Abstract
Of the so-called nonpathogenic intestinal protozoa, Endolimax nana belongs to the ones least well described. Most data on E. nana have emerged from general surveys of intestinal parasites in selected cohorts and mostly in the absence of any particular focus on Endolimax. Hence, the genus of Endolimax remains largely unexplored in terms of morphology, taxonomy, genetic diversity, host specificity, and epidemiology. In this review, we seek to provide an overview of the work that has been performed on the parasite since the genus Endolimax was described by Kuenen and Swellengrebel in 1917 and suggest activities that may pave the way for a better understanding of E. nana in a clinical and public health context.
Keywords: Diagnosis, epidemiology, infectious diseases, protozoon, public health
INTRODUCTION
The genus Endolimax appears to consist of a large number of species based on its reported occurrence in a vast range of mammals, and it has moreover been described in birds, reptiles, and amphibians. Descriptions have been based on morphology and sometimes limited to identification of a cyst stage. Analyzed specimens have been recovered from stool samples or directly from the intestinal lumen if the animal was necropsied. Recently, an ameba closely related to Endolimax was also recovered from various tissues of a fish (Solea senegalensis).[1]
An overview of fundamental information such as host specificity, pathogenicity, and epidemiology is unavailable at present. A simple search for “Endolimax” in PubMed and Web of Science on the 26th of March 2015 identified 265 and 304 articles, respectively, with an overlap of 255. The vast majority of these articles were general surveys/prevalence studies focusing on intestinal parasites in general with no particular focus on Endolimax. Narrowing down the search to “Endolimax (title),” the number of articles decreased to 19 and 25 (overlap, 19), respectively. In reality, much more work than this has been carried out on Endolimax, but most of the literature is relatively old and might not be indexed in the aforementioned search engines; this could also be the reason why such articles are not cited in the more recent literature. In this study, older articles were identified mainly by backtracking using already available articles, and it turned out that Endolimax research has been carried out by many groups that have been debating issues that can be resolved with modern day technologies. The goal of this review is therefore to provide an overview of some of the work that has been performed on Endolimax nana since the genus Endolimax was described by Kuenen and Swellengrebel[2] in 1917 and the species E. nana by Wenyon and O’Connor[3] in 1917 and Brug[4] in 1918. Central topics such as morphology, taxonomy, host specificity, epidemiology, pathogenicity, diagnosis, and treatment are reviewed and discussed. Where applicable, emphasis is given on how previous discussions in the scientific community might be elucidated and resolved using state-of-the-art technology.
MORPHOLOGY AND LIFE CYCLE
E. nana inhabits the colon and has also been found in the appendix.[5,6,7] Trophozoites (8–10 μm) move by pseudopodia and may reach a size of up to 30 μm during locomotion. They feed exclusively on bacteria and divide by binary fission. The nucleus is vesicular and spherical, measuring 2.0–2.5 μm, with a polymorphic karyosome.[6,7,8,9] Before excystation, the trophozoite divides without growing, producing stages that are smaller but with nuclei of the same size. At first, the cyst contains one nucleus that divides twice by mitotic division. When mature, cysts of Endolimax are oval and very small (6–9 μm × 5–7 μm) compared with cysts of other intestinal amebae. The cyst wall appears thin (80 nm), colorless, and smooth on the outside. In the cytoplasm, no mitochondria, Golgi apparatus, rough endoplasmic reticulum, centrioles, or microtubules are present. Uniquely among intestinal amebae, E. nana has elongated tubular structures consisting of ribosome-like particles.[7,9,10] The cyst typically contains four nuclei, but it is possible that Endolimax may produce supernucleate cysts where up to four of the four nuclei perform an additional division, producing cysts containing 5–8 nuclei; this might, however, be a somewhat rare phenomenon.[7,9] Segal[11] argued that the nuclei in excess of four were in fact chromatoid bodies that might be the same as the elongated tubular structures mentioned above. The nucleus has a thin nuclear membrane with chromatin deposits and no pores.[7,9,10] The cysts are excreted in feces and may survive for up to 2 weeks when incubated at room temperature and for up to 2 months at lower temperatures; this, however, is under optimal conditions, and survival times are lower under natural settings such as in feces or water.[6,7] Trophozoites may survive in stool for up to 1 day when feces is incubated at room temperature.[6] After ingestion, the ameba excysts by escaping through a pore in the cyst wall, divides by successive cytoplasmic bipartition into uninucleate amebae, and turn into the trophic stage.
