Abstract
Introduction:
The objective was to compare antiplatelet effect of lycopene with aspirin and to study effect of combination of the two on platelet aggregation in vitro, using platelets from healthy volunteers.
Materials and Methods:
Platelets were harvested; platelet count of platelet-rich plasma adjusted to 2.5 Χ 105/μL. Aspirin (140 μmol/L) and lycopene (4, 6, 8, 10, and 12 μmol/L) were studied in vitro against adenosine-5’- diphosphate (ADP) (2.5 μM/L) and collagen
Results:
All the concentrations of lycopene (4–12 μmol/L) exhibited reduction in maximum platelet aggregation induced by aggregating agents ADP and collagen (P < 0.01 vs. vehicle) and were comparable with aspirin. Lycopene at concentration 10 μmol/L showed maximum platelet inhibition (47.05% ± 19.56%) against ADP, whereas lycopene at concentration 8 μmol/L showed maximum platelet inhibition (54.26% ± 30.71%) against collagen. Four μmol/L of lycopene combined with 140 μmol/L and 70 μmol/L aspirin showed greater inhibition of platelets as compared to aspirin 140 μmol/L alone, against both ADP and collagen.
Conclusion:
The study favorably compares lycopene and aspirin with respect to their antiplatelet activities against ADP and collagen. Lycopene can be considered as a potential target for modifying the thrombotic and pro-inflammatory events associated with platelet activation.
KEY WORDS: Adenosine-5’- diphosphate, antiplatelet, aspirin, collagen, lycopene, optical aggregometry
Introduction
Hemostasis is a finely regulated dynamic process of repairing vascular injury and limiting blood loss while avoiding vessel occlusion (thrombosis) and inadequate perfusion of vital organs. In normal hemostasis, the activation of platelets followed by coagulation prevents hemorrhage after injury and thereby preserves vascular integrity.[1] Platelet activation and coagulation normally do not occur in an intact blood vessel.[2] However, platelets play a key pathophysiological role in formation of a thrombus when vascular injury such as rupture of an atherosclerotic plaque occurs. This may lead to vascular occlusion with resultant hypoxia and infarction of distal tissues.[3] Therefore, inhibition of platelet function is a useful prophylactic and therapeutic strategy against myocardial infarction and stroke caused by thrombosis in coronary and cerebral arteries, respectively.[1]
The antiplatelet agents in current clinical use include the cyclooxygenase inhibitors (e.g., aspirin), phosphodiesterase inhibitors (e.g., dipyridamole), adenosine-5’-diphosphate (ADP) receptor pathway inhibitors (e.g., ticlopidine and clopidogrel), and platelet glycoprotein IIb/IIIa receptor antagonists (e.g., abciximab, tirofiban, and eptifibatide).[4] They have an established role in the treatment and prevention of thrombotic vascular diseases. They also have certain limitations. These include weak and incomplete inhibition of platelet function (aspirin), blockade of only one pathway of ADP-mediated signaling, slow onset of action (clopidogrel) and interpatient variability in antiplatelet response, and inability to transform the success of intravenous GPIIb-GPIIIa antagonist therapy into successful oral therapy. In addition, there is an inability to completely separate a reduction in thrombotic events from an increase in bleeding events with all of these agents.[1]
Lycopene, a red plant pigment, is found in number of fruits such as tomatoes, watermelon, papaya, and guava. Of these, tomatoes contribute the largest to the dietary intake of lycopene. Several studies have demonstrated the antiplatelet activity of tomato in vitro as well as the ability of tomato extracts to reduce ex vivo platelet aggregation.[5,6,7,8]
The antiplatelet activity of lycopene has not been compared to any of the clinically used antiplatelet agents until now. Moreover, the influence of lycopene on the action of routinely used antiplatelet agents has not been studied. Aspirin is the cornerstone of antiplatelet therapy and is the most commonly used antiplatelet agent for prophylaxis of thrombotic vascular disorders.[1] Hence, an in vitro study was designed to compare the antiplatelet effect of lycopene with aspirin. In addition, it was proposed to study the effect of combination of the two on platelet aggregation, using platelets from healthy volunteers.
