Abstract
Metformin though primarily an antidiabetic drug, has found to play an important role in a number of cutaneous disorders. Because of its role in improving hyperinsulinemia, it has proven beneficial in hormonal acne, hidradenitis suppurativa (HS) and acanthosis nigricans. Its antiandrogenic properties further serve as an add-on to the conventional management of hirsutism associated with polycystic ovarian syndrome. Very recently, systemic usage of metformin for psoriasis and cutaneous malignancies has shown promising results. Interestingly, metformin has also been topically used in hyperpigmentary disorders with pertinent levels of improvement and happens to be the most recent addition to the list of dermatologic indications. Though an oral hypoglycemic agent to begin with, metformin today has proven to be a boon for dermatologists.
KEY WORDS: Hyperandrogenism, hyperinsulinemia, hyperpigmentary disorders, metformin, skin cancer
Introduction
(dimethylbiguanide) today is a widely used drug prescribed for diabetic patients. The history of metformin dates back to the usage of the herb Galega officinalis. This herb was found to be rich in a substance called guanidine with blood-glucose-lowering properties, which later was discovered to be the chemical basis of metformin. Though an antidiabetic drug to begin with, metformin has proven to be a drug of importance, in a number of cutaneous indications. This review will discuss the dermatologic perspective of metformin.
Pharmacokinetics
orally, metformin has a bioavailability of 40–60%. Within 6 h of drug intake, gastrointestinal absorption is complete. Gastrointestinal absorption of metformin is mediated by plasma membrane monoamine transporter.[1] Metformin does not undergo any metabolism, and it has a t1/2 of 5 h. Organic cation transporter (OCT) 1 and OCT3 facilitate hepatic uptake of metformin, whereas OCT2 plays a role in the uptake of metformin from the circulation, to renal epithelial cells.[2] Excretion of metformin occurs by active tubular secretion through the kidneys.
Applications in Dermatology
drug has shown efficacy and therapeutic applications in dermatological disorders too. A brief description of these follows:
Acanthosis Nigricans
Acanthosis nigricans (AN) is a common cutaneous condition characterized by dark, coarse and thickened skin with a velvety feel. It usually is symmetrical in distribution involving the neck, axilla, antecubital and popliteal fossa, groin folds, and rarely other sites such as face, eyelids, umbilical region, knuckles, palms, soles, nipple, and areola.[3] Recently, the association of benign AN with insulin resistance and hyperinsulinemia has been clearly established with obesity being a frequent accompaniment in these patients.[4] Further insulin levels in obese AN patients has been found to be significantly higher when compared to obese individuals who do not have AN.
Role of Metformin in Acanthosis Nigricans
The pathomechanics of AN is complex involving an interplay between various receptors and growth factors.[5,6,7] Metformin in AN brings its beneficial effects as depicted in Figure 1. Moreover, combination of metformin with thiozolidones[8] or glimepiride[9] could potentiate the effect of metformin, on AN, in cases not responding to metformin alone
Figure 1.
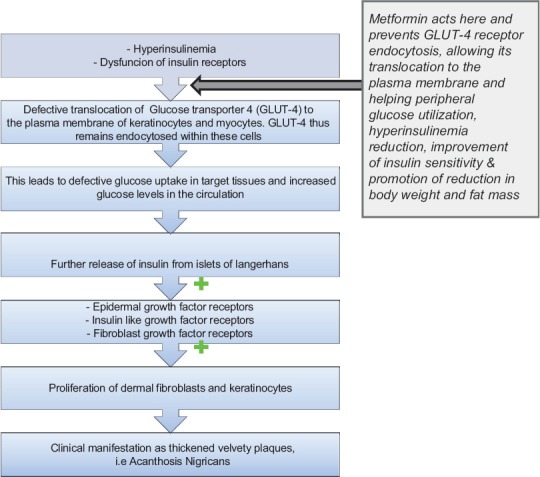
Mechanism demonstrating the antagonizing role of metformin in acanthosis nigricans
Figure 2.
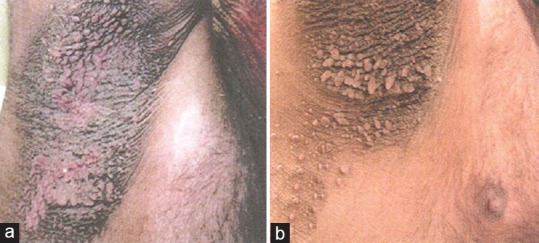
(a) Status of axillary acanthosis nigricans prior to starting therapy (b) Status of axillary acanthosis nigricans after the institution of combination therapy with metformin and tapering isotretinoin after 3 months. Considerable improvement can be visualized here. [Figure adapted from Walling HW et al. Improvement of acanthosis nigricans on isotretinoin and metformin. JDD 2003;2:677-81]
Figure 3.