Infection may last for many years exemplified by the experimental infection that Dobell performed on himself, which had been lasting for 17 years at the time of his last publication on Endolimax.[7]
TAXONOMY
Silberman et al.[12] performed the first DNA-based study on E. nana and were able to obtain a complete sequence of the Endolimax SSU rRNA gene. It is noteworthy that this sequence is still the only available sequence of E. nana in the NCBI database, despite the fact that it has been 16 years since the sequence was deposited. Compared with other amoebozoa, the SSU rRNA gene of Endolimax is relatively long (more than 2500 bases), which is in part due to AT-rich expansion regions, with no evidence of introns.[12] In their phylogenetic analysis, Silberman et al.[12] placed Endolimax as a sister taxon to the Entamoeba assemblage. Cavalier-Smith et al.[13] later performed phylogenetic analyses with more sequences, including various Mastigamoeba sequences and placed Endolimax in the family Endolimacidae, which included Endolimax and Endamoeba. Sequencing the SSU rRNA gene of Iodamoeba, Stensvold et al.[14] found that it grouped together with Endolimax, but failed to establish monophyly for Iodamoeba. To clarify the phylogenetic position of Endolimax, there was a clear need for further studies on intrageneric diversity.[14,15] Recently, and mostly due to availability of more sequences from related organisms, Zadrobílková et al.[16] were able to obtain monophyly for both Endolimax and Iodamoeba.
SSU rRNA gene sequences were recently obtained from a new species identified in a sole that was named Endolimax piscium;[1] while these sequences did cluster specifically with E. nana, they were highly divergent.[16] However, there is still a need for additional sequences of both E. nana and Endolimax isolated from nonhuman hosts to investigate intrageneric diversity and further clarify the taxonomic status of the genus.
Meanwhile, it has proved challenging to obtain complete SSU rRNA genes from Endolimax. This is mostly due to the fact that general eukaryotic primers are prone to amplifying ribosomal genes that comprise fewer bases than that of Endolimax; for instance, Blastocystis has a SSU rRNA ribosomal gene of about 1800 bp, and since Blastocystis is very often present in stool samples positive for Endolimax, general primers tend to amplify Blastocystis preferentially over Endolimax when applied to genomic DNA extracted from stool. Moreover, sequences derived from Endolimax-positive polymerase chain reaction products often turn out to be more or less unreadable, probably for the same reasons as for Iodamoeba.[14] It might therefore prove useful to develop phylogenies based on other genes; preferably genes such as actin that are also likely to be conserved in Endolimax.
HOST SPECIFICITY
The host specificity of Endolimax has been debated in the older literature.[6,17,18] Wenrich[19] stated that Endolimax species cannot be differentiated based on morphology and pointed out the need for experimental infection studies to investigate the host specificity of Endolimax. Among such studies, Dobell[6] is the only person to date to have performed experimental infections on humans, namely on himself. He had examined his own feces for many years and found it to be negative for Endolimax before experimentally infecting himself with Endolimax from what was called a Macacus sinicus. The infection established, he was later able to infect a so-called M. rhesus with his own Endolimax isolate. Some believed in a limited host range and proposed new species names for the isolates of different animals [Table 1], whereas others[18] believed that E. nana was able to infect a wide range of hosts. Dobell[6] was more cautious in drawing conclusions before having more evidence. This confusion is also evident from the great number of Endolimax species described. Constenla et al.[1] provided an overview of the different Endolimax species named to date [Table 1]. Arguments have been made against some of these species names, including the possibility that some were in fact Iodamoeba as in the case of E. kueneni.[6] Moreover, morphological descriptions disqualify the species described as E. reynoldsi, which has cysts with only one nucleus,[19] and some species described as distinct may in fact be the same species, for instance E. nana, Endolimax gildemeisteri, and Endolimax cynomolgi.[6]
Table 1.
Species of Endolimax reported to date (modified from Constenla et al. 2014)

There might be a need to revise the taxonomy of Endolimax. We have recently observed extensive genetic variation in the SSU rRNA gene among human-derived Endolimax (unpublished data). The only complete SSU rRNA sequence of E. nana available in Genbank is from a monkey (Cercocebus albigena) isolate,[33] which clusters with only some of our unpublished human-derived sequences, indicating that there may be different ribosomal lineages infecting humans and nonhuman primates, as was already observed in the closely related genus of Iodamoeba.[14] The genetic diversity might also explain the differences in host specificity observed between research groups, as discussed above. Yarinsky and Burrows[34] observed general differences in the size of trophozoites between infected individuals suggesting the existence of at least two lineages of Endolimax based on this morphological characteristic. To investigate the host specificity and classification of Endolimax spp., DNA sequence data should be obtained from animal isolates as well as humans and analyzed by phylogenetic methods.