Materials and Methods
Before the commencement of the study, approval from the Institutional Ethics Committee was obtained. The details of recruitment of healthy volunteers, the test drugs used in the study, and procedures for harvesting platelets, studying platelet viability, and aggregation are described.
Study Drugs
Test drug
Lycopene (≥98%) (Make: Extrasynthese, France) was purchased from Krishgen Biosystems, Mumbai. It was obtained in a powder form and was stored at −20°C. Lycopene was dissolved in 0.5% dimethyl sulfoxide (DMSO) and used for the study in following concentrations (in μmol/L) 4, 6, 8, 10, and 12. These concentrations were chosen as they had shown a concentration-dependent inhibition of platelet aggregation in a study conducted by Hsiao et al., 2005.[8]
Comparator
Acetylsalicylic acid (Aspirin) (Sigma Chemical Co., St. Loius, Mi, USA) was selected to compare the effect of lycopene on platelet aggregation. Aspirin was obtained in a powder form and stored at room-temperature. It was used in a concentration of 0.025 mg/ml (140 μmol/L). It was sparingly soluble in 0.5% DMSO. Hence, the solution was warmed at 80°C before use.
Vehicle
About 0.5% DMSO that was used to dissolve lycopene and aspirin served as our vehicle. Vehicle control was used in every part of the study.
Platelet aggregating agents
ADP (Sigma Chemical Co., St. Loius, Mi, USA) (2.5 μM/L) and Collagen (Chronolog Corporation, USA) (1 μg/ml) were used as inducers of platelet aggregation. These concentrations are found to cause maximum aggregation of platelet-rich plasma (PRP) (with platelet count adjusted to 2.5 × 105/μL) and have been standardized in our laboratory. ADP was stored at −20°C while collagen was stored at 4°C.
Required dilutions of all study drugs were prepared freshly before use.
Study Participants
The study was conducted on platelets harvested from healthy volunteers, after obtaining written informed consent. The presence of any disease or condition precluding participation in the study was ascertained on history, clinical examination, and laboratory evaluation. Twelve volunteers who were nonsmokers, nonalcoholics, with no history/family history of bleeding diathesis, not consuming drugs known to affect platelet function in the past 14 days, and having their hemogram, liver and renal function tests, and lipids within normal laboratory range were included in the study. Volunteers were asked to consume light diet on the preceding night to avoid lipid-rich food and diet devoid of tomato sauce, tomato ketchup, or tomato juice.
Procedure for Harvesting Platelets
Volunteers were asked to report to the laboratory after overnight fasting. Twenty-five milliliters of venous blood was collected atraumatically from these volunteers using aseptic precautions, in a citrated plastic tube (1 ml of 3.8% trisodium citrate for 9 ml of whole blood). The tubes were centrifuged at 800 rotations per min (rpm) for 8 min to obtain PRP. The PRP was carefully removed, placed in stoppered plastic tubes, and kept at room temperature. The remaining blood sample was re-centrifuged at 4000 rpm for 15 min to obtain platelet-poor plasma (PPP).
Platelets from PRP were counted using automated cell counter (Diatron Abacus Hematology Analyzer). The platelet count was adjusted to 2.5 × 105/μL using autologous PPP. The plasma with the adjusted platelet count was called as working PRP (WPRP). WPRP was used for the various parts of the study.
Study Procedures
The study was conducted in three parts as follows:
Part 1: Platelet viability
Platelet viability using lactate dehydrogenase (LDH) assay was tested in 6 of the 12 volunteers for all concentrations of lycopene as well as aspirin, 0.5% DMSO, and distilled water. Platelets contain LDH, which is released when the platelets lose their membrane permeability. This change in permeability occurs following platelet death. A significant rise in LDH release after incubation of WPRP with the study drugs for 3 min would be indicative of loss of platelet viability.[9] It was decided that any concentration of the study drugs demonstrating a significant rise in LDH release would be excluded from evaluation in further parts of the study. LDH levels were measured using commercial LDH kits, on a semi-autoanalyzer (ERBACHEM-5) at 340 nm.