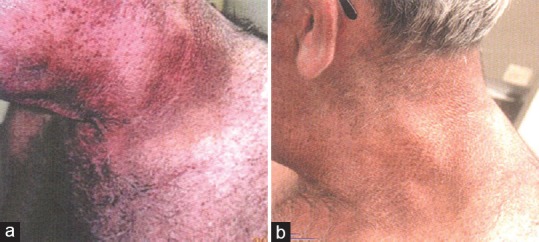
(a) Status of nuchal acanthosis nigricans prior to initiation of therapy. (b) Status of nuchal acanthosis nigricans after starting treatment with metformin and tapering isotretinoin after 3 months. Considerable improvement can be visualized here. [Figure adapted from Walling HW et al. Improvement of acanthosis nigricans on isotretinoin and metformin. JDD 2003;2:677-81]
Studies demonstrating the beneficial action of metformin in AN[5,10,11] have been summarized in Table 1.
Table 1.
Beneficial role of metformin in acanthosis nigricans - a review of published studies

To summarize, the pathogenesis of AN involves stimulation of receptors, belonging to tyrosine kinase family. Defect in glucose transporter 4 (GLUT 4) translocation to the plasma membrane of adipocytes and myocytes, further potentiate the activity of insulin-like growth factor receptors (IGF-R). Metformin improves hyperinsulinemia by its action on GLUT 4, therefore improving AN. In resistant cases of AN, combination therapy of thiozolidones or glimepiride with metformin would be worth trying.
Acne
Acne is an inflammatory disorder affecting the pilosebaceous follicles. Hyperandrogenism has been found to be a significant contributory factor in a multitude of acne patients. Increased levels of IGF-1 have been demonstrated in both males and females with acne. Furthermore, a direct correlation with reference to serum IGF-1 levels and mean facial sebum excretion rate has been observed in postadolescent individuals with acne.[12] In addition, serum levels of dehydrotestosterone and dehydroepiandrosterone sulfate correlated to serum levels of IGF-1. To elaborate further, in the human body IGF-1 is strongly expressed in sebocytes and suprabasal cells of the sebaceous ducts with IGF-R being voraciously expressed in all regions of sebaceous glands. As a result of this expression, there is stimulation of lipogenesis in sebaceous glands which potentiates the occurrence of acne.
Role of Metformin in Acne
Metformin has found to play a role, particularly in hormonal acne.[13,14,15] The exact mechanism of metformin for the same is illustrated in Figure 4.
Figure 4.
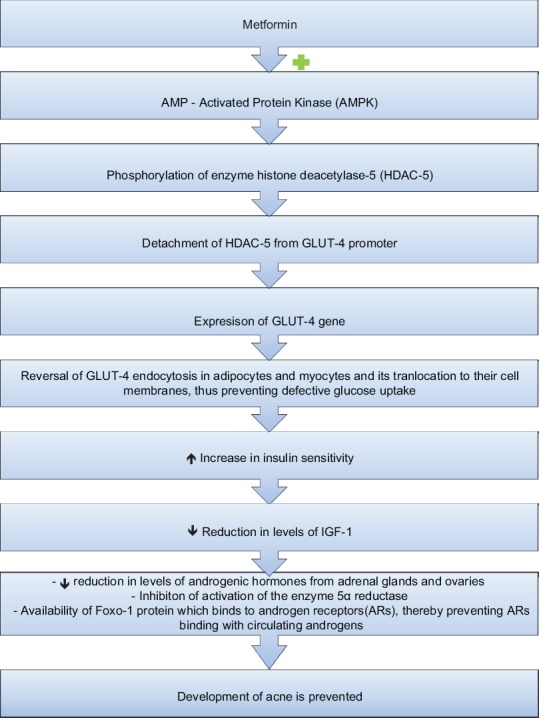
Mechanism of metformin in acne
A study conducted by Tan et al.[16] with 188 female patients suffering from polycystic ovary syndrome (PCOS) in Germany, wherein lean, overweight, and obese patients were treated with metformin in a dosage ranging from 500 to 1000 mg of metformin twice daily for a period of 6 months. At the end of the study, in all three groups, acne severity improved. Apart from this, there also was a favorable outcome with respect to menstrual irregularities, insulin resistance, and serum testosterone levels. Ibáñez et al.[17] in a randomized open labeled trial of 34 patients with hyperinsulinemic androgen excess conducted for a period of 6 months concluded that a low dose combination of metformin, pioglitazone, and flutamide, not only helped in reduction of acne, but also had beneficial effects in biochemical parameters such as hyperinsulinemia, hypercholesterolemia, and hypertriglyceridemia.
Disorders of Increased Pigmentation
Very recently, the role of metformin in treating hyperpigmentary disorders has been elaborated. Though, at present, these evidences are based on animal studies, very soon this anti-diabetic drug could be applied for this indication even in humans. Various mechanisms at the molecular level have been associated with this beneficial role of metformin and has been schematically represented in Figure 5.[18,20] Metformin acts on 3 melanogenic proteins namely tyrosinase, tyrosine-related protein (TRP)-1 and TRP-2 bringing about a reduction in their expression.[18] This is brought about by metformin initially reducing the levels of cyclic adenosine monophosphate, which antagonizes activation of protein kinase A. This, in turn, downregulates the expression of microphthalmia-associated transcription factor (MITF). MITF is an important transcription factor and has been referred to as the master gene for melanocyte survival. Therefore, when its activity is blocked, there is down-regulation in transcription of various melanogenic proteins such as tyrosinase, TRP-1, TRP-2, MART-1, and protein kinase C-beta (PKC-β).[19] Furthermore, metformin also inhibits the activity of PKC-β. Normally after getting activated by diacylglycerol (DAG), PKC-β anchors to melanosomes and phosphorylates tyrosinase, thus stimulating melanogenesis. Metformin inhibits this activation conferred by DAG to PKC-β.[20]
Figure 5.