EPIDEMIOLOGY
Endolimax is transmitted through fecal-oral contamination of food or water.[35,36,37] Endolimax cysts have been observed in drinking water from deep wells,[38] on raw consumed vegetables,[39] and on banknotes, which have been suggested to be potential fomites.[40]
The scientific literature abounds with studies that have surveyed the prevalence of Endolimax in human stools samples. This can be explained by its inclusion in studies that investigate the prevalence of intestinal parasites in general, based on for instance microscopy of fecal concentrates. Due to the overwhelming number of studies, an overview of the prevalence, study groups, and methods have been included in Supplementary Table 1 (447.5KB, pdf) . We tried to estimate the global prevalence based on data from healthy individuals and including articles that were only 20 years old or less. These studies were mainly performed on schoolchildren, minority groups, or controls. By calculating weighted averages, we estimated the global prevalence in healthy individuals to be 13.4%, yielding 950,000,000 possibly infected individuals. Based on patient samples or reports in articles older than 20 years, the global prevalence is estimated to about 3.4% [Table 2]. The relatively high estimate in healthy individuals is mainly attributable to two articles from Africa where the prevalence of Endolimax was above 80%.[41,42] The inclusion of countries in Central America in the continent of North America probably leads to overestimation of the prevalence in this continent, the opposite potentially being true by the inclusion of the Middle East in Asia. In general, apparently most carriers of E. nana are found in Africa and South America, which comprise several developing countries. A relatively low prevalence is generally observed in studies from Asia, but the very low prevalence estimate in symptomatic patients compared with controls is mainly due to the inclusion of a large study carried out in Israel.[43] It is challenging to develop a precise estimate of the prevalence of Endolimax in Asia due to the limited amount of data from India and China, the two most populous countries in the world. It is expected that the prevalence be overestimated from articles available due to publication bias, since it is unlikely that E. nana is mentioned unless observed and recorded. Likewise, there are no studies to these authors’ knowledge where the prevalence of Endolimax is described as the primary focus of the article. On the other, it is expected that some studies have not included findings of Endolimax because it was considered unimportant in relation to the study aim. In addition, investigators may lack the skills to identify this parasite, including differentiating it from other amebas, as reported by Angel Núñez et al.,[44] leading to an underestimation of the prevalence of E. nana.
Table 2.
Estimated prevalence of Endolimax nana by continent and globally
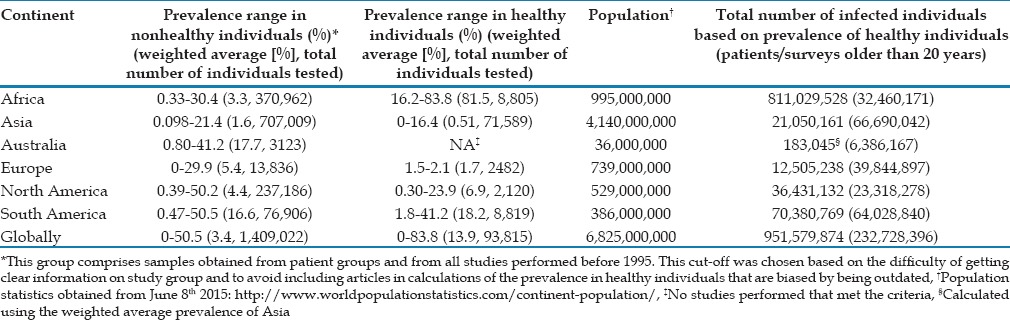
Summary of prevalence articles used to estimate the global prevalence
CLINICAL SIGNIFICANCE
Endolimax is considered a nonpathogenic commensal protozoon parasitizing the human colon;[6,9] this or a similar description is given in most textbooks.[37,45,46,47] The evidence supporting Endolimax as nonpathogenic is scarce, but in the study where Dobell[6] infected himself, the author did not experience any symptoms. Dobell[6] performed postmortem examination of infected monkeys and failed to discover any amebic lesions of the intestine. Some authors have argued that Endolimax can cause irritation of the crypts of the intestinal mucosa, referring to observations by Swerdlow and Burrows;[48] the empirical data to support such a statement are limited, however, since this report is on Dientamoeba fragilis and only one case was co-infected with Endolimax.