Part 2: Effect of lycopene on agonist-induced platelet aggregation
Platelet aggregation was studied using the turbidimetric method described by Born in 1962 on a platelet aggrecorder (490-4D Four channel automatic optical aggregation system; Chronolog Corporation, USA).[10]
WPRP (440 μL) was prewarmed at 37°C for 5 min in siliconized glass cuvettes (provided by Chronolog Corporation, USA). Ten μL of either 0.5% DMSO, different concentrations of lycopene ([in μmol/L]-4, 6, 8, 10, 12) or aspirin (140 μmol/L) was added to these WPRP cuvettes and incubated for 3 min. Then, the WPRP were transferred to the reading wells. The autologous PPP served to adjust the transmission to 100%. Platelet aggregation inducers (ADP or collagen) were added to the WPRP cuvettes in a volume of 50 μL with continuous stirring using Teflon-coated stir bars. The optical curves were allowed to run for 7 min and 10 min for ADP and collagen, respectively. The aggregation pattern was recorded as percent aggregation versus time. The maximum platelet aggregation (MPA) was recorded as an optical curve on the aggregocorder (AGGRO/LINK-8 and Vw Cofactor Software packages). The percentage inhibition (PI) shown by aspirin and lycopene was calculated from the values of MPA using the formula:

Part 3: Effect of combination of lycopene with aspirin on agonist-induced platelet aggregation
Based on the results of part 2, two concentrations of lycopene were selected for Evaluation in part 3. The selected concentrations were studied for their antiplatelet activity in full and half the concentrations in combination with 70 μmol/L and 140 μmol/L of aspirin. The study procedure followed was the same as mentioned in part 2.
Two concentrations of lycopene, i.e., 8 μmol/L and 10 μmol/L, were selected to study their effect when used in combination with full concentration aspirin i.e., 140 μmol/L. In addition, full concentration of aspirin (140 μmol/L) was combined with half the concentration of lycopene, i.e., 4 μmol/L and 5 μmol/L. Similarly, all concentrations of lycopene (4, 5, 8, and 10 μmol/L) were used in combination with half concentration (70 μmol/L) of aspirin to find out the additive effect of these two agents on MPA and PI of aggregation.
Statistical Analysis
As the study was conducted as a pilot, it was done in 12 volunteers for convenience.
LDH release caused by different concentrations of lycopene and aspirin was expressed as mean ± standard deviation (SD) and was individually compared to that caused by the vehicle using paired t-test. MPA (for each of the aggregating agents) in the presence of the vehicle, aspirin, and different concentrations of lycopene was expressed as mean ± SD and was compared using repeated measures ANOVA, followed by post-hoc Dunnett Multiple Comparisons test. PI of aggregation (for each of the aggregating agents) in the presence of vehicle, aspirin, and different combinations of aspirin and lycopene was expressed as mean ± SD and was compared using repeated measures ANOVA, followed by post-hoc Dunnett Multiple Comparisons test. A P < 0.05 was considered as significant for all parameters. Analysis was done using software GraphPad InStat (version 3.06) GraphPad Software Inc.
Results
Part 1: Effect of Lycopene on Platelet Viability
The effect of various concentrations of lycopene on LDH release from platelets of the healthy volunteers included in the study is given in Table 1.
Table 1.
Effect of various concentrations of lycopene on lactate dehydrogenase release in healthy human volunteers
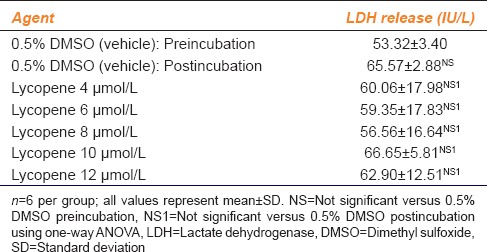
LDH release caused by the vehicle, i.e., 0.5% DMSO did not differ significantly after 3 min of incubation. In addition, when the WPRP was incubated with different concentrations of lycopene, no significant increase in LDH release was found as compared to the vehicle (P = 0.5372).
