Abstract
Introduction:
Calcified parenchymal neurocysticercosis (NCC) lesions are commonly detected in many individuals with refractory epilepsy. However, the relationship between these lesions and epilepsy is not fully determined. We sought to determine if calcified parenchymal NCC demonstrated topographic congruence with epileptiform activity in refractory epilepsy patients. Additional patients with other structural brain lesions were included for comparison.
Subjects and Methods:
Retrospective cross-sectional analysis of all patients treated at a community-based neurology clinic for refractory epilepsy during a 3-month period and with structural brain lesions detected by neuroimaging studies.
Results:
A total of 105 patients were included in the study, including 63 with calcified parenchymal NCC lesions and 42 with other structural brain lesions. No significant relationship was detected between hemispheric localization of calcified parenchymal NCC lesions and epileptiform activity. For those with other structural brain lesions, the hemispheric localization was significantly related to the side of epileptiform activity (Chi-square = 11.13, P = 0.025). In addition, logistic regression models showed that those with right-sided non-NCC lesions were more likely to have right-sided epileptiform activity (odds ratio = 4.36, 95% confidence interval [CI] =1.16–16.31, P = 0.029), and those with left-sided non-NCC lesions were more likely to have left-sided epileptiform activity (odds ratio = 7.60, 95% CI = 1.89–30.49, P = 0.004).
Conclusion:
The lack of correlation between the side of calcified parenchymal NCC lesions and the side of the epileptiform activity suggests that these lesions may be incidental findings in many patients.
Keywords: Epilepsy, etiology, neurocysticercosis
INTRODUCTION
Neurocysticercosis (NCC) is a common helminthic infection that is increasing in frequency in nonendemic countries.[1,2,3] Treatment with anthelmintics is considered useful to kill viable cysts,[4,5,6] but while antihelminthic treatment may not benefit the more commonly detected calcified parenchymal lesions that are typically considered to be remnants of already deceased organisms, when these lesions are epileptogenic, then treatment with antiepileptic medications is indicated.[7,8,9,10,11] The underlying mechanisms causing epileptogenicity of calcified parenchymal lesions are somewhat controversial.[3,12] Furthermore, seizures are often attributed to calcified parenchymal lesions without sufficient information to determine if the location of the lesion is congruent with the location of epileptic activity, and when comparisons are made, the topographic co-localization of these lesions with epileptic activity is typically poor.[13,14]
Consequently, we hypothesized that the relationship between the hemispheric localization of epileptiform activity and calcified parenchymal NCC lesions would be weaker than in other types of structural brain lesions. A reduced congruency between the side of calcified parenchymal NCC lesions and the side of epileptic activity would indicate the etiologic relationship between these lesions and seizures are potentially less robust than with other types of brain lesions.
SUBJECTS AND METHODS
Participants were adults, over the age of 18 years, who presented sequentially to a community-based neurology clinic during a 3-month period from October 1st, 2013 to December 30th, 2013, for evaluation or treatment of epilepsy. De-identified data were obtained and analyzed in a retrospective fashion for all participants. Local Institutional Review Board approval was obtained. Informed consent was waived since the project consisted of a retrospective analysis of de-identified clinical data. Demographic information and clinical factors were determined by a review of chart notes and International Classification of Diseases-9 codes.
Diagnosis of brain parenchymal NCC infection was made according to the published guidelines.[15] All the patients with NCC lesions included in this study met clinical diagnostic criteria for probable NCC based on the combination of neuroimaging findings of one or more punctuate calcifications located in the brain parenchyma without alternative etiologic explanation, presence of neurologic complications such as a headache or epilepsy and membership in a population in which NCC is endemic.[15] Other structural brain lesions were determined to be present, if noted, in the radiology report as determined by retrospective chart review.
For all participants, retrospective review of results of neuroimaging studies, either computed tomography (CT) or magnetic resonance imaging (MRI) and results of electroencephalogram (EEG) tests were used to determine the side of the structural brain lesion and the side of epileptiform activity. Brain CT images were obtained using a Siemens Somatom 64 channel multidetector CT scanner, images were volumetrically acquired through the brain without contrast. Axial, coronal, and sagittal reconstructions were performed. Brain MRI images were obtained using a GE Sigma 1.5 Tesla scanner. In all participants, coronal and sagittal T1 and T2 images were acquired without contrast and with a slice thickness of 5 mm. For all patients in which the hemisphere of the structural lesion did not match the hemisphere of the epileptiform activity, the neuroimaging scans and the EEG tracing were reviewed by one of the authors (A.M.) to confirm the accuracy of the dictated reports.