It is common to find reports on associations between diarrhea and Endolimax infections.[49,50,51,52] This association may at least in part be explained by Endolimax being an indicator of fecal contamination, which may often entail co-infection by other organisms capable of causing diarrhea. In a couple of case studies, Endolimax was associated with chronic diarrhea;[52,53,54] all cases responded well to treatment, and it was not possible to detect other infections except in the study by Shah et al.[52] where one case was co-infected with Blastocystis. It is possible that the cysts in the study published by Fitzgerald and O’Farrell[53] are not Endolimax cysts since they were described as having only one nucleus. Twelve cases were described in the study by Sanchez[55] who concluded that E. nana is possibly pathogenic.
There are also case studies that associate E. nana with urticaria[56] and polyarthritis.[57] Alarcón-Segovia and Abud-Mendoza[58] objected to the latter study, which was followed by a reply from Liakos and Burnstein[59] in the same journal issue. The objections included that no tests of reactive arthritis were performed, that Endolimax is presumably noninvasive, and that the treatment with metronidazole could eradicate other disease-causing organisms; in addition, no efforts were made to investigate whether any such organisms were present. The reply stated that testing did not reveal any other pathogenic organisms, but that such organisms could possibly be present. There is some evidence that Endolimax may give rise to an immunological response, including eosinophilia.[60,61] There are no known cases of Endolimax crossing the intestinal barrier in humans; however, E. piscium was recently described in a sole in both intestinal and nonintestinal tissue.[1]
The authors of this review are of the opinion that the sporadic articles on E. nana present too little evidence in favor of the assertion that Endolimax should be considered pathogenic with the ability to cause diarrhea or intestinal inflammation. The clinical picture may be subtle, however, and it has been suggested that symptoms may develop if a heavy infection is present[54] or that the pathogenicity might be limited to particularly virulent strains.[59]
DIAGNOSIS AND TREATMENT
The diagnosis of Endolimax traditionally relies on microscopy of cysts, which can be direct or coupled with a concentration procedure and different stains prior to analysis. Concentration can be formalin-based [Figure 1a], and when the fecal concentrate is stained with iodine, cysts of E. nana appear gibbous[7] [Figure 1b]. This gibbous appearance is however not always present and almost absent when cysts are concentrated using a sucrose gradient and stained with iodine [Figure 1c]. The cysts of Endolimax and E. hartmanni both have four nuclei but can be differentiated by E. nana having a larger punctuate karyosome and peripheral chromatin, both of which features however may be quite difficult to discern.[8,47] Endolimax stains with both Ziehl–Neelsen and trichrome. Cysts of E. nana are some of the smallest among those of the amebas, which is why it is recommended to use a microscope with at least ×400 magnification to avoid missing them but also in order to be able to distinguish them from E. hartmanni. A large number of cysts may be excreted compared with other amebae (Entamoeba coli, E. histolytica/E. dispar), with an estimate of about 8000 cysts/g, but with a few “heavy shedders.” It is possible that Endolimax is shed periodically.[62] Trophozoites are rarely observed, unless direct examination is performed on fresh stool samples [Figure 1d].
Figure 1.
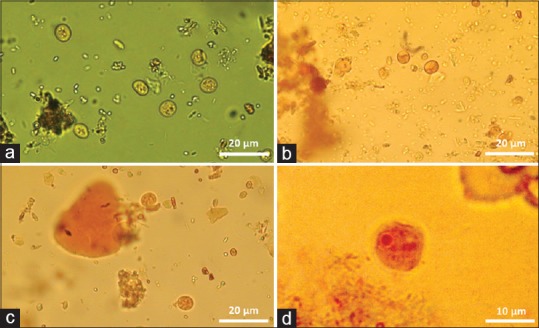
Cysts of Endolimax nana in direct smear (a), concentrated with formalin and ethyl acetate and stained with iodine showing the characteristic gibbous appearance (b), and isolated on a sucrose gradient and stained with iodine, respectively (c). Image (d) shows a Endolimax nana trophozoite
Based on the single SSU rRNA gene sequence in GenBank, in-house primers have been developed [Table 3] that have proved partially effective for diagnosing E. nana in genomic DNA extracted from fresh stool. It was from sequences generated using these primers that the high variation in the SSU rRNA genes mentioned previously was observed. Meanwhile, DNA from microscopy-positive samples have sometimes failed to show amplification with these primers. Due to the high variation in SSU rDNA (unpublished observations), designing genus-specific primers based on a single sequence or only a few sequences is problematic. There is a need for more reference sequences to develop better diagnostic primers that also eliminates selection for specific Endolimax strains. It is currently unknown whether the primers included in Table 3 will also amplify Endolimax from hosts other than humans.
