need to be considered. |
| Pattern oriented modeling (Grimm and Railsback 2005, 2012) | Provides an efficient strategy based on observed patterns for designing IBMs, developing theory and submodels for individual traits, and parameterizing models; validates models by comparing results to multiple patterns observed at levels from individual behavior to population or community processes making them more likely to capture essential mechanisms of the real system. |
| IBM software platforms | Compared with using general programming languages, IBMs can be programmed more rapidly, by users with less experience, and with more built-in observation and analysis tools. |
| Generalized IBM software | Whole classes of IBM can be developed rapidly; models do not need to be recoded for each new study system; only the parameters need to be changed. |
| Standardized submodels | IBM components such as behavioral traits, energy budgets, and interactions are standardized and can be implemented by just changing parameter values. |
Even more important than reusable and general software tools is reusable and general theory for individual-level processes, such as adaptive behavior, physiology and energetics, and interaction. For such processes, the early practice of IBE has typically required the development and testing of theory that is just complex enough to produce useful system-level dynamics; population ecology lacks such theory, whereas theory from behavioral ecology is often too limited in scope to be useful in IBMs (Railsback and Harvey 2013). Theory for IBE is now becoming available, making future studies more efficient and models more reliable. The development of theory for adaptive trade-offs between growth and risk, for example, was an important element of our trout case study.
Energetics are often critical for IBMs, because energy allocation is a key way in which organisms relate behavior (e.g., foraging rates) to fitness (growth to reduce predation risk, energy storage to avoid starvation, or offspring production). In the trout and shorebird models, different approaches were used to represent the individuals’ energy budget, but energy budget theories exist that are more generally applicable (Sibly et al. 2013). Dynamic energy budget theory (e.g., Sousa et al. 2010) is one such approach; in this case, standard equations are used for growth, maturation, and reproduction. Species differ only in parameter values, which can be determined from observations of individuals under different conditions. Generic software has been developed to implement this theory in IBMs (Martin et al. 2013).
Standard theory for interaction among individuals has also been developed. In many IBMs of plant systems, size-dependent circular zones are used to describe individual plants and their overlap as a way to represent competition for resources with neighboring plants (e.g., Monserud 1976, Schwinning and Weiner 1998). This zone-of-influence concept is well established in plant ecology and used to describe both above- (e.g., Lin et al. 2012) and below-ground competition (Lin et al. 2013). The field-of-neighborhood (figure 2c) theory (Berger and Hildenbrandt 2000) accounts for variation in the strength of competition within the zone of influence and has been used to model competition in plants (Bauer et al. 2002) and even in territorial animals (Piou et al. 2007). In individual-based forest gap models, vertical competition for light has been modeled in a standardized way for over four decades (Botkin et al. 1972, Liu and Ashton 1995, Bugmann 2001). Theory for interference competition in foraging animals has been based on kleptoparasitic (Stillman et al. 2002, Rappoldt et al. 2010) and prey disturbance interaction distances (Stillman et al. 2000b). Predation and competition for food and habitat are other kinds of interaction for which standard models are emerging (Railsback and Grimm 2012).
A vision for individual-based ecology
Especially in its early phases, IBE can require more effort, longer time frames, and more skills than traditional population modeling. There are at least two ways that the seemingly higher effort for IBE is offset to make this approach efficient and effective. First, IBE can produce models that are more general and reusable than models based more exclusively on empirical parameters. The empirical approach requires that population size and environmental conditions vary enough within the study period and that they not be strongly correlated with each other. In many species, it is difficult or impossible to measure the required population processes over a long enough time period. In contrast, once IBE reaches the third phase, diversification, IBMs based on established individual-level theory can be implemented, validated, and used in a fraction of the time needed to develop an empirical population model (Stillman and Goss-Custard 2010).
The second way in which IBE projects can be efficient is by having a high payoff. We consider IBE to be a research program in which virtual laboratories—the IBMs—are set up, calibrated, tested, and made to work. Once this laboratory exists, a wide range of specific and general questions can be answered very efficiently. The investment to get to this productive stage will, we expect, continue to decrease as long as it remains a goal to help others set up their own lab with much less effort. Producing flexible software tools and standard theory for behavior, physiology, and interaction seem to be the most promising ways forward. Getting more IBE projects into the diversification phase is therefore a priority for making ecology more predictive.