Part 2: Effect of Lycopene on Agonist-induced Platelet Aggregation and its Comparison with Aspirin
The MPA shown by various concentrations of lycopene in the presence of aggregating agents ADP and collagen are demonstrated in Figure 1a and b, respectively. It has been compared with the vehicle and aspirin.
Figure 1.
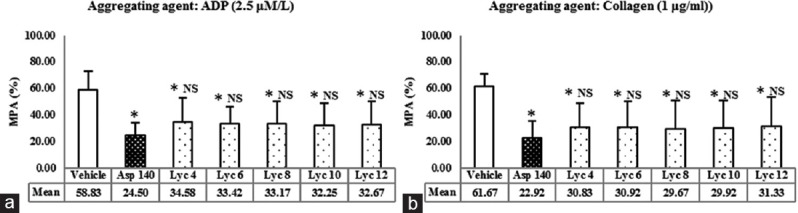
(a) Effect of lycopene on adenosine-5’-diphosphate-induced platelet aggregation. *P < 0.01 versus vehicle, NS = Not significant versus Asp 140, using one-way ANOVA followed by post-hoc Dunnett Multiple Comparisons Test. ADP: Adenosine diphosphate. (b) Effect of lycopene on collagen-induced platelet aggregation. Values represent the mean maximum platelet aggregation and error bars represent SD; n = 12 per group. Vehicle = 0.5% dimethyl sulfoxide; Asp 140 = Aspirin 140 μmol/L; Lyc 4, Lyc 6, Lyc 8, Lyc 10, and Lyc 12 corresponds to 4, 6, 8, 10, and 12 μmol/L of lycopene, respectively. MPA=Mean platelet aggregation
MPA caused by the vehicle was 58.83% ± 14.04% in the presence of ADP and 61.67% ± 9.59% in the presence of collagen. MPA caused by aspirin was 24.50% ± 9.41% in the presence of ADP and 22.92% ± 18.15% in the presence of collagen. Both the values were significantly lower as compared to the respective vehicle control (P < 0.01). MPA caused by all the concentrations of lycopene was also significantly lower as compared to the vehicle (P < 0.01) but was not significantly different from aspirin, in the presence of both ADP and collagen.
To compare the extent of antiplatelet activity of lycopene with that of aspirin, PI of platelets (PI) was calculated [Figure 2a and b]. Aspirin-induced platelet inhibition was 59.04% in the presence of ADP and 63.39% in the presence of collagen. The platelet inhibition caused by all the concentrations of lycopene was not significantly different from aspirin, in the presence of both ADP and collagen (P > 0.05).
Figure 2.
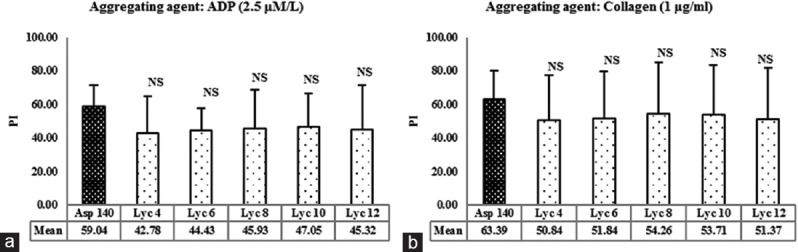
(a) Effect of lycopene on inhibition of platelet aggregation induced by adenosine-5’-diphosphate. NS = Not significant versus Asp 140, using one-way ANOVA followed by post-hoc Dunnett Multiple Comparisons Test. ADP: Adenosine diphosphate. (b) Effect of lycopene on inhibition of platelet aggregation induced by collagen. Values represent the mean percent inhibition of aggregation and error bars represent SD; n = 12 per group. Asp 140 = Aspirin 140 μmol/L; Lyc 4, Lyc 6, Lyc 8, Lyc 10 and Lyc 12 corresponds to 4, 6, 8, 10 and 12 μmol/L of lycopene, respectively. PI=Platelet inhibition’
Thus, all the concentrations of lycopene (4–12 μmol/L) tested for their effects on platelet aggregation exhibited reduction in MPA-induced by aggregating agents ADP and collagen. All the concentrations were comparable with aspirin in this respect.