Table 3.
In-house primers for detecting and characterizing Endolimax

Endolimax appears to respond well to both metronidazole and diphetarsone treatment. Stauffer and Levine[54] were able to treat two cases with metronidazole, although it appears that two courses of treatment were necessary in one of the cases. The same treatment with metronidazole was successful in a single case in the study by Burnstein and Liakos.[57] Based on these two studies, Graczyk et al.[51] recommend metronidazole for Endolimax treatment, administered as 250 mg 3 times a day for 10 days. In a study by Keystone et al.,[63] a 98% cure rate (n = 44) was observed with diphetarsone 500 mg 3 times a day for 10 days. In vitro studies have revealed little effect of streptomycin[64] and emetine[6] on Endolimax. Treatment of concurrent pathogenic parasites revealed little effect on Endolimax using emetine[6] or mebendazole.[35]
CONCLUSIONS
Based on available data, the global prevalence of E. nana in healthy individuals is estimated to be 13.9% on average, which, however, is probably an overestimation as discussed above; still, hundreds of millions are most likely infected. Very little research has been performed on Endolimax since the 1920s, 30s, and 40s. With the availability of DNA-based detection methods, resolving major issues such as host specificity, diversity, and which Endolimax species that can infect humans should be straightforward. In addition, the development of diagnostic primers will allow Endolimax to be detected with high sensitivity using fecal DNAs and distinguished easily from other amebae. The clinical significance of Endolimax is still an unresolved issue. Prior exposure (immunity), parasite load, and genetic variability might influence clinical presentation. Little evidence points toward Endolimax being pathogenic, but a few articles provide data on Endolimax-based stimulation of the immune system; whether this is a harmful or beneficial modulatory effect remains unknown. Hopefully, the present review will stimulate interest in Endolimax research, which may eventually render Endolimax a not so inconspicuous companion.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
Dr. Marianne Lebbad, The Swedish Agency of Public Health, and Dr. Maria Midgely, Liverpool School of Tropical Medicine and Hygiene, are both thanked for providing images.
REFERENCES
- 1.Constenla M, Padrós F, Palenzuela O. Endolimax piscium sp. nov. (Amoebozoa), causative agent of systemic granulomatous disease of cultured sole, Solea senegalensis Kaup. J Fish Dis. 2014;37:229–40. doi: 10.1111/jfd.12097. [DOI] [PubMed] [Google Scholar]
- 2.Kuenen W, Swellengrebel N. Korte beschrijving van enkelc minder bekendc protozoeo uit denmenschelijkcn darm. Geneesk. Tijdschr v Nederl. 1917:496. [Google Scholar]
- 3.Wenyon C, O’Connor F. London: J. Bale, Sons and Danielsson, Ltd; 1917. Human Intestinal Protozoa in the Near East. [Google Scholar]
- 4.Brug S. Batavia Geneesk: Tydsehr Ned Ind; 1918. Enkele opmerkingen over de nomenclatuur van cenige nieuwere parasieten uit den menschelyken darm; pp. 283–5. [Google Scholar]
- 5.Cerva L, Schrottenbaum M, Kliment V. Intestinal parasites: A study of human appendices. Folia Parasitol (Praha) 1991;38:5–9. [PubMed] [Google Scholar]
- 6.Dobell C. Researches on the intestinal protozoa of monkeys and man. V. The endolimax of macaques. Parasitology. 1933;25:436–67. [Google Scholar]
- 7.Dobell C. Researches on the intestinal protozoa of monkeys and man. XI. The cytology and life-history of endolimax nana. Parasitology. 1943;35:134–58. doi: 10.1017/s0031182000084225. [DOI] [PubMed] [Google Scholar]
- 8.Yarinsky A, Burrows R. Atypical nuclei of Endolimax nana in purged stool specimens. Am J Clin Pathol. 1966;46:490–3. [PubMed] [Google Scholar]