Individual-based modeling has matured over the last 25 years. Our case studies show how this approach, if it is embedded in a research program of IBE, can lead to models that are more flexible and predictive than traditional population models. Therefore, IBE implements the recommendations of Rose (2000) to combine IBMs with theory and empirical research and provides a research paradigm and tools to support biodiversity conservation in a rapidly changing world.
Acknowledgments
The trout and coastal bird IBMs were developed as part of collaborative research programs, and we are very grateful to the many contributors to these projects, including Bret Harvey, Steve Jackson, Jason White, John Goss-Custard, Richard Caldow, Sarah Durell, Selwyn McGrorty, Andy West. We thank three reviewers for their valuable comments.
References cited
- Bauer S, Klaassen M. Mechanistic models of animal migration behaviour their diversity, structure and use. Journal of Animal Ecology. 2013;82:498–508. doi: 10.1111/1365-2656.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S, Berger U, Hildenbrandt H, Grimm V. Cyclic dynamics in plant populations. Proceedings of the Royal Society B. 2002;269:2443–2450. doi: 10.1098/rspb.2002.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger U, Hildenbrandt H. A new approach to spatially explicit modelling of forest dynamics: Spacing, ageing and neighbourhood competition of mangrove trees. Ecological Modelling. 2000;132:287–302. [Google Scholar]
- Botkin DB, Janak JF, Wallis JR. Some ecological consequences of a computer model of forest growth. Journal of Ecology. 1972;60:849–872. [Google Scholar]
- Bovee KD, Lamb BL, Bartholow JM, Stalnaker CB, Taylor J, Henriksen J. Stream Habitat Analysis Using the Instream Flow Incremental Methodology. US Geological Survey, Biological Resources Division. 1998. Report no. USGS/BRD-1998–0004.
- Bugmann H. A review of forest gap models. Climatic Change. 2001;51:259–305. [Google Scholar]
- DeAngelis DL, Cox DK, Coutant CC. Cannibalism and size dispersal in young-of-the-year largemouth bass: Experiment and model. Ecological Modelling. 1980;8:133–148. [Google Scholar]
- DeAngelis DL, Gross LJ, Huston MA, Wolff WF, Fleming DM, Comiskey EJ, Sylvester SM. Landscape modeling for Everglades ecosystem restoration. Ecosystems. 1998;1:64–75. [Google Scholar]
- Durell SEAlVd, Goss-Custard JD, Stillman RA, West AD. The effect of weather and density-dependence on oystercatcher Haematopus ostralegus winter mortality. Ibis. 2001;143:498–499. [Google Scholar]
- Duriez O, Bauer S, Destin A, Madsen J, Nolet B, Stillman R, Klaassen M. What decision rules might pink-footed geese use to depart on migration? An individual-based model. Behavioural Ecology. 2009;20:560–569. [Google Scholar]
- [EPRI] Electric Power Research Institute Instream Flow Assessment Methods: Guidance for Evaluating Instream Flow Needs in Hydropower Licensing. EPRI. 2000. Report no. TR-1000554.
- Evans MR. Modelling ecological systems in a changing world. Philosophical Transactions of the Royal Society B. 2012;367:181–190. doi: 10.1098/rstb.2011.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MR, et al. Do simple models lead to generality in ecology? Trends in Ecology and Evolution. 2013;28:578–583. doi: 10.1016/j.tree.2013.05.022. [DOI] [PubMed] [Google Scholar]
- Giske J, Huse G, Fiksen Ø. Modelling spatial dynamics of fish. Reviews in Fish Biology and Fisheries. 1998;8:57–91. [Google Scholar]
- Giske J, Eliassen S, Fiksen Ø, Jakobsen PJ, Aksnes DL, Jørgensen C, Mangel M. Effects of the emotion system on adaptive behavior. American Naturalist. 2013;182:689–703. doi: 10.1086/673533. [DOI] [PubMed] [Google Scholar]
- Goss-Custard JD, Caldow RWG, Clarke RT, Durell SEAlVd, Sutherland WJ. Deriving population parameters from individual variations in foraging behaviour. 1. Empirical game-theory distribution model of oystercatchers Haematopus ostralegus feeding on mussels Mytilus edulis. Journal of Animal Ecology. 1995;64:265–276. [Google Scholar]
- Goss-Custard JD, Stillman RA, West AD, Caldow RWG, Triplet P, Durell S, McGrorty S. When enough is not enough: Shorebirds and shellfishing; Proceedings of the Royal Society B; 2004. pp. 233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm V. Ten years of agent-based modelling in ecology: What have we learned and what could we learn in the future? Ecological Modelling. 1999;115:129–148. [Google Scholar]
- Grimm V, Railsback SF. Individual-Based Modeling and Ecology. Princeton University Press; 2005. [Google Scholar]
- Grimm V, Railsback SF. Pattern-oriented modelling: A ‘multiscope’ for predictive systems ecology. Philosophical Transactions of the Royal Society B. 2012;367:298–310. doi: 10.1098/rstb.2011.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm V, Berger U, DeAngelis DL, Polhill G, Giske J, Railsback SF. The ODD protocol: A review and first update. Ecological Modelling. 2010;221:2760–2768. [Google Scholar]