Part 3: Effect of Combination of Lycopene with Aspirin on Agonist-induced Platelet Aggregation
Lycopene at concentration of 10 μmol/L showed 47.05% ±19.56% inhibition as the greatest against ADP, whereas the concentration of 8 μmol/L showed 54.26% ±30.71% inhibition as the greatest against collagen. Hence, these two concentrations were chosen for evaluation in combination with aspirin in this part of the study.
MPA and PI of platelets shown by the combination of various concentrations of lycopene with aspirin in the presence of aggregating agents ADP and collagen are demonstrated in Table 2a and b.
Table 2a.
Effect of combination of lycopene and aspirin on platelet aggregation
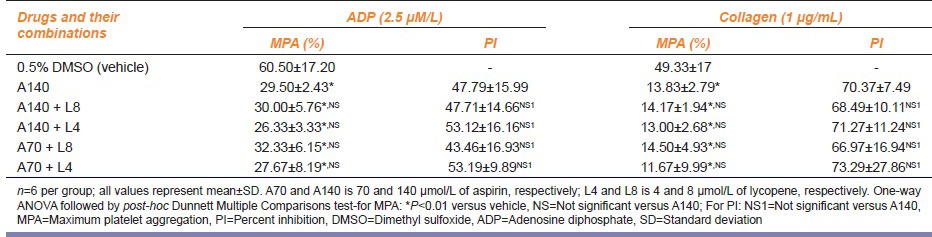
Table 2b.
Effect of combination of lycopene and aspirin on platelet aggregation
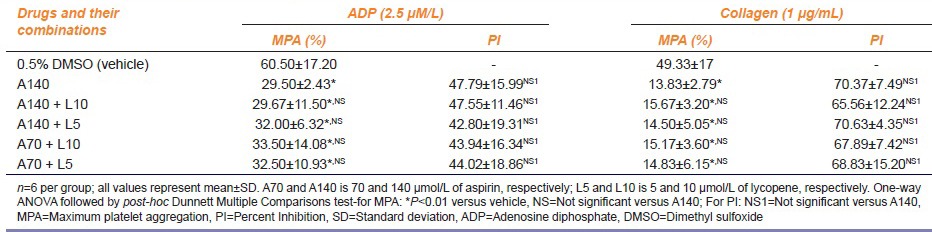
Thus, the different concentrations of lycopene and aspirin tested for their effects on platelet aggregation exhibited reduction in MPA-induced by aggregating agents ADP and collagen. This reduction in platelet aggregation was statistically significant as compared to that of the vehicle (P < 0.01). The effect of different concentrations of lycopene with respect to MPA as well as PI of platelets was comparable with aspirin 140 μmol/L (P > 0.05). The combination of lycopene in concentration of 4 μmol/L with aspirin 70 μmol/L showed the greatest inhibition of platelet aggregation namely 53.19% ± 9.89% and 73.29% ± 27.86% against ADP and collagen, respectively. This was similar to the PI shown by the combination of lycopene 4 μmol/L with aspirin 140 μmol/L namely 53.12% ± 16.16% and 71.27% ±11.24% against ADP and collagen, respectively. PI of platelets produced by aspirin 140 μmol/L alone was 47.79% ± 15.99% and 70.37% ± 7.49% against ADP and collagen, respectively.