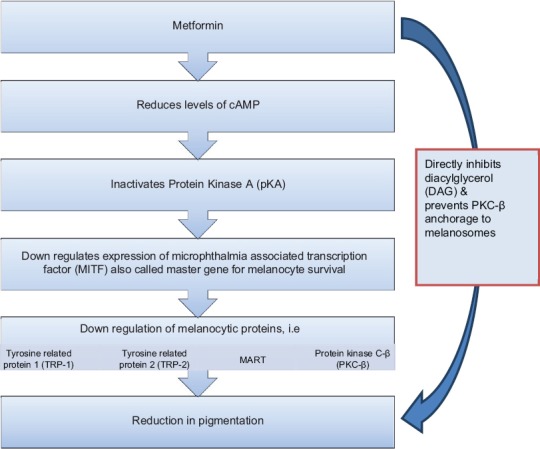
Mechanism of metformin in hyperpigmentary disorders
However, it has been only topical metformin that has demonstrated this activity and not the systemic administration of the drug. The topical preparation was obtained after crushing metformin tablets to prepare a 30% weight: Volume solution in a standard vehicle containing 70% alcohol and propylene glycol. This solution had then been applied on the tails of experimental animals for a period of 8 weeks. The topical formulation demonstrated the role of metformin for hyperpigmentary cutaneous conditions.[21]
Eruptive Xanthomas
Eruptive xanthomas (EXs) are subcutaneous lipid deposits consisting of small, yellow papules ranging from 2 to 5 mm in diameter. They are usually associated with type I, VI, and V dyslipidemia. Metformin in EXs acts by activating adenosine monophosphate-activated protein kinase (AMPK) in hepatocytes thereby reducing the activity of acetyl-CoA carboxylase thus heralding oxidation of fatty acids and reduction in expression of lipogenic enzymes, which is responsible for the beneficial activity of metformin in this indication. A successful report of resolution of EXs in a 65-year-old gentleman with type 2 diabetes on metformin has been reported [Figure 6].[22]
Figure 6.
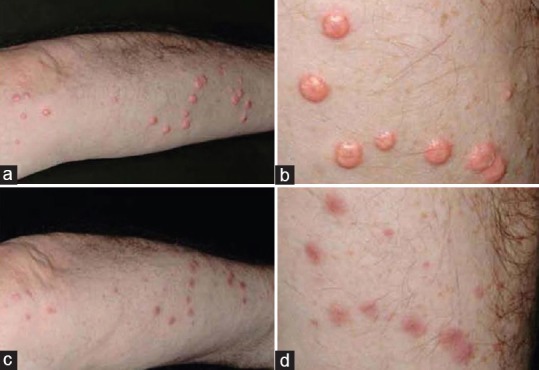
(a) Eruptive xanthomas of the right upper limb prior to treatment (b) close-up view of the same lesions prior to treatment (c) resolution of eruptive xanthomas with post inflammatory hyperpigmentation after 6 months of treatment with metformin and bezafibrate (d) close up view of the same lesions after therapy. [Figure adapted from Striet E, Helmbold P. 65 year old man with yellow orange papules on both forearms eruptive xanthomas. Hautarzt 2009;60:834-7]
Hidradenitis Suppurativa
HS, though earlier considered a disease of apocrine glands, has now been confirmed to be primarily a disorder of the pilosebaceous unit with an associated abnormality in hair structure, with apocrine gland involvement being a secondary aspect.[23] Predisposing genetic factors associated with HS heralds dilatation and distortion of the upper portion of the follicular infundibulum which is succeeded by occlusion and subsequent rupture, re-epithelialization, sinus tract formation, microbial colonization, pustulation, and fistula formation. HS has also shown to be associated with PCOS, and with the observation of decline in signs and symptoms following menopause, a relationship with regard to hormonal influences has been suggested. Further the presence of low glucose tolerance in many of these patients allows metformin to be considered as a potential treatment option in this scenario. Metformin in HS acts by a mechanism yet unknown. However, it has been postulated that metformin acts mainly by its anti-androgenic activity with a possible influence on the expression of genes involved in this condition. Second, metformin improves glucose utilization by increasing receptor sensitivity, thereby bringing about a reduction in insulin resistance.[24] Verdolini et al.[24] demonstrated the efficacy of metformin in a pilot study for treating recalcitrant HS [Figures 7 and 8]. In this study 22 females and 3 males were treated with metformin, starting with 500 mg once daily for the 1st week, and then 500 mg twice daily in the 2nd week, followed by 500 mg 3 times daily from the 3rd week onward. Treatment was continued for 24 weeks. Patients were evaluated based on the Dermatologic Life Quality Index (DLQI) and the Sartorius score at 0, 12, and 24 weeks. The average reduction of the Sartorius score at 12 weeks was 7.64 (P = 0.0055) and at 24 weeks was 12.78 (P = 0.0001), which was statistically significant. In 19 patients, DLQI improved with an average decrease in DLQI score of 7.6. In 16 of these patients, the improvement was significant (64%). In addition, depression which was earlier noted to be severe in 11 patients became nonsevere in 7 of the 11 patients. Arun and Loffeld[25] reported a case of HS with type 2 diabetes that responded exceedingly well to metformin. Here, the patient was started with metformin 500 mg once daily with the dose gradually being increased to 1 g/day in divided doses. After 4 months of treatment, the sinus tracts and leaking abscesses on the patient's left axilla showed marked resolution with no discharge [Figure 9]. Along with this, there was also considerable pain relief. A favorable tolerability profile of metformin was also witnessed in these reports.