The participants were divided into groups based on structural neuroimaging results. Nonparametric demographic data were compared between groups using the Chi-square test, and mean values for continuous demographic factors and other continuous variables were compared between groups using two-tailed t-tests. Chi-square test was then used to compare the hemisphere containing the structural brain lesions with the hemisphere showing epileptiform activity on EEG. Separate multivariate logistic regression models were then constructed for refractory epilepsy patients with NCC and other structural brain lesions to determine odds ratios describing the relationship between lesion side and side of epileptiform EEG activity.
For lateralization calculations, two ordinal variables were created, one for right-sided calcifications and one for left-sided calcifications. These ordinal variables were then given values corresponding to either present or absent for the presence of a calcification on that side. Hence, patients with bilateral calcifications would have values of present for both the right- and left-sided variables. This allowed for the greatest chance of detection of a relationship between the side of calcification and the side of epileptiform activity. The multivariate logistic regression models used the side of abnormal EEG activity as the outcome variable, lesion side as the predictor variable, and gender and alcohol/illicit substance use were included as additional independent variables in the models as well. All statistical calculations were performed using SPSS version 22 (IBM Corp., IBM SPSS statistics for windows, Version 22.0., Armonk, NY: IBM Corp.).
RESULTS
There were 583 patients who were evaluated during the study period. A total of 393 had to be excluded because they were seen for neurologic conditions other than epilepsy, 30 additional patients were excluded due to a lack of neuroimaging results, and 55 were excluded due to no structural brain abnormalities detected on neuroimaging results; leaving a total of 105 patients who were included in the study. The 105 patients who were included in the study were approximately evenly distributed between the groups, with 63 having neuroimaging findings consistent with calcified parenchymal NCC and 42 with other structural brain lesions [Table 1]. Non-NCC structural brain lesions included: Tumors (n = 8), stroke (n = 8), vascular malformations (n = 4), trauma (n = 14), and developmental abnormalities (n = 8). In addition, a total of 11 participants (17.46%) with NCC had lesions with some surrounding edema.
Table 1.
Demographic and clinical information in epilepsy patients with calcified parenchymal neurocysticercosis and other structural brain lesions
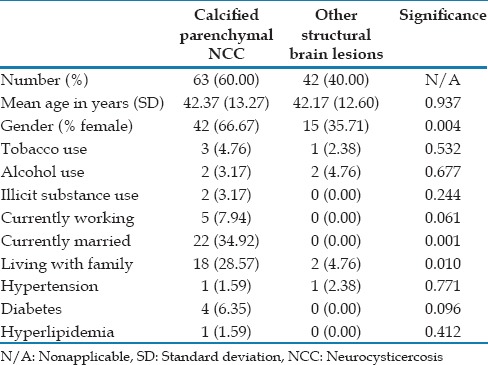
No significant difference in mean age was detected between the groups [Table 1]. The groups also did not differ significantly in the use of tobacco, alcohol, or illicit substances, which could potentially affect seizure thresholds [Table 1]. In addition, the groups did not differ significantly in frequency of comorbid medical conditions such as hypertension, diabetes, and hyperlipidemia [Table 1]. However, the groups did differ significantly in gender distribution, with more females present in the group with NCC compared to the other types of structural lesions [Table 1]. In addition, participants with calcified parenchymal NCC lesions were more likely to be currently married, currently working, and living with family than those with other types of structural brain lesions, possibly related to the differences in gender distribution between the groups [Table 1]. The majority of the participants (n = 97) had diagnoses of complex partial seizures, some with and some without a history of secondary generalization. A total of 8 patients were diagnosed with primary generalized epilepsy. Because all the participants were recruited from our active clinic patients who were currently being treated for epilepsy, all patients were being treated with antiepileptic drugs, and all had active epilepsy as defined as having had at least one seizure within the last calendar year.