- Grimm V, et al. Towards better modelling and decision support: Documenting model development, testing, and analysis using TRACE. Ecological Modelling. 2014;280:129–139. [Google Scholar]
- Hellweger FL, Bucci V. A bunch of tiny individuals—Individual-based modeling for microbes. Ecological Modelling. 2009;220:8–22. [Google Scholar]
- Hughes NF. Selection of positions by drift-feeding salmonids in dominance hierarchies: Model and test for arctic grayling (Thymallus arcticus) in subarctic mountain streams, interior Alaska. Canadian Journal of Fisheries and Aquatic Sciences. 1992;49:1999–2008. [Google Scholar]
- Huse G, Giske J. Ecology in Mare Pentium: an individual based model for fish with adapted behavior. Fisheries Research. 1998;37:163–178. [Google Scholar]
- Huse G, Strand E, Giske J. Implementing behavior in individual-based models using artificial neural networks and genetic algorithms. Evolutionary Ecology. 1999;13:469–483. [Google Scholar]
- Kreft J-U, et al. Mighty small: Observing and modeling individual microbes becomes big science; Proceedings of the National Academy of Sciences; 2013. pp. 18027–18028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Berger U, Grimm V, Ji Q-R. Differences between symmetric and asymmetric facilitation matter: Exploring the interplay between modes of positive and negative plant interactions. Journal of Ecology. 2012;100:1482–1491. [Google Scholar]
- Lin Y, Berger U, Grimm V, Huth F, Weiner J. Plant interactions alter the predictions of metabolic scaling theory. PLOS ONE. 2013;8 doi: 10.1371/journal.pone.0057612. art. e57612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ashton PS. Individual-based simulation models for forest succession and management. Forest Ecology and Management. 1995;73:157–175. [Google Scholar]
- Łomnicki A. Individual differences between animals and the natural regulation of their numbers. Journal of Animal Ecology. 1978;47:461–475. [Google Scholar]
- Mangel M, Clark CW. Toward a unified foraging theory. Ecology. 1986;67:1127–1138. [Google Scholar]
- Martin B, Jager T, Nisbet RM, Preuss TG, Grimm V. Predicting population dynamics from the properties of individuals: A cross-level test of Dynamic Energy Budget theory. American Naturalist. 2013;181:506–519. doi: 10.1086/669904. [DOI] [PubMed] [Google Scholar]
- Monserud RA. Simulation of forest tree mortality. Forest Science. 1976;22:438–444. [Google Scholar]
- Piou C, Berger U, Hildenbrandt H, Grimm V, Diele K, D'Lima C. Simulating cryptic movements of a mangrove crab: Recovery phenomena after small scale fishery. Ecological Modelling. 2007;205:110–122. [Google Scholar]
- Piou C, Berger U, Hildenbrandt H, Feller IC. Testing the intermediate disturbance hypothesis in species-poor systems: A simulation experiment for mangrove forests. Journal of Vegetation Science. 2008;19:417–424. [Google Scholar]
- Railsback SF. Concepts from complex adaptive systems as a framework for individual-based modelling. Ecological Modelling. 2001;139:47–62. [Google Scholar]
- Railsback SF, Grimm V. Agent-Based and Individual-Based Modeling: A Practical Introduction. Princeton University Press; 2012. [Google Scholar]
- Railsback S, Harvey B. Analysis of habitat selection rules using an individual-based model. Ecology. 2002;83:1817–1830. [Google Scholar]
- Railsback S, Harvey B. Importance of fish behaviour in modelling conservation problems: Food limitation as an example. Journal of Fish Biology. 2011;79:1648–1662. doi: 10.1111/j.1095-8649.2011.03050.x. [DOI] [PubMed] [Google Scholar]
- Railsback S, Harvey B. Trait-mediated trophic interactions: Is foraging theory keeping up? Trends in Ecology and Evolution. 2013;28:119–125. doi: 10.1016/j.tree.2012.08.023. [DOI] [PubMed] [Google Scholar]
- Railsback SF, Lamberson RH, Harvey BC, Duffy WE. Movement rules for spatially explicit individual-based models of stream fish. Ecological Modelling. 1999;123:73–89. [Google Scholar]
- Railsback S, Harvey B, Lamberson R, Lee D, Clausen N, Yoshihara S. Population-level analysis and validation of an individual-based cutthroat trout model. Natural Resource Modeling. 2002;14:465–474. [Google Scholar]
- Railsback SF, Stauffer HB, Harvey BC. What can habitat preference models tell us? Tests using a virtual trout population. Ecological Applications. 2003;13:1580–1594. [Google Scholar]
- Railsback SF, Harvey BC, Hayse JW, LaGory KE. Tests of theory for diel variation in salmonid feeding activity and habitat use. Ecology. 2005;86:947–959. [Google Scholar]
- Railsback SF, Harvey BC, Jackson SK, Lamberson RH. InSTREAM: The Individual-Based Stream Trout Research and Environmental Assessment Model. US Department of Agriculture Forest Service, Pacific Southwest Research Station. 2009. Report no. PSW-GTR-218.