Figure 7.
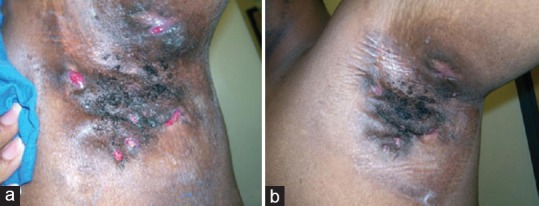
(a) Status of axillary hidradenitis suppurativa prior to starting therapy with metformin (b) status of axillary hidradenitis suppurativa 24 weeks after initiation of metformin therapy. To note here is that this patient had been recalcitrant to treatment with other antibiotics and after therapy with metformin noticed marked improvement. [Figure adapted from Verdolini et al. Metformin in the treatment of hidradenitis suppurativa: a little help along the way. JEADV 2013;27:1101-8]
Figure 8.
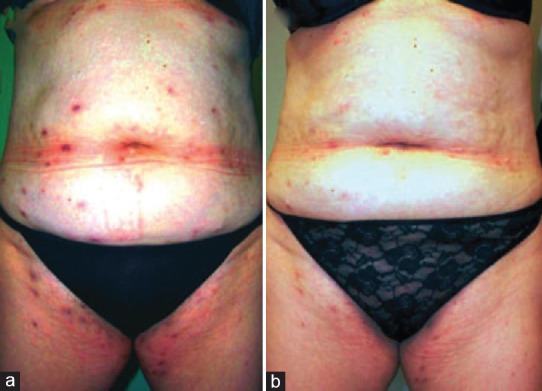
(a) Hidradenitis suppurativa of the groins and the abdominal area prior to treatment with metformin (b) similar patient 12 weeks after initiation of metformin therapy with considerable improvement. [Figure adapted from Verdolini et al. Metformin in the treatment of hidradenitis suppurativa: a little help along the way. JEADV 2013;27:1101-8]
Figure 9.
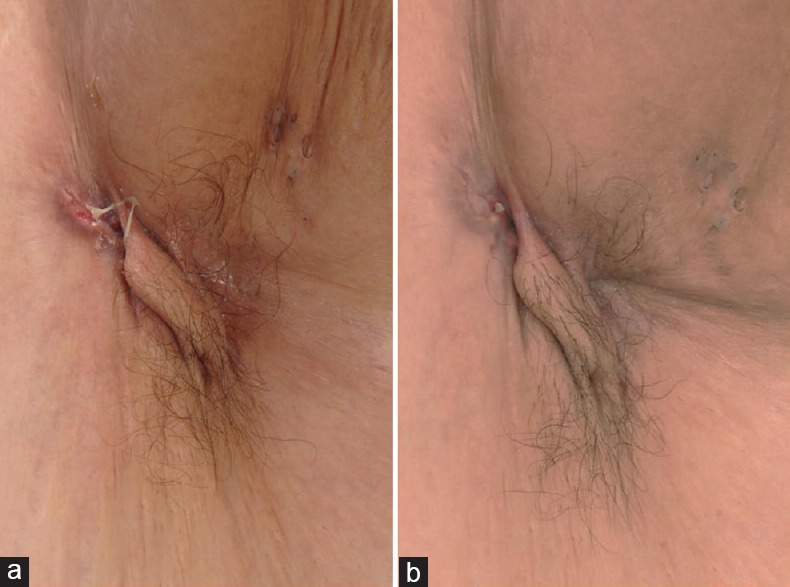
(a) Status of axillary hidradenitis suppurativa prior to starting therapy with metformin (b) Status of axillary hidradenitis suppurativa 4 months after commencement of treatment with metformin. To note here is the marked improvement in sinus tracts and leaking abscesses. [Figure adapted from Arun B, Loffeld A. Long standing hidradenitis suppurativa treated effectively with metformin. CED 2009;34:920-1]
Hirsutism
Hirsutism is defined as excessive growth of terminal hair in a male distribution on a woman's body. Specific sites included here are lips, chin, and chest. In approximately, 90% of hirsute females, there is an underlying PCOS or it may be idiopathic. It has been proposed that reduction in circulating insulin levels leads to a decrease in the concentration of free circulating levels of androgens, and, therefore, metformin may have a role in ameliorating hirsutism. There have been very few studies that have examined the influence of metformin on hirsutism as the primary end point. Kelly and Gordon[26] in a 14 months randomized, double-blind, placebo controlled crossover trial, demonstrated a modest reduction in the Ferriman–Gallwey (F-G) score at the end of treatment. In this study initially 500 mg of metformin was administered followed by a gradual increase of the drug over 3 weeks to 500 mg 3 times daily till the end of treatment. Ibáñez et al.[27] concluded that metformin therapy in girls aged 8–12 years with a combined history of low birthweight and precocious pubarche was effective in delaying development of androgen excess, oligomenorrhoea, PCOS and hirsutism than its late administration in girls aged 13–14 years. Finally, a randomized controlled trial of 70 patients with PCOS who received metformin along with intense pulse light (IPL) for hair removal when compared to IPL alone for 5 sittings over a period of 6 months, demonstrated the superiority of the regimen employing metformin in combination with IPL.[28] However, the role of metformin as a whole in hirsutism has been limited to only a few studies. Further, the F-G score prior to therapy in the studies published so far was only of a moderate grade. Therefore, ideally, how effective metformin would be in this regard as monotherapy is still questionable. More randomized studies in this aspect with a longer duration of therapy and comparison with other established therapies for the same is therefore mandated to prove this point. Currently, though, metformin should not be prioritized as a first line drug for hirsutism till more evidence is obtained in this regard.
