Abstract
Introduction:
Amebiasis is the third leading cause of death after malaria and schistosomiasis. Diagnosis is based on microscopy, culture, isoenzyme analysis, and serology-based techniques. In resource-limited nation such as India where polymerase chain reaction cannot be employed, serology is considered to be the reliable diagnostic tool. To find the seroprevalence of Entamoeba histolytica IgG antibody by enzyme-linked immunosorbent assay (ELISA) among the liver abscess cases and healthy controls.
Materials and Methods:
Commercially available RIDASCREEN Entamoeba IgG ELISA kit was used to evaluate the samples as per manufacturer's instruction.
Results:
A total of 322 samples were evaluated by ELISA. 94/157 (59.87%) were positive for amebic liver abscess cases, 2/13 (15.38%) were positive in suspected amebiasis group, 5/15 (33.3%) were positive in nonamoebic hepatic disorder group, 5/39 (12.8%) were positive in other parasitic disorders, and 2/98 (2.04%) were positive in presumed healthy controls. The sensitivity and specificity of the assay were found to be 56% and 92%, respectively.
Conclusion:
In an endemic nation such as India and other developing countries, ELISA can be used as a routine surveillance test in a clinical setup to detect amoebiasis if the cases are judicially evaluated along with the other routine tests.
Keywords: Amebiasis, Entamoeba histolytica, enzyme-linked immunosorbent assay, sensitivity, seroprevalence, specificity
INTRODUCTION
Amebiasis caused by the protozoa Entamoeba histolytica is the third leading parasitic cause of death worldwide, surpassed by malaria and schistosomiasis.[1,2] Globally, 50 million cases are reported with a significant number of deaths. The incidence of amebiasis is higher in developing countries, and 15–20% of Indians are affected by this parasite.[3,4] Currently, diagnosis of amebiasis is based on microscopy, culture, isoenzyme analysis, and serology-based techniques. In addition, nested and real-time polymerase chain reaction (RT-PCR) serves as confirmatory tests for its accurate diagnosis. Though PCR and isoenzyme analysis accurately distinguish the species, they are not practical for routine use in India where amebiasis is endemic.[5] The WHO has been emphasizing the need for the development of improved diagnostic methods specific for E. histolytica for use in the developing world.[6] Recently, RT-PCR has proven to be the most sensitive method; however, it is cumbersome for routine diagnosis because of the expensive equipment and technical expertise.[7] Therefore, in resource-limited nation such as India, where PCR cannot be routinely used, serology is recommended as the reliable diagnostic tool.[8] Antibodies are positive at the time of clinical presentation in 60–90% cases, with positive serology in endemic areas to be 5–10%. They also act as an adjunct with other tests and useful for epidemiological studies of amebiasis.[9] Thus, serological survey helps in determining the epidemiology of a disease since antibody profile in a population is a record of the present and past experience with the pathogen.[10,11] Hence, rapid serodiagnosis for suspected cases of amebiasis is often an important tool in clinical decision making and can be of help in the reduction of the costs of additional treatment and prolonged hospital stay.[12,3]
MATERIALS AND METHODS
Ethical clearance
Serum samples were collected from patients attending the Department of Medicine and Pediatrics, JIPMER during the period of 2011–2015. In total 170 subjects who were not given any treatment before collection of blood samples were included in the study. Ethical clearance from the Institute Human Ethics Committee was obtained (EC/2011/3/4 dated 03/08/2011). Informed consent was obtained from the subjects participated in the study.
Collection of serum samples
About 5 ml of venous blood was collected from diseased subjects as well as healthy controls. The blood sample was centrifuged at 2500 × g for 15 min. The supernatant containing the serum sample was collected and stored at −80°C until further use.
Patients were categorized into following groups:
Cases of amebic liver abscess (157)
Amoebic liver abscess (ALA) cases were diagnosed based on the following criteria: (i) Enlarged tender liver, febrile-associated toxemia, and abscess demonstrated on ultrasound; (ii) fever and pain in the epigastrium; (iii) bacteriologically sterile abscess aspirate; (v) improvement after treatment with an antiamoebic drug.
Suspected cases of amebic liver abscess and colitis (13)
Suspected ALA cases had enlarged palpable liver, toxemia, fever, and pain in the epigastrium. Ultrasonographically, no abscess was demonstrated, and no aspiration was made in any of these cases.
Cases of nonamoebic hepatic disorders (15)
This group comprised patients with alcoholic liver disease, hepatitis, jaundice, chronic liver disease, hepatocellular carcinoma, and cirrhosis.
Other parasitic diseases (39)
This group included other parasitic infections such as Filariasis (18), Hydatid disease (7), neurocysticercosis (4), toxoplasmosis (9), and malaria (1).
Presumed healthy controls (98)
These subjects aged between 20 and 45 years and their sex and profession matched those of the patients group. They had no recent history of fever, pain in epigastrium, diarrhea, and dysentery.