- 9.Dobell C. London: J. Bale and Danielsson; 1919. The Amoebae Living in Man: A Zoological Monograph. [Google Scholar]
- 10.Zaman V, Howe J, Ng M, Goh TK. Ultrastructure of the Endolimax nana cyst. Parasitol Res. 2000;86:54–6. doi: 10.1007/s004360050009. [DOI] [PubMed] [Google Scholar]
- 11.Segal B. Budding and other variations in Endolimax nana, a comparison with Councilmania tenuis Kofoid, 1928. Am J Hyg. 1932;15:741–52. [Google Scholar]
- 12.Silberman JD, Clark CG, Diamond LS, Sogin ML. Phylogeny of the genera Entamoeba and Endolimax as deduced from small-subunit ribosomal RNA sequences. Mol Biol Evol. 1999;16:1740–51. doi: 10.1093/oxfordjournals.molbev.a026086. [DOI] [PubMed] [Google Scholar]
- 13.Cavalier-Smith T, Chao E, Oates B. Molecular phylogeny of Amoebozoa and the evolutionary significance of the unikont Phalansterium. Eur J Protistol. 2004;40:21–48. [Google Scholar]
- 14.Stensvold CR, Lebbad M, Clark CG. Last of the human protists: The phylogeny and genetic diversity of Iodamoeba. Mol Biol Evol. 2012;29:39–42. doi: 10.1093/molbev/msr238. [DOI] [PubMed] [Google Scholar]
- 15.Ptácková E, Kostygov AY, Chistyakova LV, Falteisek L, Frolov AO, Patterson DJ, et al. Evolution of archamoebae: Morphological and molecular evidence for pelobionts including Rhizomastix, Entamoeba, Iodamoeba, and Endolimax. Protist. 2013;164:380–410. doi: 10.1016/j.protis.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Zadrobílková E, Walker G, Cepicka I. Morphological and molecular evidence support a close relationship between the free-living archamoebae Mastigella and Pelomyxa. Protist. 2015;166:14–41. doi: 10.1016/j.protis.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Hegner R. New York: The Century Co; 1927. Host-parasite Relations Between Man and His Intestinal Protozoa. [Google Scholar]
- 18.Kessel J. Host-parasite relationships of certain intestinal protozoa important to medical zoology. JAMA. 1928;90:1089–92. [Google Scholar]
- 19.Wenrich D. Host-parasite Relations Between Parasitic Protozoa and Their Hosts. American Philosophical Society: Proceedings of the American Philosophical Society. 1935:605–50. [Google Scholar]
- 20.McFall CM. Endolimax reynoldsi nov. sp. from the Intestine of the Common Swift, Sceloporus undulatus. J Parasitol. 1926;12:191–8. [Google Scholar]
- 21.Tyzzer EE. Amoebae of the caeca of the common fowl and of the Turkey.– Entamoeba gallinarum, SP. N. and Pygolimax gregariniformis, Gen. et Spec. Nov. J Med Res. 1920;41:199–210.1. [PMC free article] [PubMed] [Google Scholar]
- 22.Hegner RW. Endolimax caviae n. sp. from the Guinea-Pig and Endolimax janisae n. sp. from the Domestic Fowl. The American Society of Parasitologists. J Parasitol. 1926;12:146–7. [Google Scholar]
- 23.Kay MW. Two new amoebae from the box elder bug, Leptocoris trivittatus Say. Am Midl Nat. 1940;23:724–8. [Google Scholar]
- 24.Lucas CL. Two New Species of Amoeba found in Cockroaches: With notes on the cysts of Nyctotherus ovalis Leidy. Parasitology. 1927;19:223–35. [Google Scholar]
- 25.Momcilo I. Beiträge zur kenntnis der entwicklungsgeschichte einer im menschlichen enddarme lebenden Endolimax-amöbe (Endolimax gildemeisteri spec. nov.). Zentralblatt für Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene. 1936:377–98. [Google Scholar]
- 26.Brug S. Endolimax kueneni n. sp., Parasitic in the intestinal tract of the monkey Macacus cynomolgus. Parasitology. 1920;12:378–9. [Google Scholar]
- 27.De Mello IF, Amaral AD. Note on an ameba of the genus Endolimax, intestinal parasite of the peccary Tayassus tajacu. An Inst Med Trop (Lisb) 1952;8:615–9. [PubMed] [Google Scholar]