A total of 32 participants with NCC displayed multiple calcifications and a total of 31 participants displayed only a single calcification. Distribution of lesions among the cerebral lobes for participants with NCC was as follows: Frontal = 43 (68.25%); parietal = 36 (57.14%); temporal = 32 (50.79%); occipital = 3 (4.76%); interventricular = 3 (4.76%); and basal ganglia = 4 (6.35%). Distribution of lesions among the cerebral lobes for participants with other structural lesions was as follows: Frontal = 31 (73.81%); parietal = 9 (14.29%); temporal = 11 (26.19%); occipital = 5 (11.90%); and basal ganglia = 3 (7.14%).
For the main clinical outcome of interest, Chi-square analysis showed that the hemisphere in which epileptiform activity was detected was significantly associated with the hemispheric localization of non-NCC structural brain lesions (Chi-square = 11.13, P = 0.025), but not with the hemispheric localization of calcified parenchymal NCC lesions (Chi-square = 2.57, P = 0.632). Logistic regression models also showed no significant relationship between hemispheric localization of calcified parenchymal NCC lesions and epileptiform activity, with the odds ratio for right-sided lesions being associated with right-sided epileptiform activity being 1.936 (95% confidence interval [CI] =0.582–6.443, P = 0.282) and for left-sided lesions being associated with left-sided epileptiform activity being 1.038 (95% CI = 0.331–3.257, P = 0.948). Conversely, for non-NCC structural brain lesions, the logistic regression models showed that the hemispheric localization of the lesion was significantly related to the hemispheric localization of the epileptiform activity for both right and left hemisphere lesions, with right-sided lesions being more likely to be associated with right-sided epileptiform activity (odds ratio = 4.36, 95% CI = 1.16–16.3, P = 0.029) and left-sided lesions being more likely to be associated with left-sided epileptiform activity (odds ratio = 7.60, 95% CI = 1.89–30.49, P = 0.004).
DISCUSSION
In this study, we identified a significant relationship between hemispheric localization of non-NCC structural brain lesions and brain epileptiform activity, but failed to find a similar relationship for calcified parenchymal NCC lesions. These findings suggest that epileptiform activity co-localizes with calcified parenchymal NCC lesions less often than with other structural brain lesions, and consequently, these lesions may be relatively less epileptogenic than other types of structural brain lesions. In addition, these findings do not support spread to adjacent brain areas of increased epileptic susceptibility as an explanation for the lack of topographic co-localization between calcified parenchymal lesions and epileptic activity.
In many individuals, NCC manifests as a relatively benign disease; however, when epilepsy does develop in some cases, it can be difficult to achieve control of the patient's seizures and some of these patients may become refractory to treatment with antiepileptic medications.[12] This dichotomy, in which calcified parenchymal lesions appear to be relatively benign incidental findings in some individuals and sources of refractory seizures in others makes determining a single optimal treatment for all individuals difficult and it is likely that a more individualized approach will be necessary. Consequently, more research is needed to determine what factors predict when calcified parenchymal NCC lesions are likely to be the source of refractory seizures that warrant aggressive treatment and when they are likely to merely be incidental and nonconsequential findings.