Discussion
Aspirin in doses of 75–150 mg is the most commonly used antiplatelet agent and provides protection against occlusive, vascular events.[11] Aspirin reduces the odds of a serious arterial thrombotic event in high-risk patients by 25%. However, 10–20% of patients receiving secondary prophylaxis with aspirin have a recurrent arterial thrombotic event during long-term follow-up. The inability of aspirin to prevent an arterial thrombotic event is termed “aspirin resistance.”[12] Addition of clopidogrel to aspirin reduces the incidence of serious vascular events. However, the addition of dipyridamole has no further benefit and addition of glycoprotein IIb/IIIa antagonists over a long-term is impractical as they have to be administered intravenously.[11] Furthermore, even at the low doses used for antiplatelet activity, aspirin has gastrointestinal side effects notably gastric erosions and ulcer formation and gastrointestinal hemorrhage.[13]
Platelet function is a modifiable target for dietary interventions for primary prevention of cardiovascular disease. Dietary antiplatelets therefore can have a place in the treatment of heart diseases, along with antihypertensives and cholesterol-lowering drugs. Lycopene, present in tomato and tomato products, watermelon, papaya, and pink guava, is an example of a dietary antiplatelet which has exhibited antiplatelet activity in various studies.[5,6,7,8]
A study done by Hsiao et al. has demonstrated that lycopene at concentrations ranging from 4 μmol/L to 12 μmol/L inhibited human platelet aggregation induced by collagen, ADP, and arachidonic acid.[8] ADP and collagen are commonly used as agonists to induce platelet aggregation by light transmission aggregometry (LTA). LTA is a simple, reliable, and reproducible method to test platelet aggregation.[14] Hence, we employed this technique to test the in vitro antiplatelet activity of lycopene and compare it with that of aspirin.
The concentrations of lycopene used for the study were chosen in view of their antiplatelet effect demonstrated against agonists using LTA by Hsiao et al., where the antiplatelet effect was seen at lycopene concentrations from 4 to 12 μmol/L. Concentrations beyond 12 μmol/L showed no additional antiplatelet activity.[8] The concentration of aspirin of 0.025 mg/ml (140 μmol/L) selected for in vitro comparison corresponds to the levels achieved by low dose aspirin (75 mg) in the plasma. This was calculated as follows: It was assumed that following oral administration of 75 mg of aspirin (lowest recommended antiplatelet dose), it would get distributed into three compartments viz. Plasma (3 L), extracellular fluid (ECF) (12 L), and total body water (40 L). Concentrations of aspirin in these compartments were calculated namely 25 mg/L in plasma, 6.25 mg/L in ECF, and 1.875 mg/L in total body water, respectively. Furthermore, a study done by Stuart has shown that the maximum concentration of aspirin obtained even after administration of 300 mg of aspirin to healthy volunteers over 7 days is 0.03 mg/ml.[15] Hence, we decided to use the nearest calculated dose of 0.025 mg/ml.
In part 1 of the study, the platelet viability was studied in the presence of various concentrations of lycopene so as to ensure that the decrease in platelet aggregation which may be observed in part 2 and 3 of the study was not due to the death of the platelets at the selected concentrations of lycopene. It was observed that none of the concentrations of lycopene showed a significant change from the vehicle with respect to the LDH levels. Hence, all concentrations were considered to not hamper the platelet viability and were selected for further evaluation.
In part 2 of the study, MPA was studied using two aggregating agonists: ADP (2.5 μM/L) and collagen (1 μg/ml). To compare the antiplatelet activity of lycopene with aspirin, PI of aggregation caused by various concentrations of lycopene was calculated. Aspirin (140 μmol/L) significantly inhibited the MPA-induced by 2.5 μM/L of ADP and 1 μg/ml of collagen, when compared with the vehicle. This has been reported in literature and thus validates our assay system.[15] MPA and PI of lycopene were compared with that of the vehicle as well as aspirin.