Psoriasis
Psoriasis is a common, chronic inflammatory disease of the skin. However, now psoriasis has been recognized more as a systemic disorder. There have been many studies in recent years evaluating the role of metformin in psoriasis. Though, not very clear at the moment, the mechanism of metformin action in psoriasis has been suggested by various authors. Metformin acts on the enzyme AMPK and activates it. Once activated this enzyme has shown to demonstrate anti-inflammatory responses by down-regulating the activity of dendritic cells, T lymphocytes, macrophages, endothelial cells, and monocytes. Apart from this, metformin also has shown AMPK independent anti-inflammatory properties. By inhibiting Complex I (NADH-ubichinone-reductase) located in the inner mitochondrial membrane, metformin reduces the formation of reactive oxygen species and can therefore profoundly change T cell responses.[29,30,31] Further, in experimental studies, metformin has shown to ameliorate hepatic toxicity related to methotrexate[32] and, therefore, could serve as an add-on with methotrexate in managing psoriasis, making the usage of methotrexate safer. These benefits of metformin in psoriasis have been particularly demonstrated in patients with metabolic syndrome and/or reduced glucose tolerance.
Skin Cancer
Of late, the role of metformin for chemoprevention of skin cancer has been proposed.[33] There has been emerging evidence with regard to metformin as an antitumor agent for melanoma and squamous cell carcinoma (SCC).[34,35]
Squamous Cell Carcinoma
The role of metformin for chemoprevention of SCC is brought about by its stimulatory effect on AMPK, which inhibits the mechanistic target of rapamycin signaling pathway.[36] There has also been a recent study which demonstrated an AMPK independent role of metformin as an antitumor agent.[37] Furthermore, metformin induces apoptosis and increases Bax:Bcl2 ratio in SCC cell lines. Bax is a proapoptotic protein whereas Bcl2 is an antiapoptotic protein. Therefore, this enhancement stimulates apoptotic death of tumor cells.[38] Along with this, metformin also targets nuclear factor-kappa-beta pathway and modulates PI3K/AK+ and ERK/p38 microtubule-associated protein kinase signaling pathways, thus bringing about a decline in these pathways that are involved in bringing about cellular replication and survival. Currently, metformin has also found to play a role in inhibiting the growth of cancer stem cells and antagonizing the inflammation seen following cellular transformation in carcinoma breast.[39,40] To add to the beneficial properties of metformin in SCC, it has also shown to potentiate effects of standard chemotherapeutic agents such as doxorubicin, paclitaxel, and cisplatin in breast cancer xenografts.[41]
Melanoma
Metformin activates AMPK, which brings about anti-cancer signaling in melanoma, by stimulating the p53 tumor suppressor gene.[35] Further metformin represses transcription factors Snail and Slug, blocks epithelial-mesenchymal transition in melanoma and decreases the activity of matrix metalloproteinase, therefore promoting anti-invasive and antimetastatic effects. A recent study by Martin et al.[40] demonstrated that metformin by upregulating vascular endothelial growth factor (VEGF) could accelerate the growth of BRAFV600E mutated melanoma cells in vitro. Interestingly, when metformin was combined with VEGF inhibitors, the growth of these malignant cells was suppressed in vivo. Therefore, with this data, it could be prudent to conclude that combination therapy of BRAF mutant melanoma resistant to BRAF inhibitors, with metformin and VEGF antagonists, could be a valuable alternative.