However, before predictive factors can be established, it is necessary to first have a reliable method for determining when comorbid calcified parenchymal lesions are etiologically related to seizures. One method for establishing evidence of an etiologic relationship would be topographic co-localization, where the location of the calcified parenchymal lesion approximates the location of seizure activity. Unfortunately, the literature yields few reports on co-localization of these lesions with epileptic activity and the reports consist of two relatively small case series that have found poor congruence between radiologic location of the lesion and electroclinical localization of the epileptic activity, with a positive correlation being present only 33–55% of the time.[13,14]
The larger of the two studies, published in 2000 by Singh et al., evaluated EEG and CT data from 40 patients and found hemispheric co-localization in 22 (55%) patients.[13] This study was limited by a high rate (30%) of inadequate EEG measurements that did not allow for determination of the location of epileptic activity.[13] Furthermore, 12.5% of the patients in the study displayed primary generalized seizures rather than focal onset seizures, and consequently, a focal lesion could not be considered to be an adequate explanation for the generalized epileptic activity present in these patients.[13]
An earlier report by Cukiert et al. in 1994 presented similar results from a series of 34 patients.[14] This study divided the patients into two groups, one group with partial onset seizures and a second group with generalized epilepsies.[14] The results for the first group showed that 15 of the 29 patients (51.7%) displayed a positive correlation between the side of the lesion and side of the epileptic activity, while 14 of the 29 patients (48.3%) displayed a negative correlation.[14] For the second group, as expected, no correlation could be established for the patients who had generalized epilepsies.[14] In addition, when they looked at only patients with single calcific lesions (n = 21), they found that 67% actually had a negative correlation between the side of the lesion and the side of the epileptic activity.[14] This group is particularly interesting because when evaluating patients with only a single calcific lesion, it would necessarily be restricted to only one brain region and thereby reduce the chance of serendipitous associations. Consequently, if the results from the group with single lesions are considered to be the most accurate representation, then a correlation between lesion location and epileptic activity would be present only about one-third of the time,[14] indicating that for the majority of patients with comorbid epilepsy and calcified parenchymal lesions, these lesions are not etiologically related to the epileptic activity.
The lack of co-localization between calcified parenchymal lesions and epileptic activity may indicate that the direct stimulation of surrounding brain tissue by the foreign body is a less likely explanation for continued epileptogenicity of these lesions. Previously published theories describing how calcified parenchymal lesions may initiate seizures have included essentially two types of processes: (1) Intermittent activation of the immune system causing a lowered seizure threshold and (2) direct toxicity either from calcium or granuloma formation.[12] Because direct toxicity as the mechanism of epileptogenicity would be expected to consistently produce co-localizing epileptic activity through direct stimulation of surrounding tissue, this would be less likely to explain the frequent lack of topographic correlation identified in this and other studies.[13,14] The alternative process, whereby intermittent release or recognition of residual antigens induces an immune response that lowers the seizure threshold would inherently be less likely to produce co-localizing seizures, since reduction of the seizure threshold would affect the propensity for generation of seizures across the entire brain and consequently increase the odds for occurrence of seizures that do not co-localize with the lesion location.
This study has several limitations. First, the retrospective nature of the study did not allow for a direct determination of causality between the presence of parenchymal NCC and the patient's epilepsy. In addition, for the diagnosis of calcified parenchymal NCC, we relied on the results of neuroimaging studies without serologic confirmation which may have increased the rate of false attribution of calcified parenchymal lesions to NCC. However, while the lack of serological confirmation may have resulted in other etiologies of parenchymal calcifications inadvertently being attributed to NCC, this is consistent with clinical practice and the current diagnostic guidelines, and in many cases, patients with calcified NCC may have negative serology results and so the diagnostic sensitivity would not likely have improved significantly by having this information.[15] Consequently, this would increase the value of this study to treat clinicians who commonly make a diagnosis of calcified parenchymal NCC in this manner. Another limitation is the lack of information regarding previous treatment with antihelminthic medications which may have decreased the rate of development of chronic epilepsy in patients with NCC. However, because the patients included in this study all suffered from refractory epilepsy, this is not expected to have affected the results or conclusions of this study.