- Railsback SF, Gard M, Harvey BC, White JL, Zimmerman JKH. Contrast of degraded and restored stream habitat using an individual-based salmon model. North American Journal of Fisheries Management. 2013;33:384–399. [Google Scholar]
- Rappoldt C, Stillman RA, Ens BJ. A geometrical model for the effect of interference on food intake. Ecological Modelling. 2010;221:147–151. [Google Scholar]
- Rose KA. Why are quantitative relationships between environmental quality and fish populations so elusive? Ecological Applications. 2000;10:367–385. [Google Scholar]
- Schwinning S, Weiner J. Mechanisms determining the degree of size asymmetry in competition among plants. Oecologia. 1998;113:447–455. doi: 10.1007/s004420050397. [DOI] [PubMed] [Google Scholar]
- Sibly RM, Grimm V, Martin BT, Johnston ASA, Kulakowska K, Topping CJ, Calow P, Nabe-Nielsen J, Thorbek P, DeAngelis DL. Representing the acquisition and use of energy by individuals in agent-based models of animal populations. Methods in Ecology and Evolution. 2013;4:151–161. [Google Scholar]
- Sousa T, Domingos T, Poggiale J-C, Kooijman SALM. Dynamic energy budget theory restores coherence in biology. Philosophical Transactions of the Royal Society B. 2010;365:3413–3428. doi: 10.1098/rstb.2010.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman RA. MORPH: An individuals-based model to predict the effect of environmental change on foraging animal populations. Ecological Modelling. 2008;216:265–276. [Google Scholar]
- Stillman RA, Goss-Custard JD. Individual-based ecology of coastal birds. Biological Reviews. 2010;85:413–434. doi: 10.1111/j.1469-185X.2009.00106.x. [DOI] [PubMed] [Google Scholar]
- Stillman RA, Goss-Custard JD, West AD, Durell S, Caldow RWG, McGrorty S, Clarke RT. Predicting mortality in novel environments: tests and sensitivity of a behaviour-based model. Journal of Applied Ecology. 2000a;37:564–588. [Google Scholar]
- Stillman RA, Goss-Custard JD, Alexander MJ. Predator search pattern and the strength of interference through prey depression. Behavioral Ecology. 2000b;11:597–605. [Google Scholar]
- Stillman RA, et al. Predicting shorebird mortality and population size under different regimes of shellfishery management. Journal of Applied Ecology. 2001;38:857–868. [Google Scholar]
- Stillman RA, Poole AE, Goss-Custard JD, Caldow RWG, Yates MG, Triplet P. Predicting the strength of interference more quickly using behaviour-based models. Journal of Animal Ecology. 2002;71:532–541. [Google Scholar]
- Stillman RA, et al. An individual behaviour-based model can predict shorebird mortality using routinely collected shellfishery data. Journal of Applied Ecology. 2003;40:1090–1101. [Google Scholar]
- Van Winkle W, Jager HI, Railsback SF, Holcomb BD, Studley TK, Baldrige JE. Individual-based model of sympatric populations of brown and rainbow trout for instream flow assessment: Model description and calibration. Ecological Modelling. 1998;110:175–207. [Google Scholar]