Lycopene at concentrations of 4, 6, 8, 10, and 12 μmol/L significantly inhibited aggregation induced by both aggregating agents, as compared to vehicle control. Of these, the concentration of 10 μmol/L showed maximum antiplatelet activity against ADP and the concentration of 8 μmol/L showed maximum antiplatelet activity against collagen. A study done by Hsiao et al. in 2005 had demonstrated similar antiplatelet activity of lycopene (2–12 μmol/L) against ADP and collagen. The antiplatelet activity of lycopene was concentration dependent.[8] Literature search also revealed significant reductions in ex vivo platelet aggregation induced by ADP and collagen 3 h after supplementation of apparently healthy human volunteers with various doses of tomato extract, without affecting the clotting time variables, implying that supplementation with tomato extract may not result in a prolonged bleeding time under normal conditions.[6,7] However, as these extracts were prepared from ripe tomatoes and administered to the volunteers, we were unable to compare the results of these studies with the dose and effect observed in our study.
When the antiplatelet activity of various concentrations of lycopene was compared to that of aspirin, it was observed that though the antiplatelet activity was less than aspirin, there was no statistically significant difference between any of the concentrations of lycopene and aspirin. The concentrations of 8 μmol/L and 10 μmol/L of lycopene were chosen to study the effect of their combination with aspirin 140 μmol/L, as these concentrations had shown maximum inhibition of platelet activity against collagen and ADP, respectively. Moreover, it was of interest to study if both lycopene and aspirin combined at lower concentrations would exhibit an additive effect. Thus, when combinations of 8 μmol/L and 4 μmol/L with aspirin 140 μmol/L and 70 μmol/L were studied, it was found that the concentration of 4 μmol/L of lycopene combined with 140 μmol/L and 70 μmol/L aspirin showed marginally greater inhibition of platelets as compared to aspirin 140 μmol/L alone, against both ADP and collagen. The combination of 8 μmol/L of lycopene with aspirin 140 μmol/L and 70 μmol/L was similar to that of aspirin 140 μmol/L alone. When compared statistically, however, none of the groups were found to be different from each other. Furthermore, the effect of combinations of 10 μmol/L and 5 μmol/L with aspirin 140 μmol/L and 70 μmol/L was comparable to aspirin 140 μmol/L alone. These findings demonstrate that perhaps, the lower concentrations of lycopene potentiated the platelet inhibition caused by both 140 μmol/L and 70 μmol/L of aspirin. However, this synergistic effect was not seen at concentrations >4 μmol/L as the concentrations of 5, 8, and 10 μmol/L did not show additional inhibition of platelet aggregation in combination with any of the concentrations of aspirin against both ADP and collagen. In summary, 8 μmol/L and 10 μmol/L of lycopene had maximum antiplatelet effect and 4 μμmol/L of lycopene had maximal additive effect with aspirin.
It is the limitation of our study that we could not delve into the mechanism of antiplatelet activity. The exact mechanism of antiplatelet activity has not been elucidated. Tomatoes being the richest source of lycopene, studies done with tomato extracts, and evaluating the antiplatelet activity of lycopene have proposed various mechanisms for the platelet inhibition that is observed namely interaction with tissue factor, potentiation of nitric oxide pathway, interference with calcium flux, and cyclic guanosine monophosphate signaling.[6,7] The antiplatelet activity of lycopene and its potentiation of aspirin at low concentrations raise the possibility of an interaction between the two, as lycopene is a constituent of various dietary products. Whether individuals on secondary prophylaxis with aspirin should be advised to consume a low lycopene diet due to the potential bleeding risk is questionable as the comparative bioavailability of lycopene from different fruits and vegetables is unknown, as is the proportion of such food products in individual diet. In addition, it may have a potential use in patients with aspirin resistance syndrome, in whom the expected antiplatelet effects of aspirin are not observed and which accounts for 20–30% of aspirin-treated patients.[7]
Conclusion
This study favorably compares lycopene and aspirin with respect to their antiplatelet activities against aggregation agonists ADP and collagen implying that it may be interfering with the subsequent steps of platelet activation and aggregation. Lycopene therefore can be considered as a potential target for modifying the thrombotic and pro-inflammatory events associated with platelet activation. Further, investigations into the mechanism of antiplatelet activity of lycopene and its in vitro and in vivo effects on platelets of hyperlipidemic and hypertensive patients warrant research.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
Acknowledgment
We are grateful to the Research Society, Seth G.S. Medical College and K.E.M. Hospital, for providing funds to conduct this project.