- 28.Simitch T, Chibalitch D, Petrovitch Z, Heneberg N. Contribution to the knowledge of the fauna of intestinal protozoa of Yugoslavian pigs. Their experimental identification. Arch Inst Pasteur Alger. 1959;37:401–8. [PubMed] [Google Scholar]
- 29.Chiang SF. The rat as a possible carrier of the dysentery amoeba. Proc Natl Acad Sci USA. 1925;11:239–46. doi: 10.1073/pnas.11.5.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirby H. Studies on some amoebae from the termite Mirptermes with notes on some other Protozoa from the Termitidae. Q J Microsc Sci. 1927;71:189–223. [Google Scholar]
- 31.Gutierrez-Ballesteros E, Wenrich DH. Endolimax clevelandi, n. sp. from turtles. J Parasitol. 1950;36:489–93. [PubMed] [Google Scholar]
- 32.Stark D, Phillips O, Peckett D, Munro U, Marriott D, Harkness J, et al. Gorillas are a host for Dientamoeba fragilis: An update on the life cycle and host distribution. Vet Parasitol. 2008;151:21–6. doi: 10.1016/j.vetpar.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Clark CG, Diamond LS. Intraspecific variation and phylogenetic relationships in the genus Entamoeba as revealed by riboprinting. J Eukaryot Microbiol. 1997;44:142–54. doi: 10.1111/j.1550-7408.1997.tb05951.x. [DOI] [PubMed] [Google Scholar]
- 34.Yarinsky A, Burrows R. Increase in number of Endolimax nana trophozoites with typical nuclei in serial samples of purged stool specimens. Am J Clin Pathol. 1966;46:494–5. [PubMed] [Google Scholar]
- 35.Hostetler LD. Microbiology problem. Am J Med Technol. 1981;47:328–9. [PubMed] [Google Scholar]
- 36.Sard BG, Navarro RT, Esteban Sanchis JG. Non-pathogenic intestinal amoebae: A clinical-analytical overview. Enferm Infecc Microbiol Clin. 2011;29(Suppl 3):20–8. doi: 10.1016/S0213-005X(11)70023-4. [DOI] [PubMed] [Google Scholar]
- 37.Garcia L, Bruckner D. 2nd ed. Washington, DC: ASM Press; 1993. Diagnostic Medical Parasitology. [Google Scholar]
- 38.Guillen A, Gonzalez M, Gallego L, Suarez B, Heredia H, Hernandez T, et al. Presence of intestinal protozoans in water of consumption in “18 de Mayo Community”. Aragua State-Venezuela. 2011. Bol Malariol Salud Ambient. 2013;53:29–36. [Google Scholar]
- 39.Monge R, Chinchilla M, Reyes L. Seasonality of parasites and intestinal bacteria in vegetables that are consumed raw in Costa Rica. Rev Biol Trop. 1996;44:369–75. [PubMed] [Google Scholar]
- 40.Moreno P, Perfetti D, Antequera I, Navas P, Acosta M. Contamination of banknotes with enteric parasites in Coro, Falcon state, Venezuela. Bol Malariol Salud Ambient. 2014;54:38–46. [Google Scholar]
- 41.Raso G, Utzinger J, Silué KD, Ouattara M, Yapi A, Toty A, et al. Disparities in parasitic infections, perceived ill health and access to health care among poorer and less poor schoolchildren of rural Côte d’Ivoire. Trop Med Int Health. 2005;10:42–57. doi: 10.1111/j.1365-3156.2004.01352.x. [DOI] [PubMed] [Google Scholar]
- 42.Ouattara M, Silué KD, N’Guéssan AN, Yapi A, Barbara M, Raso G, et al. Prevalence and polyparasitism of intestinal protozoa and spatial distribution of Entamoeba histolytica, E. dispar and Giardia intestinalis from pupils in the rural zone of Man in Côte d’Ivoire. Sante. 2008;18:215–22. [PubMed] [Google Scholar]
- 43.Ben-Shimol S, Sagi O, Greenberg D. Differences in prevalence of parasites in stool samples between three distinct ethnic pediatric populations in Southern Israel, 2007-2011. Parasitol Int. 2014;63:456–62. doi: 10.1016/j.parint.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 44.Angel Núñez F, Ginorio DE, Finlay CM. External quality assessment in coproparasitology in Havana City Province, Cuba. Cad Saude Publica. 1997;13:67–72. [PubMed] [Google Scholar]
- 45.Roberts L, Schmidt G, Janovy J, Gerald D. 8th ed. Boston: McGraw-Hill Higher Education; 2009. Schmidt and Larry S. Roberts’ Foundations of Parasitology. [Google Scholar]