Contraindications to Metformin
should be used with caution if serum creatinine is 150 μmols/l or higher, in patients with hypersensitivity to the drug, in conditions such as myocardial infarction or sepsis where tissue hypoxia is suspected. In case of administration of iodine-containing contrast media, metformin is to be withdrawn for 3 days and restarted only after normal renal function has been confirmed.[41]
Drug Interactions
Cationic drugs such as cimetidine, nifedipine, and frusemide that are eliminated by renal tubular secretion have a strong potential of interacting with metformin. They compete with OCT2 in the tubular epithelial cells and, therefore, may play a role in increasing the concentration of metformin in the circulation. Therefore, in patients simultaneously receiving these drugs, careful monitoring of patients, along with dose adjustments of metformin is warranted. These drugs if not appropriately monitored may increase the risk of lactic acidosis.[42]
Dosing and Monitoring
In adults, metformin is initiated in dose of 500 mg twice daily with an increment of 500 mg every week or 850 mg every other week to reach a maximum daily dose of 2550 mg. In children from the age of 10 to 16 years also, treatment is initiated with metformin 500 mg twice daily, which is increased with 500 mg of the drug every week to reach a maximum dose of 2000 mg/day in divided doses. Hemoglobin, hematocrit, red blood cell indices, and renal function tests need to be monitored periodically in all patients receiving metformin, at least once a year after the initial check-up.[43]
Adverse Effects
Follwing Adverse Effects of the Drug Have Been Reported
Cutaneous
There has been a report of bullous pemphigoid developing after metformin use in a patient who was also receiving gliptins. However, whether metformin was responsible for the dermatoses, in this case, was not clear.[44] Metformin-induced lichen planus had been elaborated in a case report by Azzam et al.[45] There have been several reports indicating metformin responsible for the development of leukocytoclastic vasculitis.[46]
Noncutaneous
These include flatulence, indigestion, myalgia, nausea, vomiting, diarrhea, heartburn, palpitations, lightheadedness, dyspnea, lactic acidosis, and flu-like symptoms.
Conclusion
Although primarily metformin plays a role in the management of type 2 diabetes mellitus, the drug demonstrates a therapeutic potential of various cutaneous disorders, especially those linked with hyperinsulinemia and hyperandrogenism. Very recently, there have been encouraging reports of metformin in cutaneous malignancies and hyperpigmentary disorders. Surprisingly, in disorders associated with increased pigmentation, in animal studies done metformin could only execute its antagonistic role in pigmentation as a topical preparation. Thus, metformin may have both systemic and topical utility in Dermatology.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Gong L, Goswami S, Giacomini KM, Altman RB, Klein TE. Metformin pathways: Pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2012;22:820–7. doi: 10.1097/FPC.0b013e3283559b22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50:81–98. doi: 10.2165/11534750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Phiske MM. An approach to acanthosis nigricans. Indian Dermatol Online J. 2014;5:239–49. doi: 10.4103/2229-5178.137765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hermanns-Lê T, Scheen A, Piérard GE. Acanthosis nigricans associated with insulin resistance: Pathophysiology and management. Am J Clin Dermatol. 2004;5:199–203. doi: 10.2165/00128071-200405030-00008. [DOI] [PubMed] [Google Scholar]
- 5.Walling HW, Messingham M, Myers LM, Mason CL, Strauss JS. Improvement of acanthosis nigricans on isotretinoin and metformin. J Drugs Dermatol. 2003;2:677–81. [PubMed] [Google Scholar]
- 6.Jensterle M, Janez A, Mlinar B, Marc J, Prezelj J, Pfeifer M. Impact of metformin and rosiglitazone treatment on glucose transporter 4 mRNA expression in women with polycystic ovary syndrome. Eur J Endocrinol. 2008;158:793–801. doi: 10.1530/EJE-07-0857. [DOI] [PubMed] [Google Scholar]
- 7.Atabek ME, Pirgon O. Use of metformin in obese adolescents with hyperinsulinemia: A 6-month, randomized, double-blind, placebo-controlled clinical trial. J Pediatr Endocrinol Metab. 2008;21:339–48. doi: 10.1515/jpem.2008.21.4.339. [DOI] [PubMed] [Google Scholar]
- 8.Barbato MT, Criado PR, Silva AK, Averbeck E, Guerine MB, Sá NB. Association of acanthosis nigricans and skin tags with insulin resistance. An Bras Dermatol. 2012;87:97–104. doi: 10.1590/s0365-05962012000100012. [DOI] [PubMed] [Google Scholar]