References
- 1.Michelson AD. Advances in antiplatelet therapy. Hematology Am Soc Hematol Educ Program 2011. 2011:62–9. doi: 10.1182/asheducation-2011.1.62. [DOI] [PubMed] [Google Scholar]
- 2.Sangkuhl K, Shuldiner AR, Klein TE, Altman RB. Platelet aggregation pathway. Pharmacogenet Genomics. 2011;21:516–21. doi: 10.1097/FPC.0b013e3283406323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Born G, Patrono C. Antiplatelet drugs. Br J Pharmacol. 2006;147(Suppl 1):S241–51. doi: 10.1038/sj.bjp.0706401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weitz JI. Blood coagulation and anticoagulant, fibrinolytic and anti-platelet drugs. In: Brunton L, Chabner B, Knollman B, editors. Goodman and Gillman's the Pharmacologic Basis of Therapeutics. 12th ed. China: McGraw Hill; pp. 849–77. [Google Scholar]
- 5.Yamamoto J, Taka T, Yamada K, Ijiri Y, Murakami M, Hirata Y, et al. Tomatoes have natural anti-thrombotic effects. Br J Nutr. 2003;90:1031–8. doi: 10.1079/bjn2003994. [DOI] [PubMed] [Google Scholar]
- 6.O’Kennedy N, Crosbie L, van Lieshout M, Broom JI, Webb DJ, Duttaroy AK. Effects of antiplatelet components of tomato extract on platelet function in vitro and ex vivo: A time-course cannulation study in healthy humans. Am J Clin Nutr. 2006;84:570–9. doi: 10.1093/ajcn/84.3.570. [DOI] [PubMed] [Google Scholar]
- 7.O’Kennedy N, Crosbie L, Whelan S, Luther V, Horgan G, Broom JI, et al. Effects of tomato extract on platelet function: A double-blinded crossover study in healthy humans. Am J Clin Nutr. 2006;84:561–9. doi: 10.1093/ajcn/84.3.561. [DOI] [PubMed] [Google Scholar]
- 8.Hsiao G, Wang Y, Tzu NH, Fong TH, Shen MY, Lin KH, et al. Inhibitory effects of lycopene on in vitro platelet activation and in vivo prevention of thrombus formation. J Lab Clin Med. 2005;146:216–26. doi: 10.1016/j.lab.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 9.Snyder EL, Hezzey A, Katz AJ, Bock J. Occurrence of the release reaction during preparation and storage of platelet concentrates. Vox Sang. 1981;41:172–7. doi: 10.1111/j.1423-0410.1981.tb01032.x. [DOI] [PubMed] [Google Scholar]
- 10.Born GV. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962;194:927–9. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- 11.Antithrombotic Trialists’ Collaboration. Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michelson AD. Platelet function testing in cardiovascular diseases. Circulation. 2004;110:E489–93. doi: 10.1161/01.CIR.0000147228.29325.F9. [DOI] [PubMed] [Google Scholar]
- 13.Grosser T, Smyth EM, FitzGerald GA. Anti-inflammatory, anti-pyretic and analgesic agents; pharmacotherapy of gout. In: Brunton L, Chabner B, Knollman B, editors. Goodman and Gillman's the Pharmacologic Basis of Therapeutics. 12th ed. China: McGraw Hill; 2011. pp. 959–1004. [Google Scholar]
- 14.Harrison P, Frelinger AL, 3rd, Furman MI, Michelson AD. Measuring antiplatelet drug effects in the laboratory. Thromb Res. 2007;120:323–36. doi: 10.1016/j.thromres.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Stuart RK. Platelet function studies in human beings receiving 300 mg. of aspirin per day. J Lab Clin Med. 1970;75:463–71. [PubMed] [Google Scholar]