Serological evaluation
A commercially available enzyme-linked immunosorbent assay (ELISA) kit (RIDASCREEN E. histolytica IgG, R-Biopharm, Germany, K-1721) was used for qualitative determination of IgG antibodies of E. histolytica in human serum. The kit includes 96 well plate coated with purified antigens, protein A conjugate, tetramethylbenzidine chromogenic substrate, buffers, and control solutions.
The test was performed as per the manufacturer's recommendation. Briefly, all serum samples were diluted by sample diluent in 1:50 ratio before employing for the test. 100 μl of diluted samples added into the microtiter plate along with the controls, incubated at room temperature for 15 min, then 5 times washed with wash buffer. 100 μl of protein A conjugate was added, incubated for 15 min and washed five times with buffer. 100 μl of substrate was added, incubated for 15 min, to which 50 μl of stop solution was added. Optical density was measured at 450 nm (reference filter 620 nm).
Statistics
The Z-test (converted to P value) and unpaired Student's t-test were used to determine the significance of differences. Sensitivity was calculated as follows: Number of patients with positive test results/total number of patients ×100. Specificity was calculated as follows: Number of controls with negative test results/total number of controls ×100. The positive predictive value was calculated as follows: Number of true positives/(number of true positives + number of false positives) ×100. The negative predictive value was calculated as follows: Number of true negatives/(number of true negatives + number of false negatives) ×100. The positive predictive value defines the probability of patients having a disease if the test is positive. The negative predictive value defines the probability of patients not having amebiasis if the test is negative.
RESULTS
The sample index was interpreted by calculating the average absorbance of the negative control. 0.15 was added to the average absorbance which yielded the cutoff value for the test. Sample index was obtained by dividing the absorbance for the sample by the cutoff value.
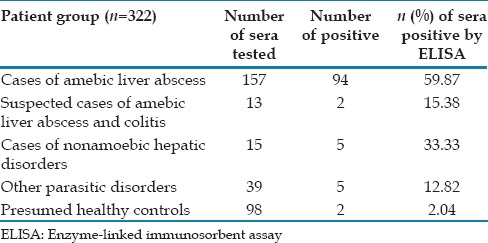
Out of 322 samples tested, 108 were positive and 214 were negative by RIDASCREEN IgG antibody ELISA. Among the cases of confirmed liver abscess, 94 (59.87%) were positive. In suspected liver abscess group, 2 (15.38%) out of 13 were positive. 5 (33.33%) out of 15 were positive among the cases of nonamoebic hepatic disorders while 5 (12.82%) out of 39 were positive in other parasitic infection group. Among the presumed healthy controls, 2 (2%) out of 98 were positive [Table 1].
Table 1.
Recognition of Entamoeba histolytica IgG antibody in different groups of patient sera
At 95% confidence interval, the overall sensitivity of RIDASCREEN E. histolytica IgG antibody ELISA was evaluated to be 56.47% while specificity to be around 92.11%. The positive predictive value was found to be 88.89% and negative predictive value 65.42%. The overall disease prevalence was calculated to be 52.8%.
DISCUSSION
The traditional way for diagnosis of amebiasis still depends on microscopy in laboratories where molecular techniques are still not employed. This technique is tedious, time-consuming, and requires a highly skillful technician. However, extraintestinal amebiasis is often characterized by the absence of cyst in stool. In such cases diagnosis mainly depends on the clinical picture. Hence, often cases are either misdiagnosed or missed out.[13] Therefore, in developing nation such as India, where molecular techniques cannot be used routinely, serological methods comes as an aid in the diagnosis of amebiasis.
Serological assays include indirect hemagglutination assay, Latex agglutination assay, complement fixation test, counterimmunoelectrophoresis, gel diffusion, indirect fluorescence assay, and ELISA. Among them, ELISA is considered to be one of the most popular diagnostic methods, and the kinetics of antibody response of E. histolytica has been well-elucidated in the recent past. It has been reported that sensitivity of detection to specific antibodies from serum in E. histolytica is 100%. Furthermore, in 95% cases of amebic colitis and ALA, serum IgG antibody was found to be present within 1 week after the onset of symptoms. Hence, ELISA has been used to study the epidemiology of asymptomatic and symptomatic amebiasis after fecal examination. It is also useful in the evaluation of intestinal and extraintestinal infections where amebiasis is suspected, but organism cannot be detected in feces.[14,15]
Many commercially available ELISA kits with varied sensitivity and specificity have been reported. In our study, RIDASCREEN Entamoeba IgG ELISA kit which has been reported with 97–100% sensitivity and 95–97% specificity has been employed. Our study reported 94 positive out of 157 in ALA group and 2 positive out of 13 suspected amebiasis group though most of the cases have been clinically proven to be ALA. In a previous study reported from JIPMER using TECHLAB ELISA kit, 29/45 (64.4%) cases were reported to be positive.[11] The reason for low detection rate of IgG antibody may be because samples have been collected from patient immediately after the onset of the disease. In control group of nonamoebic hepatic disorders 5/15, other parasitic disorders 5/39, and healthy control 2/98 were seropositive for E. histolytica IgG antibody. In an endemic country such as India, most of the people are exposed to Entamoeba infection and remain asymptomatic. Though some cases are positive in the control groups, there is a chance of these people being exposed to the infection. Though the kit has been reported to have very high sensitivity and specificity, our study reported to have sensitivity of 56% and specificity of 92%. Thus, ELISA can be used as a routine surveillance test in a clinical setup to detect amebiasis if the cases are judicially evaluated along with the other routine tests.