- 9.Bermudez-Pirila VJ, Cano C, Medina MT, Souki A, Lemus MA, Leal EM, et al. Metformin plus low dose glimepiride significantly improves homeostasis model assessment for insulin resistance (HOMA(IR)) and beta-cell function HOMA(beta-cell) without hyperinsulinemia in patients with type-2 diabetes mellitus. Am J Ther. 2007;14:194–202. doi: 10.1097/01.pap.0000249909.54047.0e. [DOI] [PubMed] [Google Scholar]
- 10.Bellot-Rojas P, Posades-Sanchez R, Carcas-Portilla N, Zamona-Gonzalez J, Cardoso-Saldana G, Jurado-Santacruz F, et al. Comparison of metformin versus rosiglitazone in patients with acanthosis nigricans: A pilot study. J Drugs Dermatol. 2006;5:884–9. [PubMed] [Google Scholar]
- 11.Hermanns-Lê T, Hermanns JF, Piérard GE. Juvenile acanthosis nigricans and insulin resistance. Pediatr Dermatol. 2002;19:12–4. doi: 10.1046/j.1525-1470.2002.00013.x. [DOI] [PubMed] [Google Scholar]
- 12.Vora S, Ovhal A, Jerajani H, Nair N, Chakrabortty A. Correlation of facial sebum to serum insulin-like growth factor-1 in patients with acne. Br J Dermatol. 2008;159:990–1. doi: 10.1111/j.1365-2133.2008.08764.x. [DOI] [PubMed] [Google Scholar]
- 13.Guercio G, Rivarola MA, Chaler E, Maceiras M, Belgorosky A. Relationship between the growth hormone/insulin-like growth factor-I axis, insulin sensitivity, and adrenal androgens in normal prepubertal and pubertal girls. J Clin Endocrinol Metab. 2003;88:1389–93. doi: 10.1210/jc.2002-020979. [DOI] [PubMed] [Google Scholar]
- 14.Fan W, Yanase Y, Morinaga H, Okabe T, Nomura M, Daitoku H, et al. Insulin like growth factor 1/insulin signalling activates androgen signalling through direct interactions of Foxo 1 with androgen receptor. J Biol Chem. 2007;282:7329–38. doi: 10.1074/jbc.M610447200. [DOI] [PubMed] [Google Scholar]
- 15.Karnilli E, Armoni M. Transcriptional regulation of the insulin responsive glucose transporter GLUT 4 gene: From physiology to pathology. Am J Physiol Endocrinol Metabol. 2008;295:E38–45. doi: 10.1152/ajpendo.90306.2008. [DOI] [PubMed] [Google Scholar]
- 16.Tan S, Hahn S, Benson S, Dietz T, Lahner H, Moeller LC, et al. Metformin improves polycystic ovary syndrome symptoms irrespective of pre-treatment insulin resistance. Eur J Endocrinol. 2007;157:669–76. doi: 10.1530/EJE-07-0294. [DOI] [PubMed] [Google Scholar]
- 17.Ibáñez L, Diaz M, Sebastiani G, Sánchez-Infantes D, Salvador C, Lopez-Bermejo A, et al. Treatment of androgen excess in adolescent girls: Ethinylestradiol-cyproteroneacetate versus low-dose pioglitazone-flutamide- metformin. J Clin Endocrinol Metab. 2011;96:3361–6. doi: 10.1210/jc.2011-1671. [DOI] [PubMed] [Google Scholar]
- 18.Belisle ES, Park HY. Metformin: A potential drug to treat hyperpigmentation disorders. J Invest Dermatol. 2014;134:2488–91. doi: 10.1038/jid.2014.245. [DOI] [PubMed] [Google Scholar]
- 19.Park HY, Lee J, González S, Middelkamp-Hup MA, Kapasi S, Peterson S, et al. Topical application of a protein kinase C inhibitor reduces skin and hair pigmentation. J Invest Dermatol. 2004;122:159–66. doi: 10.1046/j.0022-202X.2003.22134.x. [DOI] [PubMed] [Google Scholar]
- 20.Batchuluun B, Inoguchi T, Sonoda N, Sasaki S, Inoue T, Fujimura Y, et al. Metformin and liraglutide ameliorate high glucose-induced oxidative stress via inhibition of PKC-NAD(P) H oxidase pathway in human aortic endothelial cells. Atherosclerosis. 2014;232:156–64. doi: 10.1016/j.atherosclerosis.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 21.Lehraiki A, Abbe P, Cerezo M, Rouaud F, Regazzetti C, Chignon-Sicard B, et al. Inhibition of melanogenesis by the antidiabetic metformin. J Invest Dermatol. 2014;134:2589–97. doi: 10.1038/jid.2014.202. [DOI] [PubMed] [Google Scholar]
- 22.Streit E, Helmbold P. 65-year-old man with yellow-orange papules on both forearms. Eruptive xanthomas. Hautarzt. 2009;60:834–7. doi: 10.1007/s00105-009-1847-5. [DOI] [PubMed] [Google Scholar]
- 23.Danby FW, Jemec GB, Marsch WC, Von Laffert M. Preliminary findings suggest hidradenitis suppurativa maybe due to defective follicular support. Br J Dermatol. 2013;168:1034–9. doi: 10.1111/bjd.12233. [DOI] [PubMed] [Google Scholar]
- 24.Verdolini R, Clayton N, Smith A, Alwash N, Mannello B. Metformin for the treatment of hidradenitis suppurativa: A little help along the way. J Eur Acad Dermatol Venereol. 2013;27:1101–8. doi: 10.1111/j.1468-3083.2012.04668.x. [DOI] [PubMed] [Google Scholar]
- 25.Arun B, Loffeld A. Long-standing hidradenitis suppurativa treated effectively with metformin. Clin Exp Dermatol. 2009;34:920–1. doi: 10.1111/j.1365-2230.2008.03121.x. [DOI] [PubMed] [Google Scholar]
- 26.Kelly CJ, Gordon D. The effect of metformin on hirsutism in polycystic ovary syndrome. Eur J Endocrinol. 2002;147:217–21. doi: 10.1530/eje.0.1470217. [DOI] [PubMed] [Google Scholar]