CONCLUSION
As the rates of new NCC cases continue to rise, it is important to continue to evaluate the relationship between all forms of this disease and epilepsy. Already, parenchymal NCC lesions are identified in patients presenting to Emergency Rooms for seizures and epilepsy with increasing frequency,[16] and as this trend continues, the frequency of incidentally detected calcified parenchymal lesions should also be expected to increase as well. This raises the issue that many of these lesions may be merely unassociated bystanders that are present in people with epilepsy due to other etiologies, and greater care should be taken when attributing the etiology of a patient's seizures to these lesions. Overall, the results of this study suggest that not all patients with comorbid epilepsy and calcified parenchymal lesions actually have seizures that can be attributed directly to the NCC infection. Consequently, additional research is needed to further clarify the relationship between parenchymal and other forms of NCC with the development of refractory epilepsy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Garcia HH. Cysticercosis Working Group in Peru. Neurocysticercosis in immigrant populations. J Travel Med. 2012;19:73–5. doi: 10.1111/j.1708-8305.2011.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montano SM, Villaran MV, Ylquimiche L, Figueroa JJ, Rodriguez S, Bautista CT, et al. Neurocysticercosis: Association between seizures, serology, and brain CT in rural Peru. Neurology. 2005;65:229–33. doi: 10.1212/01.wnl.0000168828.83461.09. [DOI] [PubMed] [Google Scholar]
- 3.Earnest MP, Reller LB, Filley CM, Grek AJ. Neurocysticercosis in the United States: 35 cases and a review. Rev Infect Dis. 1987;9:961–79. doi: 10.1093/clinids/9.5.961. [DOI] [PubMed] [Google Scholar]
- 4.García HH, Evans CA, Nash TE, Takayanagui OM, White AC, Jr, Botero D, et al. Current consensus guidelines for treatment of neurocysticercosis. Clin Microbiol Rev. 2002;15:747–56. doi: 10.1128/CMR.15.4.747-756.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baird RA, Wiebe S, Zunt JR, Halperin JJ, Gronseth G, Roos KL. Evidence-based guideline: Treatment of parenchymal neurocysticercosis: Report of the guideline development subcommittee of the American Academy of Neurology. Neurology. 2013;80:1424–9. doi: 10.1212/WNL.0b013e31828c2f3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu W, Jia F, Wang W, Huang Y, Huang Y. Antiparasitic treatment of cerebral cysticercosis: Lessons and experiences from China. Parasitol Res. 2013;112:2879–90. doi: 10.1007/s00436-013-3459-3. [DOI] [PubMed] [Google Scholar]
- 7.Del Brutto OH. Neurocysticercosis: New thoughts on controversial issues. Curr Opin Neurol. 2013;26:289–94. doi: 10.1097/WCO.0b013e32836027fa. [DOI] [PubMed] [Google Scholar]
- 8.Abba K, Ramaratnam S, Ranganathan LN. Anthelmintics for people with neurocysticercosis. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD000215.pub4. CD000215. doi: 10.1002/14651858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramírez-Zamora A, Alarcón T. Management of neurocysticercosis. Neurol Res. 2010;32:229–37. doi: 10.1179/016164110X12644252260592. [DOI] [PubMed] [Google Scholar]
- 10.Salinas R, Counsell C, Prasad K, Gelband H, Garner P. Treating neurocysticercosis medically: A systematic review of randomized, controlled trials. Trop Med Int Health. 1999;4:713–8. doi: 10.1046/j.1365-3156.1999.00477.x. [DOI] [PubMed] [Google Scholar]
- 11.Carpio A, Santillán F, León P, Flores C, Hauser WA. Is the course of neurocysticercosis modified by treatment with antihelminthic agents? Arch Intern Med. 1995;155:1982–8. [PubMed] [Google Scholar]
- 12.Nash TE, Del Brutto OH, Butman JA, Corona T, Delgado-Escueta A, Duron RM, et al. Calcific neurocysticercosis and epileptogenesis. Neurology. 2004;62:1934–8. doi: 10.1212/01.wnl.0000129481.12067.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh G, Sachdev MS, Tirath A, Gupta AK, Avasthi G. Focal cortical-subcortical calcifications (FCSCs) and epilepsy in the Indian subcontinent. Epilepsia. 2000;41:718–26. doi: 10.1111/j.1528-1157.2000.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 14.Cukiert A, Puglia P, Scapolan HB, Vilela MM, Marino Júnior R. Congruence of the topography of intracranial calcifications and epileptic foci. Arq Neuropsiquiatr. 1994;52:289–94. doi: 10.1590/s0004-282x1994000300001. [DOI] [PubMed] [Google Scholar]
- 15.Del Brutto OH, Rajshekhar V, White AC, Jr, Tsang VC, Nash TE, Takayanagui OM, et al. Proposed diagnostic criteria for neurocysticercosis. Neurology. 2001;57:177–83. doi: 10.1212/wnl.57.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cantey PT, Coyle CM, Sorvillo FJ, Wilkins PP, Starr MC, Nash TE. Neglected parasitic infections in the United States: Cysticercosis. Am J Trop Med Hyg. 2014;90:805–9. doi: 10.4269/ajtmh.13-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]