- 46.Levine N. 1st ed. Minneapolis: Burgess Pub; 1961. Protozoan Parasites of Domestic Animals and of Man. [Google Scholar]
- 47.Ash LR, Orihel TC. 5th ed. Chicago: ASCP Press; 2007. Atlas of Human Parasitology. [Google Scholar]
- 48.Swerdlow MA, Burrows RB. Dientamoeba fragilis, an intestinal pathogen. J Am Med Assoc. 1955;158:176–8. doi: 10.1001/jama.1955.02960030026008. [DOI] [PubMed] [Google Scholar]
- 49.Peters C, Kocka F, Chittom A, Sable R, Janda W. High carriage of Endolimax-nana in diarrheal specimens from homosexual men. Lett Appl Microbiol. 1987;5:65–6. [Google Scholar]
- 50.Iqbal J, Hira PR, Al-Ali F, Philip R. Cryptosporidiosis in Kuwaiti children: Seasonality and endemicity. Clin Microbiol Infect. 2001;7:261–6. doi: 10.1046/j.1198-743x.2001.00254.x. [DOI] [PubMed] [Google Scholar]
- 51.Graczyk TK, Shiff CK, Tamang L, Munsaka F, Beitin AM, Moss WJ. The association of Blastocystis hominis and Endolimax nana with diarrheal stools in Zambian school-age children. Parasitol Res. 2005;98:38–43. doi: 10.1007/s00436-005-0003-0. [DOI] [PubMed] [Google Scholar]
- 52.Shah M, Tan CB, Rajan D, Ahmed S, Subramani K, Rizvon K, et al. Blastocystis hominis and Endolimax nana co-infection resulting in chronic diarrhea in an immunocompetent male. Case Rep Gastroenterol. 2012;6:358–64. doi: 10.1159/000339205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fitzgerald O, O’farrell TT. Chronic diarrhoea due to Endolimax nana infestation. Ir J Med Sci. 1954;346:467–8. doi: 10.1007/BF02952047. [DOI] [PubMed] [Google Scholar]
- 54.Stauffer JQ, Levine WL. Chronic diarrhea related to Endolimax nana: Response to treatment with metronidazole. Am J Dig Dis. 1974;19:59–63. doi: 10.1007/BF01073354. [DOI] [PubMed] [Google Scholar]
- 55.Salazar Sanchez A. Infestation with Endolimax nana; preliminary report. Rev Fac Med Univ Nac Colomb. 1957;25:214–8. [PubMed] [Google Scholar]
- 56.Veraldi S, Schianchi-Veraldi R, Gasparini G. Urticaria probably caused by Endolimax nana. Int J Dermatol. 1991;30:376. doi: 10.1111/j.1365-4362.1991.tb03884.x. [DOI] [PubMed] [Google Scholar]
- 57.Burnstein SL, Liakos S. Parasitic rheumatism presenting as rheumatoid arthritis. J Rheumatol. 1983;10:514–5. [PubMed] [Google Scholar]
- 58.Alarcón-Segovia D, Abud-Mendoza C. Parasitic rheumatism by Endolimax nana. Objections. J Rheumatol. 1985;12:184–5. [PubMed] [Google Scholar]
- 59.Liakos S, Burnstein S. Parasitic rheumatism by Endolimax-nana – Reply. J Rheumatol. 1985;12:184–5. [PubMed] [Google Scholar]
- 60.Cerva L, Kliment V. Contribution to the problem of the so-called nonpathogenic amoebae in the intestine of man. Folia Parasitol (Praha) 1978;25:367–70. [PubMed] [Google Scholar]
- 61.Yamaguchi N, Takeuchi T, Kobayashi S, Tanabe M, Miura S, Asami K, et al. Health status of Indochinese refugees in Japan: Statistical analyses on anemia, eosinophilia and serum alkaline phosphatase. Southeast Asian J Trop Med Public Health. 1984;15:209–16. [PubMed] [Google Scholar]
- 62.Garrido-Gonzalez E, Zurabian R, Acuna-Soto R. Cyst production and transmission of Entamoeba and Endolimax. Trans R Soc Trop Med Hyg. 2002;96:119–23. doi: 10.1016/s0035-9203(02)90275-0. [DOI] [PubMed] [Google Scholar]
- 63.Keystone JS, Proctor E, Glenn C, McIntyre L. Safety and efficacy of diphetarsone in the treatment of amoebiasis, non-pathogenic amoebiasis and trichuriasis. Trans R Soc Trop Med Hyg. 1983;77:84–6. doi: 10.1016/0035-9203(83)90022-6. [DOI] [PubMed] [Google Scholar]
- 64.Pfeiffer AV. Effect of streptomycin on Endolimax nana in vitro. J Parasitol. 1948;34:142–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of prevalence articles used to estimate the global prevalence