- 27.Ibáñez L, López-Bermejo A, Díaz M, Marcos MV, de Zegher F. Early metformin therapy (age 8-12 years) in girls with precocious pubarche to reduce hirsutism, androgen excess, and oligomenorrhea in adolescence. J Clin Endocrinol Metab. 2011;96:E1262–7. doi: 10.1210/jc.2011-0555. [DOI] [PubMed] [Google Scholar]
- 28.Rezvanian H, Adibi N, Siavash M, Kachuei A, Shojaee-Moradie F, Asilian A. Increased insulin sensitivity by metformin enhances intense-pulsed-light-assisted hair removal in patients with polycystic ovary syndrome. Dermatology. 2009;218:231–6. doi: 10.1159/000187718. [DOI] [PubMed] [Google Scholar]
- 29.Glossmann H, Reider N. A marriage of two “Methusalem” drugs for the treatment of psoriasis. Arguments for a pilot trial with metformin as add-on for methotrexate? Dermatoendocrinol. 2013;5:252–63. doi: 10.4161/derm.23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaminski MM, Sauer SW, Klemke CD, Süss D, Okun JG, Krammer PH, et al. Mitochondrial reactive oxygen species control T cell activation by regulating IL-2 and IL-4 expression: Mechanism of ciprofloxacin-mediated immunosuppression. J Immunol. 2010;184:4827–41. doi: 10.4049/jimmunol.0901662. [DOI] [PubMed] [Google Scholar]
- 31.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–23. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hadi NR, Al-Amran FG, Swadi A. Metformin ameliorates methotrexate-induced hepatotoxicity. J Pharmacol Pharmacother. 2012;3:248–53. doi: 10.4103/0976-500X.99426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reddi A, Powers MA, Dellavalle RP. Therapeutic potential of the anti-diabetic agent metformin in targeting the skin cancer stem cell diaspora. Exp Dermatol. 2014;23:345–6. doi: 10.1111/exd.12349. [DOI] [PubMed] [Google Scholar]
- 34.Sikka A, Kaur M, Agarwal C, Deep G, Agarwal R. Metformin suppresses growth of human head and neck squamous cell carcinoma via global inhibition of protein translation. Cell Cycle. 2012;11:1374–82. doi: 10.4161/cc.19798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cerezo M, Tichet M, Abbe P, Ohanna M, Lehraiki A, Rouaud F, et al. Metformin blocks melanoma invasion and metastasis development in AMPK/p53-dependent manner. Mol Cancer Ther. 2013;12:1605–15. doi: 10.1158/1535-7163.MCT-12-1226-T. [DOI] [PubMed] [Google Scholar]
- 36.Chaudhary SC, Kurundkar D, Elmets CA, Kopelovich L, Athar M. Metformin, an antidiabetic agent reduces growth of cutaneous squamous cell carcinoma by targeting mTOR signaling pathway. Photochem Photobiol. 2012;88:1149–56. doi: 10.1111/j.1751-1097.2012.01165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iliopoulos D, Hirsch HA, Wang G, Struhl K. Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via IL6 secretion. Proc Natl Acad Sci U S A. 2011;108:1397–402. doi: 10.1073/pnas.1018898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirsch HA, Iliopoulos D, Struhl K. Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth. Proc Natl Acad Sci U S A. 2013;110:972–7. doi: 10.1073/pnas.1221055110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iliopoulos D, Hirsch HA, Struhl K. Metformin decreases the dose of chemotherapy for prolonging tumor remission in mouse xenografts involving multiple cancer cell types. Cancer Res. 2011;71:3196–201. doi: 10.1158/0008-5472.CAN-10-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin MJ, Hayward R, Viros A, Marais R. Metformin accelerates the growth of BRAF V600E-driven melanoma by upregulating VEGF-A. Cancer Discov. 2012;2:344–55. doi: 10.1158/2159-8290.CD-11-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones GC, Macklin JP, Alexander WD. Contraindications to the use of metformin. BMJ. 2003;326:4–5. doi: 10.1136/bmj.326.7379.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Powers AC, D’Alessio D. Endocrine pancreas and pharmacotherapy of diabetes mellitus and hypoglycaemia. In: Brunton L, Chabner B, Knollman B, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 12th ed. New York: McGraw Hill; 2011. pp. 1237–73. [Google Scholar]
- 43.Scarpello JH, Howlett HC. Metformin therapy and clinical uses. Diab Vasc Dis Res. 2008;5:157–67. doi: 10.3132/dvdr.2008.027. [DOI] [PubMed] [Google Scholar]
- 44.Skandalis K, Spirova M, Gaitanis G, Tsartsarakis A, Bassukas ID. Drug-induced bullous pemphigoid in diabetes mellitus patients receiving dipeptidyl peptidase-IV inhibitors plus metformin. J Eur Acad Dermatol Venereol. 2012;26:249–53. doi: 10.1111/j.1468-3083.2011.04062.x. [DOI] [PubMed] [Google Scholar]
- 45.Azzam H, Bergman R, Friedman-Birnbaum R. Lichen planus associated with metformin therapy. Dermatology. 1997;194:376. doi: 10.1159/000246152. [DOI] [PubMed] [Google Scholar]
- 46.Czarnowicki T, Ramot Y, Ingber A, Maly A, Horev L. Metformin-induced leukocytoclastic vasculitis: A case report. Am J Clin Dermatol. 2012;13:61–3. doi: 10.2165/11593230-000000000-00000. [DOI] [PubMed] [Google Scholar]