Financial support and sponsorship
The study was financially supported by JIPMER Intramural fund.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Fadeyi A, Nwabuisi C, Adegboro B. Antigen detection of E. histolytica intestinal infection: Cost-associated challenge in a resource poor country. Int J Health Res. 2009;2:171–5. [Google Scholar]
- 2.Fadeyi A, Nwabuisi C, Adegboro B, Akanbi AA, Fowotade A, Odimayo MS. Apparent rarity of E. histolytica and other intestinal parasites in acute and persistent diarrhoeic patients attending Ilorin hospitals: Time for ELISA antigen based amoebiasis diagnosis. European journal of scientific research. 2009;31:388–97. [Google Scholar]
- 3.Parija SC, Karki BM. Detection of circulating antigen in amoebic liver abscess by counter-current immunoelectrophoresis. J Med Microbiol. 1999;48:99–101. doi: 10.1099/00222615-48-1-99. [DOI] [PubMed] [Google Scholar]
- 4.Parija SC, Khairnar K. Entamoeba moshkovskii and Entamoeba dispar-associated infections in Pondicherry, India. J Health Popul Nutr. 2005;23:292–5. [PubMed] [Google Scholar]
- 5.Fotedar R, Stark D, Beebe N, Marriott D, Ellis J, Harkness J. Laboratory diagnostic techniques for Entamoeba species. Clin Microbiol Rev. 2007;20:511–32. doi: 10.1128/CMR.00004-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Wkly Epidemiol Rec. 1997;72:97–100. [Google Scholar]
- 7.Leo M, Haque R, Kabir M, Roy S, Lahlou RM, Mondal D, et al. Evaluation of Entamoeba histolytica antigen and antibody point-of-care tests for the rapid diagnosis of amebiasis. J Clin Microbiol. 2006;44:4569–71. doi: 10.1128/JCM.01979-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalkan IH, Dagli U. What is the most accurate method in the diagnosis of amebic dysentery? Turk J Gastroenterol. 2010;21:87–90. doi: 10.4318/tjg.2010.0062. [DOI] [PubMed] [Google Scholar]
- 9.Arianpour N, Mohapatra TM. Study of antiamoebic antibody in amoebiasis using ELISA and RID techniques. Iran J Public Health. 2003;32:13–8. [Google Scholar]
- 10.Khairnar K, Parija SC. A novel nested multiplex polymerase chain reaction (PCR) assay for differential detection of Entamoeba histolytica, E. moshkovskii and E. dispar DNA in stool samples. BMC Microbiol. 2007;7:47. doi: 10.1186/1471-2180-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parija SC, Khairnar K. Detection of excretory Entamoeba histolytica DNA in the urine, and detection of E. histolytica DNA and lectin antigen in the liver abscess pus for the diagnosis of amoebic liver abscess. BMC Microbiol. 2007;7:41. doi: 10.1186/1471-2180-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Doorn HR, Hofwegen H, Koelewijn R, Gilis H, Peek R, Wetsteyn JC, et al. Use of rapid dipstick and latex agglutination tests and enzyme-linked immunosorbent assay for serodiagnosis of amebic liver abscess, amebic colitis, and Entamoeba histolytica cyst passage. J Clin Microbiol. 2005;43:4801–6. doi: 10.1128/JCM.43.9.4801-4806.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanyuksel M, Petri WA., Jr Laboratory diagnosis of amebiasis. Clin Microbiol Rev. 2003;16:713–29. doi: 10.1128/CMR.16.4.713-729.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Punthuprapasa P, Thammapalerd N, Chularerk U, Charoenlarp K, Bhaibulaya M. Diagnosis of intestinal amebiasis using salivary IgA antibody detection. Southeast Asian J Trop Med Public Health. 2001;32(Suppl 2):159–64. [PubMed] [Google Scholar]
- 15.Sehgal R, Devi R, Singh K, Ganguly NK, Mahajan RC. Secretory IgA as a marker of invasive amoebiasis. Rev Infect. 2010;1:235–8. [Google Scholar]


